Structure Determination, Mechanical Properties, Thermal Stability of Co2MoB4 and Fe2MoB4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Method
2.3. Characterizations
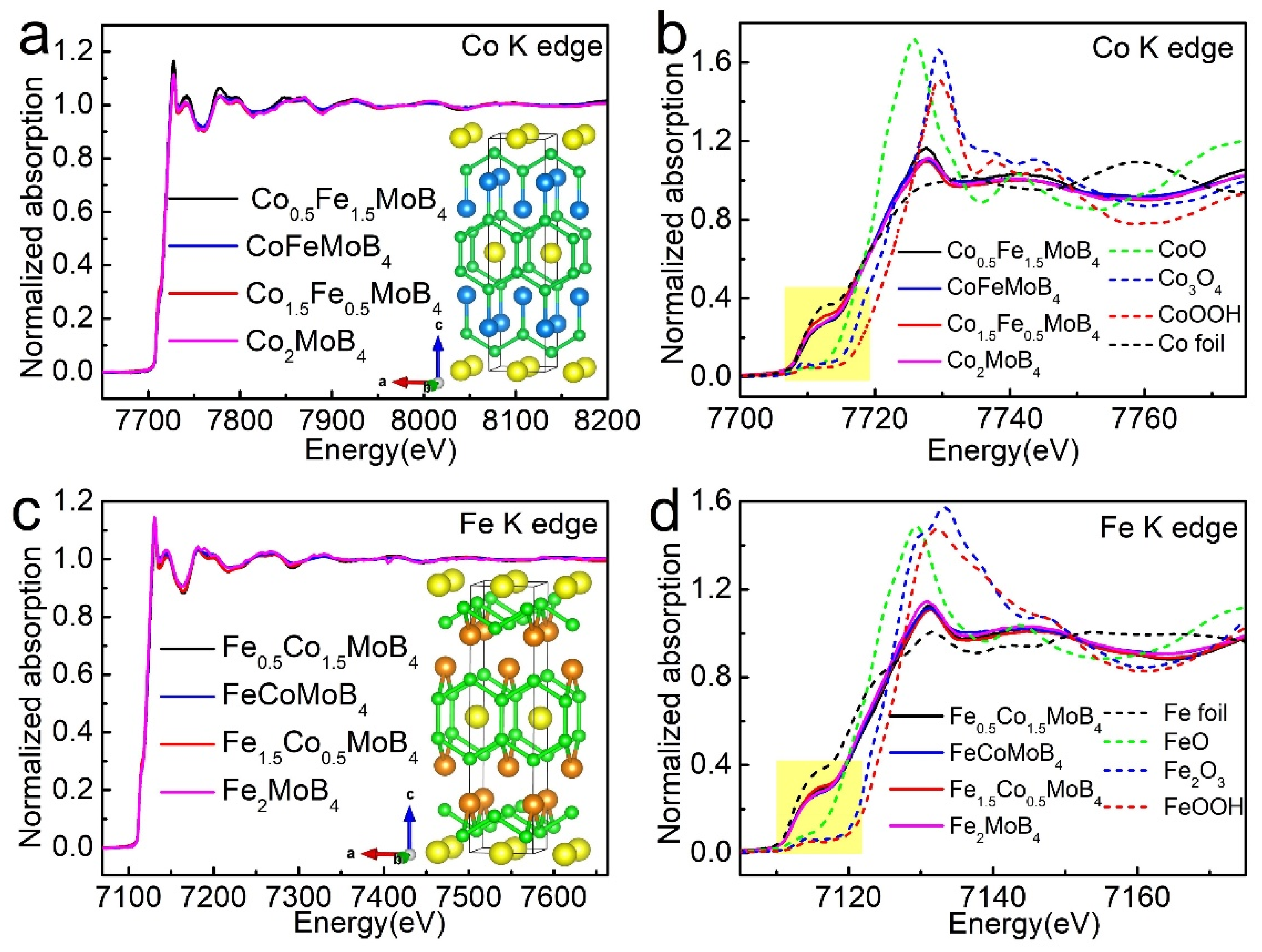
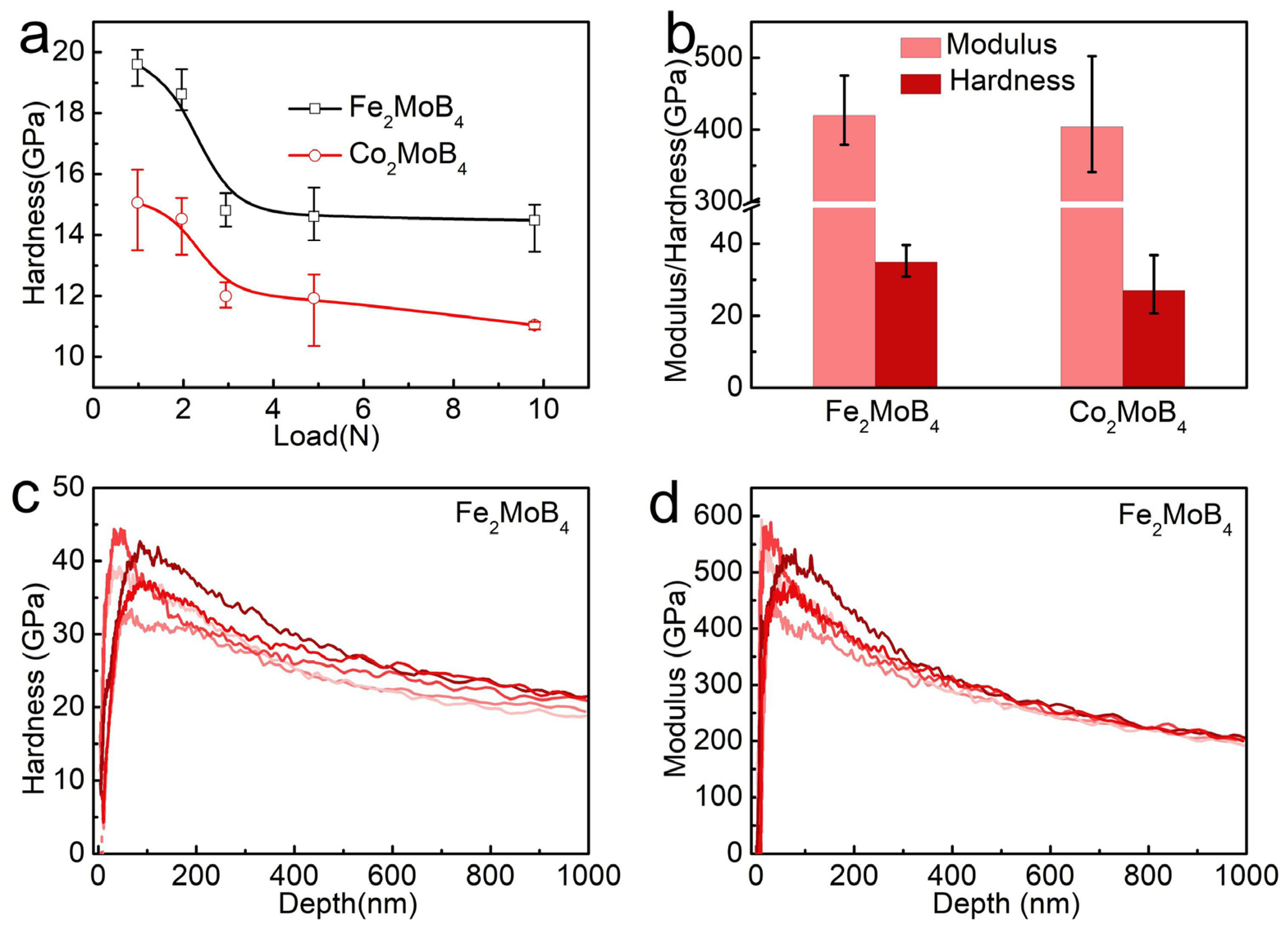
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carenco, S.; Portehault, D.; Boissiere, C.; Mezailles, N.; Sanchez, C. Nanoscaled metal borides and phosphides: Recent developments and perspectives. Chem. Rev. 2013, 113, 7981–8065. [Google Scholar] [CrossRef] [PubMed]
- Akopov, G.; Yeung, M.T.; Kaner, R.B. Rediscovering the Crystal Chemistry of Borides. Adv. Mater. 2017, 29, 1604506. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, N.N.; Parish, R.V.; Thornton, P. Metal borides. Q. Rev. Chem. Soc. 1966, 20, 441–464. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Zhang, Z.; Ni, B.J. Boride-based electrocatalysts: Emerging candidates for water splitting. Nano Res. 2020, 13, 293–314. [Google Scholar] [CrossRef]
- Park, H.; Encinas, A.; Scheifers, J.P.; Zhang, Y.; Fokwa, B.P.T. Boron-Dependency of Molybdenum Boride Electrocatalysts for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2017, 56, 5575–5578. [Google Scholar] [CrossRef]
- Gou, H.; Dubrovinskaia, N.; Bykova, E.; Tsirlin, A.A.; Kasinathan, D.; Schnelle, W.; Richter, A.; Merlini, M.; Hanfland, M.; Abakumov, A.M.; et al. Discovery of a superhard iron tetraboride superconductor. Phys. Rev. Lett. 2013, 111, 157002. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yu, G.; Chen, W.; Liu, Y.; Li, G.D.; Zhu, P.; Tao, Q.; Li, Q.; Liu, J.; Shen, X.; et al. Highly Active, Nonprecious Electrocatalyst Comprising Borophene Subunits for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 12370–12373. [Google Scholar] [CrossRef]
- Park, H.; Zhang, Y.; Scheifers, J.P.; Jothi, P.R.; Encinas, A.; Fokwa, B.P.T. Graphene- and Phosphorene-like Boron Layers with Contrasting Activities in Highly Active Mo2B4 for Hydrogen Evolution. J. Am. Chem. Soc. 2017, 139, 12915–12918. [Google Scholar] [CrossRef]
