A Review of Polylactic Acid as a Replacement Material for Single-Use Laboratory Components
Abstract
:1. Introduction
2. Common Labware Polymers and Production
2.1. Polymer Properties
2.1.1. Polystyrene
2.1.2. Polyethylene Terephthalate G Copolyester
2.1.3. Polycarbonate
2.1.4. Polypropylene
2.2. Plastic Labware Production Techniques
3. Polylactic Acid (PLA)
3.1. Mechanical Properties of PLA
3.2. Thermal Properties of PLA
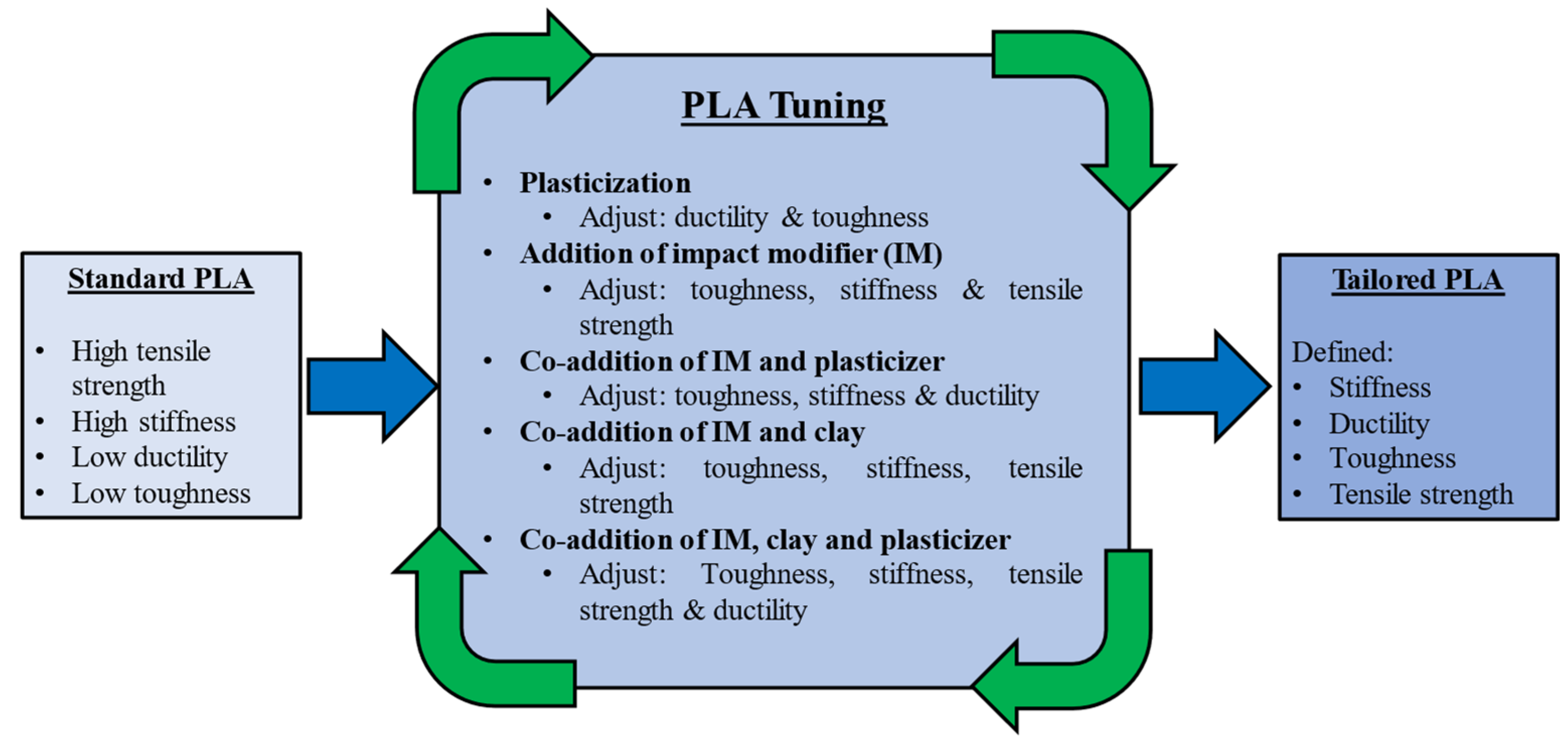
3.3. Mitigating PLA’s Functional Limitations
3.4. Biological Compatibility Requirements of PLA
3.5. Solvent Interaction with PLA
3.6. Effect of Temperature on Leachables from PLA
3.7. Additives to PLA
Sustainable, Compostable PLA Additives
3.8. PLA Industrial Synthesis Processes
3.9. PLA Current Applications
PLA for Labware Applications
4. Labware Needs Assessment
Standards
5. Environmental Impact of Laboratory Plastics
5.1. Plastic Labware End of Life
End of Life for PLA
5.2. Carbon Dioxide Emissions of Plastic Labware Manufacture
5.3. Environmental Considerations of PLA Feedstock
5.4. Economic Considerations of PLA Production
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbina, M.A.; Watts, A.J.; Reardon, E.E. Labs should cut plastic waste too. Nature 2015, 528, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, E.; Rader, R. Biopharmaceutical Manufacturing Is Shifting to Single-Use Systems. Are the Dinosaurs, the Large Stainless Steel Facilities, Becoming Extinct? Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/354820-Biopharmaceutical-Manufacturing-is-Shifting-to-Single-Use-Systems-Are-the-Dinosaurs-the-Large-Stainless-Steel-Facilities-Becoming-Extinct/ (accessed on 15 December 2021).
- Frank, G.T. Transformation of biomanufacturing by single-use systems and technology. Curr. Opin. Chem. Eng. 2018, 22, 62–70. [Google Scholar] [CrossRef]
- Shukla, A.A.; Gottschalk, U. Single-use disposable technologies for biopharmaceutical manufacturing. Trends Biotechnol. 2013, 31, 147–154. [Google Scholar] [CrossRef]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Hwang, K.-R.; Jeon, W.; Lee, S.Y.; Kim, M.-S.; Park, Y.-K. Sustainable bioplastics: Recent progress in the production of bio-building blocks for the bio-based next-generation polymer PEF. Chem. Eng. J. 2020, 390, 124636. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Plastic Materials Selection. Available online: https://www.thermofisher.com/ie/en/home/life-science/lab-plasticware-supplies/plastic-material-selection.html (accessed on 15 December 2021).
- Wang, Y.; Balowski, J.; Phillips, C.; Phillips, R.; Sims, C.E.; Allbritton, N.L. Benchtop micromolding of polystyrene by soft lithography. Lab Chip 2011, 11, 3089–3097. [Google Scholar] [CrossRef]
- Fry, B. Working with Polyethylene; Society of Manufacturing Engineers: Dearborn, MI, USA, 1999. [Google Scholar]
- Gray, J.E. Polystyrene Properties Performance and Applications; Nova Science Publishers, Inc.: New York, NY, USA, 2011. [Google Scholar]
- Thermo Fisher Scientific. Chemical Resistance Summary. Available online: https://assets.thermofisher.com/TFS-Assets/LCD/Specification-Sheets/D20481.pdf (accessed on 15 December 2021).
