Enhancement in Electrical and Thermal Properties of LDPE with Al2O3 and h-BN as Nanofiller
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Analytical Methods
3. Results and Discussion
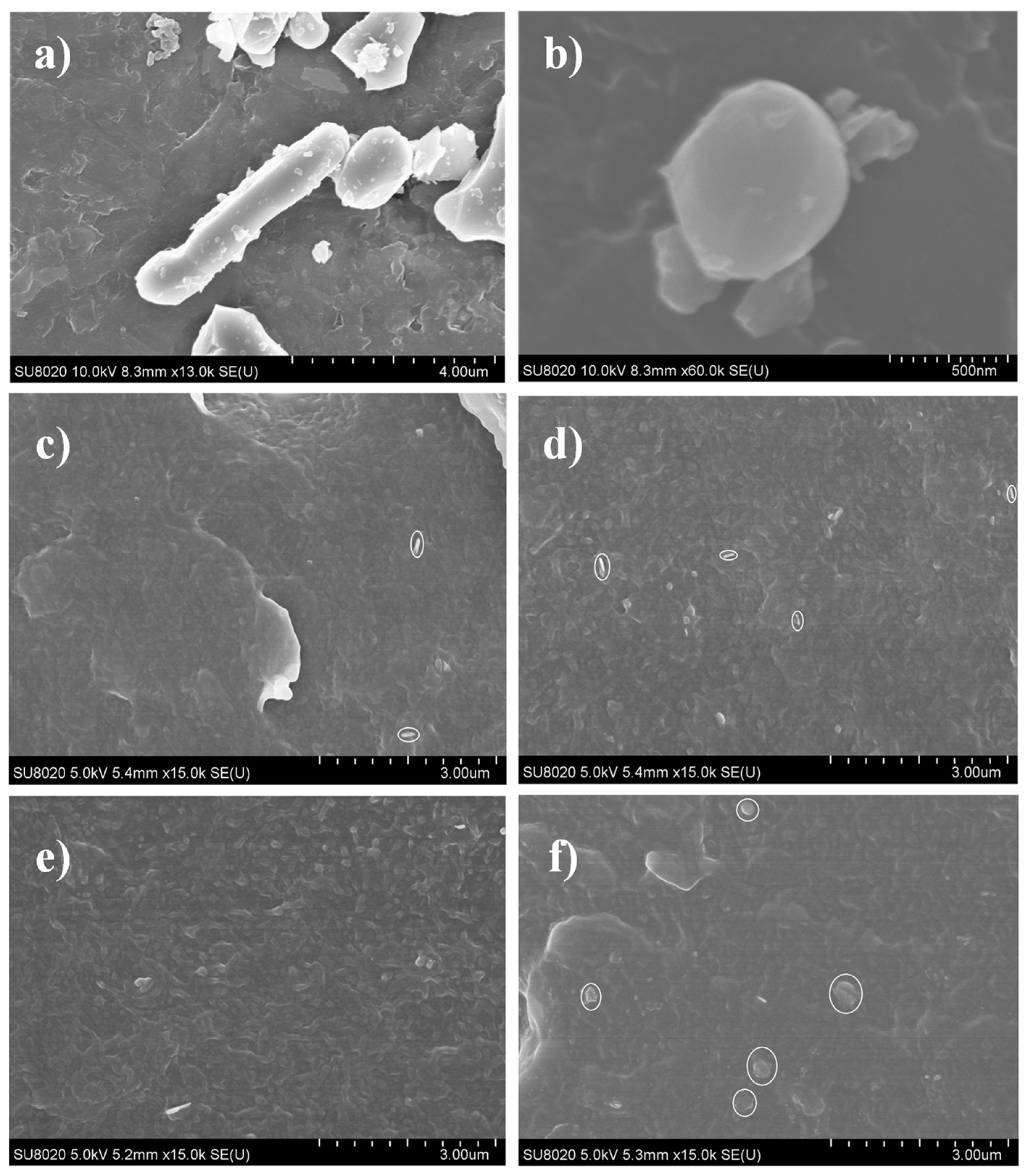
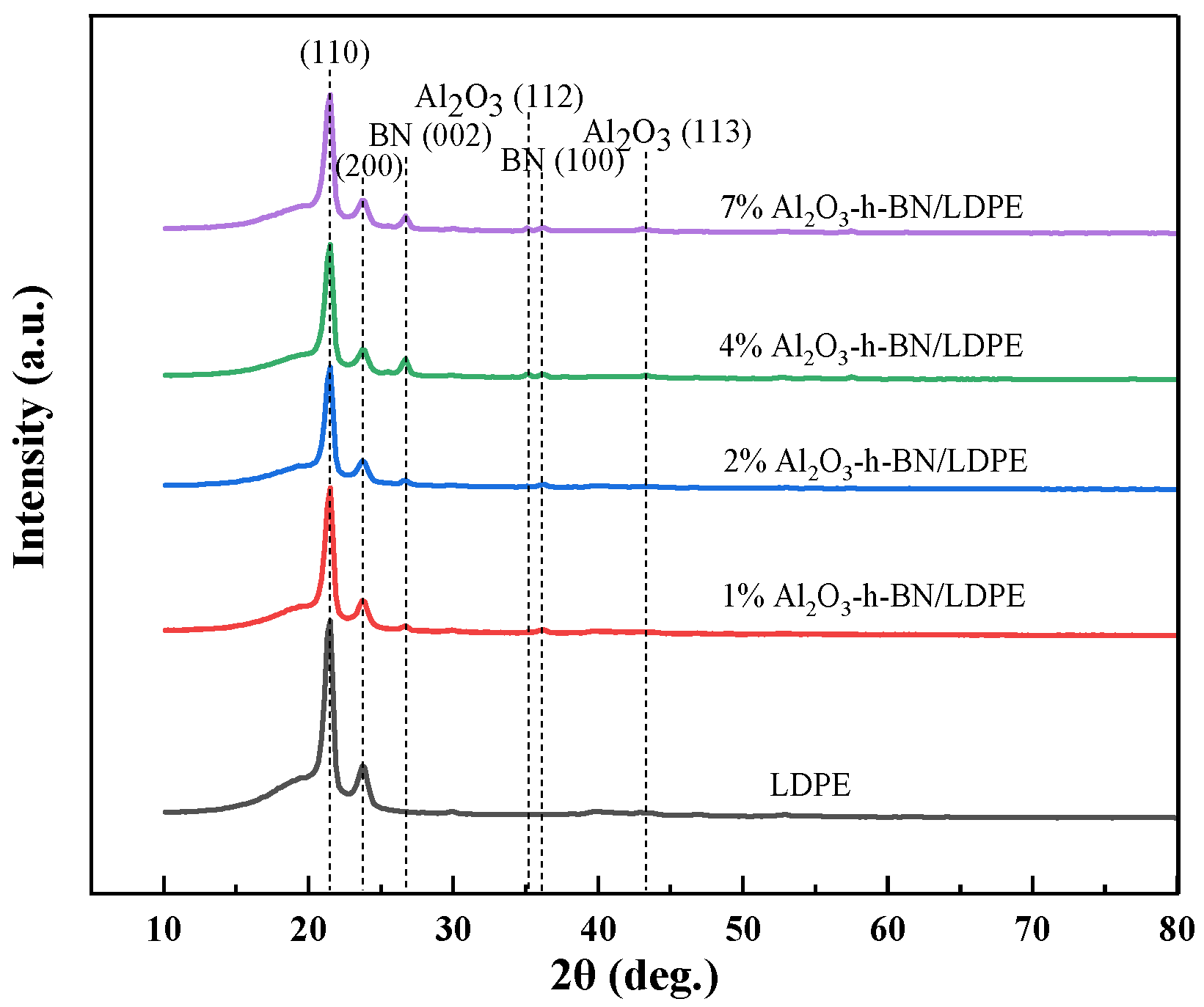
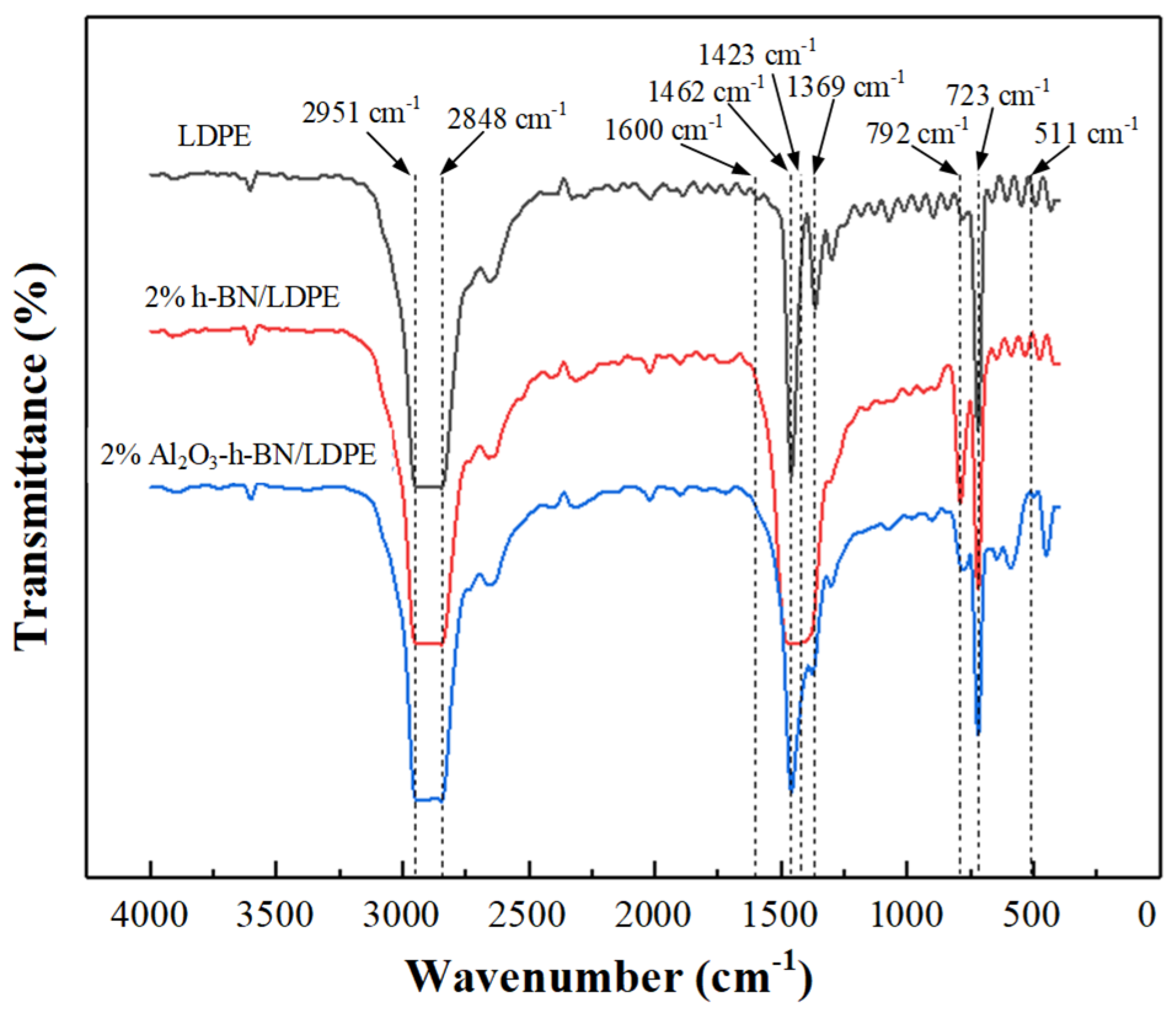
3.1. Microstructure Characterization
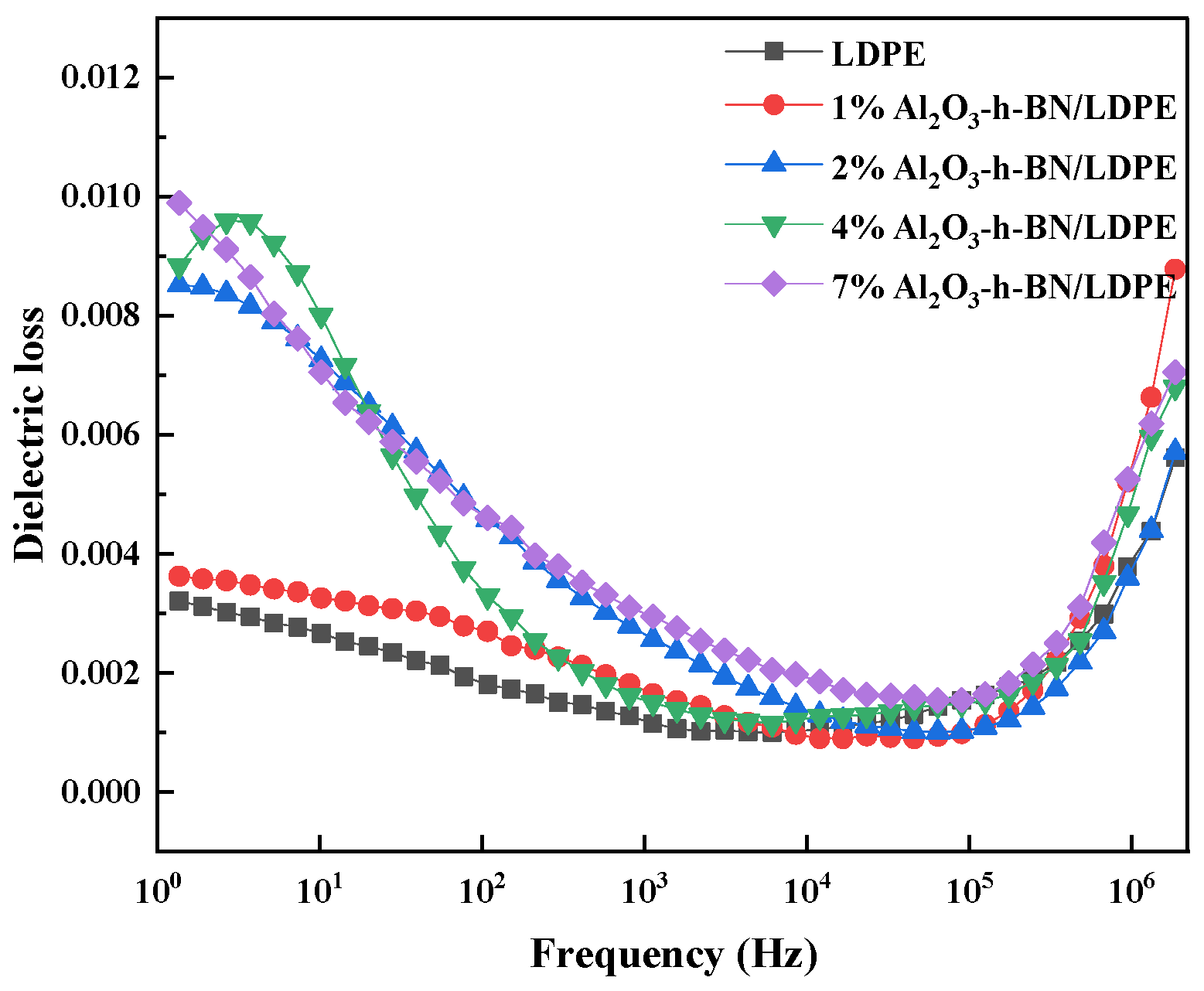
3.2. Dielectric Property Testing
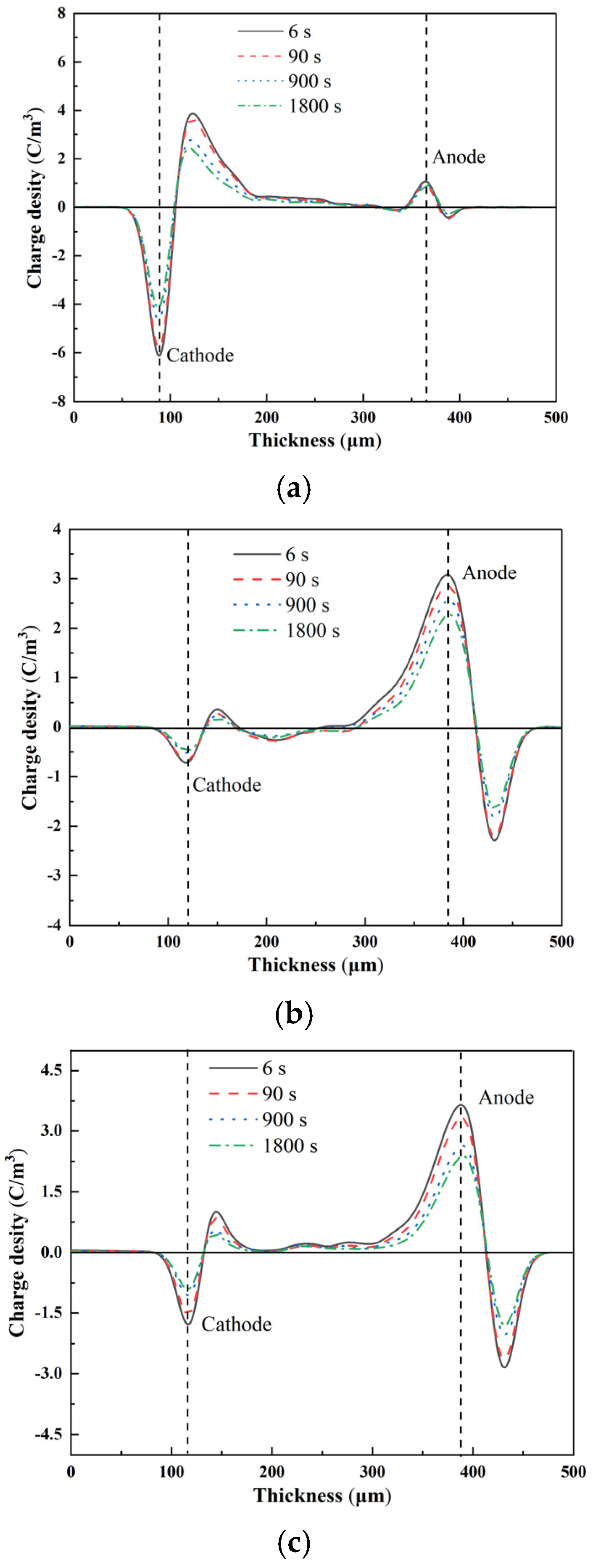
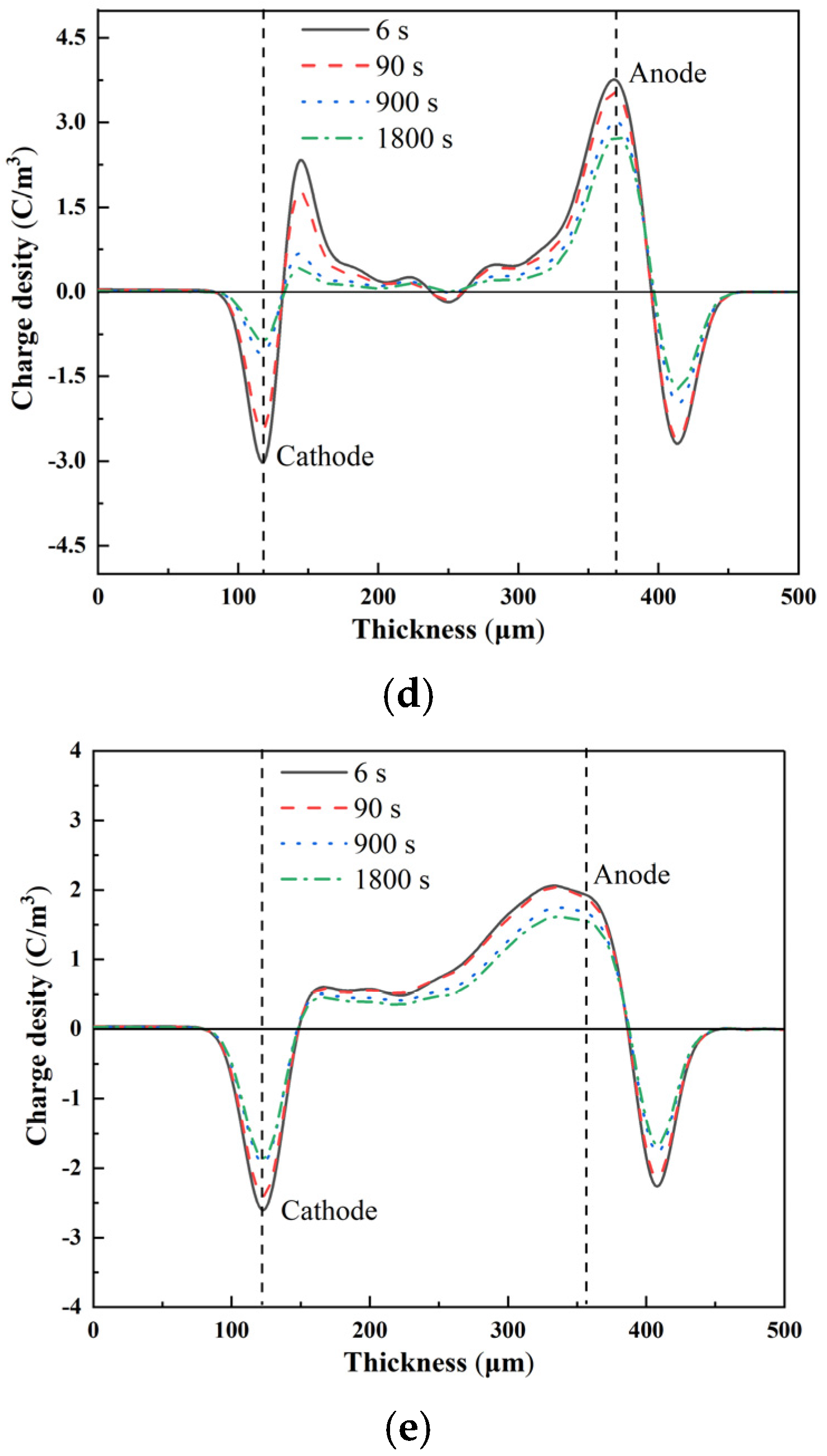
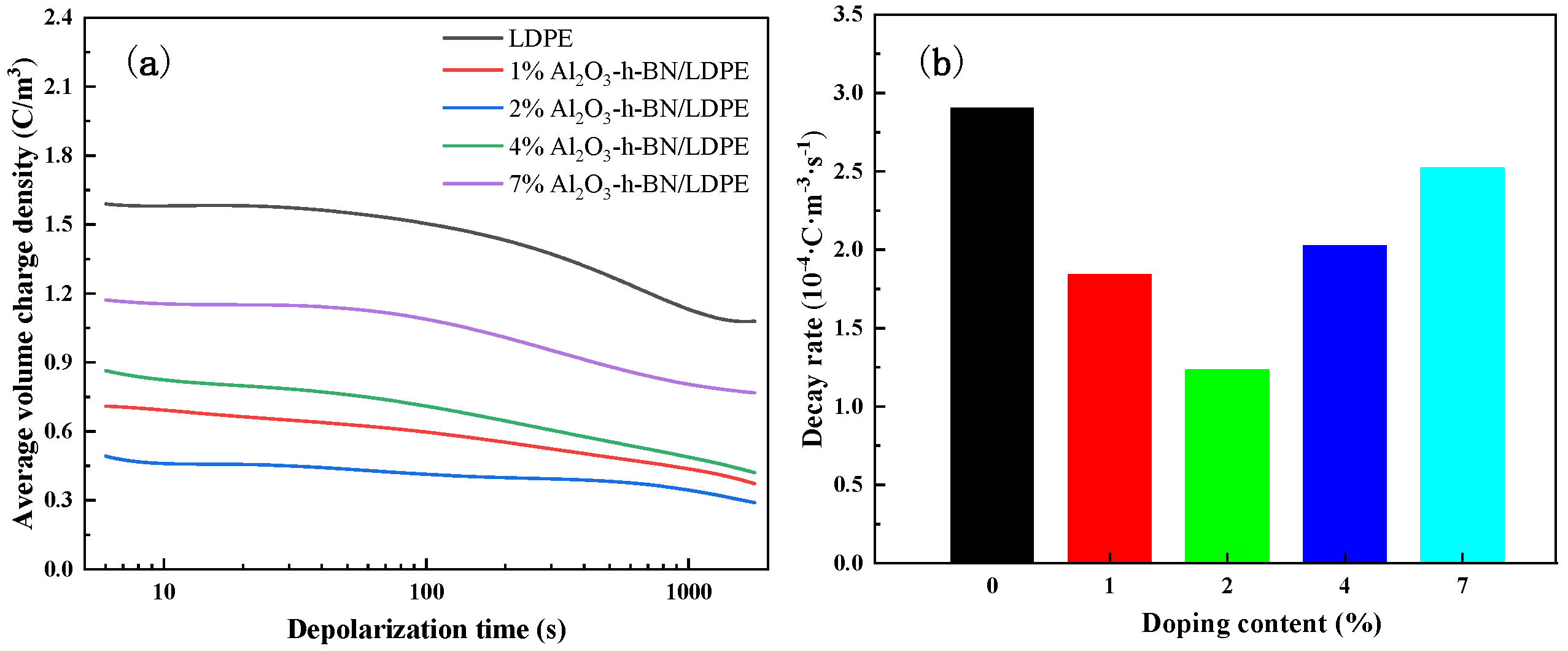
3.3. Space-Charge Distribution Measurement
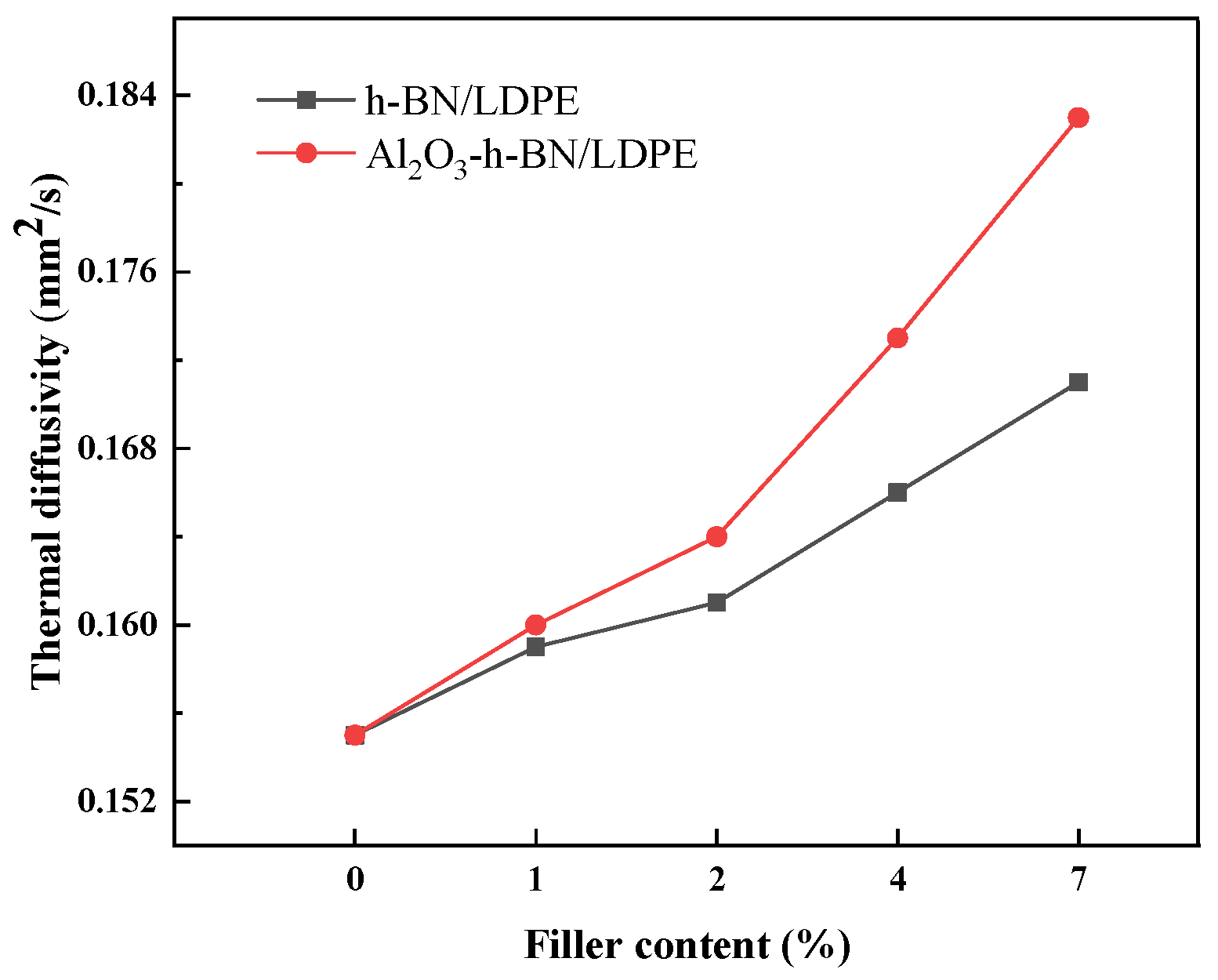
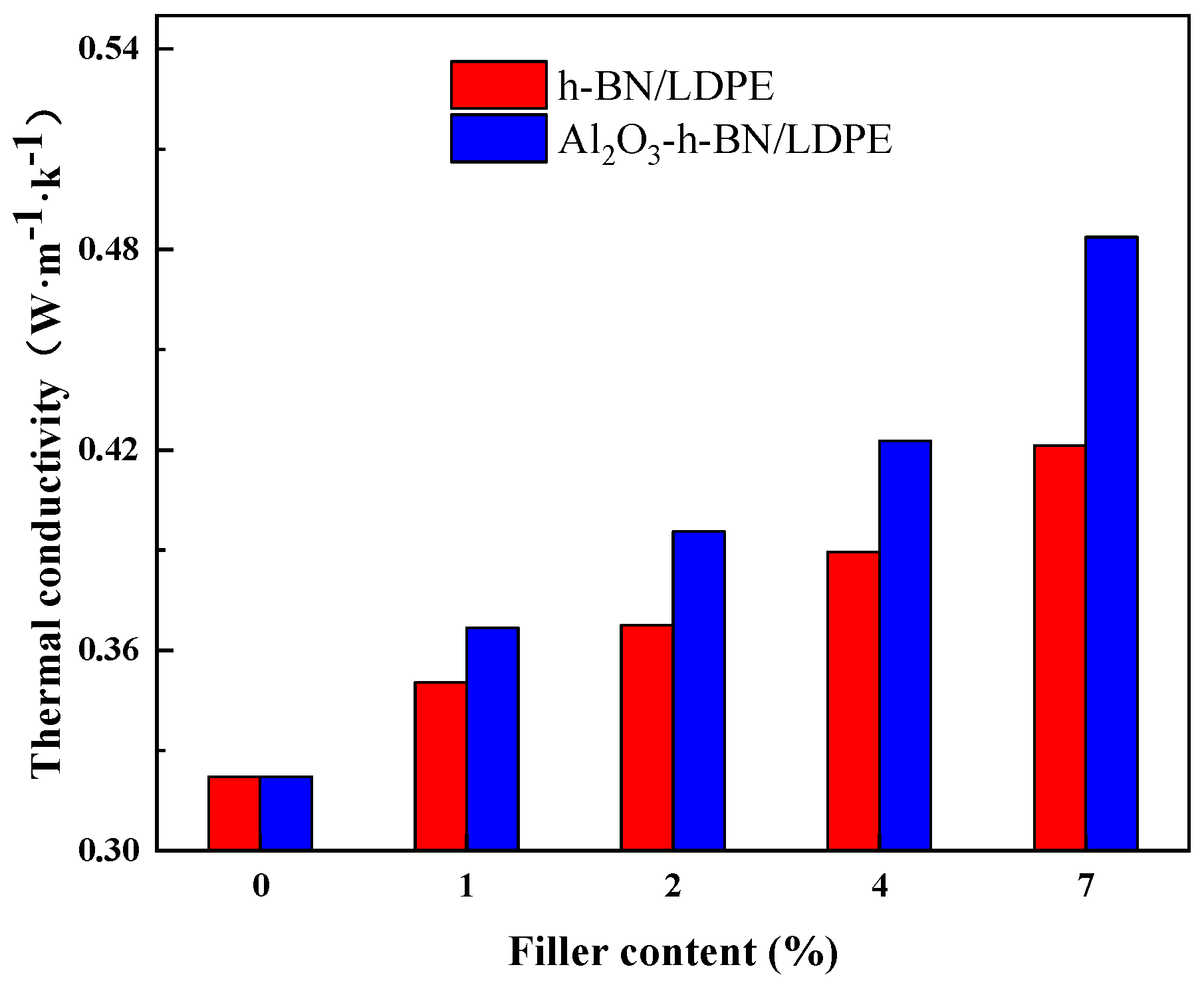
3.4. Thermal Conductivity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Chen, G.; Tu, Y.; Wang, C.; Li, C. Environment-friendly Insulating Gases for HVDC Gas-insulated Transmission Lines. J. Power Energy Syst. 2021, 7, 510–529. [Google Scholar]
- He, L.J.; Zeng, J.J.; Huang, Y.W.; Yang, X.; Li, D.W.; Chen, Y.; Yang, X.Y.; Wang, D.B.; Zhang, Y.X.; Fu, Z.D. Enhanced Thermal Conductivity and Dielectric Properties of h-BN/LDPE Composites. Materials 2020, 13, 4738. [Google Scholar] [CrossRef] [PubMed]
