Mechanical, Leaching, and Microstructure Properties of Mine Waste Rock Reinforced and Stabilised with Waste Oyster Shell for Road Subgrade Use
Abstract
:1. Introduction
2. Materials and Methods
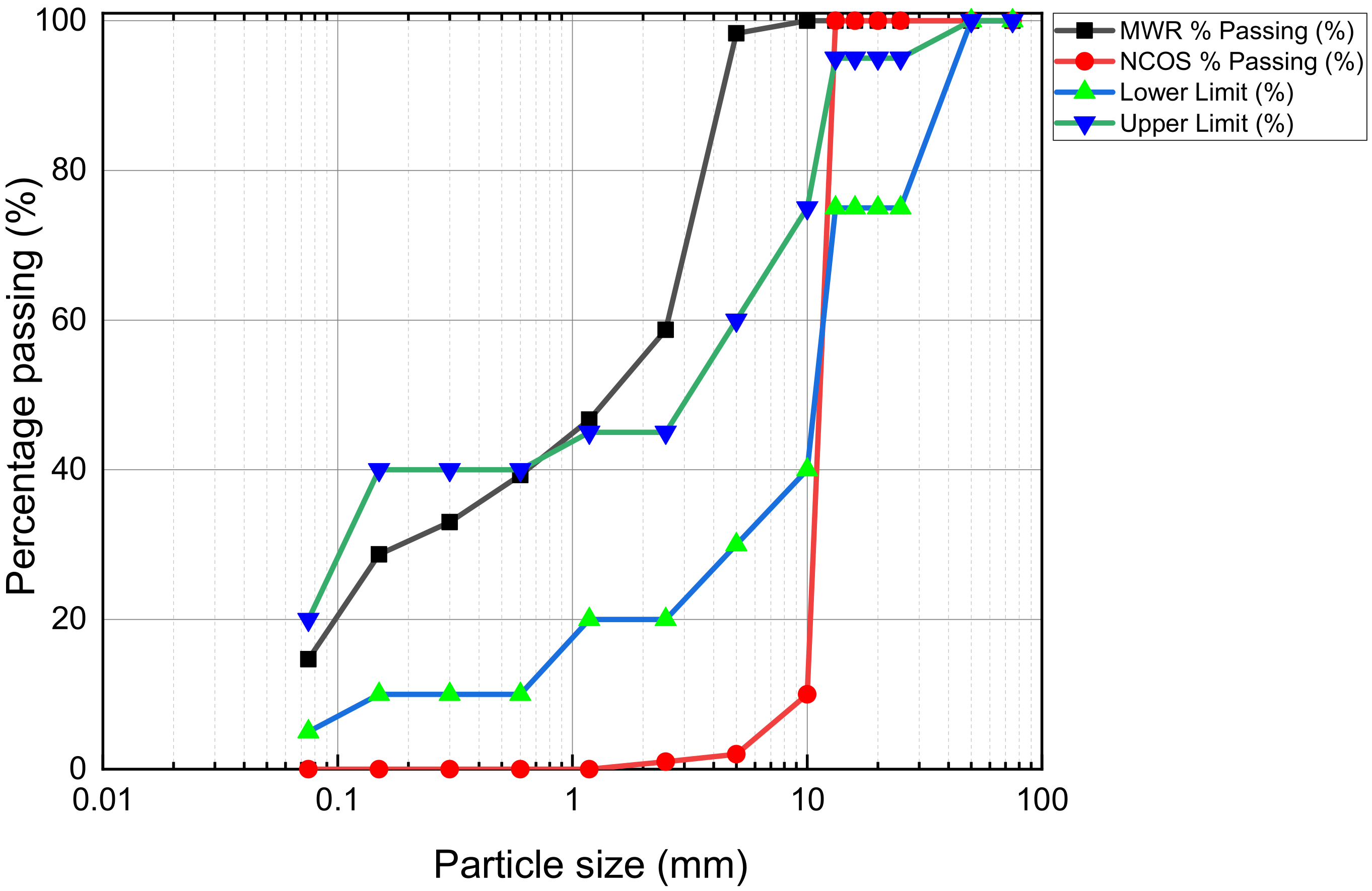
2.1. Materials
Optimal Material Parameters for Civil and Waste Treatment Works (Using Binders and Seashells)
2.2. Methods: Mechanism of Stabilisation and Immobilisation, Tests and Experiments
2.2.1. Mechanism of Stabilisation and Immobilisation of Heavy Metal Contaminated Soils
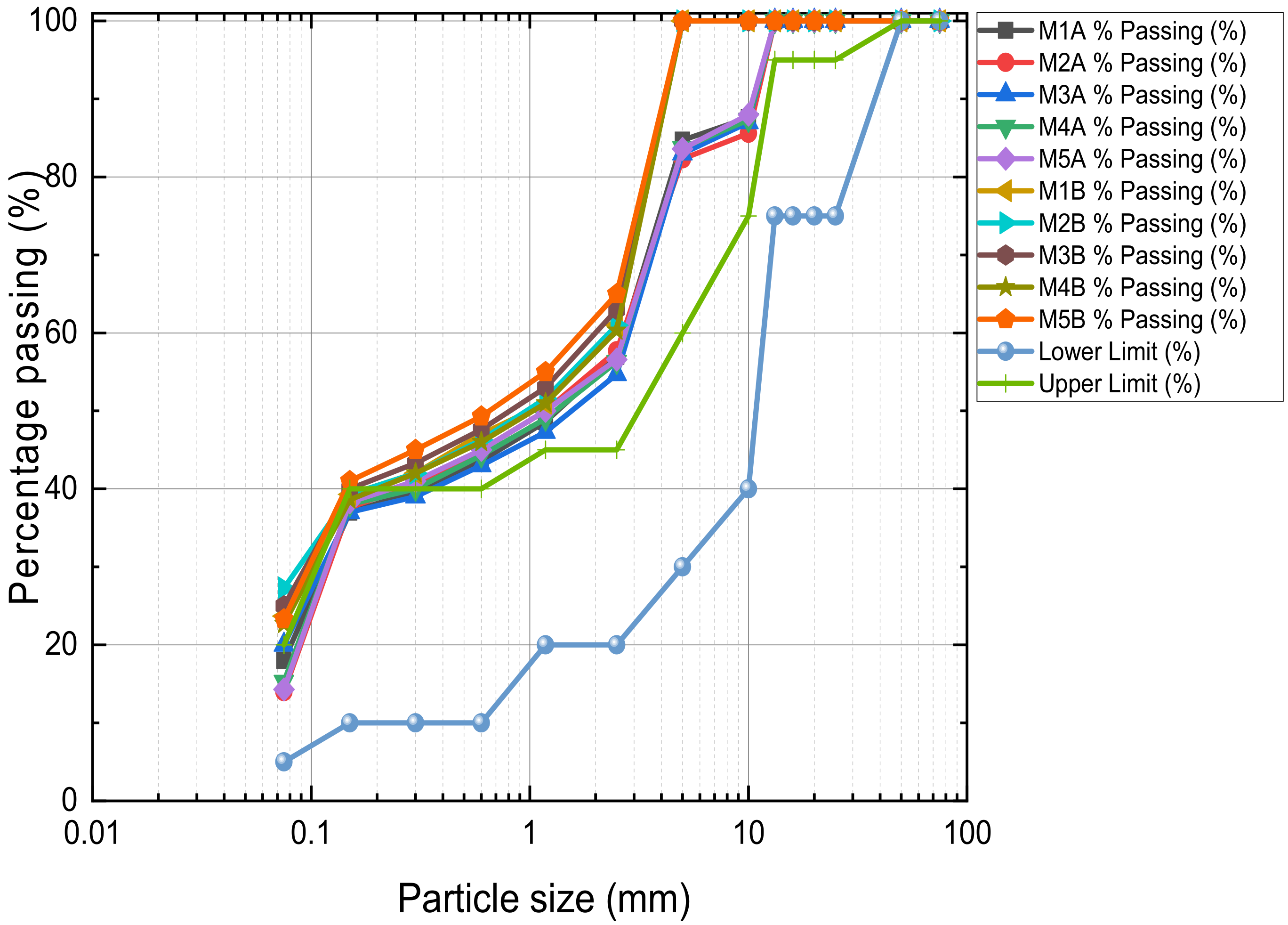
2.2.2. Preliminary Tests and Sample Preparation
2.2.3. Unconfined Compressive Strength (UCS) Test
2.2.4. Toxicity Characteristic Leaching Procedure (TCLP)
2.2.5. X-ray Diffractometry (XRD) and Scanning Electron Microscopy (SEM)
3. Results and Discussions
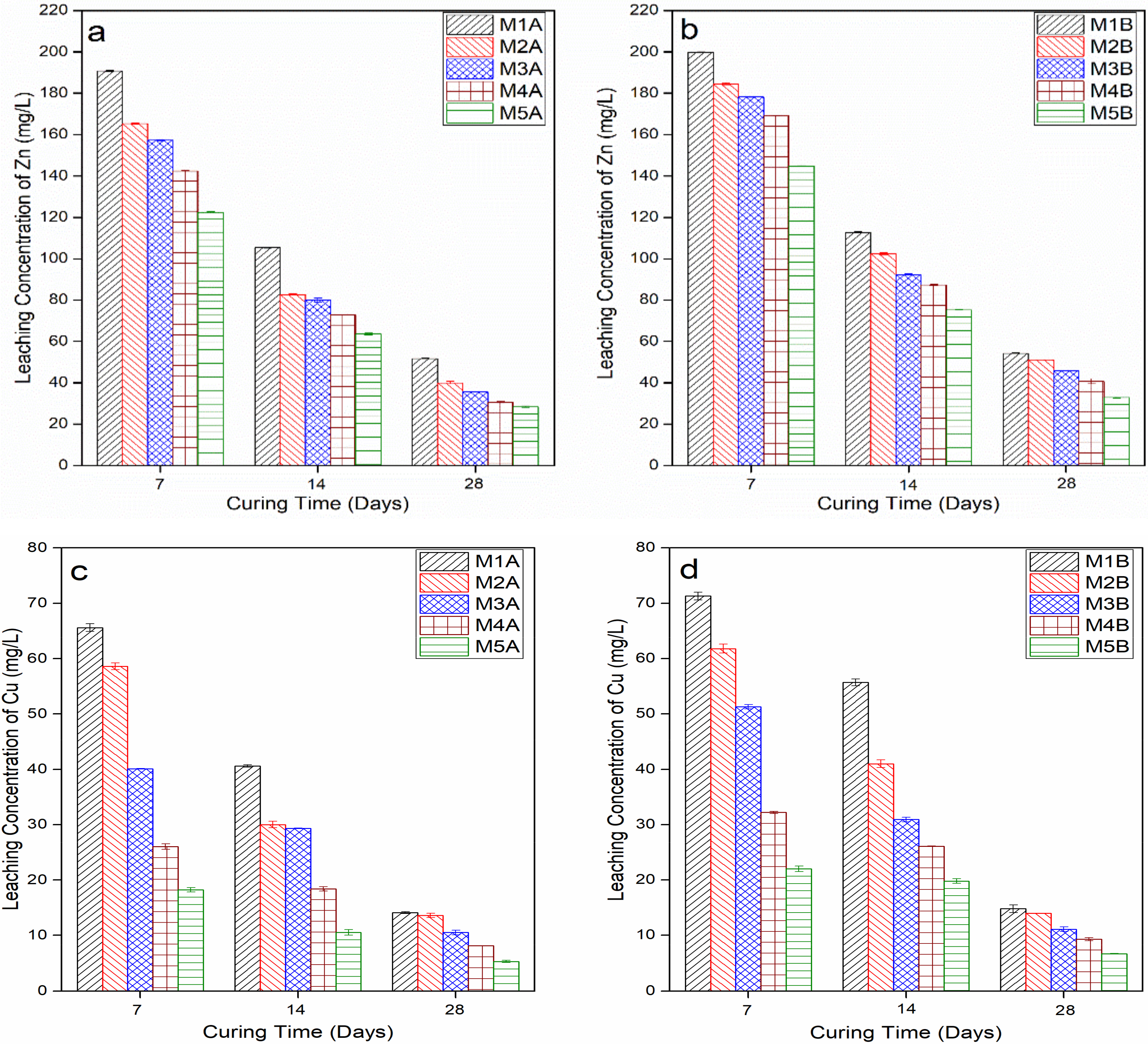
3.1. Heavy Metal Leaching Behaviour of Stabilised MWR
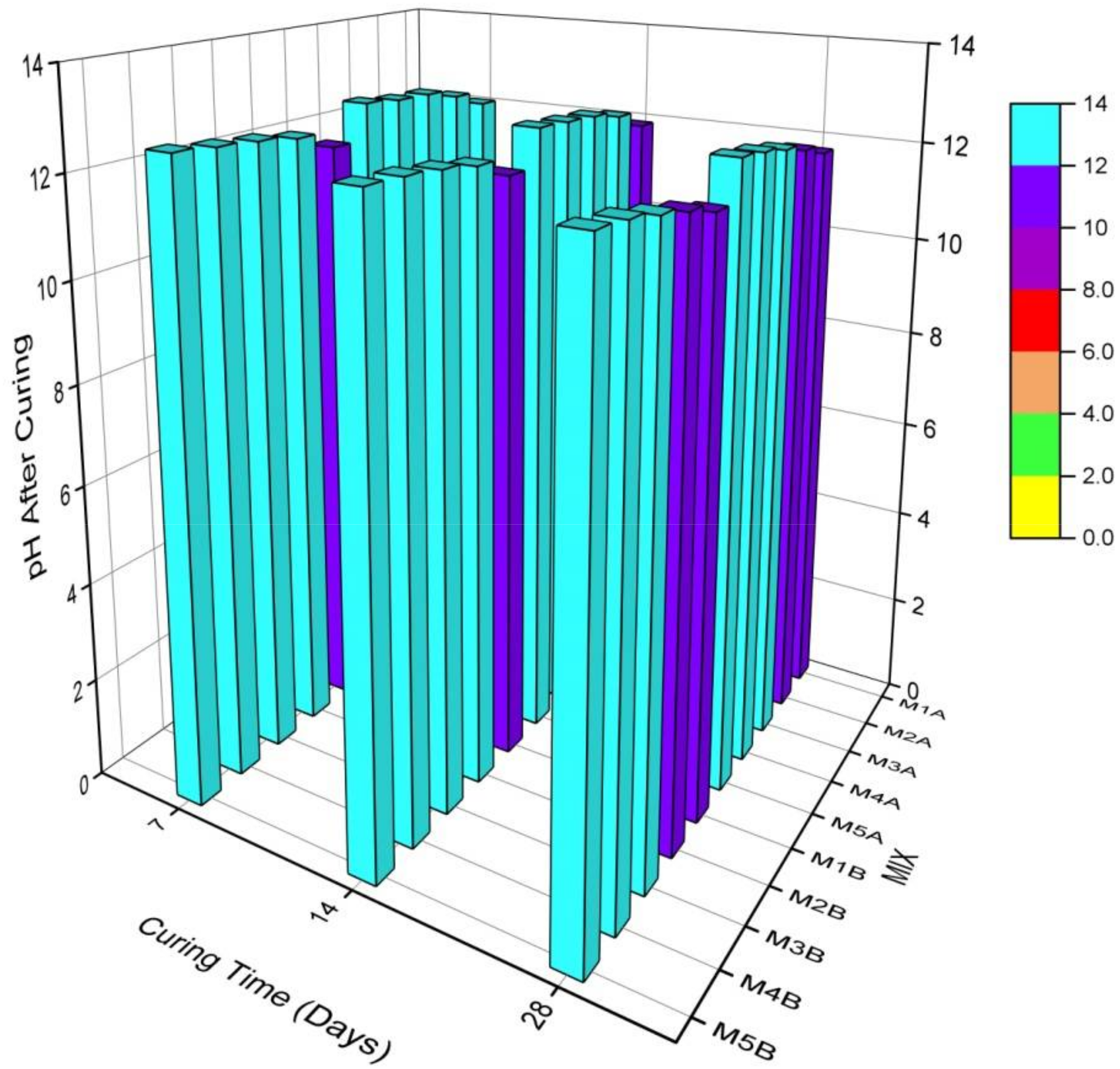
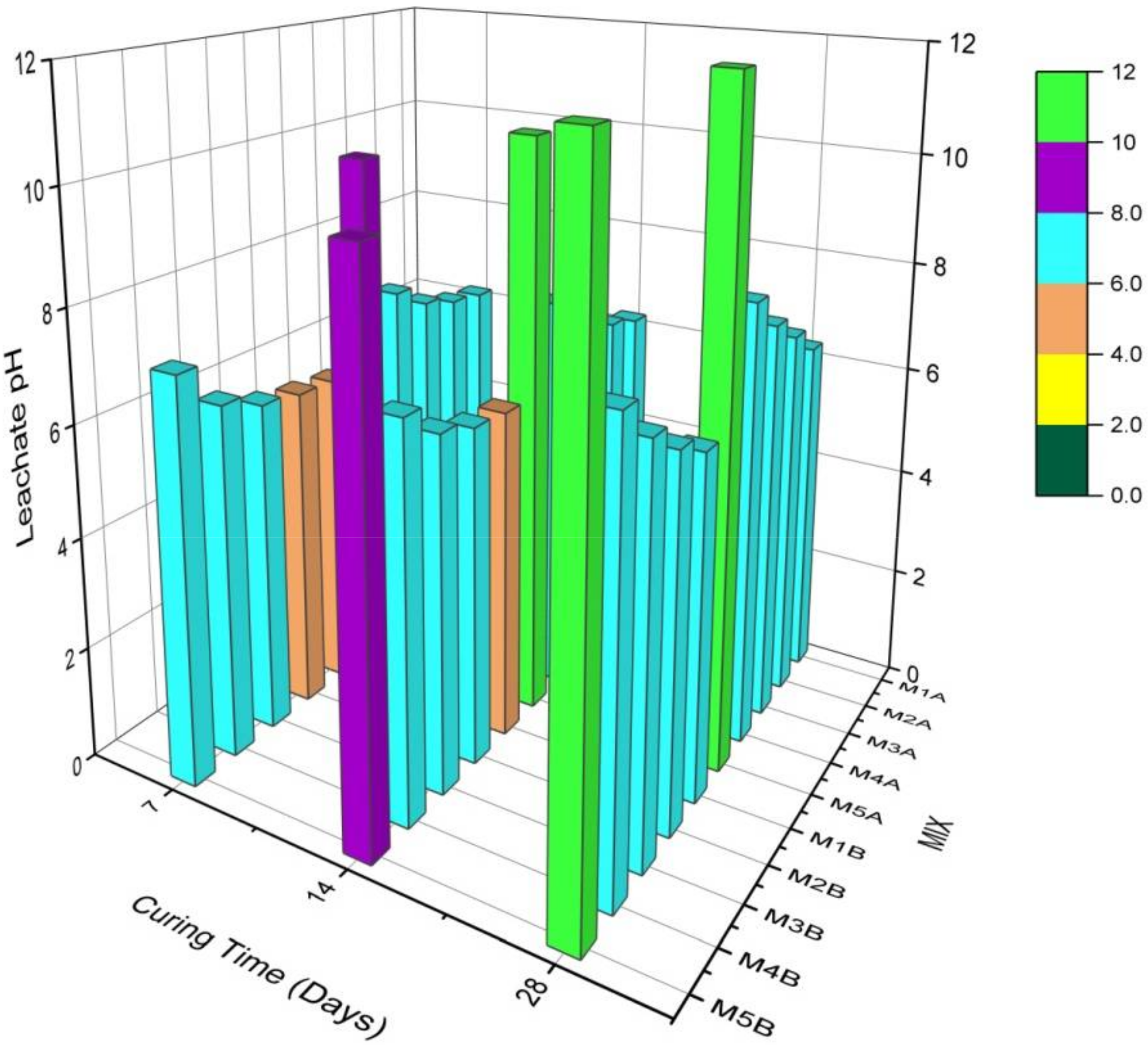
3.1.1. Curing and Leachate pH
3.1.2. Heavy Metal Leachate Concentration
3.2. Unconfined Compressive Strength (UCS)
3.2.1. Unsoaked UCS of Stabilised MWR
3.2.2. Soaked 28-Day UCS of Stabilised MWR
3.3. XRD Analysis of Stabilised MWR
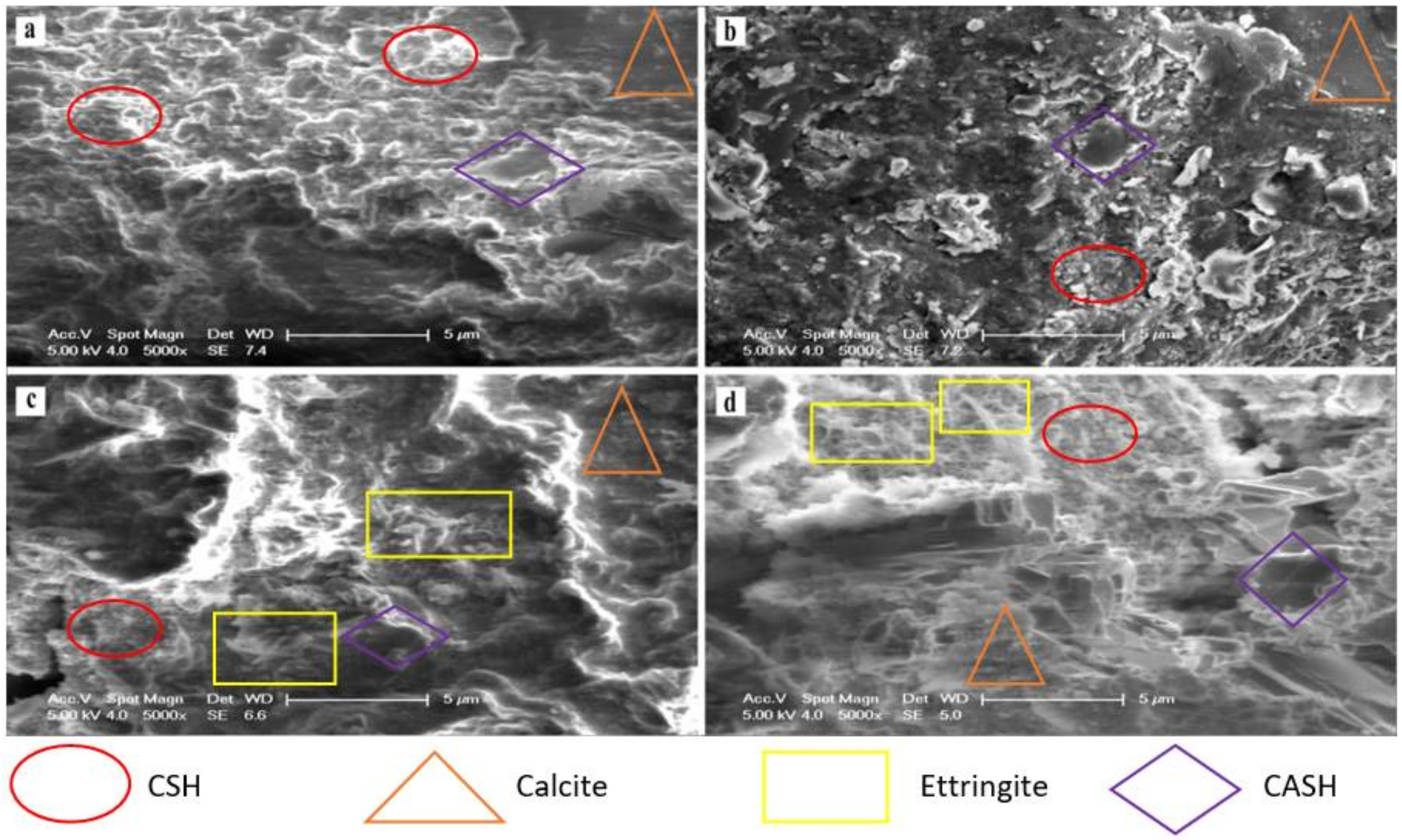
3.4. SEM Analysis of Stabilised MWR
3.5. Inter-Relationships between Mechanical, Leaching, and Microstructural Properties of Reinforced and Stabilised MWR
4. Conclusions
- The presence of NCOS and the increase in COSP content and curing age increased curing and leachate pH, decreased the leachability of Zn, Cu, and Pb, and decreased their concentrations in the stabilized composite to almost zero (0), or below contamination levels. This is due to the high-pH environment ensured by NCOS and COSP, thus stabilising and immobilising these heavy metals;
