Promising Results of the Comparison of Coatings on Aged Bridges and of Same Coatings in Laboratory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bridge Structure Assessed
- (1)
- Without active fillers;
- (2)
- With zinc filler 75 and 94% by weight;
- (3)
- In a dry shell and with an aluminum filler 2–4% by weight;
- (4)
- Made of epoxy interlayer without active or barrier fillers;
- (5)
- With aluminum filler 2–4% by weight and iron flake 12, 36 and 58 wt.%;
- (6)
- With a polyurethane topcoat, predominantly acrylic;
- (7)
- In one case acrylic/polyester.
2.1.1. Coating Damage Assessment
2.1.2. EIS Studies of Coatings on Objects
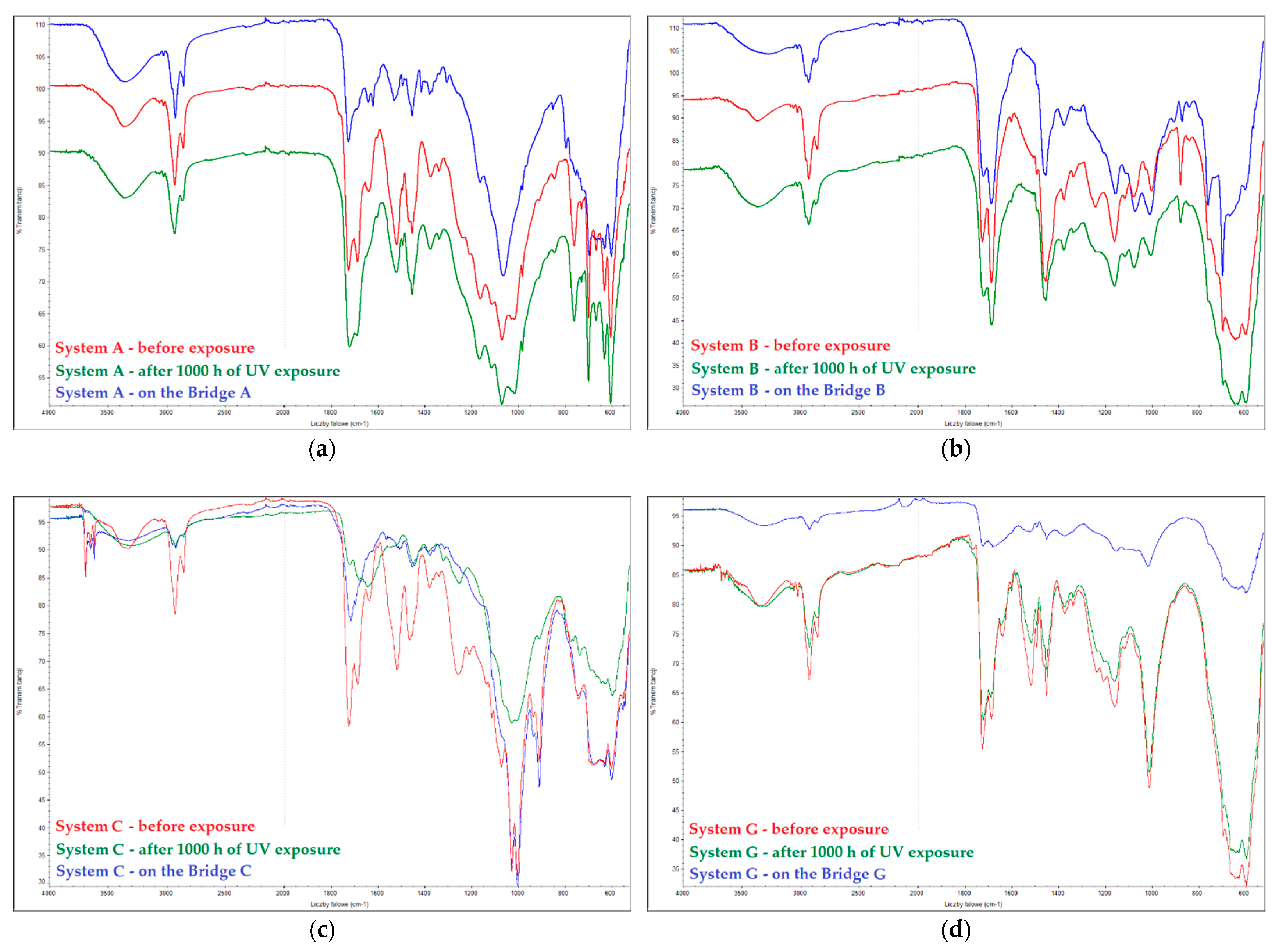
2.1.3. Assessment of Coating Degradation Using FTIR
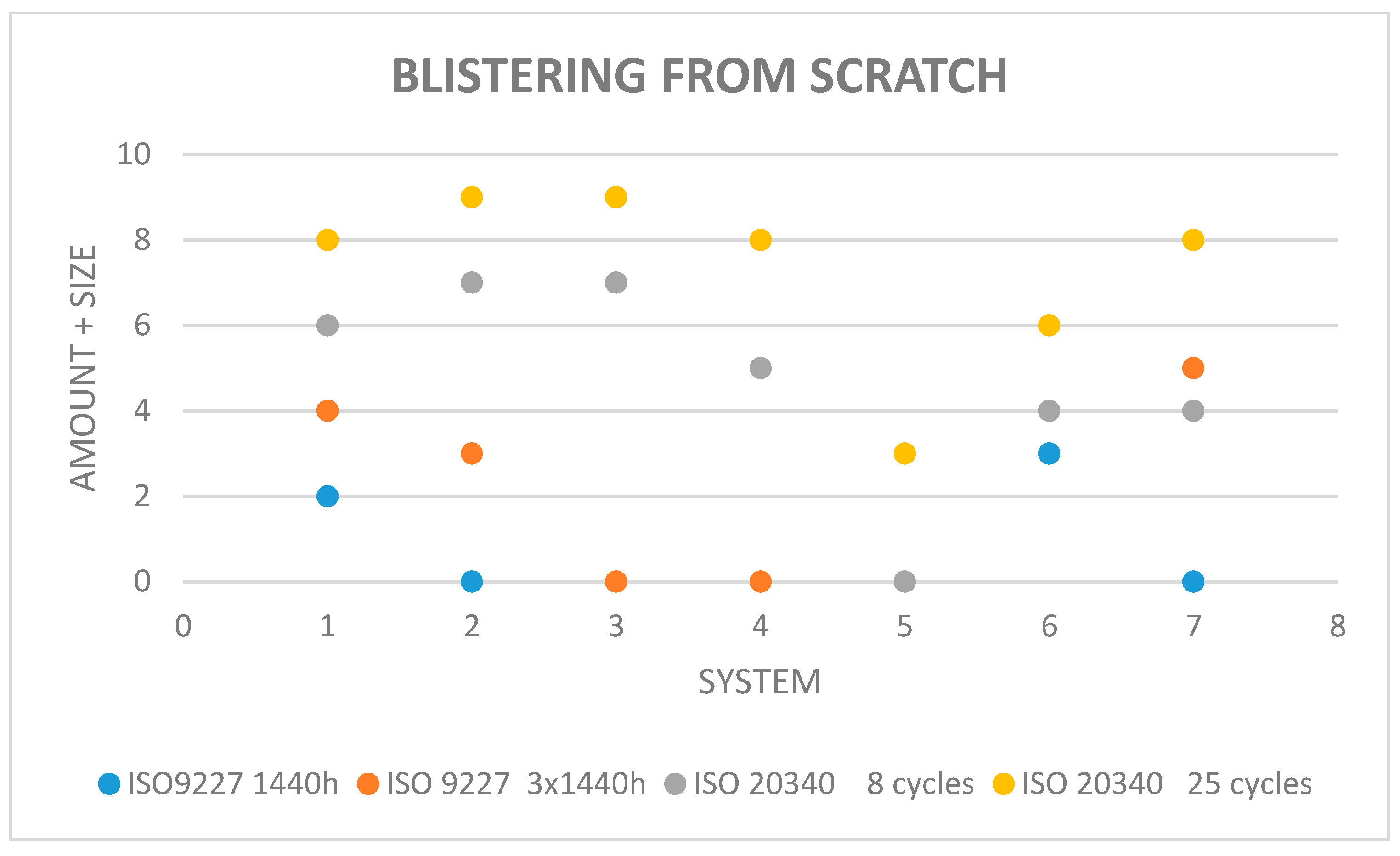
2.2. Accelerated Corrosion and Aging Testing of Coatings on Test Panels and Evaluation of Coating Damage
2.3. Assessment of Coating Degradation Using FTIR an EIS
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, A.K.; Seetharamaiah, N.; Pichumani, M.; Shilpa Chakra, C. Research progress in organic zinc rich primer coatings for cathodic protection of metals—A comprehensive review. Prog. Org. Coat. 2021, 153, 106040. [Google Scholar] [CrossRef]
- General Directorate for National Roads and Motorways Regulation. Recommendations for the Execution and Acceptance of Anti-Corrosion Protection of Bridge Steel Structures; Warsaw, Poland. 2022. Available online: https://www.gov.pl/web/infrastruktura/wr-m (accessed on 29 March 2022).