- Zarubin, V.S.; Kuvyrkin, G.N.; Savelyeva, I.Y. A Variation Model of Thermal Breakdown of a High-Voltage DC Cable Electrical Insulation. Russ. Electr. Eng. 2020, 91, 279–285. [Google Scholar] [CrossRef]
- Zhang, D.L.; Zha, J.W.; Li, C.Q.; Li, W.K.; Wang, S.J.; Wen, Y.; Dang, Z.M. High thermal conductivity and excellent electrical insulation performance in double-percolated three-phase polymer nanocomposites. Compos. Sci. Technol. 2017, 144, 36–42. [Google Scholar] [CrossRef]
- Du, B.X.; Hang, X. Effects of thermal conductivity on dc resistance to erosion of silicone rubber/BN nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 511–518. [Google Scholar] [CrossRef]
- Zou, D.; Huang, X.; Zhu, Y.; Chen, J.; Jiang, P. Boron nitride nanosheets endow the traditional dielectric polymer composites with advanced thermal management capability. Compos. Sci. Technol. 2019, 177, 88–95. [Google Scholar] [CrossRef]
- Fei, C.; Liu, Y.X. Preparation and Characterization of Al2O3 Heat Conduction Enhanced Polyimide Film. Fiber. Compos. 2020, 37, 3. [Google Scholar]