- Kawabata, N. Biodegradability of Polystyrene that Contains N-Benzyl-4-Vinylpyridinium Chloride in the Main Chain. In Polystyrene: Properties, Performance, and Applications; Gray, J.E., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 39–64. [Google Scholar]
- Thakur, S.; Verma, A.; Sharma, B.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent developments in recycling of polystyrene based plastics. Curr. Opin. Green Sustain. Chem. 2018, 13, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Maldas, D.; Kokta, B.; Daneault, C. Thermoplastic composties of polystyrene: Effect of different wood species on mechanical properties. J. Appl. Polym. Sci. 1989, 38, 413–439. [Google Scholar] [CrossRef]
- Reza Barzegari, M.; Alemdar, A.; Zhang, Y.; Rodrigue, D. Mechanical and rheological behavior of highly filled polystyrene with lignin. Polym. Compos. 2012, 33, 353–361. [Google Scholar] [CrossRef]
- Selvin, T.P.; Kuruvilla, J.; Sabu, T. Mechanical properties of titanium dioxide-filled polystyrene microcomposites. Mater. Lett. 2004, 58, 281–289. [Google Scholar] [CrossRef]
- Southern Labware. EZBio Single Use Bottles Assembly. Available online: https://www.southernlabware.com/single-use-bioprocessing/ezbior-single-use-bottles-assembly.html (accessed on 15 December 2021).
- Corning Life Sciences. Corning Erlenmeyer Shake Flasks. Available online: https://www.corning.com/worldwide/en/products/life-sciences/products/bioprocess/corning-erlenmeyer-shake-flasks.html (accessed on 15 December 2021).
- Ciardelli, F.; Bertoldo, M.; Bronco, S.; Passaglia, E. Polymers from Fossil and Renewable Resources Scientific and Technological Comparison of Plastic Properties; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Koerner, G.R.; Koerner, R.M. Puncture resistance of polyester (PET) and polypropylene (PP) needle-punched nonwoven geotextiles. Geotext. Geomembr. 2011, 29, 360–362. [Google Scholar] [CrossRef]
- United States Plastic Corporation. Physical Properties for VIVAK PETG Sheet. Available online: https://www.usplastic.com/knowledgebase/article.aspx?contentkey=537 (accessed on 15 December 2021).
- Singh, A.K.; Bedi, R.; Kaith, B.S. Composite materials based on recycled polyethylene terephthalate and their properties–A comprehensive review. Compos. Part B Eng. 2021, 219, 108928. [Google Scholar] [CrossRef]
- Foxx Life Sciences. PC EZBio Single Use Bottles Assembly. Available online: https://www.foxxlifesciences.com/collections/pc-ezbio-single-use-bottles-assembly (accessed on 15 December 2021).
- Drummond Scientific Company. DNA Sequencing Pipette Microdispenser. Available online: https://www.drummondsci.com/microinjection/sequencing-pipette/ (accessed on 15 December 2021).
- Bostick, E.E. Introduction and Historical Background. In Handbook of Polycarbonate Science and Technology; LeGrand, D.G., Bendler, J.T., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2000. [Google Scholar]
- Joseph, A.; King, J. Synthesis of Polycarbonates. In Handbook of Polycarbonate Science and Technology; LeGrand, D.G., Bendler, J.T., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2000. [Google Scholar]
- Fukuoka, S.; Fukawa, I.; Adachi, T.; Fujita, H.; Sugiyama, N.; Sawa, T. Industrialization and Expansion of Green Sustainable Chemical Process: A Review of Non-phosgene Polycarbonate from CO2. Org. Process Res. Dev. 2019, 23, 145–169. [Google Scholar] [CrossRef]
- Artham, T.; Doble, M. Biodegradation of aliphatic and aromatic polycarbonates. Macromol. Biosci. 2008, 8, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, J.; Park, M.; Choe, C.R.; Lee, J.; Kim, D. Thermal characterization and morphological study of polyphenylene sulfide–polycarbonate blends. Polym. Eng. Sci. 1996, 36, 2502–2508. [Google Scholar] [CrossRef]
- Long, T.; Sokol, R. Molding polycarbonate: Moisture degradation effect on physical and chemical properties. Polym. Eng. Sci. 1974, 14, 817–822. [Google Scholar] [CrossRef]
- Ullrich, F.; Singh, S.P.V.; McDonald, S.; Krueger, A.; Vera-Sorroche, J.; Amirkhizi, A.; Masato, D. Effect of strain rate on the mechanical properties of polycarbonate processed by compression and injection molding. Polym. Eng. Sci. 2022, 62, 174–184. [Google Scholar] [CrossRef]
- Corning Life Sciences. Centrifuge Tubes. Available online: https://ecatalog.corning.com/life-sciences/b2b/US/en/Liquid-Handling/Tubes%2C-Liquid-Handling/Centrifuge-Tubes/c/centrifugeTubes (accessed on 15 December 2021).
- Merck-Sigma-Aldrich. BRAND Disposal Bag. Available online: https://www.sigmaaldrich.com/catalog/substance/branddisposalbag1234598765?lang=en®ion=IE (accessed on 15 December 2021).
- SP Bel-Art. Disposable Polypropylene Pestles and 1.5 mL Tubes. Available online: https://www.belart.com/disposable-polypropylene-pestles-and-1-5ml-tubes-21228.html (accessed on 15 December 2021).
