Shock-Induced Energy Release Performances of PTFE/Al/Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
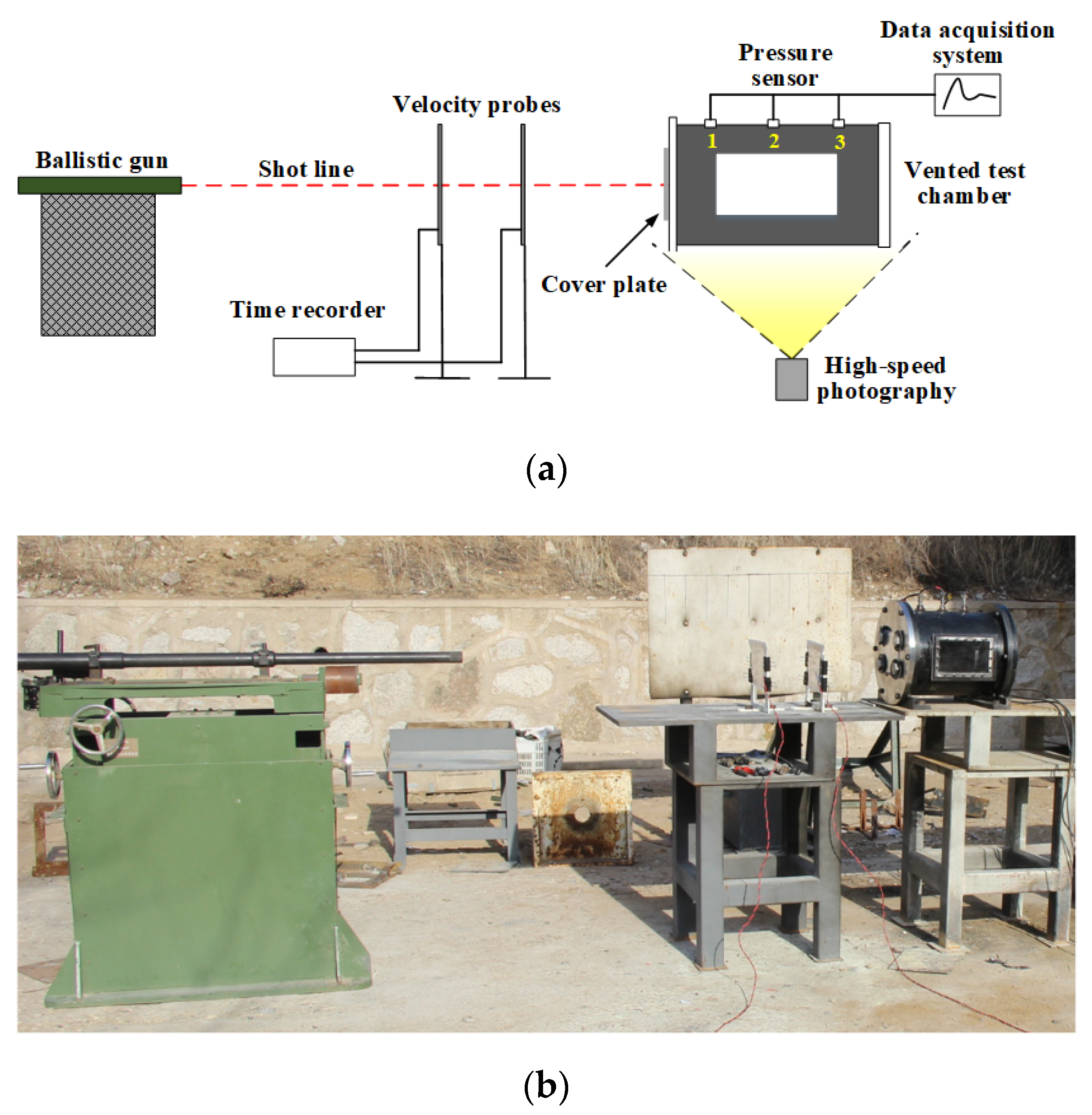
2.2. Experimental Setup
3. Results
4. Discussion
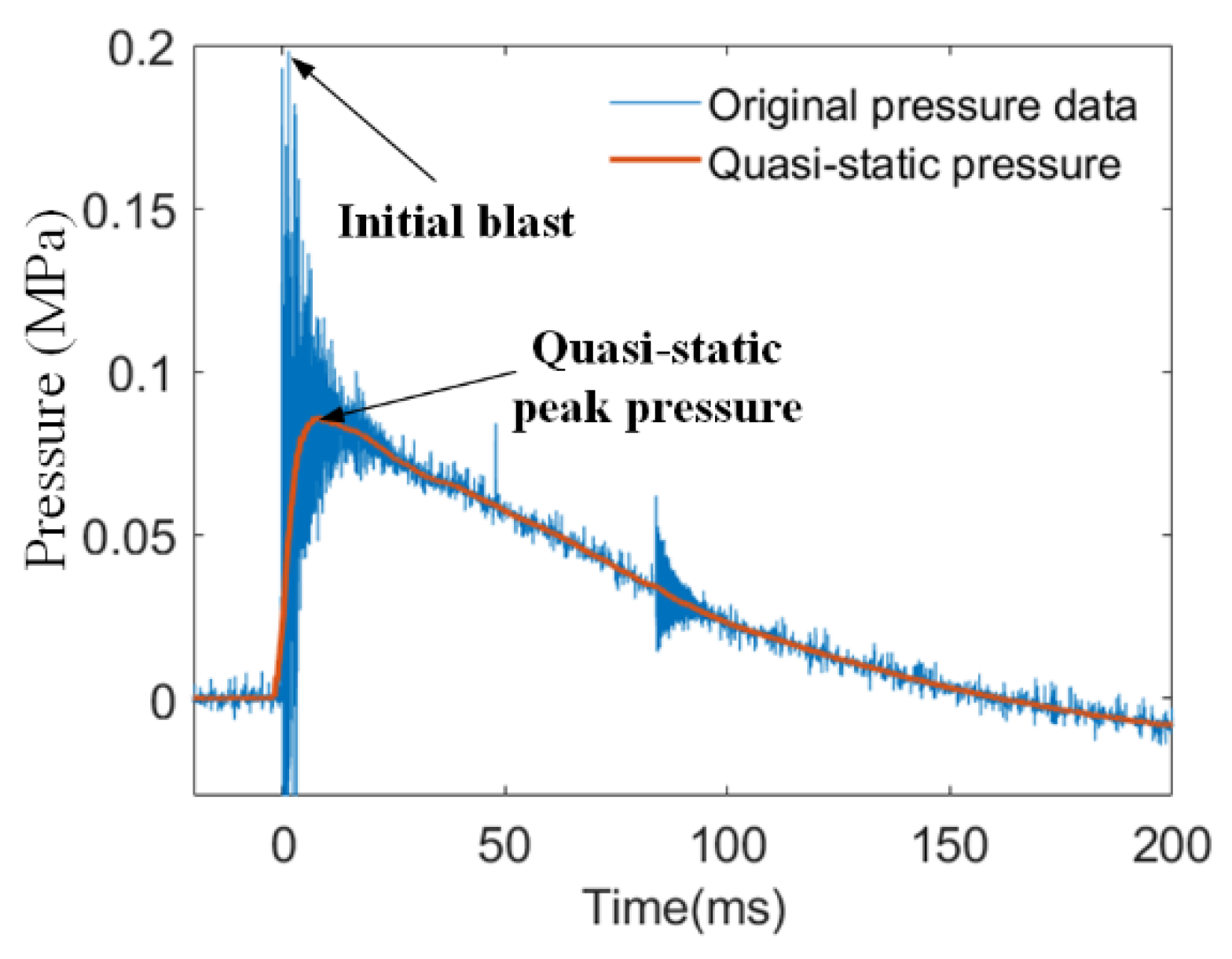