- Tao, Q.; Zhao, X.; Chen, Y.; Li, J.; Li, Q.; Ma, Y.; Li, J.; Cui, T.; Zhu, P.; Wang, X. Enhanced Vickers hardness by quasi-3D boron network in MoB2. RSC Adv. 2013, 3, 18317–18322. [Google Scholar] [CrossRef]
- Tao, Q.; Chen, Y.; Lian, M.; Xu, C.; Li, L.; Feng, X.; Wang, X.; Cui, T.; Zheng, W.; Zhu, P. Modulating Hardness in Molybdenum Monoborides by Adjusting an Array of Boron Zigzag Chains. Chem. Mater. 2018, 31, 200–206.11. [Google Scholar] [CrossRef]
- Saad, A.; Gao, Y.; Owusu, K.A.; Liu, W.; Wu, Y.; Ramiere, A.; Guo, H.; Tsiakaras, P.; Cai, X. Ternary Mo2NiB2 as a Superior Bifunctional Electrocatalyst for Overall Water Splitting. Small 2022, 18, e2104303. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, T.; Zhang, G.; Liu, X.; Gauthier, M.A.; Chen, Z.; Sun, S. Nanostructured Metal Borides for Energy-Related Electrocatalysis: Recent Progress, Challenges, and Perspectives. Small Methods 2021, 5, e2100699. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, Z.; Jiao, L. Development Strategies in Transition Metal Borides for Electrochemical Water Splitting. Energy Environ. Mater. 2021. [Google Scholar] [CrossRef]
- Liu, C.; Hou, Z.; Jia, Q.; Liu, X.; Zhang, S. Low Temperature Synthesis of Phase Pure MoAlB Powder in Molten NaCl. Materials 2020, 13, 785. [Google Scholar] [CrossRef] [Green Version]
- Burrage, K.C.; Lin, C.M.; Chen, W.C.; Chen, C.C.; Vohra, Y.K. Experimental and Computational Studies on Superhard Material Rhenium Diboride under Ultrahigh Pressures. Materials 2020, 13, 1657. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Xu, S.; Yao, J.; Chen, N.; Gong, Y.; Zhang, X.; Hao, X.; Zhang, L.; Pei, C.; Tian, R.; et al. Elucidating the reaction pathway of crystalline multi-metal borides for highly efficient oxygen-evolving electrocatalysts. J. Mater. Chem. A 2022, 10, 1569–1578. [Google Scholar] [CrossRef]
- Nych, O.V.; Skolozdra, R.V.; Kuz’ma, Y.B. Molybdenum-cobalt-boron system. Izv. Akad. Nauk. SSSR Neorg. Mater. 1966, 2, 1709–1712. [Google Scholar]