- The addition of 15% NCOS affected UCS positively with an evident increase in UCS. Samples without NCOS had relatively lower UCSs. Moreover, as cement content decreased and COSP content increased, UCS increased up to 10% cement replacement with increases in curing age. At COSP contents higher than 10%, UCS decreased slightly. Nevertheless, higher COSP content mixes with little or no cement still yielded UCSs that satisfy specifications for both subgrade and sub-base use. The twenty-eight (28)-day UCS for the A mixes are 1.24-times greater than their corresponding B counterparts. The average 28-day UCSs of M1A, M2A, and M3A are 2.55-times greater than those of M4A and M5A. Also, the average 28-day UCSs of M1B, M2B, and M3B are 2.59-times greater than those of M4B and M5B. Statistical analysis, using Minitab 21.1.0.0 (x64) one-way ANOVA, confirmed that the results were statistically significant for all curing periods, with p values < 0.05 (p = 0.000);
- The UCS results of the 28-day samples soaked in water for 48 h were just slightly lower than their corresponding unsoaked counterparts. The 28-day unsoaked UCSs are, on average, 1.33-times greater than their soaked counterparts. Cementitious hydration, pozzolanic reaction and carbonation products, COSP, and NCOS refined the pore structure of the composites by filling pores, densifying the composites and, subsequently, yielding improved mechanical properties. Therefore, even saturation in water did not adversely affect UCSs;
- In addition to the main hydration and reaction products (CSH, CASH, and ettringite), XRD patterns revealed that NCOS and COSP supplied extra CaO, which facilitated the formation of Calcite. CaO reacts with atmospheric Co2 to form calcite. Calcite, an insoluble carbonate with considerable strength, was visibly seen. The increased formation of calcite resulted in the improved mechanical strength of high-COSP–NCOS mixes, which would have otherwise had much lower UCSs. Calcite can also be seen to contribute greatly to higher Zn, Cu, and Pb stabilisation efficiency in M4, as it precipitated these heavy metals as insoluble complexes. The presence of all these hydration, reaction and carbonation products will ensure the permanence of treatment;
