Decomposition Characteristics of the TTIP (Tetraisopropyl Orthotitanate) Precursor for Atomic Layer Deposition
Abstract
:1. Introduction
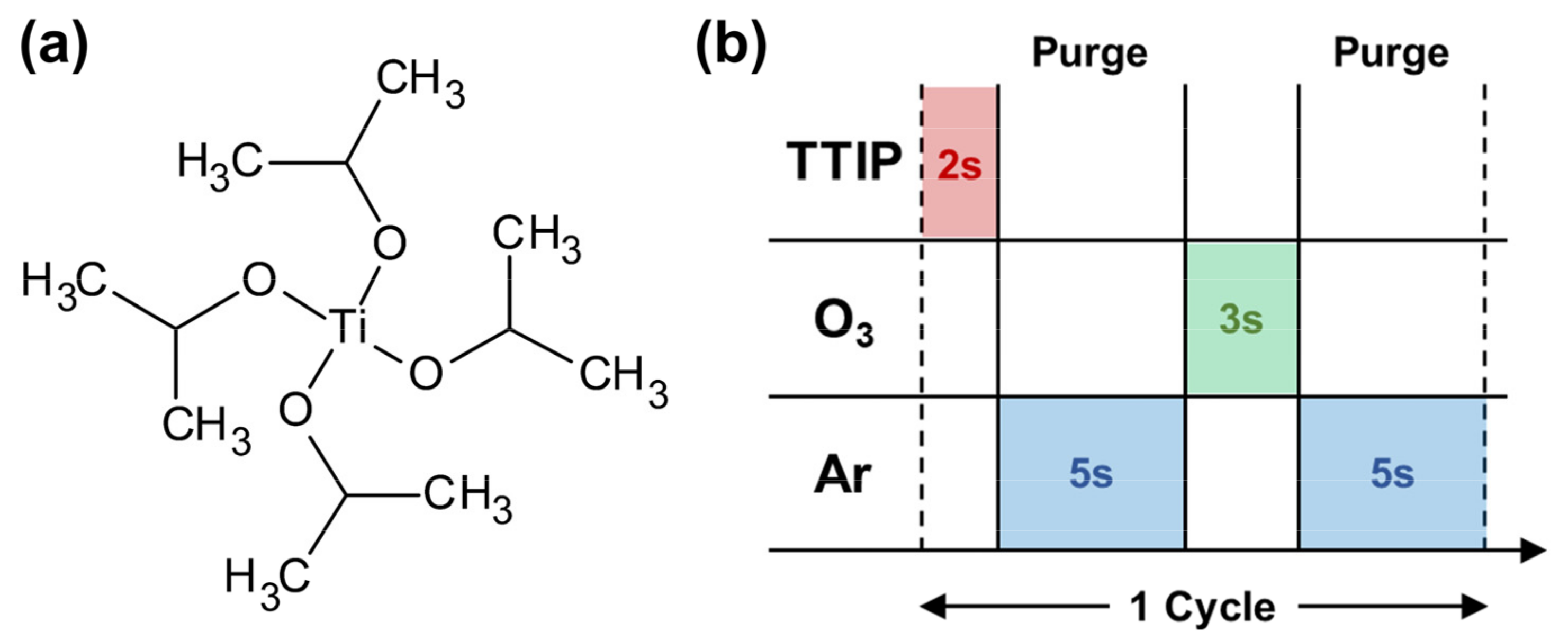
2. Materials and Methods
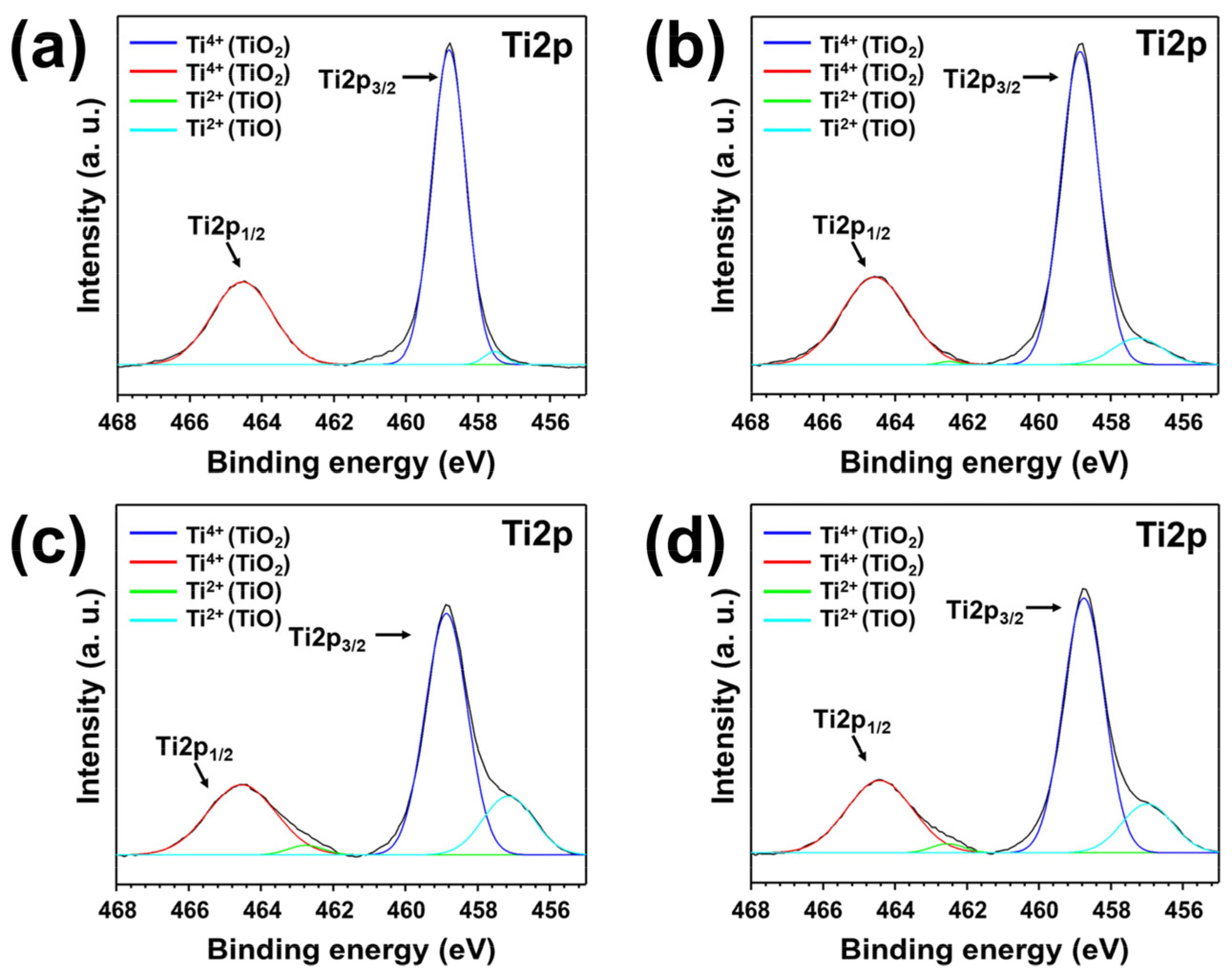
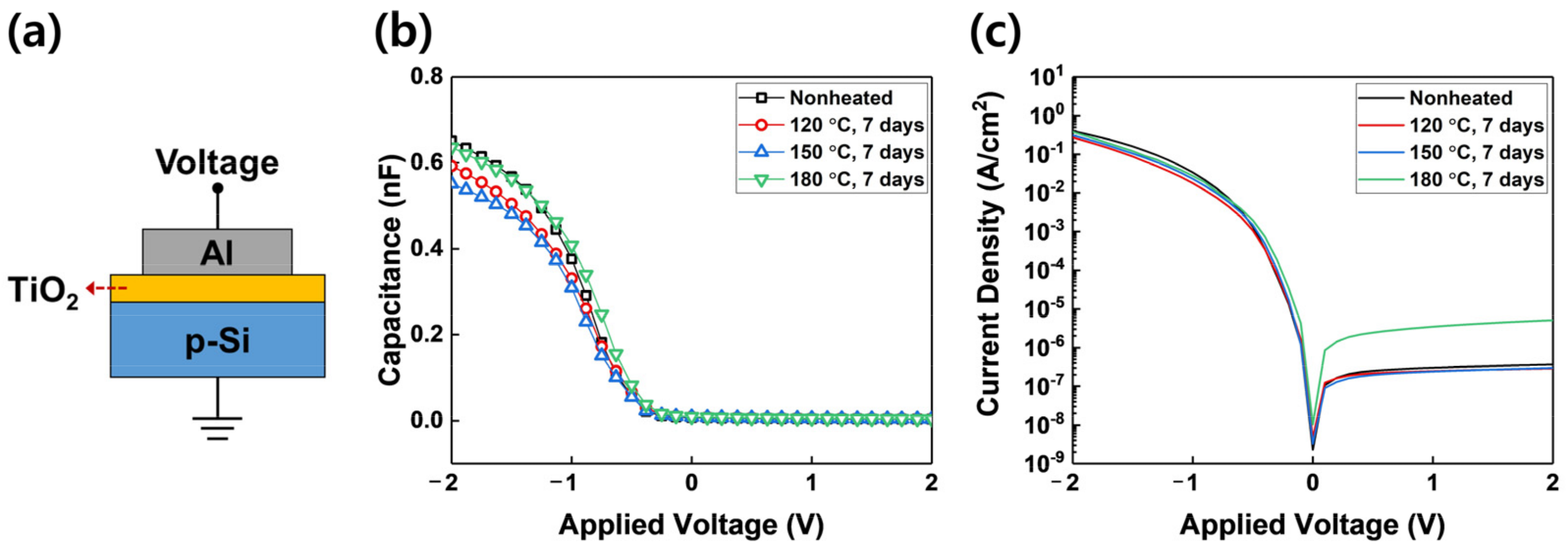
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atanassova, E.; Spasov, D. Thermal Ta2O5—Alternative to SiO2 for storage capacitor application. Microelectron. Reliab. 2002, 42, 1171–1177. [Google Scholar] [CrossRef]
- Yu, T.; Jin, C.G.; Dong, Y.J.; Cao, D.; Zhuge, L.J.; Wu, X.M. Temperature dependence of electrical properties for MOS capacitor with HfO2/SiO2 gate dielectric stack. Mater. Sci. Semicond. Process. 2013, 16, 1321–1327. [Google Scholar] [CrossRef]
- Hourdakis, E.; Nassiopoulou, A.G.; Casanova, A.; Larrieu, G. In Model 3D MOS capacitor system using regular arrays of vertical Si nanowires. In Proceedings of the 2017 Joint International EUROSOI Workshop and International Conference on Ultimate Integration on Silicon (EUROSOI-ULIS), Athens, Greece, 3–5 April 2017. [Google Scholar] [CrossRef]
- Singh, R.; Paily, R.; DasGupta, A.; DasGupta, N.; Misra, P.; Kukreja, L.M. Optimized dual temperature pulsed laser deposition of TiO2 to realize MTOS (metal-TiO2–SiO2–Si) capacitors with ultrathin gate dielectric. Semicond. Sci. Technol. 2004, 20, 38. [Google Scholar] [CrossRef]
- Houssa, M.; Mertens, P.W.; Heyns, M.M. Relation between stress-induced leakage current and time-dependent dielectric breakdown in ultra-thin gate oxides. Semicond. Sci. Technol. 1999, 14, 892. [Google Scholar] [CrossRef]