- Maier, C.; Calafut, T. Polypropylene—The Definitive User’s Guide and Databook; Plastics Design Library: New York, NY, USA, 1998. [Google Scholar]
- Farotti, E.; Natalini, M. Injection molding. Influence of process parameters on mechanical properties of polypropylene polymer. A first study. Procedia Struct. Integr. 2018, 8, 256–264. [Google Scholar] [CrossRef]
- Sayeed, M.; Rawal, A.; Onal, L.; Karaduman, Y. Mechanical properties of surface modified jute fiber/polypropylene nonwoven composites. Polym. Compos. 2014, 35, 1044–1050. [Google Scholar] [CrossRef]
- Agüero, A.; Morcillo, M.d.C.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Lascano, D.; Torres-Giner, S.; Fenollar, O. Study of the influence of the reprocessing cycles on the final properties of polylactide pieces obtained by injection molding. Polymers 2019, 11, 1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dizon, J.R.C.; Valino, A.D.; Souza, L.R.; Espera, A.H.; Chen, Q.; Advincula, R.C. Three-dimensional-printed molds and materials for injection molding and rapid tooling applications. MRS Commun. 2019, 9, 1267–1283. [Google Scholar] [CrossRef]
- Sang, Z.-H.; Xie, X.-L.; Zhou, S.-Y.; Li, Y.; Yan, Z.; Xu, L.; Zhong, G.-J.; Li, Z.-M. Gradient structure of crystalline morphology in injection-molded polylactide parts tuned by oscillation shear flow and its influence on thermomechanical performance. Ind. Eng. Chem. Res. 2017, 56, 6295–6306. [Google Scholar] [CrossRef]
- Schubert, C.; Van Langeveld, M.C.; Donoso, L.A. Innovations in 3D printing: A 3D overview from optics to organs. Br. J. Ophthalmol. 2014, 98, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-M.; Tseng, Y.-Y.; Lee, D.; Lin, Y.-T.; Lin, S.-H.; Lee, T.-Y.; Liu, S.-J.; Ito, H. A robust experimental model to explore the three-dimensional printing of polylactide parts: Solution versus melt extrusion. Appl. Sci. 2020, 10, 509. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhang, D.; Qi, S.; Wen, X.; Su, Y. Mechanical properties of 3D parts fabricated by fused deposition modeling: Effect of various fillers in polylactide. J. Appl. Polym. Sci. 2019, 136, 47824. [Google Scholar] [CrossRef]
- Ferràs-Tarragó, J.; Sabalza-Baztán, O.; Sahuquillo-Arce, J.M.; Angulo-Sánchez, M.Á.; Amaya-Valero, J.; De-La-Calva Ceinos, C.; Baixauli-García, F. Security of 3D-printed polylactide acid piece sterilization in the operating room: A sterility test. Eur. J. Trauma Emerg. Surg. 2021, 1–6. [Google Scholar] [CrossRef]
- Diep, T.T.; Ray, P.P.; Daniel Edwards, A. Methods for Rapid Prototyping Novel Labware: Using CAD and Desktop 3D Printing in the Microbiology Laboratory. Lett. Appl. Microbiol. 2021, 74, 247–257. [Google Scholar] [CrossRef]
- Baden, T.; Chagas, A.M.; Gage, G.; Marzullo, T.; Prieto-Godino, L.L.; Euler, T. Open Labware: 3-D printing your own lab equipment. PLoS Biol. 2015, 13, e1002086. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [Green Version]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. Polylactic Acid: PLA Biopolymer Technology and Applications; William Andrew: Oxford, UK, 2012. [Google Scholar]
- EuropeanBioplastics. Available online: https://www.european-bioplastics.org/market/ (accessed on 16 December 2021).
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef] [PubMed]
- Vink, E.T.; Rabago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of life cycle assessment to NatureWorks™ polylactide (PLA) production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- Djukić-Vuković, A.; Mladenović, D.; Ivanović, J.; Pejin, J.; Mojović, L. Towards sustainability of lactic acid and poly-lactic acid polymers production. Renew. Sustain. Energy Rev. 2019, 108, 238–252. [Google Scholar] [CrossRef]
- Tábi, T.; Ageyeva, T.; Kovács, J.G. Improving the ductility and heat deflection temperature of injection molded Poly (lactic acid) products: A comprehensive review. Polym. Test. 2021, 101, 107282. [Google Scholar] [CrossRef]
- Sangeetha, V.; Deka, H.; Varghese, T.; Nayak, S. State of the art and future prospectives of poly (lactic acid) based blends and composites. Polym. Compos. 2018, 39, 81–101. [Google Scholar] [CrossRef]
- Anderson, K.S.; Schreck, K.M.; Hillmyer, M.A. Toughening polylactide. Polym. Rev. 2008, 48, 85–108. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Perego, G.; Cella, G.D.; Bastioli, C. Effect of molecular weight and crystallinity on poly (lactic acid) mechanical properties. J. Appl. Polym. Sci. 1996, 59, 37–43. [Google Scholar] [CrossRef]
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, properties and green packaging technology: A review. Int. J. Food Prop. 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Avinc, O.; Khoddami, A. Overview of poly (lactic acid)(PLA) fibre. Fibre Chem. 2010, 42, 68–78. [Google Scholar] [CrossRef]
- Kaiser, M.; Anuar, H.; Razak, S. Ductile–brittle transition temperature of polylactic acid-based biocomposite. J. Thermoplast. Compos. Mater. 2013, 26, 216–226. [Google Scholar] [CrossRef]
- Janorkar, A.V.; Metters, A.T.; Hirt, D.E. Degradation of poly (L-lactide) films under ultraviolet-induced photografting and sterilization conditions. J. Appl. Polym. Sci. 2007, 106, 1042–1047. [Google Scholar] [CrossRef]
- Man, C.; Zhang, C.; Liu, Y.; Wang, W.; Ren, W.; Jiang, L.; Reisdorffer, F.; Nguyen, T.P.; Dan, Y. Poly (lactic acid)/titanium dioxide composites: Preparation and performance under ultraviolet irradiation. Polym. Degrad. Stab. 2012, 97, 856–862. [Google Scholar] [CrossRef]
- Mohr, L.; Capelezzo, A.; Baretta, C.; Martins, M.; Fiori, M.; Mello, J. Titanium dioxide nanoparticles applied as ultraviolet radiation blocker in the polylactic acid bidegradable polymer. Polym. Test. 2019, 77, 105867. [Google Scholar] [CrossRef]
- Ho, M.-P.; Lau, K.-T.; Wang, H.; Hui, D. Improvement on the properties of polylactic acid (PLA) using bamboo charcoal particles. Compos. Part B Eng. 2015, 81, 14–25. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.; Ni, L.; Pei, Y.; Zhang, H.; Zhang, H. Applied organic-inorganic nanocomposite of PLA-TiO2 for preparing polysulfone membrane: Structure, performance and UV-assisted cleaning strategy. Water Sci. Technol. 2021, 83, 198–211. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, P.; Lv, P.; Lemstra, P.J.; Cai, X.; Yang, W.; Dong, W.; Chen, M.; Liu, T.; Du, M. Excellent UV resistance of polylactide by interfacial stereocomplexation with double-shell-structured TiO2 nanohybrids. ACS Appl. Mater. Interfaces 2020, 12, 49090–49100. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Qin, Y. 14—Biocompatibility testing for medical textile products. In Medical Textile Materials; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Anderson, J. Biocompatibility. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Moller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 9, pp. 363–383. [Google Scholar]