- Martinez-Oviedo, A.; Kshetri, Y.K.; Joshi, B.; Lee, S.W. Surface modification of blue TiO2 with silane coupling agent for NOx abatement. Prog. Nat. Sci. Mater. Int. 2021, 31, 230–238. [Google Scholar]
- Suzuoki, Y.; Cai, G.X.; Mizutani, T.; Idea, M. TSC Study on Interfacial Phenomena in PE (Polyethylene)-EVA (Ethylene-Vinylacetate Copolymer) Laminated Films. Jpn. J. Appl. Phys. 1982, 21, 1759. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.; Kim, J. Enhancement of the thermal and mechanical properties of a surface-modified boron nitride–polyurethane composite. Int. J. Hydrogen. Energy 2014, 25, 791–798. [Google Scholar] [CrossRef]
- Shan, F.R.; Yu, Z.M.; Luo, L.S.; Zhang, Y. Study on surface modification of nano-alumina by silane coupling agent KH550. New Chem. Mater. 2013, 41, 169–170. [Google Scholar]
- Reddy, M.P.; Manakari, V.; Parande, G.; Ubaid, F.; Shakoor, R.A.; Mohamed, A.M.; Gupta, M. Enhancing compressive, tensile, thermal and damping response of pure Al using BN nanoparticles. J. Alloys Compd. 2018, 762, 398–408. [Google Scholar] [CrossRef]