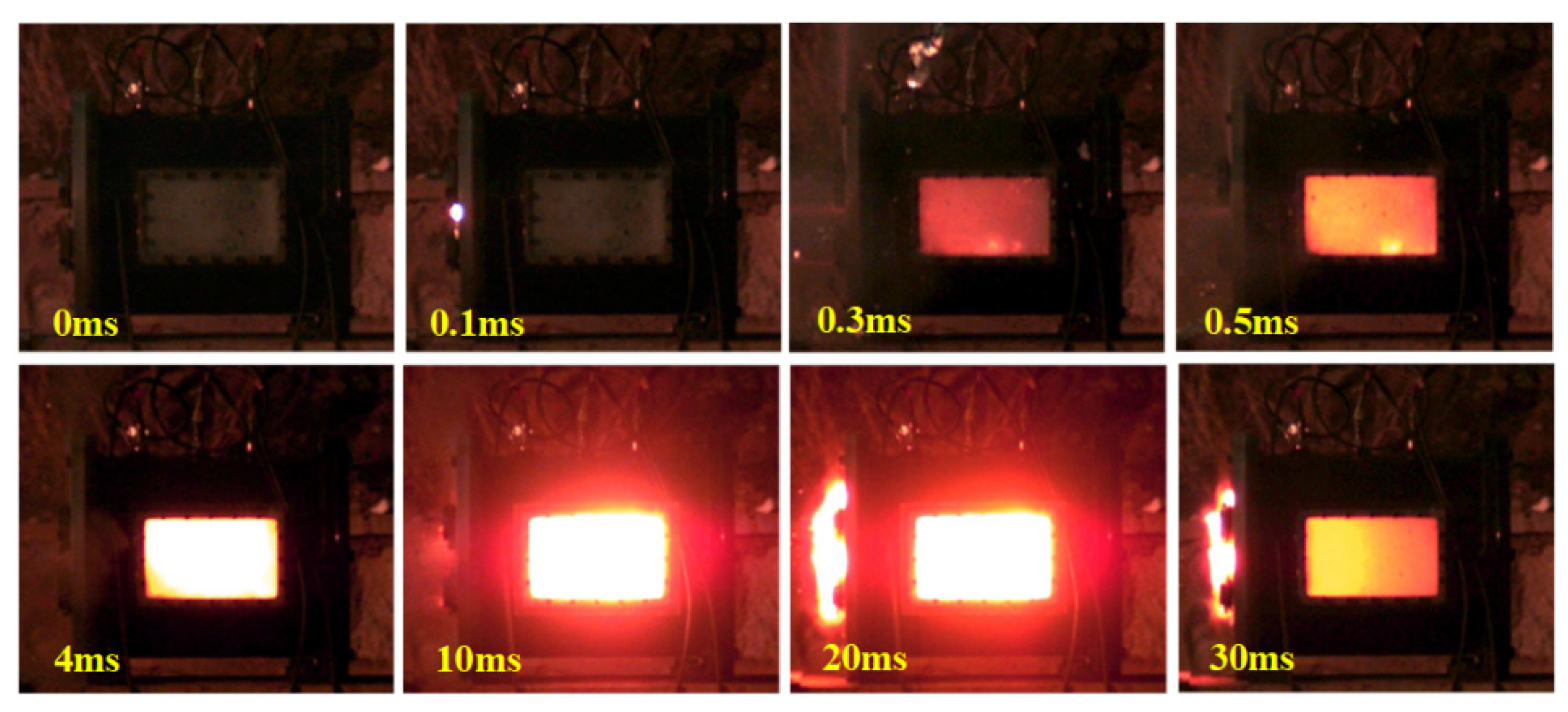
4.1. Typical Shock-Induced Energy Release Behavior
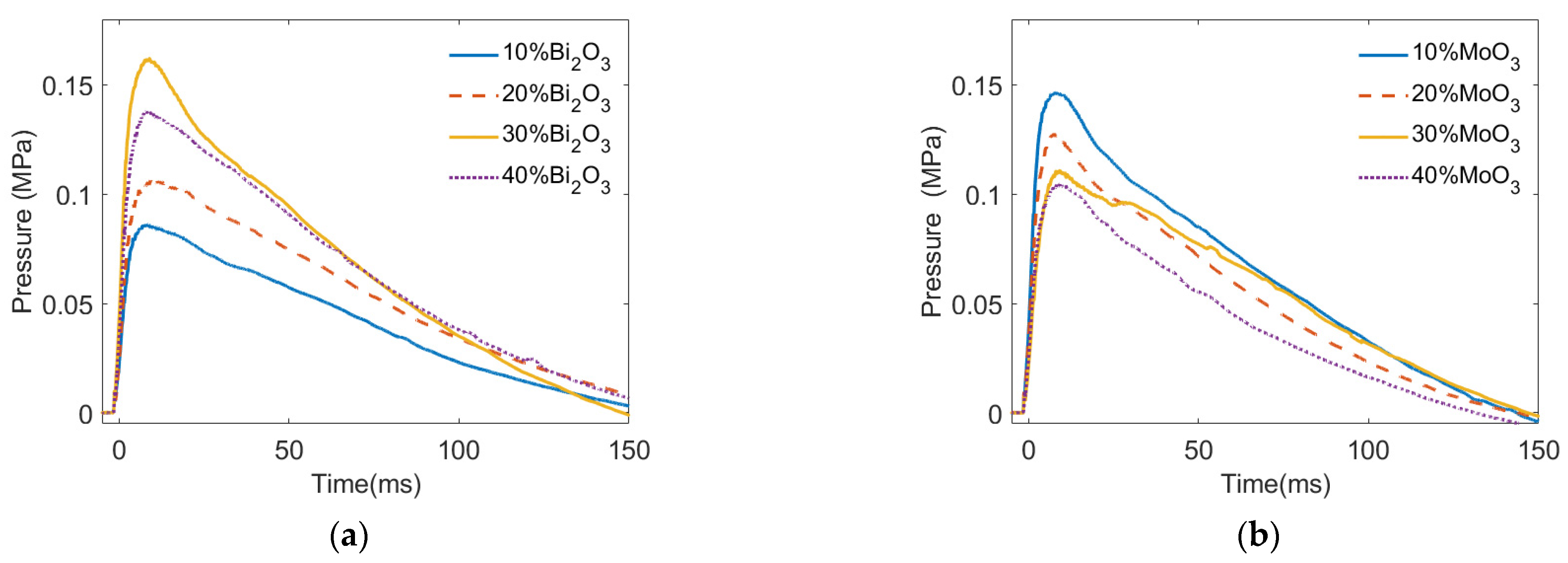
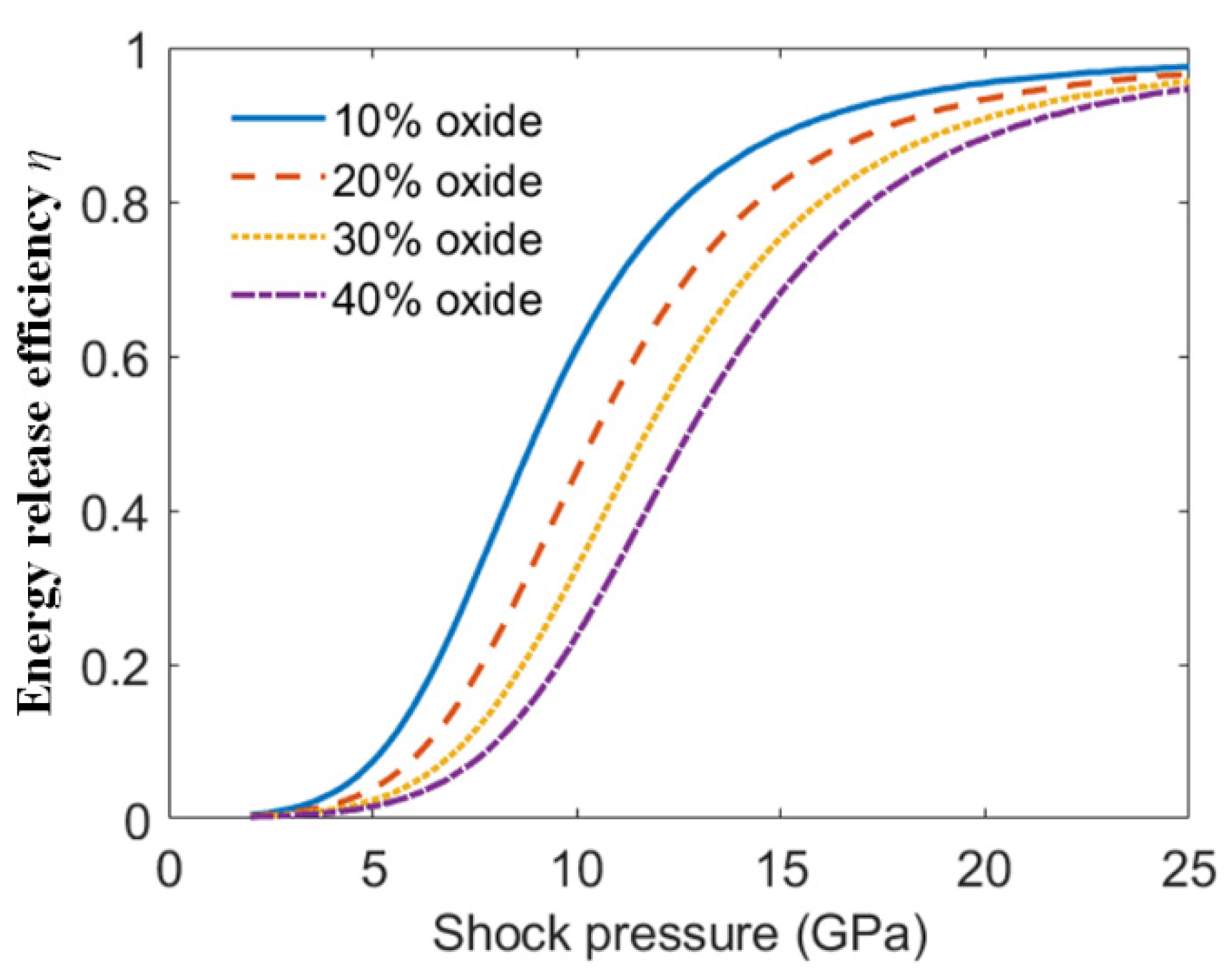
4.2. Shock Pressure Distribution of the PTFE/Al/Oxide