- Jenke, D. Evaluation of the chemical compatibility of plastic contact materials and pharmaceutical products; safety considerations related to extractables and leachables. J. Pharm. Sci. 2007, 96, 2566–2581. [Google Scholar] [CrossRef]
- Massey, L.K. Introduction to Sterilization Methods. In The Effect of Sterilization Methods on Plastics and Elastomers, 2nd ed.; William Andrew: Norwich, NY, USA, 2005; pp. 5–18. [Google Scholar]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Agrawal, A.; Saran, A.D.; Rath, S.S.; Khanna, A. Constrained nonlinear optimization for solubility parameters of poly (lactic acid) and poly (glycolic acid)—validation and comparison. Polymer 2004, 45, 8603–8612. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Hansen, C.M.; Smith, A.L. Using Hansen solubility parameters to correlate solubility of C60 fullerene in organic solvents and in polymers. Carbon 2004, 42, 1591–1597. [Google Scholar] [CrossRef]
- Welker, R.W. Basics and sampling of particles for size analysis and identification. In Developments in Surface Contamination and Cleaning; Kohli, R., Mittal, K., Eds.; William Andrew: Norwich, NY, USA, 2012; pp. 1–80. [Google Scholar]
- Mutsuga, M.; Kawamura, Y.; Tanamoto, K. Migration of lactic acid, lactide and oligomers from polylactide food-contact materials. Food Addit. Contam. 2008, 25, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, R. The case for polylactic acid as a commodity packaging plastic. J. Macromol. Sci. Part A Pure Appl. Chem. 1996, 33, 585–597. [Google Scholar] [CrossRef]
- Hiljanen-Vainio, M.; Varpomaa, P.; Seppälä, J.; Törmälä, P. Modification of poly (L-lactides) by blending: Mechanical and hydrolytic behavior. Macromol. Chem. Phys. 1996, 197, 1503–1523. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Gross, R.; McCarthy, S. Reactive compatibilization of biodegradable blends of poly (lactic acid) and poly (ε-caprolactone). Polym. Degrad. Stab. 1998, 59, 161–168. [Google Scholar] [CrossRef]
- Notta-Cuvier, D.; Odent, J.; Delille, R.; Murariu, M.; Lauro, F.; Raquez, J.; Bennani, B.; Dubois, P. Tailoring polylactide (PLA) properties for automotive applications: Effect of addition of designed additives on main mechanical properties. Polym. Test. 2014, 36, 1–9. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Kawamoto, N.; Sakai, A.; Horikoshi, T.; Urushihara, T.; Tobita, E. Physical and mechanical properties of poly (l-lactic acid) nucleated by dibenzoylhydrazide compound. J. Appl. Polym. Sci. 2007, 103, 244–250. [Google Scholar] [CrossRef]
- Tábi, T.; Kovács, N.; Sajó, I.; Czigány, T.; Hajba, S.; Kovács, J. Comparison of thermal, mechanical and thermomechanical properties of poly (lactic acid) injection-molded into epoxy-based Rapid Prototyped (PolyJet) and conventional steel mold. J. Therm. Anal. Calorim. 2016, 123, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Qi, S.; Zhang, D.; Su, Y.; Wang, D. The role of poly (ethylene glycol) on crystallization, interlayer bond and mechanical performance of polylactide parts fabricated by fused filament fabrication. Addit. Manuf. 2020, 35, 101414. [Google Scholar] [CrossRef]
- Hriţuc, A.; Slătineanu, L.; Mihalache, A.; Dodun, O.; Coteaţă, M.; Nagîţ, G. Accuracy of polylactide parts made by 3D printing. In Proceedings of the Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2020; p. 1900064. [Google Scholar]
- Cheon, S.S.; Jeong, K.S. Composite side-door impact beams for passenger cars. Compos. Struct. 1997, 38, 229–239. [Google Scholar] [CrossRef]
- Joo, S.-J.; Yu, M.-H.; Kim, W.S.; Lee, J.-W.; Kim, H.-S. Design and manufacture of automotive composite front bumper assemble component considering interfacial bond characteristics between over-molded chopped glass fiber polypropylene and continuous glass fiber polypropylene composite. Compos. Struct. 2020, 236, 111849. [Google Scholar] [CrossRef]
- Poulikidou, S.; Jerpdal, L.; Björklund, A.; Åkermo, M. Environmental performance of self-reinforced composites in automotive applications—Case study on a heavy truck component. Mater. Des. 2016, 103, 321–329. [Google Scholar] [CrossRef]
- Yakout, M.; Elbestawi, M. Additive manufacturing of composite materials: An overview. In Proceedings of the 6th International Conference on Virtual Machining Process Technology (VMPT), Montréal, QC, Canada, 29 May–2 June 2017. [Google Scholar]
- Brøndsted, P.; Lilholt, H.; Lystrup, A. Composite materials for wind power turbine blades. Annu. Rev. Mater. Res. 2005, 35, 505–538. [Google Scholar] [CrossRef]
- Su, H.; Kam, T. Reliability analysis of composite wind turbine blades considering material degradation of blades. Compos. Struct. 2020, 234, 111663. [Google Scholar] [CrossRef]
- Rahimizadeh, A.; Kalman, J.; Henri, R.; Fayazbakhsh, K.; Lessard, L. Recycled glass fiber composites from wind turbine waste for 3D printing feedstock: Effects of fiber content and interface on mechanical performance. Materials 2019, 12, 3929. [Google Scholar] [CrossRef] [Green Version]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Misra, M.; Mohanty, A.K. Injection-moulded biocomposites from polylactic acid (PLA) and recycled carbon fibre: Evaluation of mechanical and thermal properties. J. Thermoplast. Compos. Mater. 2014, 27, 1286–1300. [Google Scholar] [CrossRef]
- Agüero, Á.; Lascano, D.; Garcia-Sanoguera, D.; Fenollar, O.; Torres-Giner, S. Valorization of linen processing by-products for the development of injection-molded green composite pieces of polylactide with improved performance. Sustainability 2020, 12, 652. [Google Scholar] [CrossRef] [Green Version]
- Quiles-Carrillo, L.; Montanes, N.; Garcia-Garcia, D.; Carbonell-Verdu, A.; Balart, R.; Torres-Giner, S. Effect of different compatibilizers on injection-molded green composite pieces based on polylactide filled with almond shell flour. Compos. Part B Eng. 2018, 147, 76–85. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. On the use of acrylated epoxidized soybean oil as a reactive compatibilizer in injection-molded compostable pieces consisting of polylactide filled with orange peel flour. Polym. Int. 2018, 67, 1341–1351. [Google Scholar] [CrossRef]
- Calì, M.; Pascoletti, G.; Gaeta, M.; Milazzo, G.; Ambu, R. A new generation of bio-composite thermoplastic filaments for a more sustainable design of parts manufactured by FDM. Appl. Sci. 2020, 10, 5852. [Google Scholar] [CrossRef]
- Matsuzaki, R.; Ueda, M.; Namiki, M.; Jeong, T.-K.; Asahara, H.; Horiguchi, K.; Nakamura, T.; Todoroki, A.; Hirano, Y. Three-dimensional printing of continuous-fiber composites by in-nozzle impregnation. Sci. Rep. 2016, 6, 23058. [Google Scholar] [CrossRef]
- López-Rodríguez, N.; López-Arraiza, A.; Meaurio, E.; Sarasua, J. Crystallization, morphology, and mechanical behavior of polylactide/poly (ε-caprolactone) blends. Polym. Eng. Sci. 2006, 46, 1299–1308. [Google Scholar] [CrossRef]