- Han, S.J.; Gross, L.H. Electroluminescence test to evaluate dielectric property at the interface between semiconductive shield and insulation. In Proceedings of the Conference Record of the 2004 IEEE International Symposium on Electrical Insulation, Indianapolis, IN, USA, 19–22 September 2004. [Google Scholar]
- Huang, X.; Zhi, C.; Jiang, P.; Colberg, D.; Bando, Y.; Tanaka, T. Polyhedral Oligosilsesquioxane-Modified Boron Nitride Nanotube Based Epoxy Nanocomposites: An Ideal Dielectric Material with High Thermal Conductivity. Adv. Funct. Mater. 2013, 23, 1824–1831. [Google Scholar] [CrossRef]
- Chi, Q.; Ma, T.; Zhang, Y.; Chen, Q.; Zhang, C.; Cui, Y.; Zhang, T.; Lin, J.; Wang, X.; Lei, Q. Excellent energy storage of sandwich-structured PVDF-based composite at low electric field by introduced the hybrid CoFe2O4@BZT-BCT nanofibers. Acs Sustain. Chem. Eng. 2017, 6, 403–412. [Google Scholar] [CrossRef]
- Winell, S.; Annersten, H.; Prakapenka, V. The high-pressure phase transformation and breakdown of MgFe2O4. Am. Mineral. 2006, 91, 560–567. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, Y.; Li, X.J.; Song, H. High thermoelectric performance in nano-SiC dispersed Bi1.6Pb0.4Sr2Co2Oy compounds. J. Alloys Compd. 2020, 825, 154065. [Google Scholar] [CrossRef]