- ISO 12944; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems (Part 1—General introduction, Part 2—Classification of environments, Part 3—Design considerations, Part 4—Types of surface and surface preparation, Part 5—Protective paint systems, Part 6—Laboratory performance test methods, Part 7—Execution and supervision of paint work, Part 8—Development of specifications of new work and maintenance, Part 9—Protective paint systems and laboratory performance test methods for offshore and related structures). The International Organization for Standardization: Geneva, Switzerland, 1998–2019.
- Kodumuri, P.; Lee, S.-K. Federal Highway Administration 100-Year Coating Study; Federal Highway Administration Report No. FHWA HRT-12-044 2012; Research, Development, and Technology Turner-Fairbank Highway Research Center 6300 Georgetown Pike; Federal Highway Administration: McLean, VA, USA, 2012. [Google Scholar]
- Liu, R.; Runion, A. Coating Performance on Existing Steel Bridge Superstructures; Publication No. FHWA-HRT-20-065 2020; Research, Development, and Technology Turner-Fairbank Highway Research Center 6300 Georgetown Pike; Federal Highway Administration: McLean, VA, USA, 2020. [Google Scholar]
- Regulation of the Minister of Infrastructure and Construction of 17 November 2016 on the Method of Declaring the Performance of Construction Products and the Method of Marking Them with a Construction Mark (Polish Journal of Laws, Dz. U. of 6 December 2016, Item 1966). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20160001966/O/D20161966.pdf (accessed on 29 March 2022).
- Królikowska, A. Requirements for paint systems for the steel bridges in Poland. Prog. Org. Coat. 2000, 39, 137–139. [Google Scholar] [CrossRef]
- ISO 4628; Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance (Part 1—General introduction and designation system, Part 2—Assessment of degree of blistering, Part 3—Assessment of degree of rusting, Part 4—Assessment of degree of cracking, Part 5—Assessment of degree of flaking, Part 6—Assessment of degree of chalking by tape method, Part 7—Assessment of degree of chalking by velvet method, Part 8—Assessment of degree of delamination and corrosion around a scribe or other artificial defect). The International Organization for Standardization: Geneva, Switzerland, 2003–2012.
- ISO 16276-2; Corrosion Protection of Steel Structures by Protective Paint Systems—Assessment of, and Acceptance Criteria for, the Adhesion/Cohesion (Fracture Strength) of a Coating—Part 2: Cross-Cut Testing and X-Cut Testing. The International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 19840; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Measurement of, and Acceptance Criteria for, the Thickness of Dry Films on Rough Surfaces. The International Organization for Standardization: Geneva, Switzerland, 2012.