- SEM micrographs revealed denser microstructures for mixes with NCOS and COSP, as a result of the additional formation of calcite, which fills pores more effectively. This resulted in a denser stabilised composite. SEM–EDX results further confirmed increased Ca, and comparatively lower Si and Al, in the M4 mixes, which yielded more calcite and less CSH and CASH, respectively, thereby densifying these mixes but with slightly lower UCSs, precipitating more insoluble metal complexes, and consequently decreasing the concentrations of heavy metals—all confirming further the TCLP, UCS, and XRD results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tayeh, B.A.; Hasaniyah, M.W.; Zeyad, A.M.; Yusuf, M.O. Properties of concrete containing recycled seashells as cement partial replacement: A review. J. Clean. Prod. 2019, 237, 117723. [Google Scholar] [CrossRef]
- Hart, A. Mini-review of waste shell-derived materials’ applications. Waste Manag. Res. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Luhar, S.; Cheng, T.-W.; Luhar, I. Incorporation of natural waste from agricultural and aquacultural farming as supplementary materials with green concrete: A review. Compos. Part B 2019, 175, 107076. [Google Scholar] [CrossRef]
- James, J.; Pandian, P.K. Industrial Wastes as Auxiliary Additives to Cement/Lime Stabilization of Soils. Adv. Civ. Eng. 2016, 2016, 1267391. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.H.; Park, S.M.; Yang, B.J.; Jang, J.G. Calcined Oyster Shell Powder as an Expansive Additive in Cement Mortar. Materials 2019, 12, 1322. [Google Scholar] [CrossRef] [Green Version]
- Yoon, G.-L.; Kim, B.-T.; Kim, B.-O.; Han, S.H. Chemical-mechanical characteristics of crushed oyster shell. Waste Manag. 2003, 23, 825–834. [Google Scholar] [CrossRef]