- Zhu, H.; Feng, X.; Zhang, H.; Guo, Y.; Zhang, J.; Chen, J. Structural characteristics and properties of silk fibroin/poly (lactic acid) blend films. J. Biomater.Sci. Polym. Ed. 2009, 20, 1259–1274. [Google Scholar] [CrossRef]
- Chen, G.-X.; Kim, H.-S.; Kim, E.-S.; Yoon, J.-S. Compatibilization-like effect of reactive organoclay on the poly (l-lactide)/poly (butylene succinate) blends. Polymer 2005, 46, 11829–11836. [Google Scholar] [CrossRef]
- Wang, R.; Wan, C.; Wang, S.; Zhang, Y. Morphology, mechanical properties, and durability of poly (lactic acid) plasticized with Di (isononyl) cyclohexane-1, 2-dicarboxylate. Polym. Eng. Sci. 2009, 49, 2414–2420. [Google Scholar] [CrossRef]
- Park, J.W.; Im, S.S. Phase behavior and morphology in blends of poly (L-lactic acid) and poly (butylene succinate). J. Appl. Polym. Sci. 2002, 86, 647–655. [Google Scholar] [CrossRef]
- Yokohara, T.; Yamaguchi, M. Structure and properties for biomass-based polyester blends of PLA and PBS. Eur. Polym. J. 2008, 44, 677–685. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(butylene succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas-Martínez, L.; Flores-Hernandez, C.; López-Marín, L.; Martinez-Hernandez, A.; Thorat, S.; Vasquez, C.R.; Del Rio-Castillo, A.; Velasco-Santos, C. 3D printing of PLA composites scaffolds reinforced with keratin and chitosan: Effect of geometry and structure. Eur. Polym. J. 2020, 141, 110088. [Google Scholar] [CrossRef]
- Brounstein, Z.; Yeager, C.M.; Labouriau, A. Development of Antimicrobial PLA Composites for Fused Filament Fabrication. Polymers 2021, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Ray, S.S. An overview of the recent advances in polylactide-based sustainable nanocomposites. Polym. Eng. Sci. 2021, 61, 617–649. [Google Scholar] [CrossRef]
- Dedukh, N.; Makarov, V.; Pavlov, A. Polylactide-based biomaterial and its use as bone implants (analytical literature review). Pain Jt. Spine 2019, 9, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Moetazedian, A.; Gleadall, A.; Han, X.; Silberschmidt, V.V. Effect of environment on mechanical properties of 3D printed polylactide for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 102, 103510. [Google Scholar] [CrossRef]
- Bulanda, K.; Oleksy, M.; Oliwa, R.; Budzik, G.; Gontarz, M. Biodegradable polymer composites based on polylactide used in selected 3D technologies. Polimery 2020, 65, 557–562. [Google Scholar] [CrossRef]
- Bergström, J.S.; Hayman, D. An overview of mechanical properties and material modeling of polylactide (PLA) for medical applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Duart, S.; Montanes, N.; Torres-Giner, S.; Balart, R. Enhancement of the mechanical and thermal properties of injection-molded polylactide parts by the addition of acrylated epoxidized soybean oil. Mater. Des. 2018, 140, 54–63. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iniguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly (lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masutani, K.; Kimura, Y. PLA Synthesis. From the Monomer to the Polymer. In Poly(Lactic Acid) Science and Technology Processing, Properties, Additives and Applications; Jimenez, A., Peltzer, M., Ruseckaite, R., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2015; pp. 3–36. [Google Scholar]
- Gupta, A.; Kumar, V. New emerging trends in synthetic biodegradable polymers–Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Wintergerste, T. Great advances in bioplastics. Sulzer Tech. Rev. 2012, 13–14. [Google Scholar]
- Andrzejewska, A. One Year Evaluation of material properties changes of polylactide parts in various hydrolytic degradation conditions. Polymers 2019, 11, 1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunt, J.; Shafer, A.L. Polylactic acid polymers from com. Applications in the textiles industry. J. Ind. Text. 2000, 29, 191–205. [Google Scholar] [CrossRef]
- Swiftpak. Eco-friendly Packaging—PLA Packaging: The Ultimate Guide. Available online: https://www.swiftpak.co.uk/insights/pla-packaging-the-ultimate-guide (accessed on 15 December 2021).
- EU, P.B. LCA of PLA Bottle Solutions for Extended Shelf-Life (ESL) Milk. Available online: https://plabottles.eu/lca-of-pla-bottle-for-milk/ (accessed on 15 December 2021).
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E. Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties. e-Polymers 2020, 20, 571–599. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Wu, B.; Cui, C.; Guo, Y.; Yan, C. A critical review of fused deposition modeling 3D printing technology in manufacturing polylactic acid parts. Int. J. Adv. Manuf. Technol. 2019, 102, 2877–2889. [Google Scholar] [CrossRef]
- Gad, S.C. Integrated Safety and Risk Assessment for Medical Devices and Combination Products; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- SP Bel-Art. Earth-Friendly 1001 Sticks. Available online: https://www.belart.com/earth-friendly-1001-sticks.html (accessed on 15 December 2021).
- SP Bel-Art. Earth-Friendly Long Handle Sampling Spoon. Available online: https://www.belart.com/earth-friendly-long-handle-sampling-spoons.html (accessed on 15 December 2021).
- Gordeev, E.; Degtyareva, E.; Ananikov, V. Analysis of 3D printing possibilities for the development of practical applications in synthetic organic chemistry. Russ. Chem. Bull. 2016, 65, 1637–1643. [Google Scholar] [CrossRef]
- ISO. ISO—International Organization for Standardization. Available online: https://www.iso.org/ (accessed on 15 December 2021).
- ISO 6706:1981; Plastics Laboratory Ware—Graduated Measuring Cylinders. International Organization for Standardization: Geneva, Switzerland, 1981.
- ISO 7056:1981; Plastics Laboratory Ware—Beakers. International Organization for Standardization: Geneva, Switzerland, 1981.
- ISO 7057:1981; Plastic Laboratory Ware—Filter Funnels. International Organization for Standardization: Geneva, Switzerland, 1981.
- ISO 12771:1997; Plastics Laboratory Ware—Disposable Serological Pipettes. International Organization for Standardization: Geneva, Switzerland, 1981.
- ISO 24998:2008; Plastics Laboratory Ware—Single-Use Petri Dishes for Microbiological Procedures. International Organization for Standardization: Geneva, Switzerland, 1981.
- Thermo Fisher Scientific. Centrifuge Tubes. Available online: https://www.fishersci.nl/nl/en/products/I9C8L7UU/centrifuge-tubes.html (accessed on 15 December 2021).
- ISO 6427:2013; Plastics—Determination of Matter Extractable by Organic Solvents (conventional Methods). International Organization for Standardization: Geneva, Switzerland, 2013.
- SR CEN/TR 15932; Plastics—Recommendation for Terminology and Characterisation of Biopolymers and Bioplastics. Nation Standards Authority of Ireland: Dublin, Ireland, 2010.