- Kovchavtsev, A.P.; Tsarenko, A.V.; Guzev, A.A.; Aksenov, M.S.; Polovinkin, V.G.; Nastovjak, A.E.; Valisheva, N.A. The influence of electron energy quantization in a space-charge region on the accumulation capacitance of InAs metal-oxide-semiconductor capacitors. J. Appl. Phys. 2015, 118, 125708. [Google Scholar] [CrossRef]
- Mizutani, T. Behavior of Charge Carriers in Organic Insulating Materials. In Proceedings of the 2006 IEEE Conference on Electrical Insulation and Dielectric Phenomena, Kansas City, MO, USA, 15–18 October 2006. [Google Scholar]
- He, L.J.; Niu, H.Q.; Ma, Y.; Li, D.W.; Zhao, L.; Chen, C.T. Effect of maleic anhydride on trap levels of alumina/low-density polyethylene by photo-stimulated discharge. Integr. Ferroelectr. 2018, 191, 20–29. [Google Scholar] [CrossRef]
- Simmons, J.G.; Tam, M.C. Theory of isothermal currents and the direct determination of trap parameters in semiconductors and insulators containing arbitrary trap distributions. Phys. Rev. B 1972, 7, 3706–3713. [Google Scholar] [CrossRef]
- Papathanassiou, A.N.; Sakellis, I.; Vitoratos, E.; Sakkopoulos, S. Interfacial and space charge dielectric effects in Polypyrrole/Zinc Oxide composites. Synth. Met. 2017, 228, 41–44. [Google Scholar] [CrossRef]
- Mitsumoto, S.; Nagao, M.; Fu, M.; Dissado, L.A.; Fothergill, J.C. The space charge decay of high density polyethylene under different temperatures and accumulation fields. In Proceedings of the 2007 IEEE International Conference on Solid Dielectrics, Winchester, UK, 8–13 July 2007; pp. 470–473. [Google Scholar]
- Huang, X.; Jiang, P.; Yin, Y. Nanoparticle surface modification induced spacecharge suppression in linear low density polyethylene. Appl. Phys. Lett. 2009, 95, 1546. [Google Scholar] [CrossRef]
- Li, S.; Min, D.; Wang, W.; Chen, G. Modelling of dielectric breakdown through chargedynamics for polymer nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3476–3485. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Yin, Y.; Han, T. Effect of micro and nano-size boron nitride and silicon carbide on thermal properties and partial discharge resistance of silicone elastomer composite. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 377–385. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Liu, H.; Zhang, M.; Zhou, S. Hollow polymeric microsphere-filled silicone-modified epoxy as an internally insulated material for composite cross-arm applications. Compos. Sci. Technol. 2020, 200, 108418. [Google Scholar] [CrossRef]
- Nakajima, D.; Kikuchi, T.; Natsui, S.; Sakaguchi, N.; Suzuki, R.O. Fabrication of a novel aluminum surface covered by numerous high-aspect-ratio anodic alumina nanofibers. Appl. Surf. Sci. 2015, 356, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Naovaratpong, S.; Boonyaroonate, I.; Nathakaranakule, A. Plasma Density Control in a Dielectric Barrier Discharge (DBD) Ozone Generator Using a Laser Engraved Dielectric Layer and Alumina Sand Filled Discharge Channel. Lasers Eng. 2014, 28, 189–200. [Google Scholar]
- Tian, Z.; Kim, S.; Sun, Y.; White, B. A Molecular Dynamics Study of Thermal Conductivity in Nanocomposites via the Phonon Wave Packet Method. Proc. ASME InterPack Conf. 2009, 2009, 607–615. [Google Scholar]




| Sample | A (wt%) | B (wt%) | C (wt%) |
|---|---|---|---|
| LDPE | 100 | 0 | 0 |
| 1 wt% Al2O3-h-BN/LDPE | 99 | 0.5 | 0.5 |
| 2 wt% Al2O3-h-BN/LDPE | 98 | 1.0 | 1.0 |