- Gladyshevskii, E.I.; Fedorov, T.F.; Kuz’ma, Y.B.; Skolozdra, R.V. Isothermal section of the molbdenum-iron-boron system. Sov. Powder Metall. Met. Ceram. 1966, 5, 305–309. [Google Scholar] [CrossRef]
- Minyaev, R.M.; Hoffmann, R. Transition-metal borides with the tantalum boride (Ta3B4) crystal structure: Their electronic and bonding properties. Chem. Mater. 1991, 3, 547–557. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Xu, S.; Wang, M.; Saranya, G.; Chen, N.; Zhang, L.; He, Y.; Wu, L.; Gong, Y.; Yao, Z.; Wang, G.; et al. Pressure-driven catalyst synthesis of Co-doped Fe C@Carbon nano-onions for efficient oxygen evolution reaction. Appl. Catal. B 2020, 268, 118385. [Google Scholar] [CrossRef]
- Qin, G.; Wu, L.; Gou, H. Diamane: Design, synthesis, properties, and challenges. Funct. Diamond 2021, 1, 83–92. [Google Scholar] [CrossRef]
- Wu, B.; Lei, L.; Zhang, F.; Tang, Q.; Liu, S.; Pu, M.; He, D.; Xia, Y.; Fang, L.; Ohfuji, H.; et al. Pressure-induced disordering of site occupation in iron–nickel nitrides. Matter Radiat. Extremes 2021, 6, 038401. [Google Scholar] [CrossRef]
- Xiang, X.; Song, G.; Zhou, X.; Liang, H.; Xu, Y.; Qin, S.; Wang, J.; Hong, F.; Dai, J.; Zhou, B.; et al. Congruent melting of tungsten phosphide at 5 GPa and 3200 °C for growing its large single crystals. Chin. Phys. B 2020, 29, 088202. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, C.; Liu, J.; Wei, J.; Ye, H. Diamond with nitrogen: States, control, and applications. Funct. Diamond 2021, 1, 63–82. [Google Scholar] [CrossRef]
- Knappschneider, A.; Litterscheid, C.; Dzivenko, D.; Kurzman, J.A.; Seshadri, R.; Wagner, N.; Beck, J.; Riedel, R.; Albert, B. Possible superhardness of CrB4. Inorg. Chem. 2013, 52, 540. [Google Scholar] [CrossRef]
- Gou, H.; Tsirlin, A.A.; Bykova, E.; Abakumov, A.M.; Van Tendeloo, G.; Richter, A.; Ovsyannikov, S.V.; Kurnosov, A.V.; Trots, D.M.; Konôpková, Z.; et al. Peierls distortion, magnetism, and high hardness of manganese tetraboride. Phys. Rev. B 2014, 89, 064108. [Google Scholar] [CrossRef] [Green Version]