- I.S. EN 13432:2001; Packaging—Requirements for Packaging Recoverable through Composting and Biodegradation—Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. Nation Standards Authority of Ireland: Dublin, Ireland, 2001.
- I.S. EN 14995:2006; Plastics—Evaluation of Compostability—Test Scheme and Specifications. Nation Standards Authority of Ireland: Dublin, Ireland, 2007.
- Scaffaro, R.; Morreale, M.; Mirabella, F.; La Mantia, F.P. Preparation and recycling of plasticized PLA. Macromol. Mater. Eng. 2011, 296, 141–150. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. The effect of natural additives on the composting properties of aliphatic polyesters. Polymers 2020, 12, 1856. [Google Scholar] [CrossRef]
- De Andrade, M.F.C.; Souza, P.M.; Cavalett, O.; Morales, A.R. Life cycle assessment of poly (lactic acid)(PLA): Comparison between chemical recycling, mechanical recycling and composting. J. Polym. Environ. 2016, 24, 372–384. [Google Scholar] [CrossRef]
- Piemonte, V.; Sabatini, S.; Gironi, F. Chemical recycling of PLA: A great opportunity towards the sustainable development? J. Polym. Environ. 2013, 21, 640–647. [Google Scholar] [CrossRef]
- Aryan, V.; Maga, D.; Majgaonkar, P.; Hanich, R. Valorisation of polylactic acid (PLA) waste: A comparative life cycle assessment of various solvent-based chemical recycling technologies. Resour. Conserv. Recycl. 2021, 172, 105670. [Google Scholar] [CrossRef]
- Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y. (Micro) plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020, 254, 120138. [Google Scholar] [CrossRef]
- Dormer, A.; Finn, D.P.; Ward, P.; Cullen, J. Carbon footprint analysis in plastics manufacturing. J. Clean. Prod. 2013, 51, 133–141. [Google Scholar] [CrossRef]
- Posen, I.D.; Jaramillo, P.; Landis, A.E.; Griffin, W.M. Greenhouse gas mitigation for US plastics production: Energy first, feedstocks later. Environ. Res. Lett. 2017, 12, 034024. [Google Scholar] [CrossRef]
- USDA. Departmental Regulation; Biobased Products Procurement Program. Available online: http://www.ocio.usda.gov/directives/doc/DR5023-002.htm (accessed on 5 December 2021).
- Álvarez-Chávez, C.R.; Edwards, S.; Moure-Eraso, R.; Geiser, K. Sustainability of bio-based plastics: General comparative analysis and recommendations for improvement. J. Clean. Prod. 2012, 23, 47–56. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Wellenreuther, C.; Wolf, A.; Zander, N. Cost Structure of Bio-Based Plastics: A Monte-Carlo-Analysis for PLA; HWWI Research Paper: Hamburg, Germany, 2021. [Google Scholar]
- Crutchik, D.; Franchi, O.; Caminos, L.; Jeison, D.; Belmonte, M.; Pedrouso, A.; Val del Rio, A.; Mosquera-Corral, A.; Campos, J.L. Polyhydroxyalkanoates (PHAs) production: A feasible economic option for the treatment of sewage sludge in municipal wastewater treatment plants? Water 2020, 12, 1118. [Google Scholar] [CrossRef]
- Leong, Y.K.; Show, P.L.; Lan, J.C.-W.; Loh, H.-S.; Lam, H.L.; Ling, T.C. Economic and environmental analysis of PHAs production process. Clean Technol. Environ. Policy 2017, 19, 1941–1953. [Google Scholar] [CrossRef]
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Techno-economic analysis of a food waste valorisation process for lactic acid, lactide and poly (lactic acid) production. J. Clean. Prod. 2018, 181, 72–87. [Google Scholar] [CrossRef]



| Polymer | Typical Items | General Properties |
|---|---|---|
| Polymethyl pentene (PMP) | Beakers, Cylinders, Erlenmeyer Flasks, Jars | Rigid, translucent, fair UV resistance |
| High Density Polyethylene (HDPE) | Bottles, Carboys, Pans | Semi-rigid, translucent, poor UV resistance |
| Low Density Polyethylene (LDPE) | Bottles, Carboys, Wash or Dropper Bottles | Flexible, translucent, fair UV resistance |
| Polypropylene (PP) | Autoclave baskets, Carboys, Funnels, Vacuum Flasks | Rigid, translucent, fair UV resistance |
| Polypropylene Copolymer (PPCO) | Bottles, Beakers, Centrifuge Tubes, Graduated Cylinders | Semi-rigid, translucent, fair UV resistance |
| Polyvinyl Chloride (PVC) | Tubing | Very flexible, transparent |
| Polyethylene Terephthalate G Copolyester (PETG) | Bioprocessing Containers, Bottles, Erlenmeyer Flasks | Moderately flexible, transparent, fair UV resistance |
| Polytetrafluoroethylene (PTFE) | Stirrers, Test Tubes, Vessels | High thermal stability and chemical inertness |
| Polystyrene (PS) | Filtration Units, Pipettes/Tips, Single-Use Petri Dishes | Rigid, transparent, fair chemical resistance, poor UV resistance |
| Polycarbonate (PC) | Bottles, Culture Flasks, Desiccators, Jars | Rigid, transparent, fair UV resistance |
| Polysulfone (PSF) | Bottles, Centrifuge Tubes, Filtration Units | Rigid, transparent, poor UV resistance |
| Teflon (FEP) | Bottles, Centrifuge Tubes, Wash bottles | Very flexible, translucent, good UV resistance |
| Teflon (PFA) | Beakers, Bottles, Cylinders, Tubing | Very flexible, translucent, fair UV resistance |
| Properties | General Purpose |