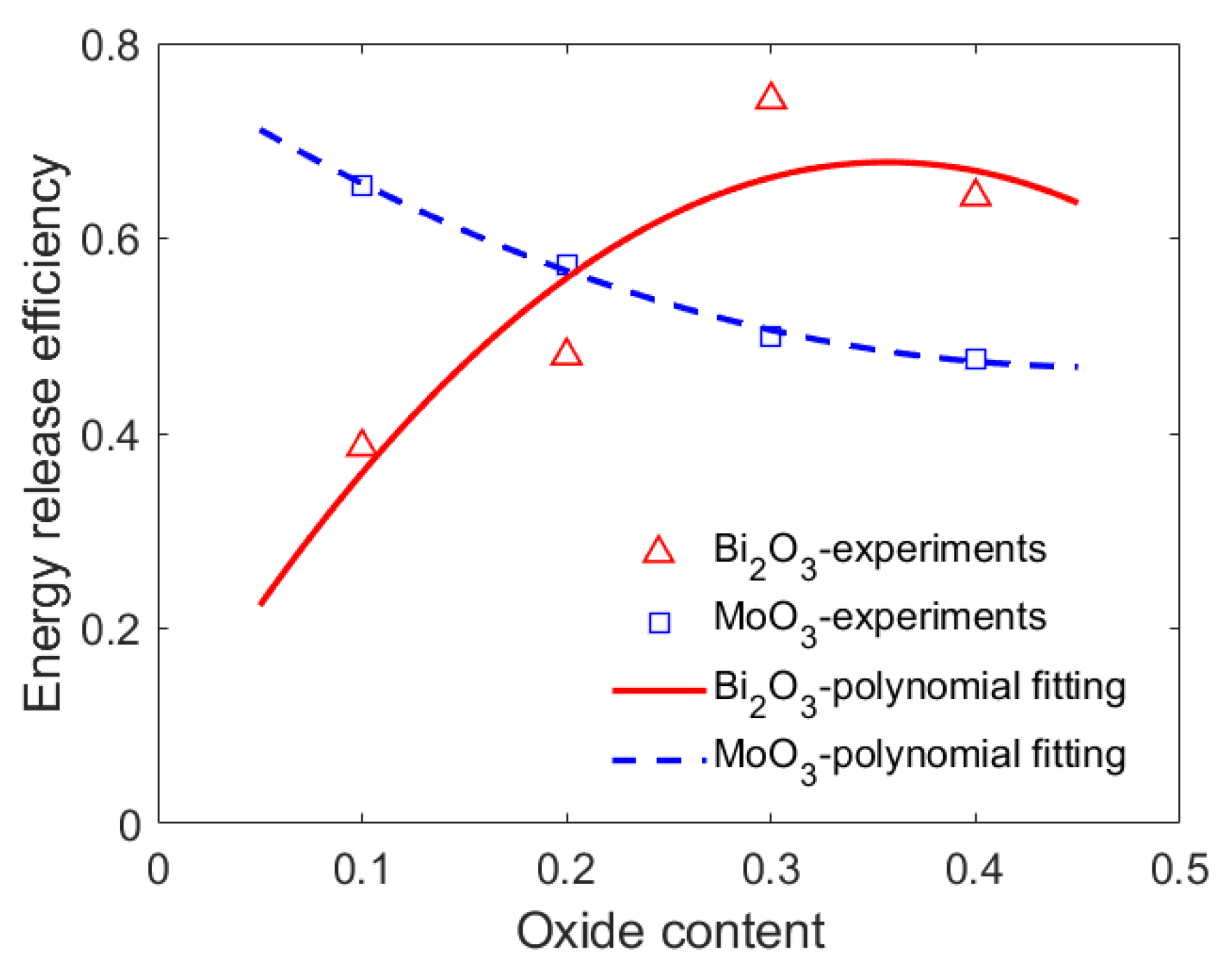
4.3. Energy Release Efficiency of the PTFE/Al/Oxide
5. Conclusions
- (1)
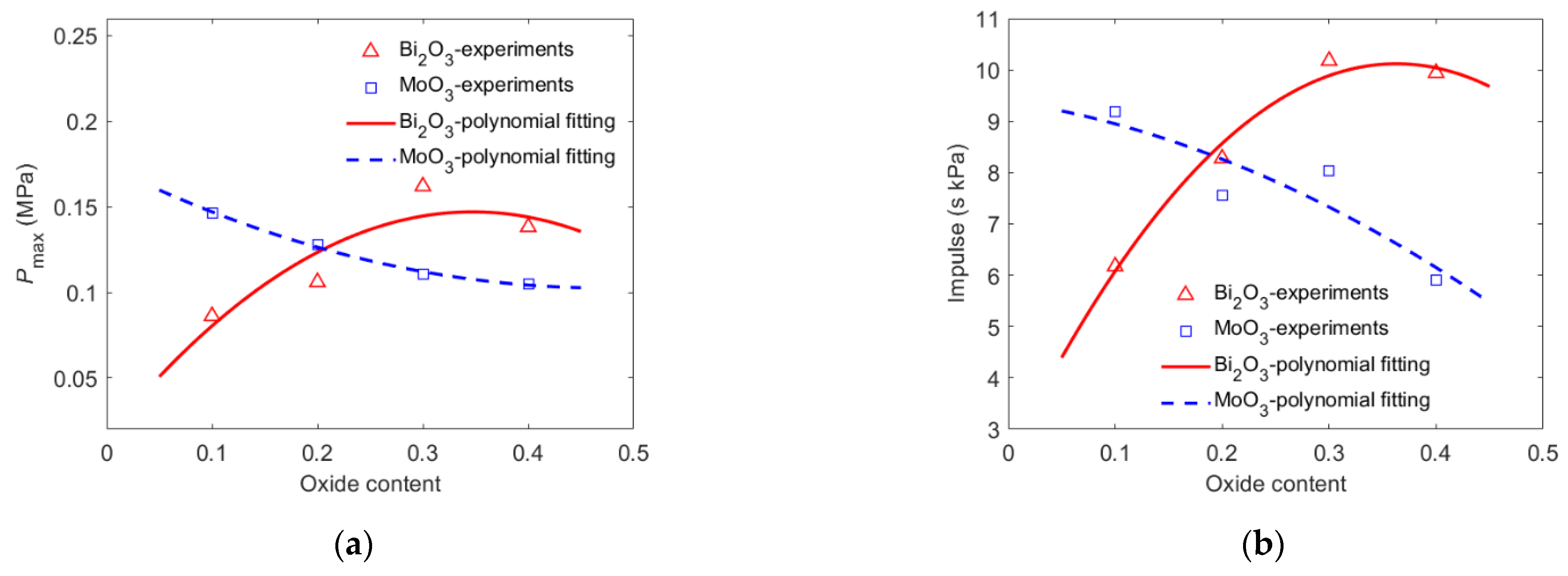
- With the Bi2O3 content increasing from 10% to 40%, the energy release of PTFE/Al/Bi2O3 increased first and then decreased. When the PTFE/Al had 30% Bi2O3 added, the pressure peak generated by the energetic material reached 0.162 MPa, which was 1.88 times higher than the one with 10% Bi2O3 (0.0862 MPa).
- (2)
- The oxide content that caused the PTFE/Al/oxide release of maximum energy is called the optimal oxide content, and the optimal oxide content of PTFE/Al/Bi2O3 was approximately 30%.
- (3)
- The PTFE/Al/oxide energy release mechanism analysis presented that the shock pressure and energy release efficiency under the corresponding shock pressure were affected by the oxide content, which depended on the energy release efficiency of PTFE/Al/oxide.