- Bordziłowski, J.; Darowicki, K.; Krakowiak, S.; Królikowska, A. Impedance measurements of coating properties on bridge structures. Prog. Org. Coat. 2003, 46, 216–219. [Google Scholar] [CrossRef]
- Popova, K.; Prošek, T. Corrosion Monitoring in Atmospheric Conditions: A Review. Metals 2022, 12, 171. [Google Scholar] [CrossRef]
- Gray, L.G.S.; Appleman, B.R. EIS: Electrochemical Impedance Spectroscopy a Tool to Predict Remaining Coating Life? J. Prot. Coat. Linings 2003, 20, 66–74. [Google Scholar]
- Jaśniok, T.; Jaśniok, M.; Skórkowski, A. Diagnostics of Large Non-Conductive Anti-Corrosion Coatings on Steel Structures by Means of Electrochemical Impedance Spectroscopy. Materials 2021, 14, 3959. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.J.; Jamali, S.S. The best tests for anti-corrosive paints. And why: A personal viewpoint. Prog. Org. Coat. 2017, 102, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Bickham, R.A. Comparing Global Standards for Offshore Athmospheric Coatings, Material Performance 2/1/2022. Available online: https://www.materialsperformance.com/articles/material-selection-design/2022/02/comparing-global-standards-for-offshore-atmospheric-coatings (accessed on 29 March 2022).
- Hernández, H.H.; Ruiz Reynoso, A.M.; Trinidad González, J.C.; González Morán, C.O.; Miranda Hernández, J.G.; Ruiz, A.M.; Hernández, J.M.; Cruz, R.O. Electrochemical Impedance Spectroscopy (EIS): A Review Study of Basic Aspects of the Corrosion Mechanism Applied to Steels. In Electrochemical Impedance Spectroscopy; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Carlozzo, B.J.; Andrews, J.; Anwari, F.; DiLorenzo, M.; Glover, R.; Grossman, S.; Harding, C.; McCarthy, J.; Mysza, B.; Raymond, R.; et al. Correlation of Accelerated Exposure Testing and Exterior Exposure Sites. 2. One-Year Results. J. Coat. Technol. 1994, 68, 47–61. [Google Scholar]
- Claydon, D.A. Performance testing of anticorrosive coatings. Coat. World 2003, 2, 26–33. [Google Scholar]
- Cremer, N.D. From continuous to cyclic salt spray testing. Polym. Paint. Colour J. 1998, 188, 31–32. [Google Scholar]
- Crewdson, M.J.; Brennan, P. Outdoor Weathering. Basic Exposure Procedures. J. Prot. Coat. Linings. 1995, 12, 17–25. [Google Scholar]
- Ferlauto, E.C.; Emami, M.; Galantefox, J.; Grivna, M.; Habeck, E.; Jones, L.; Prevost, O.J.; Vetter, G.; Wood, L. Selection of corrosion test methods based on mechanism principles. J. Coat. Technol. 1994, 66, 85–97. [Google Scholar]
- Schulz, U. Accelerated Testing. In Nature and Artificial Weathering in the Coatings Industry; Vincentz: Hannover, Germany, 1994. [Google Scholar]
- Białomazur, M.; Jasinska, I.; Kowalczyk, K.; Musik, M.; Pasierbiewicz, K.; Wrobel, R. Acrylic polyurethane coatings durability under outdoor weathering in an industrial area. Polimery 2021, 66, 503–517. [Google Scholar] [CrossRef]
- Skerry, B.S.; Simpson, C.H. Accelerated test method for assessing corrosion and weathering of paints for atmospheric corrosion control. Corrosion 1993, 49, 663–674. [Google Scholar] [CrossRef]
- Tait, W.S. Using Electrochemical Measurements to Estimate Coating and Polymer Film Durability. J. Coat. Technol. 2003, 942, 45–50. [Google Scholar] [CrossRef]
- Vincent, L.D. (Ed.) Ask the Coatings Experts: Coating Performance Test Methods for Offshore Service Compared to Actual Service Life. Mater. Perform. 2009, 48, 54–58. [Google Scholar]
- ISO 20340; Paints and Varnishes—Performance Requirements for Protective Paint Systems for Offshore and Related Structures. The International Organization for Standardization: Geneva, Switzerland, 2009.