- Knappschneider, A.; Litterscheid, C.; George, N.C.; Brgoch, J.; Wagner, N.; Beck, J.; Kurzman, J.A.; Seshadri, R.; Albert, B. Peierls-distorted monoclinic MnB4 with a Mn-Mn bond. Angew. Chem. Int. Ed. 2014, 53, 1684–1688. [Google Scholar] [CrossRef]
- Xu, S.; Gao, X.; Deshmukh, A.; Zhou, J.; Chen, N.; Peng, W.; Gong, Y.; Yao, Z.; Finkelstein, K.D.; Wan, B.; et al. Pressure-promoted irregular CoMoP2 nanoparticles activated by surface reconstruction for oxygen evolu-tion reaction electrocatalysts. J. Mater. Chem. A 2020, 8, 2001–2007. [Google Scholar] [CrossRef]
- Huang, K.; Wang, R.; Zhao, S.; Du, P.; Wang, H.; Wei, H.; Long, Y.; Deng, B.; Lei, M.; Ge, B.; et al. Atomic species derived CoOx clusters on nitrogen doped mesoporous carbon as advanced bifunctional electro-catalysts for Zn-air battery. Energy Stor. Mater. 2020, 29, 156–162. [Google Scholar] [CrossRef]
- Peng, W.; Li, J.; Shen, K.; Zheng, L.; Tang, H.; Gong, Y.; Zhou, J.; Chen, N.; Zhao, S.; Chen, M.; et al. Iron-regulated NiPS for enhanced oxygen evolution efficiency. J. Mater. Chem. A 2020, 8, 23580–23589. [Google Scholar] [CrossRef]
- Chen, X.; Guo, J.; Deng, J.; Wang, R.; Zhao, S.; Chen, N.; Gou, H.; Song, B.; Chen, X. Highly Active Sites in Quaternary LnPdAsO (Ln = La, Ce, Pr) with Excellent Catalytic Activity for Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2021, 4, 4302–4307. [Google Scholar] [CrossRef]
- Bai, R.; Zhang, M.; Zhang, X.; Zhao, S.; Chen, W.; Chen, N.; Ji, P.; Kurbanov, M.S.; Wang, H.; Gou, H.; et al. A Multi-dimensional Topotactic Host Composite Anode Toward Transparent Flexible Potassium-Ion Microcapacitors. ACS Appl. Mater. Interfaces 2022, 14, 1478–1488. [Google Scholar] [CrossRef]
- Tian, R.; Zhao, S.; Li, J.; Chen, Z.; Peng, W.; He, Y.; Zhang, L.; Yan, S.; Wu, L.; Ahuja, R.; et al. Pressure-promoted high-ly-ordered Fe-doped-Ni2B for effective oxygen evolution reaction and overall water splitting. J. Mater. Chem. A 2021, 9, 6469–6475. [Google Scholar] [CrossRef]