- Ha, S.; Lee, J.W.; Choi, S.-H.; Kim, S.-H.; Kim, K.; Kim, Y. Calcination characteristics of oyster shells and their comparison with limestone from the perspective of waste recycling. J. Mater. Cycles Waste Manag. 2019, 21, 1075–1084. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, J.; Clark, M.; Yu, Y. Adsorption of copper to different biogenic oyster shell structures. Appl. Surf. Sci. 2014, 311, 264–272. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Kuo, W.-T.; Lin, C.-C.; Po-Yo, C. Study of the material properties of fly ash added to oyster cement mortar. Constr. Build. Mater. 2013, 41, 532–537. [Google Scholar] [CrossRef]
- Eo, S.-H.; Yi, S.-T. Effect of oyster shell as an aggregate replacement on the characteristics of concrete. Mag. Concr. Res. 2015, 67, 833–842. [Google Scholar] [CrossRef]
- Li, G.; Xu, X.; Chen, E.; Fan, J.; Xiong, G. Properties of cement-based bricks with oyster-shells ash. J. Clean. Prod. 2015, 91, 279–287. [Google Scholar] [CrossRef]
- Hsu, T.C. Experimental assessment of adsorption of Cu2+ and Ni2+ from aqueous solution by oyster shell powder. J. Hazard. Mater. 2009, 171, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Ok, Y.S.; Lim, J.E.; Moon, D.H. Stabilization of Pb and Cd contaminated soils and soil quality improvements using waste oyster shells. Environ. Geochem. Health 2011, 33, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Etim, R.K.; Attah, I.C.; Yohanna, P. Experimental study on potential of oyster shell ash in structural strength improvement of lateritic soil for road construction. Int. J. Pavement Res. Technol. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- International Institute for Environment and Development (IIED). Mining for the Future–Appendix A: Large Volume Waste Working Paper. 2002. Available online: http://pubs.iied.org/pdfs/G00883.pdf (accessed on 15 December 2021).