- Xu, Z.; Houssa, M.; De Gendt, S.; Heyns, M. Polarity effect on the temperature dependence of leakage current through HfO2/SiO2 gate dielectric stacks. Appl. Phys. Lett. 2002, 80, 1975–1977. [Google Scholar] [CrossRef]
- Park, S.-U.; Kang, C.-Y.; Kwon, H.-M.; Park, B.-S.; Choi, W.-H.; Han, I.-S.; Bersuker, G.; Jammy, R.; Lee, H.-D. Analysis of reliability characteristics of high capacitance density MIM capacitors with SiO2–HfO2–SiO2 dielectrics. Microelectron. Eng. 2011, 88, 3389–3392. [Google Scholar] [CrossRef]
- Jakschik, S.; Avellan, A.; Schroeder, U.; Bartha, J.W. Influence of Al2O3 dielectrics on the trap-depth profiles in MOS devices investigated by the charge-pumping method. IEEE Trans. Electron. Devices 2004, 51, 2252–2255. [Google Scholar] [CrossRef]
- Nandi, S.K.; Chakraborty, S.; Bera, M.K.; Maiti, C.K. Structural and optical properties of ZnO films grown on silicon and their applications in MOS devices in conjunction with ZrO2 as a gate dielectric. Bull. Mater. Sci. 2007, 30, 247–254. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Z.G. Structure and dielectric properties of ultra-thin ZrO2 films for high-k gate dielectric application prepared by pulsed laser deposition. Appl. Phys. A 2004, 78, 741–744. [Google Scholar] [CrossRef]
- Yang, B.L.; Wong, H.; Kakushima, K.; Iwai, H. Improving the electrical characteristics of MOS transistors with CeO2/La2O3 stacked gate dielectric. Microelectron. Reliab. 2012, 52, 1613–1616. [Google Scholar] [CrossRef]
- Lo, G.Q.; Kwong, D.L.; Lee, S. Reliability characteristics of metal-oxide-semiconductor capacitors with chemical vapor deposited Ta2O5 gate dielectrics. Appl. Phys. Lett. 1993, 62, 973–975. [Google Scholar] [CrossRef]