- Peng, W.; Deshmukh, A.; Chen, N.; Lv, Z.; Zhao, S.; Li, J.; Yan, B.; Gao, X.; Shang, L.; Gong, Y.; et al. Deciphering the Dynamic Structure Evolution of Fe- and Ni-Codoped CoS2 for Enhanced Water Oxidation. ACS Catal. 2022, 12, 3743–3751. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, B.; Wu, L.; Li, Z.; Wang, Z.; Zhang, J.; Gou, H.; Gao, F. Revealing phase relations between Fe2B7 and FeB4 and hypothetical Fe2B7-type Ru2B7 and Os2B7: First-principles calculations. RSC Adv. 2017, 7, 44860–44866. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Bao, K.; Tao, Q.; Xu, C.; Feng, X.; Zhao, X.; Ge, Y.; Zhu, P.; Cui, T. Double-zigzag boron chain-enhanced Vickers hardness and manganese bilayers-induced high d-electron mobility in Mn3B4. Phys. Chem. Chem. Phys. 2019, 21, 2697–2705. [Google Scholar] [CrossRef]
- Gao, F.; He, J.; Wu, E.; Liu, S.; Yu, D.; Li, D.; Zhang, S.; Tian, Y. Hardness of covalent crystals. Phys. Rev. Lett. 2003, 91, 015502. [Google Scholar] [CrossRef]
- Mazhnik, E.; Oganov, A.R. Application of machine learning methods for predicting new superhard materials. J. Appl. Phys. 2020, 128, 075102. [Google Scholar] [CrossRef]
- Simunek, A.; Vackar, J. Hardness of covalent and ionic crystals: First-principle calculations. Phys. Rev. Lett. 2006, 96, 085501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balogh, J.; Bujdosó, L.; Faigel, G.; Gránásy, L.; Kemény, T.; Vincze, I.; Szabó, S.; Bakker, H. Nucleation controlled transfor-mation in ball milled FeB. Nanostruct. Mater. 1993, 2, 11–18. [Google Scholar] [CrossRef]
- Anik, M.; Küçükdeveci, N. Discharging characteristics of CoB nano powders. Int. J. Hydrogen Energy 2013, 38, 1501–1509. [Google Scholar] [CrossRef]


| Co2MoB4 | Co2MoB4 (Ref. [17]) | Fe2MoB4 | Fe2MoB4 (Ref. [18]) | |
|---|---|---|---|---|
| Space group | Immm | Immm | Immm | Immm |
| a (Å) | 3.0129 | 3.079 | 2.9869 | 3.128 |
| b (Å) | 3.0725 | 12.57 | 3.0972 | 12.7 |
| c (Å) | 12.5240 | 3.018 | 12.750 | 2.984 |