| 4 wt% Al2O3-h-BN/LDPE | 96 | 2.0 | 2.0 |
| 7 wt% Al2O3-h-BN/LDPE | 93 | 3.5 | 3.5 |
| Sample | Quantity/N | Weibull Parameter | |
|---|---|---|---|
| E0/(kV/mm) | |||
| LDPE | 12 | 302.8 | 9.78 |
| 1 wt%Al2O3-h-BN/LDPE | 12 | 319.6 | 9.07 |
| 2 wt%Al2O3-h-BN/LDPE | 12 | 340.1 | 10.72 |
| 4 wt%Al2O3-h-BN/LDPE | 12 | 278.2 | 9.26 |
| 7 wt%Al2O3-h-BN/LDPE | 12 | 255.1 | 8.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Ye, Z.; Zeng, J.; Yang, X.; Li, D.; Yang, X.; Chen, Y.; Huang, Y. Enhancement in Electrical and Thermal Properties of LDPE with Al2O3 and h-BN as Nanofiller. Materials 2022, 15, 2844. https://doi.org/10.3390/ma15082844
He L, Ye Z, Zeng J, Yang X, Li D, Yang X, Chen Y, Huang Y. Enhancement in Electrical and Thermal Properties of LDPE with Al2O3 and h-BN as Nanofiller. Materials. 2022; 15(8):2844. https://doi.org/10.3390/ma15082844
Chicago/Turabian StyleHe, Lijuan, Zhanzheng Ye, Junji Zeng, Xiong Yang, Dawei Li, Xiangyu Yang, Yu Chen, and Yuewu Huang. 2022. "Enhancement in Electrical and Thermal Properties of LDPE with Al2O3 and h-BN as Nanofiller" Materials 15, no. 8: 2844. https://doi.org/10.3390/ma15082844