- Novkovski, N. Physical modeling of electrical and dielectric properties of high-k Ta2O5 based MOS capacitors on silicon. FU Elec. Energ. 2014, 27, 259–273. [Google Scholar] [CrossRef]
- Wu, J.-R.; Wu, Y.-H.; Lin, C.-C.; Ou, W.-Y.; Wu, M.-L.; Chen, L.-L. Effect of Nitrogen Passivation on the Performance of MIM Capacitors with a Crystalline TiO2/SiO2 Stacked Insulator. IEEE Electron Device Lett. 2012, 33, 878–880. [Google Scholar] [CrossRef]
- Paily, R.; DasGupta, A.; DasGupta, N.; Bhattacharya, P.; Misra, P.; Ganguli, T.; Kukreja, L.M.; Balamurugan, A.K.; Rajagopalan, S.; Tyagi, A.K. Pulsed laser deposition of TiO2 for MOS gate dielectric. Appl. Surf. Sci. 2002, 187, 297–304. [Google Scholar] [CrossRef]
- Chandra Sekhar, M.; Kondaiah, P.; Jagadeesh Chandra, S.V.; Mohan Rao, G.; Uthanna, S. Substrate temperature influenced physical properties of silicon MOS devices with TiO2 gate dielectric. Surf. Interface Anal. 2012, 44, 1299–1304. [Google Scholar] [CrossRef]
- Ahmad, T.; Shahazad, M.; Ubaidullah, M.; Ahmed, J. Synthesis, characterization and dielectric properties of TiO2–CeO2 ceramic nanocomposites at low titania concentration. Bull. Mater. Sci. 2018, 41, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Ajimsha, R.S.; Rajiv, K.; Mukherjee, C.; Gupta, M.; Misra, P.; Kukreja, L.M. Spectroscopic ellipsometry characterization of amorphous and crystalline TiO2 thin films grown by atomic layer deposition at different temperatures. Appl. Surf. Sci. 2014, 315, 116–123. [Google Scholar] [CrossRef]
- Triyoso, D.H.; Hegde, R.I.; Grant, J.; Fejes, P.; Liu, R.; Roan, D.; Ramon, M.; Werho, D.; Rai, R.; La, L.B.; et al. Film properties of ALD HfO2 and La2O3 gate dielectrics grown on Si with various pre-deposition treatments. J. Vac. Sci. Technol. 2004, 22, 2121–2127. [Google Scholar] [CrossRef]