|---|---|
| Specific Gravity | 1.04 |
| Specific Heat (J/kg K) | 1256–1465 |
| Thermal Conductivity (W/mK) | 0.100–0.156 |
| Thermal Expansion (K−1) | 5.94–8.64 × 10−5 |
| Ultimate Tensile Strength (MPa) | 34.5–68.9 |
| Yield Strength (MPa) | 34.5–68.9 |
| Flexural Strength (MPa) | 68.9–103 |
| Impact Strength-Izod notched (J/m) | - |
| Tensile Elastic Modulus (GPa) | 3.17–3.45 |
| Flexural Elastic Modulus (GPa) | 2.76–3.45 |
| Yield Elongation (%) | 1–2.3 |
| Max Elongation (%) | 1.0–2.3 |
| Hardness (Rockwell) | M72 |
| Refractive Index | 1.6 |
| Water Absorption (% in 24 h) | 0.03–0.2 |
| Properties | PET | PETG |
|---|---|---|
| Specific Gravity | 1.38 | 1.27 |
| Thermal Conductivity (W/mK) | 0.29 | - |
| Glass Transition Temperature | 340–413 | 354 |
| Thermal Expansion (m/mK) | 6.84 | 6.84 |
| Tensile Strength (MPa) | 58.6–72.4 | 53.1 |
| Flexural Strength (MPa) | 96.5–124.1 | 77.2 |
| Impact Strength-Izod notched (J/m) | 13.34–34.68 | 90.8 |
| Tensile Elastic Modulus (GPa) | 2.7–4.1 | 2.21 |
| Flexural Elastic Modulus (GPa) | 2.4–3.1 | 2.14 |
| Max Elongation (%) | 30–80 | - |
| Hardness (Rockwell) | M50–100 | 115 (R Scale) |
| Refractive Index | 1.58 | 1.57 |
| Water Absorption (% in 24 h) | 0.1–0.2 | 0.2 |
| Properties | Polycarbonate |
|---|---|
| Specific Gravity | 1.2 |
| Specific Heat (J/kg K) | 1260 |
| Thermal Conductivity (W/mK) | 0.190 |
| Thermal Expansion (K−1) | 6.75 × 10−6 |
| Ultimate Tensile Strength (MPa) | 65.5 |
| Yield Strength (MPa) | 58.6 |
| Flexural Strength (MPa) | 93.1 |
| Impact Strength-Izod notched (J/m) | 641–854 |
| Tensile Elastic Modulus (GPa) | 2.38 |
| Flexural Elastic Modulus (GPa) | 2.34 |
| Yield Elongation (%) | 5 |
| Max Elongation (%) | 110 |
| Hardness (Rockwell) | M70 |
| Refractive Index | 1.586 |
| Water Absorption (% in 24 h) | 0.15 |
| Properties | General Purpose |
|---|---|
| Specific Gravity | 0.90–0.91 |
| Specific Heat (J/kg K) | 1880 |
| Thermal Conductivity (W/mK) | 2.09–2.35 |
| Thermal Expansion (K−1) | 6.84–10.44 × 10−5 |
| Tensile Strength (MPa) | 31.0–41.4 |
| Yield Strength (MPa) | 31.0–41.4 |
| Flexural Strength (MPa) | 41.4–48.3 |
| Impact Strength-Izod notched (J/m) | 21.4–117 |
| Tensile Elastic Modulus (GPa) | - |
| Flexural Elastic Modulus (GPa) | 1.17–1.72 |
| Yield Elongation (%) | 9–15 |
| Max Elongation (%) | 100–600 |
| Hardness (Rockwell) | R80–100 |
| Refractive Index | Opaque |
| Water Absorption (% in 24 h) | <0.01–0.03 |
| Properties | PLA |
|---|---|
| Specific Gravity | 1.24 |
| Tensile Strength (MPa) | 62.1 |
| Tensile Elongation (%) | 3.5 |
| Impact Strength-Izod notched (J/m) | 16 |
| Flexural Strength (MPa) | 108 |
| Flexural Modulus (MPa) | 3600 |
| Glass Transition Temperature (K) | 328 |
| Melting Temperature (K) | 428 |
| Heat Distortion Temperature (K) | 328 |
| Clarity | Transparent |
| Properties | PLA | PS | PET |
|---|---|---|---|
| Density (kg/m3) | 1.26 | 1.05 | 1.40 |
| Ultimate Tensile Strength (MPa) | 59 | 45 | 57 |
| Elastic Modulus (GPa) | 3.8 | 3.2 | 2.8–4.1 |
| Max Elongation (%) | 4–7 | 3 | 300 |
| Impact Strength-Izod notched (J/m) | 26 | 21 | 59 |
| Heat Deflection (°C) | 55 | 75 | 67 |
| PLLA | ||||
| Molecular Weight (g/mol) | 23,000 | 31,000 | 58,000 | 67,000 |
| Ultimate Tensile Strength (MPa) | 59 | 55 | 58 | 59 |
| Yield Strength (MPa) | 65 | 68 | 70 | |
| Max Elongation (%) | 1.5 | 5.5 | 5.0 | 7.0 |
| Yield Elongation (%) | 2.2 | 2.3 | 2.2 | |
| Tensile Elastic Modulus (GPa) | 3.55 | 3.55 | 3.75 | 4.75 |
| Flexural Strength (MPa) | 64 | 97 | 100 | 106 |
| Max Flexural Strain (%) | 2.0 | 4.2 | 4.1 | 4.7 |
| Flexural Elastic Modulus (GPa) | 3.65 | 3.60 | 3.60 | 3.65 |
| Impact Strength-notched (J/m) | 19 | 22 | 25 | 26 |
| Impact Strength-unnotched (J/m) | 135 | 175 | 185 | 195 |
| Heat Deflection Temperature (°C) | 57 | 55 | 55 | |
| Vicat Penetration (°C) | 60 | 59 | 59 | 59 |
| Rockwell Hardness (HR) | 85 | 84 | 83 | 88 |
| Annealed PLLA | ||||
| Molecular Weight (g/mol) | 20,000 | 33,500 | 47,000 | 71,000 |
| Ultimate Tensile Strength (MPa) | 47 | 54 | 59 | 66 |
| Yield Strength (MPa) | 63 | 68 | 70 | |
| Max Elongation (%) | 1.3 | 3.3 | 3.5 | 4.0 |
| Yield Elongation (%) | 1.8 | 2.2 | 2 | |
| Tensile Elastic Modulus (GPa) | 4.10 | 4.10 | 4.05 | 4.15 |
| Flexural Strength (MPa) | 51 | 83 | 113 | 119 |
| Max Flexural Strain (%) | 1.6 | 2.3 | 4.8 | 4.6 |
| Flexural Elastic Modulus (GPa) | 4.20 | 4.00 | 4.05 | 4.15 |
| Impact Strength-notched (J/m) | 32 | 55 | 70 | 66 |
| Impact Strength-unnotched (J/m) | 180 | 360 | 340 | 350 |
| Heat Deflection Temperature (°C) | 66 | 60 | 61 | |
| Vicat Penetration (°C) | 157 | 159 | 163 | 165 |
| Rockwell Hardness (HR) | 84 | 82 | 84 | 88 |
| PDLLA | ||||
| Molecular Weight (g/mol) | 47,500 | 75,000 | 114,000 | |
| Ultimate Tensile Strength (MPa) | 40 | 44 | 44 | |
| Yield Strength (MPa) | 49 | 53 | 53 | |
| Max Elongation (%) | 7.5 | 4.8 | 5.4 | |
| Yield Elongation (%) | 1.7 | 1.4 | 1.5 | |
| Tensile Elastic Modulus (GPa) | 3.65 | 4.05 | 3.90 | |