- Luo, Y. Environmental problems in the mining of metal minerals. IOP Conf. Ser. Earth Environ. Sci. 2019, 384, 012195. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.K.; Ban, H.J.; Im, I.R. Recycling of Waste Rock as a Filler to Prevent Ground Subsidence. Mater. Sci. Forum 2007, 544–545, 617–620. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Moon, D.H.; Wazne, M.; Cheong, K.H.; Chang, Y.-Y.; Baek, K.; Ok, Y.S.; Park, J.-H. Stabilization of As-, Pb-, and Cu-contaminated soil using calcined oyster shells and steel slag. Environ. Sci. Pollut. Res. 2015, 22, 11162–11169. [Google Scholar] [CrossRef]
- Moon, D.H.; Cheong, K.H.; Khim, J.; Wazne, M.; Hyun, S.; Park, J.-H.; Chang, Y.-Y.; Ok, Y.S. Stabilization of Pb2+ and Cu2+ contaminated firing range soil using calcined oyster shells and waste cow bones. Chemosphere 2013, 91, 1349–1354. [Google Scholar] [CrossRef]
- Moon, D.H.; Cheong, K.H.; Koutsospyros, A.; Chang, Y.Y.; Hyun, S.; Ok, Y.S.; Park, J.H. Assessment of waste oyster shells and coal mine drainage sludge for the stabilization of As-, Pb-, and Cu-contaminated soil. Environ. Sci. Pollut. R 2016, 23, 2362–2370. [Google Scholar] [CrossRef]
- Moon, D.H.; Park, J.W.; Cheong, K.H.; Hyun, S.; Koutsospyros, A.; Park, J.H.; Ok, Y.S. Stabilization of lead and copper contaminated firing range soil using calcined oyster shells and fly ash. Environ. Geochem. Health 2013, 35, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Doran, E., Jr. Shell Roads in Texas. American Geographical Society. Geogr. Rev. 1965, 55, 223–240. Available online: http://www.jstor.org/stable/212711 (accessed on 15 December 2021). [CrossRef]
- United States Environmental Protection Agency (USEPA). Report: Recent Developments for In Situ Treatment of Metals Contaminated Soils; United States Environmental Protection Agency, Office of Solid Waste and Emergency Response: Washington, DC, USA, 1996.