- Niemelä, J.-P.; Marin, G.; Karppinen, M. Titanium dioxide thin films by atomic layer deposition: A review. Semicond. Sci. Technol. 2017, 32, 093005. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Film. 2002, 409, 138–146. [Google Scholar] [CrossRef]
- Zydor, A.; Elliott, S.D. Thermal Stability of Precursors for Atomic Layer Deposition of TiO2, ZrO2, and HfO2: An Ab Initio Study of α-Hydrogen Abstraction in Bis-Cyclopentadienyl Dimethyl Complexes. J. Phys. Chem. A 2010, 114, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Blanquart, T.; Niinistö, J.; Gavagnin, M.; Longo, V.; Pallem, V.R.; Dussarrat, C.; Ritala, M.; Leskelä, M. Novel heteroleptic precursors for atomic layer deposition of TiO2. Chem. Mater. 2012, 24, 3420–3424. [Google Scholar] [CrossRef]
- Niinistö, J.; Mäntymäki, M.; Kukli, K.; Costelle, L.; Puukilainen, E.; Ritala, M.; Leskelä, M. Growth and phase stabilization of HfO2 thin films by ALD using novel precursors. J. Cryst. Growth 2010, 312, 245–249. [Google Scholar] [CrossRef]
- Rushworth, S.; Coward, K.; Davies, H.; Heys, P.; Leese, T.; Kempster, L.; Odedra, R.; Song, F.; Williams, P. Thermal stability studies for advanced Hafnium and Zirconium ALD precursors. Surf. Coat. Technol. 2007, 201, 9060–9065. [Google Scholar] [CrossRef]
- Lee, B.; Choi, K.J.; Hande, A.; Kim, M.J.; Wallace, R.M.; Kim, J.; Senzaki, Y.; Shenai, D.; Li, H.; Rousseau, M.; et al. A novel thermally-stable zirconium amidinate ALD precursor for ZrO2 thin films. Microelectron. Eng. 2009, 86, 272–276. [Google Scholar] [CrossRef]