- (4)
- With the same impact velocity, as the oxide content increased the shock pressure of PTFE/Al/oxide increased and the chemical reaction extent of the material under the corresponding shock wave intensity decreased.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.X.; Fang, X.; Gao, Z.R.; Wang, H.X.; Huang, J.Y.; Wu, S.Z.; Li, Y.C. Investigation on mechanical properties and reaction characteristics of Al-PTFE composites with different Al particle size. Adv. Mater. Sci. Eng. 2018, 2018, 2767563. [Google Scholar] [CrossRef] [Green Version]
- Raftenberg, M.N.; Mock, W.; Kirby, G.C. Modeling the impact deformation of rods of a pressed PTFE/Al composite mixture. Int. J. Impact Eng. 2008, 35, 1735–1744. [Google Scholar] [CrossRef]
- Mock, W., Jr.; Holt, W.H. Impact initiation of rods of pressed Polytetrafluoroethylene(PTFE) and aluminum powders. AIP Conf. Proc. 2006, 845, 1097. [Google Scholar] [CrossRef]
- Feng, B.; Li, Y.C.; Hao, H.; Wang, H.X.; Hao, Y.F.; Fang, X. A Mechanism of Hot-spots Formation at the Crack Tip of Al-PTFE under Quasi-static Compression. Propell. Explos. Pyrot. 2017, 42, 1366–1372. [Google Scholar] [CrossRef]
- Ames, R. Energy Release Characteristics of Impact-Initiated Energetic Materials. Mater. Res. Soc. Symp. Proc. 2006, 18, 21–24. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Mao, Y.; Gong, F. An effective way to enhance energy output and combustion characteristics of Al/PTFE. Combust. Flame 2020, 214, 419–425. [Google Scholar] [CrossRef]
- Yu, Z.; Fang, X.; Li, Y.; Wu, J.; Wu, S.; Zhang, J.; Ren, J.; Zhong, M.; Chen, L.; Yao, M. Investigation on the Reaction Energy, Dynamic Mechanical Behaviors, and Impact-Induced Reaction Characteristics of PTFE Al with Different TiH2 Percentages. Materials 2018, 11, 2008. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, J.; Yang, M.; Wang, L.; Lan, J.; Li, S.; He, C.; Xue, X. Effects of multicomponent co-addition on reaction characteristics and impact damage properties of reactive material. Mater. Des. 2018, 153, 1–8. [Google Scholar] [CrossRef]
- Ding, L.L.; Zhou, J.Y.; Tang, W.H.; Ran, X.W.; Hu, Y.X. Impact Energy Release Characteristics of PTFE/Al/CuO Reactive Materials Measured by a New Energy Release Testing Device. Polymers 2019, 11, 149. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Huang, J.Y.; Fang, X.; Li, Y.C.; Yu, Z.S.; Gao, Z.R.; Wu, S.Z.; Yang, L.; Wu, J.X.; Kui, J.Y. Thermal Decomposition and Thermal Reaction Process of PTFE/Al/MnO2 Fluorinated Thermite. Materials 2018, 11, 2451. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.Y.; Fang, X.; Wu, S.Z.; Yang, L.; Yu, Z.S.; Li, Y.C. Mechanical Response and Shear-Induced Initiation Properties of PTFE/Al/MoO3 Reactive Composites. Materials 2018, 11, 1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Geng, B.; Sun, T.; Yu, Q.; Wang, H. Impact-Induced Reaction Characteristic and the Enhanced Sensitivity of PTFE/Al/Bi2O3 Composites. Polymers 2019, 11, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, J.; Liu, J.; Zhang, S.; Xue, X.; He, C.; Wu, Z.; Yang, M.; Li, S. Influence of multi-oxidants on reaction characteristics of PTFE-Al-XmOY reactive material. Mater. Des. 2020, 186, 108325. [Google Scholar] [CrossRef]
- Glavier, L.; Taton, G.; Ducere, J.; Baijot, V.; Pinon, S.; Calais, T.; Esteve, A.; Rouhani, M.D.; Rossi, C. Nanoenergetics as pressure generator for nontoxic impact primers: Comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE. Combust. Flame 2015, 162, 1813–1820. [Google Scholar] [CrossRef]
- Ames, R. Vented Chamber Calorimetry for Impact-Initiated Energetic Materials. In Proceedings of the 43rd AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 10 January 2005; p. AIAA 2005-279. [Google Scholar] [CrossRef]