| V (Å3) | 115.94 | 116.806 | 117.95 | 118.541 |
| Atomic parameters (x/a, y/b, z/c) | ||||
| Co/Fe | 0.5, 0.5, 0.3140 | 0, 0.18, 0 | 0.5, 0.5, 0.3151 | 0, 0.18, 0 |
| B1 | 1.0, 0, 0.3508 | 0, 0.375, 0 | 0.5, 0, 0.4267 | 0, 0.375, 0 |
| B2 | 0.5, 0, 0.4259 | 0, 0.444, 0.5 | 0, 0, 0.3513 | 0, 0.444, 0.5 |
| Mo | 1.0, 0.5, 0.5 | 0.5, 0.5, 0 | 0, 0.5, 0.5 | 0.5, 0.5, 0 |
| Co2MoB4 | Co2MoB4 (Ref. [17]) | Fe2MoB4 | Fe2MoB4 (Ref. [18]) | ||
|---|---|---|---|---|---|
| Bond | Bond length (Å) | Bond | Bond length (Å) | ||
| B-B | 1.776 (5) | 1.41 | B-B | 1.776 (11) | 1.42 |
| B-B | 1.856 (15) | 1.74 | B-B | 1.87 (3) | 1.73 |
| Co-B | 2.064 (7) | 2.19 | Fe-B | 2.103 (10) | 2.22 |
| Co-B | 2.2005 (14) | 2.45 | Fe-B | 2.200 (3) | 2.48 |
| Co-B | 2.080 (5) | 2.26 | Fe-B | 2.122 (15) | 2.27 |
| Co-Co | 2.6830 (10) | 2.78 | Fe-Fe | 2.718 (2) | 2.8 |
| Mo-B | 2.343 (3) | 2.2 | Mo-B | 2.346 (6) | 2.23 |
| Mo-B | 2.419 (5) | 2.27 | Mo-B | 2.448 (11) | 2.28 |
| Sample | Bulk Modulus (GPa) | Shear Modulus (GPa) | Young’s Modulus (GPa) | Poisson’s Ratio | Hardness (GPa) |
|---|---|---|---|---|---|
| Co2MoB4 | 311.6 | 181.2 | 455.3 | 0.256 | 19.2 |
| Fe2MoB4 | 314.6 | 178.2 | 449.6 | 0.262 | 18.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Zhou, W.; Xiang, X.; Cao, X.; Chen, N.; Chen, W.; Yu, X.; Yan, B.; Gou, H. Structure Determination, Mechanical Properties, Thermal Stability of Co2MoB4 and Fe2MoB4. Materials 2022, 15, 3031. https://doi.org/10.3390/ma15093031
Zhao S, Zhou W, Xiang X, Cao X, Chen N, Chen W, Yu X, Yan B, Gou H. Structure Determination, Mechanical Properties, Thermal Stability of Co2MoB4 and Fe2MoB4. Materials. 2022; 15(9):3031. https://doi.org/10.3390/ma15093031
Chicago/Turabian StyleZhao, Shijing, Wenju Zhou, Xiaojun Xiang, Xuyan Cao, Ning Chen, Weifeng Chen, Xiaohui Yu, Bingmin Yan, and Huiyang Gou. 2022. "Structure Determination, Mechanical Properties, Thermal Stability of Co2MoB4 and Fe2MoB4" Materials 15, no. 9: 3031. https://doi.org/10.3390/ma15093031