- Niinistö, J.; Kukli, K.; Kariniemi, M.; Ritala, M.; Leskelä, M.; Blasco, N.; Pinchart, A.; Lachaud, C.; Laaroussi, N.; Wang, Z.; et al. Novel mixed alkylamido-cyclopentadienyl precursors for ALD of ZrO2 thin films. J. Mater. Chem. 2008, 18, 5243–5247. [Google Scholar] [CrossRef]
- Pinchart, A.; Blasco, N.; Lachaud, C.; Schleisman, A.; Dussarrat, C.; Suzuki, I.; Yanagita, K. Novel thermally-stable hafnium and zirconium ALD precursors. In Proceedings of the 2007 IEEE/SEMI Advanced Semiconductor Manufacturing Conference, Stresa, Italy, 11–12 June 2007. [Google Scholar] [CrossRef]
- Blanquart, T.; Niinisto, J.; Aslam, N.; Banerjee, M.; Tomczak, Y.; Gavagnin, M.; Longo, V.; Puukilainen, E.; Wanzenboeck, H.D.; Kessels, W.M.M.; et al. [Zr(NEtMe)2(guan-NEtMe)2] as a novel atomic layer deposition precursor: ZrO2 film growth and mechanistic studies. Chem. Mater. 2013, 25, 3088–3095. [Google Scholar] [CrossRef]
- Niinistö, J.; Rahtu, A.; Putkonen, M.; Ritala, M.; Leskelä, M.; Niinistö, L. In Situ Quadrupole Mass Spectrometry Study of Atomic-Layer Deposition of ZrO2 using Cp2Zr(CH3)2 and water. Lanmuir 2005, 21, 7321–7325. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Uustare, T.; Ritala, M.; Leskelä, M. Titanium isopropoxide as a precursor for atomic layer deposition: Characterization of titanium dioxide growth process. Appl. Surf. Sci. 2000, 161, 385–395. [Google Scholar] [CrossRef]
- Yun, J.-Y.; Heo, S.-W.; Kang, S.-W.; Na, J.-G.; Park, Y.-J.; Shin, Y.-H.; Lee, J.-H.; Kim, T.-S.; Moon, D.-K. A study on the real-time decomposition monitoring of a metal organic precursor for metal organic chemical vapor deposition processes. Meas. Sci. Technol. 2008, 20, 025701. [Google Scholar] [CrossRef]