- Ruiz, G.; Farfán, P. Use of Crushed Seashell by-Products for Sandy Subgrade Stabilization for Pavement Purpose. In Proceedings of the LACCEI International Multi-Conference for Engineering, Education and Technology, San Jose, Costa Rica, 20–22 July 2016; pp. 1–6. [Google Scholar] [CrossRef] [Green Version]
- Eziefula, U.G.; Ezeh, J.C.; Eziefula, B.I. Properties of seashell aggregate concrete: A review. Constr. Build. Mater. 2018, 192, 287–300. [Google Scholar] [CrossRef]
- Kogbara, R.B. Interrelationships among geotechnical and leaching properties of a cement-stabilized contaminated soil. J. Environ. Sci. Health 2016, 52, 149–157. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 2018, 8, 216. [Google Scholar] [CrossRef]
- Du, Y.-J.; Wei, M.-L.; Reddy, K.R.; Jin, F.; Wu, H.-L.; Liu, Z.-B. New phosphate based binder for stabilisation of soils contaminated with heavy metals: Leaching, strength and microstructure characterisation. J. Environ. Manag. 2014, 146, 179–188. [Google Scholar] [CrossRef]
- Negi, A.S.; Faizan, M.; Siddharth, D.P. Soil stabilisation using lime. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 448–453. [Google Scholar]
- Firoozi, A.A.; Guney, O.C.; Firoozi, A.A.; Baghini, M.S. Fundamentals of soil stabilization. Int. J. Geo. Eng. 2017, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Nnochiri, E.S.; Ogundipe, O.M.; Oluwatuyi, O.E. Effects of Palm Kernel Shell Ash on Lime-Stabilized Lateritic Soil. Slovak J. Civ. Eng. 2017, 25, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dash, S.K.; Hussain, M. Lime Stabilization of Soils: Reappraisal. J. Mater. Civ. Eng. ASCE 2012, 24, 707–714. [Google Scholar] [CrossRef]
- Mosa, A.M.; Taher, A.H.; Al-Jaberi, L.A. Improvement of Poor Subgrade Soils Using Cement Kiln Dust. Case Stud. Constr. Mater. 2017, 7, 138–143. [Google Scholar] [CrossRef]
- Nnochiri, E.S. Effects of corn cob ash on lime stabilized lateritic soil. SSP J. Civ. Eng. 2018, 13, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Dhakal, S.K. Stabilization of Very Weak Subgrade Soil with Cementitious Stabilizers. Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2012. [Google Scholar]
- Puppala, A.J. Advances in ground modification with chemical additives: From theory to practice. Transp. Geotech. 2016, 9, 123–138. [Google Scholar] [CrossRef]
- Gross, J.; Adaska, W. Guide to Cement-Stabilized Subgrade Soils; PCA Special Report SR1007P; National Concrete Pavement Technology Center Iowa State University: Ames, IA, USA, 2020. [Google Scholar]
- Mohammad, L.N.; Saadeh, S. Performance evaluation of stabilized base and subbase material. In Proceedings of the GeoCongress 2008, New Orleans, LA, USA, 9–12 March 2008; pp. 1073–1080. [Google Scholar]
- Little, D.N.; Nair, S. Recommended Practice for Stabilization of Subgrade Soils and Base Materials. National Cooperative Highway Research Program; Transportation Research Board of the National Academies: Washington, DC, USA, 2009; p. 144. Available online: http://onlinepubs.trb.org/onlinepubs/nchrp/nchrp_w144.pdf (accessed on 15 December 2021).
- Kumar, P.S.; Kumar, C.S.; Yuvaraj, P. A Partial Replacement for Coarse Aggregate by Sea Shell and Cement by Lime in Concrete. Imp. J. Interdiscip. Res. 2016, 2, 1131–1136. [Google Scholar]
- Ojuri, O.O.; Adavi, A.A.; Oluwatuyi, O.E. Geotechnical and Environmental Evaluation of Lime-cement Stabilized Soil-mine Tailing Mixtures for Highway Construction. Transp. Geotech. 2016, 10, 1–12. [Google Scholar] [CrossRef]
- McManis, K. Identification and Stabilization Methods for Problematic Silt Soils: A Laboratory Evaluation of Modification and Stabilization Additives; FHWA/LA.02/371; Louisiana Transportation Research Center: Baton Rouge, LA, USA, 2003. [Google Scholar]
- Gupta, D.; Kumar, A. Performance evaluation of cement-stabilized pond ash-rice husk ash-clay mixture as a highway construction material. J. Rock Mech. Geotech. Eng. 2017, 9, 159–169. [Google Scholar] [CrossRef]
- Athanasopoulou, A. The role of curing period on the engineering characteristics of a cement-stabilised soil. Rom. J. Transp. Infrastruct. 2016, 5, 38–52. [Google Scholar] [CrossRef] [Green Version]
- Bell, F.G. Lime stabilization of clay minerals and soil. Eng. Geol. 1996, 42, 223–237. [Google Scholar] [CrossRef]
- Consoli, N.C.; Lopes, L.D.S., Jr.; Prietto, P.D.M. Variables controlling stiffness and strength of lime-stabilized soils. J. Geotech. Geoenviron. Eng. 2010, 137, 628–632. [Google Scholar] [CrossRef]
- Hilt, G.H.; Davidson, D.T. Lime fixation in clayey soils. Highw. Resour. Board Bull. 1960, 262, 20–32. [Google Scholar]
- Shi, C.; Fernandez-Jimenez, A. Stabilization/Solidification of Hazardous and Radioactive Wastes with Alkali-activated Cements. J. Hazard. Mater. 2006, B137, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.-Y.; Mahmud, H.B.; Shaaban, M.G. Stabilization/solidification of lead- contaminated soil using cement and rice husk ash. J. Hazard. Mater. 2006, B137, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.H.; Lee, J.-R.; Grubb, D.G. An assessment of Portland cement, cement kiln dust and Class C fly ash for the immobilization of Zn in contaminated soils. Environ. Earth Sci. 2010, 61, 1745–1750. [Google Scholar] [CrossRef]
- Yoon, I.H.; Moon, D.H.; Kim, K.-W. Mechanism for the stabilization/solidification of arsenic-contaminated soils with Portland cement and cement kiln dust. J. Environ. Manag. 2010, 91, 2322–2328. [Google Scholar] [CrossRef]