| Flexural Strength (MPa) | 84 | 86 | 88 | |
| Max Flexural Strain (%) | 4.8 | 4.1 | 4.2 | |
| Flexural Elastic Modulus (GPa) | 3.50 | 3.55 | 3.60 | |
| Impact Strength-notched (J/m) | 18 | 17 | 18 | |
| Impact Strength-unnotched (J/m) | 135 | 140 | 150 | |
| Heat Deflection Temperature (°C) | 51 | 50 | 50 | |
| Vicat Penetration (°C) | 52 | 53 | 52 | |
| Rockwell Hardness (HR) | 78 | 72 | 76 | |
| Isomer Type | Mn | Mw/Mn | Tg | Tm | ΔHm | Tc | ΔHc |
|---|---|---|---|---|---|---|---|
| L | 4700 | 1.09 | 45.6 | 157.8 | 55.5 | 98.3 | 47.8 |
| DL | 4300 | 1.9 | 44.7 | - | - | - | - |
| L | 7000 | 1.09 | 67.9 | 159.9 | 58.8 | 108.3 | 48.3 |
| DL | 7300 | 1.16 | 44.1 | - | - | - | - |
| D | 13,800 | 1.19 | 65.7 | 170.3 | 67.0 | 107.6 | 52.4 |
| L | 14,000 | 1.12 | 66.8 | 173.3 | 61.0 | 110.3 | 48.1 |
| D | 16,500 | 1.2 | 69.1 | 173.5 | 64.6 | 109.0 | 51.6 |
| L | 16,800 | 1.32 | 58.6 | 173.4 | 61.4 | 105.0 | 38.1 |
| Techniques | Conditions | Advantages | Disadvantages |
|---|---|---|---|
| Steam | High steam pressure, 120–135 °C | No toxic residue | Deformation or degradation due to water attack, limited usage for lactic acid-based polymers |
| Dry heat | 160–190 °C | No toxic residue | Melting and softening of polymer, not usable for lactic acid-based polymers |
| Radiation | Ionising or gamma | High penetration, low chemical reactivity, and quick effect | Instability and deterioration, crosslinking or breaking of polymer chains |
| Gas | Ethylene oxide | Low temperature range | Lengthy process due to degassing, residues are toxic |
| Solvents | δd | δp | δh | δt |
|---|---|---|---|---|
| Acetone | 15.0 | 10.4 | 7.0 | 19.6 |
| Acetonitrile | 15.3 | 18.0 | 6.1 | 24.4 |
| Benzene | 18.4 | 0.0 | 2.0 | 18.5 |
| Chloroform | 17.8 | 3.1 | 5.5 | 18.9 |
| m-Cresol | 18.0 | 5.1 | 12.9 | 22.7 |
| Dimethyl formamide | 17.4 | 13.7 | 11.3 | 24.9 |
| Dimethyl sulfoxide | 18.4 | 16.4 | 10.0 | 26.6 |
| 1-4 Dioxane | 19.0 | 1.8 | 7.4 | 20.5 |
| 1-3 Dioxolane | 18.1 | 6.6 | 9.3 | 21.4 |
| Ethyl acetate | 15.8 | 5.3 | 7.2 | 18.2 |
| Furan | 17.8 | 1.8 | 5.3 | 18.7 |
| Hexafluoro isopropanol | 17.2 | 4.5 | 14.7 | 23.1 |
| Isoamyl alcohol | 15.8 | 5.2 | 13.3 | 21.3 |
| Methylene dichloride | 18.2 | 6.3 | 6.1 | 20.2 |
| Methyl ethyl ketone | 16.0 | 9.0 | 5.1 | 19.1 |
| N-Methyl pyrrolidone | 18.0 | 12.3 | 7.2 | 23.0 |
| Pyridine | 19.0 | 8.8 | 5.9 | 31.8 |
| Tetrahydrofuran | 16.8 | 5.7 | 8.0 | 19.5 |
| Toluene | 18.0 | 1.4 | 2.0 | 18.2 |
| Xylene | 17.6 | 1.0 | 3.1 | 17.9 |
| Isopropyl ether | 13.7 | 3.9 | 2.3 | 14.4 |
| Cyclohexane | 16.5 | 0.0 | 0.2 | 16.5 |
| Hexane | 14.9 | 0.0 | 0.0 | 14.9 |
| Ethanol | 15.8 | 8.8 | 19.4 | 26.5 |
| Methanol | 15.1 | 12.3 | 22.3 | 29.6 |
| Water | 15.5 | 16.0 | 42.3 | 47.8 |
| Diethyl ether | 14.5 | 2.9 | 5.1 | 15.6 |
| Method | δd | δp | δh | δt |
|---|---|---|---|---|
| Intrinsic 3D viscosity method | 17.61 | 5.30 | 5.80 | 19.28 |
| Intrinsic 1D viscosity method | - | - | - | 19.16 |
| Classical 3D geometric method | 16.85 | 9.00 | 4.05 | 19.53 |
| Fedors group contribution | - | - | - | 21.42 |
| Van Krevelen group contribution | - | - | - | 17.64 |
| Optimisation method | 18.50 | 9.70 | 6.00 | 21.73 |
| ISO No. | ISO Name |
|---|---|
| 384:2015 | Laboratory glass and plastics ware—Principles of design and construction of volumetric instruments |
| 6706:1981 | Plastics Laboratory Ware-Graduated Measuring Cylinders |
| 7056:1981 | Plastics Laboratory Ware-Beakers |
| 7057:1981 | Plastics Laboratory Ware-Filter Funnels |
| 12771:1997 | Plastics Laboratory Ware-Disposable Serological Pipettes |
| 24998:2008 | Plastics Laboratory Ware-Single-Use Petri Dishes for Microbiological Procedures |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freeland, B.; McCarthy, E.; Balakrishnan, R.; Fahy, S.; Boland, A.; Rochfort, K.D.; Dabros, M.; Marti, R.; Kelleher, S.M.; Gaughran, J. A Review of Polylactic Acid as a Replacement Material for Single-Use Laboratory Components. Materials 2022, 15, 2989. https://doi.org/10.3390/ma15092989
Freeland B, McCarthy E, Balakrishnan R, Fahy S, Boland A, Rochfort KD, Dabros M, Marti R, Kelleher SM, Gaughran J. A Review of Polylactic Acid as a Replacement Material for Single-Use Laboratory Components. Materials. 2022; 15(9):2989. https://doi.org/10.3390/ma15092989
Chicago/Turabian StyleFreeland, Brian, Eanna McCarthy, Rengesh Balakrishnan, Samantha Fahy, Adam Boland, Keith D. Rochfort, Michal Dabros, Roger Marti, Susan M. Kelleher, and Jennifer Gaughran. 2022. "A Review of Polylactic Acid as a Replacement Material for Single-Use Laboratory Components" Materials 15, no. 9: 2989. https://doi.org/10.3390/ma15092989
APA StyleFreeland, B., McCarthy, E., Balakrishnan, R., Fahy, S., Boland, A., Rochfort, K. D., Dabros, M., Marti, R., Kelleher, S. M., & Gaughran, J. (2022). A Review of Polylactic Acid as a Replacement Material for Single-Use Laboratory Components. Materials, 15(9), 2989. https://doi.org/10.3390/ma15092989