- Soulet, A.; Duquesne, L.; Jursich, G.; Inman, R.; Misra, A.; Blasco, N.; Lachaud, C.; Marot, Y.; Prunier, R.; Vautier, M.; et al. Optimizing the selection and supply of Hf precursor candidates for gate oxide. Semicond. Fabtech 2005, 27, 74–81. [Google Scholar]
- Wang, C.; Yang, S.; Chen, Y. Determination of the vapour pressure curves and vaporization enthalpies of hafnium alkoxides using thermogravimetric analysis. R. Soc. Open Sci. 2019, 6, 181193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackburn, B.J.; Drosos, C.; Brett, D.B.; Parkes, M.A.; Carmalt, C.J.; Parkin, I.P. In situ mass spectrometry analysis of chemical vapour deposition of TiO2 thin films to study gas phase mechanisms. RSC Adv. 2016, 6, 111797–111805. [Google Scholar] [CrossRef]
- Determination of Sublimation Enthalpy and Vapor Pressure for Inorganic and Metal-Organic Compounds by Thermogravimetric Analysis. Available online: http://cnx.org/content/m33649/1.2/ (accessed on 21 March 2022).
- Hannula, M.; Ali-Löytty, H.; Lahtonen, K.; Sarlin, E.; Saari, J.; Valden, M. Improved Stability of Atomic Layer Deposited Amorphous TiO2 Photoelectrode Coatings by Thermally Induced Oxygen Defects. Chem. Mater. 2018, 30, 1199–1208. [Google Scholar] [CrossRef] [Green Version]
- Zafar, M.; Yun, J.-Y.; Kim, D.-H. Performance of inverted organic photovoltaic cells with nitrogen doped TiO2 films by atomic layer deposition. Korean J. Chem. Eng. 2018, 35, 567–573. [Google Scholar] [CrossRef]
- Kumar, A.; Mondal, S.; Rao, K.K. Low temperature solution processed high-κ ZrO2 gate dielectrics for nanoelectonics. Appl. Surf. Sci. 2016, 370, 373–379. [Google Scholar] [CrossRef]