- Li, X.D.; Poon, C.S.; Sun, H. Heavy metal speciation and leaching behaviors in cement based solidified/stabilized waste materials. J. Hazard. Mater. 2001, 82, 215–230. [Google Scholar] [CrossRef]
- Bandara, N.; Jensen, E.; Binoy, T. Performance Evaluation of Subgrade Stabilization with Recycled Materials (Report No. RC-1635); Michigan Department of Transportation: Lansing, MI, USA, 2016. [Google Scholar]
- Nikraz, H.R. Engineering properties and performance of lime kiln dust for subgrade stabilisation of soft clay. In Proceedings of the 3rd International Conference on Road and Airfield Pavement Technology, Beijing, China, 28–30 April 1998; pp. 1466–1472. [Google Scholar]
- Parsons, R.L.; Kneebone, E.; Milburn, J.P. Use of Cement Kiln Dust for Subgrade Stabilization (Report No. KS-04-3); Kansas Department of Transportation: Topeka, KS, USA, 2004. [Google Scholar]
- Moon, D.H.; Grubb, D.G.; Reilly, T.L. Stabilization/solidification of selenium-impacted soils using Portland cement and cement kiln dust. J. Hazard. Mater. 2009, 162, 944–951. [Google Scholar] [CrossRef]
- Moon, D.H.; Cheong, K.H.; Choi, S.B. Assessment of waste oyster shells for the stabilization of Pb-contaminated mine tailings in the Republic of Korea. In Proceedings of the 10th International Symposium on Environmental Geotechnology and Sustainable Development, Bochum, Germany, 7–11 September 2009. [Google Scholar]
- Burlakovs, J.; Kasparinskis, R.; Klavins, M. Leaching of Contamination from Stabilization/Solidification Remediated Soils of Different Texture. Envir. Climate Tech. 2012, 9, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Ok, Y.S.; Arshad, M.A.; Chang, S.X. Distribution of exchangeable Al, Fe and Mn in forest soils from two contrasting watersheds in the oil sands region in northern Alberta. Can. J. Appl. Biol. Chem. 2007, 50, 32–34. [Google Scholar]
- Lee, M.; Park, I.S.; Kim, I. Remediation of heavy metal contaminated groundwater originated from abandoned mine using lime and calcium carbonate. J. Hazard. Mater. 2007, 144, 208–214. [Google Scholar] [CrossRef]
- Lee, C.W.; Kwon, H.B.; Jeon, H.P. A new recycling material for removing phosphorous from water. J. Clean. Prod. 2009, 17, 683–687. [Google Scholar] [CrossRef]
- Ok, Y.S.; Lee, H.; Jung, J. Chemical characterization and bioavailability of cadmium in artificially and naturally contaminated soils. Agric. Chem. Biotechnol. 2004, 47, 143–146. [Google Scholar]
- Edalat-Behbahani, A.; Soltanzadeh, F.; Emam-Jomeh, M. Sustainable approaches for developing concrete and mortar using waste seashell. Eur. J. Environ. Civil Eng. 2019, 25, 1874–1893. [Google Scholar] [CrossRef]
- Du, Y.-J.; Jiang, N.-J.; Liu, S.-Y.; Jin, F.; Singh, D.; Puppala, A.J. Engineering properties and microstructural characteristics of cement-stabilized zinc-contaminated kaolin. Can. Geotech. J. 2014, 51, 289–302. [Google Scholar] [CrossRef]
- Environmental Quality Standards for Soils; MEP (2008); National Standard of the People’s Republic of China: Beijing, China, 2008. (In Chinese)
- Aldeeky, H.; Al Hattamleh, O. Experimental Study on the Utilization of Fine Steel Slag on Stabilizing High Plastic Subgrade Soil. Adv. Civ. Eng. 2017, 2017, 9230279. [Google Scholar] [CrossRef]
- EPA Method 1311-Toxicity Characteristic Leaching Procedure. US EPA 1992; United States Environmental Protection Agency: Washington, DC, USA, 1992.
- Islam, M.N.; Taki, G.; Nguyen, X.P.; Jo, Y.-T.; Kim, J.; Park, J.-H. Heavy metal stabilization in contaminated soil by treatment with calcined cockle shell. Environ. Sci. Pollut. Res. 2017, 24, 7177–7183. [Google Scholar] [CrossRef]
- Jade Version 7.1.; Materials Data Inc. MDI: Livermore, CA, USA, 2005.
- Powder Diffraction File, PDF-2 Database Release; International Centre for Diffraction Data, ICDD: Newtown Square, PA, USA, 2002.
- Jha, A.K.; Sivapullaiah, P.V. Lime Stabilization of Soil: A Physico-Chemical and Micro-Mechanistic Perspective. Indian Geotech. J. 2020, 50, 339–347. [Google Scholar] [CrossRef]
- Peyronnard, O.; Benzaazoua, M. Estimation of the cementitious properties of various industrial by-products for applications requiring low mechanical strength. Resour. Conserv. Recycl. 2011, 56, 22–33. [Google Scholar] [CrossRef]
- Varhen, C.; Carrillo, S.; Ruiz, G. Experimental investigation of Peruvian scallop used as fine aggregate in concrete. Constr. Build. Mater. 2017, 136, 533–540. [Google Scholar] [CrossRef]
- American Concrete Pavement Association. Concrete Pavement Research and Technology Update; Number 3.06; American Concrete Pavement Association: Rosemont, IL, USA, 2002. [Google Scholar]
- ODOT Pavement Design Guide; Oregon Department of Transportation, Pavement Services Unit: Salem, OR, USA, 2007.
- Lertwattanaruk, J.P.; Makul, N.; Siripattarapravat, C. Utilization of ground waste seashells in cement mortars for masonry and plastering. J. Environ. Manag. 2012, 111, 133–141. [Google Scholar] [CrossRef]
- Lo, I.M.C.; Tang, C.I.; Li, X.D.; Poon, C.S. Leaching and microstructural analysis of cement-based solidified wastes. Env. Ment. Sci. Technol. 2000, 34, 5038–5042. [Google Scholar] [CrossRef]
- Bisanal, M.J.; Badiger, R. Study on Stabilization of Soil Using Sea Shell and Bitumen Emulsion. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 5875–5882. [Google Scholar] [CrossRef]
- Overseas Road Note 31. A Guide to the Structural Design of Bitumen Surfaced Roads in Tropical and Sub-Tropical Countries, 4th ed.; Transport Research Laboratory: Berkshire, UK, 1993.