- Salasnich, L. Shock waves in quasi one-dimensional Bose-Einstein condensate. Eur. Phys. J. Plus 2016, 131, 66. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.Y.; Yu, Q.B.; Zheng, Y.F.; Lei, M.A.; Wang, H.F. Damage effects of double-spaced aluminum plates by reactive material projectile impact. Int. J. Impact Eng. 2017, 104, 13–20. [Google Scholar] [CrossRef]
- Fredenburg, D.A.; Thadhani, N.N. High-pressure equation of state properties of bismuth oxide. J. Appl. Phys. 2011, 110, 063510. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, X.F.; He, Y.; Shi, A.S.; Guan, Z.W. Mesoscale simulation on the shock compression behaviour of Al–W-Binder granular metal mixtures. Mater. Des. 2013, 47, 341–349. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Liu, Y.; Huang, F.L.; Lv, Z. Dynamic Behavior of Materials; National Defense Industry Press: Beijing, China, 2006; pp. 80–97. [Google Scholar]
- Ortega, A.; Perez Maqueda, L.; Criado, J.M. The problem of discerning Avrami-Erofeev kinetic models from the new controlled rate thermal analysis with constant acceleration of the transformation. Thermochim. Acta 1995, 254, 147–152. [Google Scholar] [CrossRef]
- Zhang, X.F.; Shi, A.S.; Qiao, L.; Zhang, J.; Zhang, Y.G.; Guan, Z.W. Experimental study on impact-initiated characters of multifunctional energetic structural materials. J. Appl. Phys. 2013, 113, 083508. [Google Scholar] [CrossRef]
- Umbrajkar, S.M.; Schoenitz, M.; Dreizin, E.L. Exothermic Reactions in Al-CuO Nanocomposites. Thermochim. Acta 2006, 451, 34–43. [Google Scholar] [CrossRef]


| Element | Manufacturer | Density (g·cm−3) | Measure Size (μm) | Shape | Enthalpy of Formation (kJ/mol) |
|---|---|---|---|---|---|
| PTFE | Donghu (Shanghai, China) | 2.20 | ≈24 | Sphere | −854 |
| Al | Xingrongyuan (Beijing, China) | 2.78 | ≈24 | Sphere | 0 |
| Bi2O3 | Xingrongyuan | 8.90 | ≈38 | Ovoid | −573.9 |
| MoO3 | Xingrongyuan | 4.69 | ≈45 | Random shape | −745.2 |
| Material | Composition | Mass Fraction | TMD 1 (g/cm3) | TED 2 (kJ/g) |
|---|---|---|---|---|
| B1 | PTFE/Al/Bi2O3 | 65.30/24.70/10 | 2.520 | 7.716 |
| B2 | 57.10/22.90/20 | 2.744 | 7.013 | |
| B3 | 48.89/21.11/30 | 3.013 | 6.309 | |
| B4 | 40.69/19.31/40 | 3.341 | 5.606 | |
| M1 | PTFE/Al/MoO3 | 63.39/26.61/10 | 2.468 | 7.908 |
| M2 | 53.29/26.71/20 | 2.625 | 7.396 | |
| M3 | 43.18/26.82/30 | 2.803 | 6.885 | |
| M4 | 33.07/26.92/40 | 3.008 | 6.373 |
| Material | v (m/s) | Pmax (MPa) | Impulse (s kPa) | Duration (ms) |
|---|---|---|---|---|
| B1 | 970.1 | 0.086 | 6.175 | 144.54 |
| B2 | 944.8 | 0.106 | 8.276 | 160.62 |
| B3 | 940.3 | 0.162 | 10.177 | 139.52 |
| B4 | 944.3 | 0.138 | 9.940 | 153.36 |
| M1 | 959.0 | 0.147 | 9.184 | 134.50 |
| M2 | 909.8 | 0.128 | 7.555 | 130.32 |
| M3 | 970.9 | 0.111 | 8.034 | 137.66 |
| M4 | 904.5 | 0.105 | 5.914 | 120.24 |
| Material | ρ (g cm−3) | C (m/s) | S | Cv (J K−1) | γ |
|---|---|---|---|---|---|
| PTFE | 2.20 | 1680 | 1.123 | 890 | 2.0 |
| Al | 2.78 | 5250 | 1.370 | 1020 | 0.6 |
| Bi2O3 | 8.90 | 3432 | 0.275 | 235.7 | 0.796 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Shi, D.; He, S.; Guo, H.; Zheng, Y.; Zhang, Y.; Wang, H. Shock-Induced Energy Release Performances of PTFE/Al/Oxide. Materials 2022, 15, 3042. https://doi.org/10.3390/ma15093042
Yuan Y, Shi D, He S, Guo H, Zheng Y, Zhang Y, Wang H. Shock-Induced Energy Release Performances of PTFE/Al/Oxide. Materials. 2022; 15(9):3042. https://doi.org/10.3390/ma15093042
Chicago/Turabian StyleYuan, Ying, Dongfang Shi, Suo He, Huanguo Guo, Yuanfeng Zheng, Yong Zhang, and Haifu Wang. 2022. "Shock-Induced Energy Release Performances of PTFE/Al/Oxide" Materials 15, no. 9: 3042. https://doi.org/10.3390/ma15093042