- Jin, H.S.; Kim, D.H.; Kim, S.K.; Wallace, R.M.; Kim, J.; Park, T.J. Strategic Selection of the Oxygen Source for Low Temperature-Atomic Layer Deposition of Al2O3 Thin Film. Adv. Electron. Mater. 2019, 5, 1800680. [Google Scholar] [CrossRef]
- Shibata, T.; Uenuma, M.; Yamada, T.; Yoshitsugu, K.; Higashi, M.; Nishimura, K.; Uraoka, Y. Effects of carbon impurity in ALD-Al2O3 film on HAXPES spectrum and electrical properties of Al2O3/AlGaN/GaN MIS structure. Jpn. J. Appl. Phys. 2022. accepted. [Google Scholar] [CrossRef]

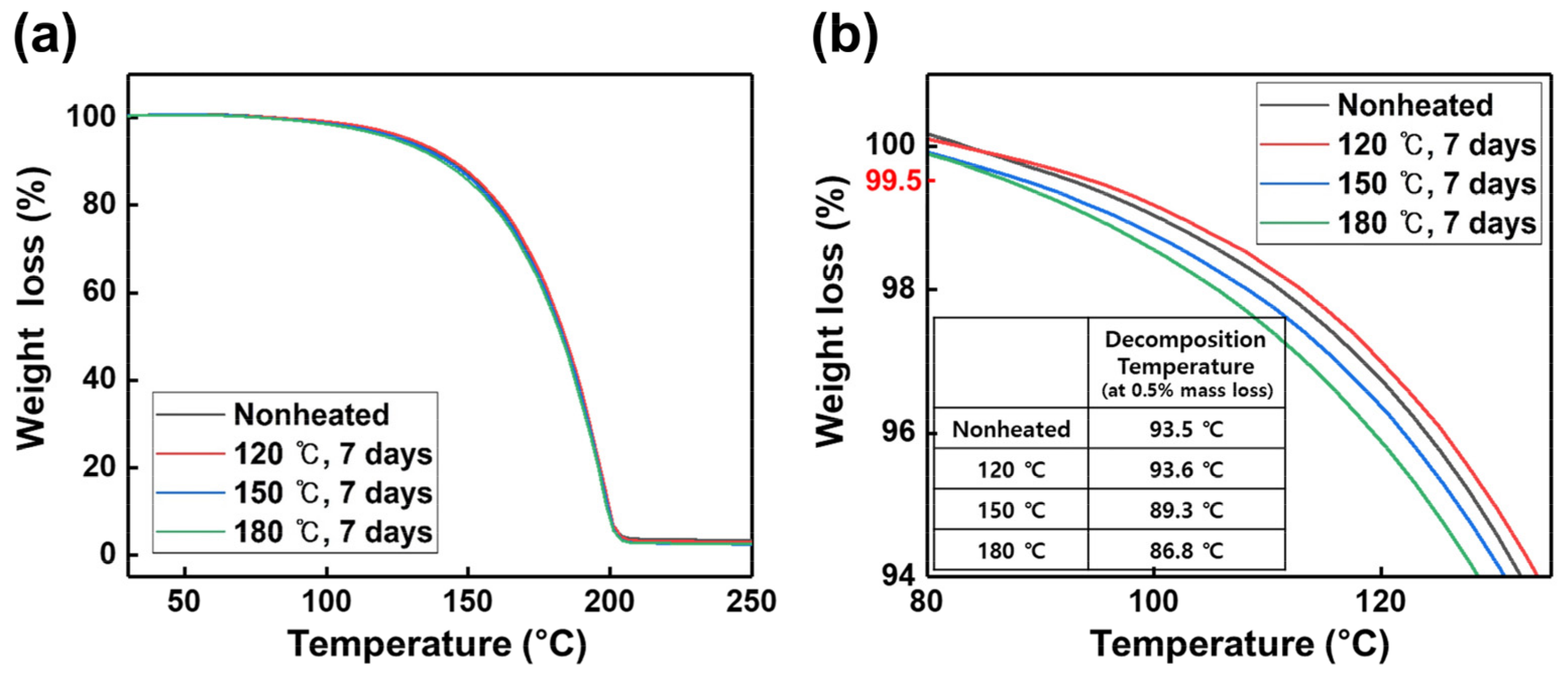


| Antoine Equation Parameters | Vaporization Enthalpy | ||
|---|---|---|---|
| A | B | ||
| Nonheated | 32.659 | 9449.569 | 78.6 |
| 120 °C, 7 days | 33.223 | 9626.489 | 80.0 |
| 150 °C, 7 days | 30.784 | 8844.900 | 73.5 |
| 180 °C, 7 days | 33.052 | 9609.026 | 79.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; An, J.; Maeng, S.; Shin, J.-S.; Choi, E.; Yun, J.-Y. Decomposition Characteristics of the TTIP (Tetraisopropyl Orthotitanate) Precursor for Atomic Layer Deposition. Materials 2022, 15, 3021. https://doi.org/10.3390/ma15093021
Kim H, An J, Maeng S, Shin J-S, Choi E, Yun J-Y. Decomposition Characteristics of the TTIP (Tetraisopropyl Orthotitanate) Precursor for Atomic Layer Deposition. Materials. 2022; 15(9):3021. https://doi.org/10.3390/ma15093021
Chicago/Turabian StyleKim, Hayeong, Jihyeok An, SeonJeong Maeng, Jae-Soo Shin, Eunmi Choi, and Ju-Young Yun. 2022. "Decomposition Characteristics of the TTIP (Tetraisopropyl Orthotitanate) Precursor for Atomic Layer Deposition" Materials 15, no. 9: 3021. https://doi.org/10.3390/ma15093021