- Lim, J.E.; Ahmad, M.; Lee, S.S.; Shope, C.L.; Hashimoto, Y.; Kim, K.-R.; Usman, A.R.A.; Yang, J.E.; Ok, Y.S. Effects of lime-based waste mate rials on immobilization and phyto availability of cadmium and lead in contaminated soil. Clean Soil Air Water 2013, 41, 1235–1241. [Google Scholar] [CrossRef]
- Niu, M.; Li, G.; Wang, Y.; Li, Q.; Han, L.; Song, Z. Comparative study of immobilization and mechanical properties of sulfoalu- minate cement and ordinary Portland cement with different heavy metals. Constr. Build. Mater. 2018, 193, 332–343. [Google Scholar] [CrossRef]
- Goodarzi, A.R.; Movahedrad, M. Stabilization/solidification of zinc-contaminated kaolin clay using ground granulated blast- furnace slag and different types of activators. Appl. Geochem. 2017, 81, 155–165. [Google Scholar] [CrossRef]
- Kogbara, R.B. A review of the mechanical and leaching performance of stabilized/solidified contaminated soils. Environ. Rev. 2014, 22, 66–86. [Google Scholar] [CrossRef] [Green Version]
- Radovanovi, D.D.; Kamberovi, Z.J.; Kora, M.S.; Rogan, J.R. Solidified structure and leaching properties of metallurgical waste water treatment sludge after solidification/stabilization process. J. Environ. Sci. Health 2016, 51, 34–43. [Google Scholar] [CrossRef]
- Gougar, M.L.D.; Scheetz, B.E.; Roy, D.M. Ettringite and C–S–H Portland cement phases for waste ion immobilization: A review. Waste Manag. 1996, 4, 295–303. [Google Scholar] [CrossRef]
- Islam, K.N.; Bakar, M.Z.B.A.; Noordin, M.M.; Hussein, M.Z.B.; Rahman, N.S.B.A.; Ali, M.E. Characterisation of calcium carbonate and its polymorphs from cockle shells (Anadara granosa). Powder Technol. 2011, 213, 188–191. [Google Scholar] [CrossRef]
- Żak, R.; Deja, J. Spectroscopy study of Zn, Cd, Pb and Cr ions immobilization on C–S–H phase. Spectro Chemical. Acta. 2015, 134, 614–620. [Google Scholar] [CrossRef]
- Locat, J.; Bérubé, M.A.; Choquette, M. Laboratory investigations on the lime stabilization of sensitive clays: Shear strength development. Can. Geotech. J. 1990, 27, 294–304. [Google Scholar] [CrossRef]
- Kamruzzaman, A.H.M.; Chew, S.H.; Lee, F.H. Microstructure of cement-treated Singapore marine clay. Ground Improv. 2006, 10, 113–123. [Google Scholar] [CrossRef]
- Yousuf, M.; Mollah, A.; Vempati, R.K.; Lin, T.C.; Cocke, D.L. The interfacial chemistry of solidification/stabilization of metals in cement and pozzolanic material systems. Waste Manag. 1995, 15, 137–148. [Google Scholar] [CrossRef]






| Property | Index | MWR | NCOS | OPC | COSP |
|---|---|---|---|---|---|
| Physical properties | Natural moisture content (%) | 3.49 | / | / | / |
| Specific gravity, Gs | 2.62 | / | 3.14 | 2.39 | |
| Liquid limit (%) | 34.74 | / | / | / | |
| Plastic limit (%) | 21.85 | / | / | / | |
| Plasticity index | 12.9 | / | / | / | |
| Gravel fraction: 2–5 mm (%) | 43.00 | / | / | / | |
| Sand fraction: 0.06–2 mm (%) | 47.00 | / | / | / | |
| Silt and Clay fraction: <0.06 mm (%) | 10.00 | / | / | / | |
| Soil classification: Poorly-graded gravelly sand | GP | / | / | / | |
| Unconfined compressive strength (Mpa) | 0.13 | / | / | / | |
| pH | 8.70 | 9.60 | 12.30 | 12.70 | |
| Water absorption (%) | / | 2.10 | / | / | |
| Organic matter (%) | / | 1.57 | / | / | |
| Size (mm) | ≤5 | 10–13 | ≈0.04 | ≈0.01 | |
| Chemical compositions (Wt. %) | Calcium oxide (CaO) | 56.7 | 95.54 | 59.80 | 97.50 |
| Silicon oxide (SiO2) | 17.9 | 0.25 | 22.31 | 0.21 | |
| Aluminium oxide (Al2O3) | 7.08 | 0.90 | 6.29 | 0.07 | |
| Magnesium oxide (MgO) | 1.50 | 0.42 | 3.40 | 0.42 | |
| Potassium oxide (K2O) | 2.02 | 0.01 | 0.70 | 0.02 | |
| Sulfate oxide (SO3) | 0.02 | 0.60 | 4.01 | 0.02 | |
| Sodium oxide (Na2O) | / | 0.36 | 0.69 | 0.13 | |
| Ferric oxide (Fe2O3) | 14.6 | 1.10 | 2.55 | 0.15 | |
| Chloride (Cl) | / | 0.74 | / | 0.11 | |
| Others | 0.05 | / | / | / | |
| Loss of ignition | 0.06 | 0.08 | 0.25 | 1.19 |
| Design Mix | Mix 1A | Mix 2A | Mix 3A | Mix 4A | Mix 5A | Mix 1B | Mix 2B | Mix 3B | Mix 4B | Mix 5B |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement (% by total Wt.) | 20 | 15 | 10 | 5 | 0 | 20 | 15 | 10 | 5 | 0 |
| COSP (% by total Wt.) | 0 | 5 | 10 | 15 | 20 | 0 | 5 | 10 | 15 | 20 |
| NCOS (% by total Wt.) | 15 | 15 | 15 | 15 | 15 | 0 | 0 | 0 | 0 | 0 |
| Element | Mix 1A Atomic % | Mix 4A Atomic % | Mix 1B Atomic % | Mix 4B Atomic % |
|---|---|---|---|---|
| O | 65.25 | 69.82 | 67.32 | 70.49 |
| Mg | 2.42 | 1.39 | 2.63 | 1.47 |
| Al | 3.67 | 2.01 | 4.42 | 3.71 |
| Si | 8.71 | 3.07 | 9.91 | 6.14 |
| Ca | 16.68 | 21.46 | 10.16 | 14.76 |
| Fe | 2.32 | 1.53 | 3.03 | 2.24 |
| Cu | 0.24 | 0.2 | 0.72 | 0.24 |
| Zn | 0.43 | 0.37 | 0.86 | 0.47 |
| Pb | 0.28 | 0.15 | 0.95 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wurie, N.N.; Zheng, J.; Traore, A.F. Mechanical, Leaching, and Microstructure Properties of Mine Waste Rock Reinforced and Stabilised with Waste Oyster Shell for Road Subgrade Use. Materials 2022, 15, 2916. https://doi.org/10.3390/ma15082916
Wurie NN, Zheng J, Traore AF. Mechanical, Leaching, and Microstructure Properties of Mine Waste Rock Reinforced and Stabilised with Waste Oyster Shell for Road Subgrade Use. Materials. 2022; 15(8):2916. https://doi.org/10.3390/ma15082916
Chicago/Turabian StyleWurie, Nadia N., Junjie Zheng, and Abdoul Fatah Traore. 2022. "Mechanical, Leaching, and Microstructure Properties of Mine Waste Rock Reinforced and Stabilised with Waste Oyster Shell for Road Subgrade Use" Materials 15, no. 8: 2916. https://doi.org/10.3390/ma15082916