- NORSOK M-501:2012 Surface Preparation and Protective Coating. Edition 6. Available online: https://www.standard.no/Global/PDF/Petroleum/Norsok%20M-501%20ed%206%20Clarification%20log%202015%20rev%201_finished.pdf (accessed on 29 March 2022).
- ISO 9227; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. The International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 4624; Paints and Varnishes—Pull-Off Test for Adhesion. The International Organization for Standardization: Geneva, Switzerland, 2002.
- ISO 16773-3; Electrochemical Impedance Spectroscopy (EIS) on Coated and Uncoated Metallic Specimens—Part 3: Processing and Analysis of Data from Dummy Cells. The International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 16474-3; Paints and Varnishes—Methods of Exposure to Laboratory Light Sources: Fluorescent UV Lamps. The International Organization for Standardization: Geneva, Switzerland, 2013.
- EN 13523-10; Coil Coated Metals—Test Methods—Part 10: Resistance to Fluorescent UV Radiation and Water Condensation. European Committee For Standardization: Brussels, Belgium, 2010.
- GSB ST 663-4; International Quality Regulations for the Coating of Building Componenta of Steel and Galvanised Steel Building Components. Material Approval for Coating Materials: Aluminium, Steel and Galvanized Steel. Available online: https://gsb-international.de/media/pdftoimage/6F011541552F4BBFBFD187D5E71B70A9.PDF (accessed on 29 March 2022).
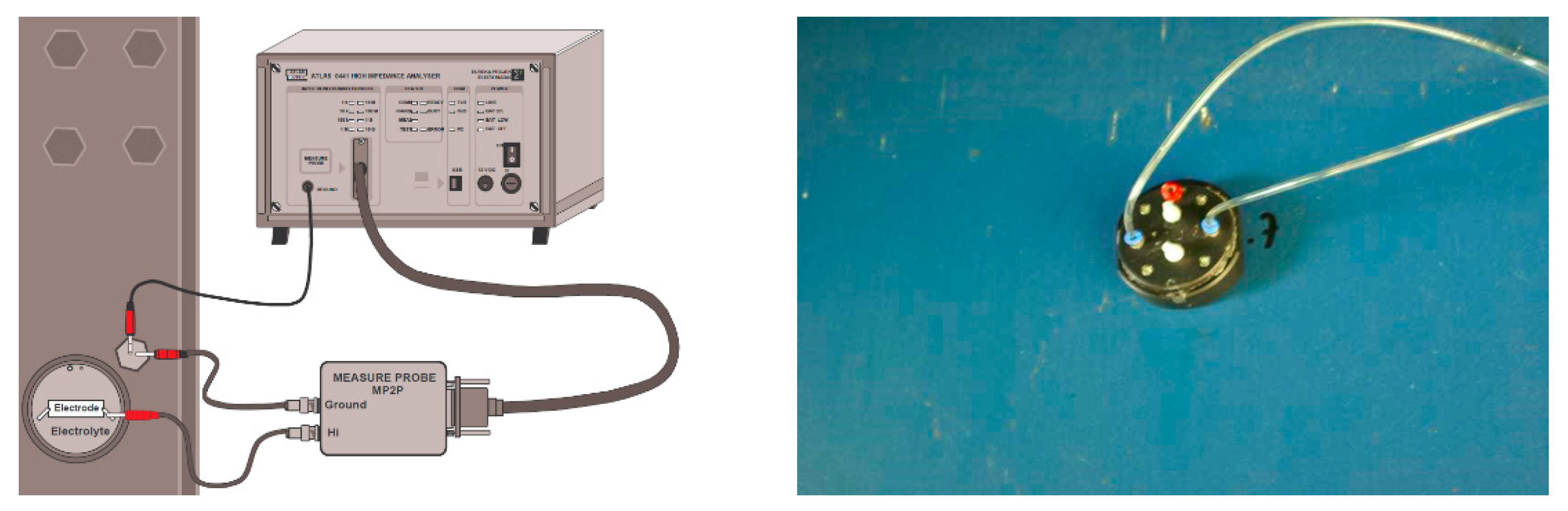


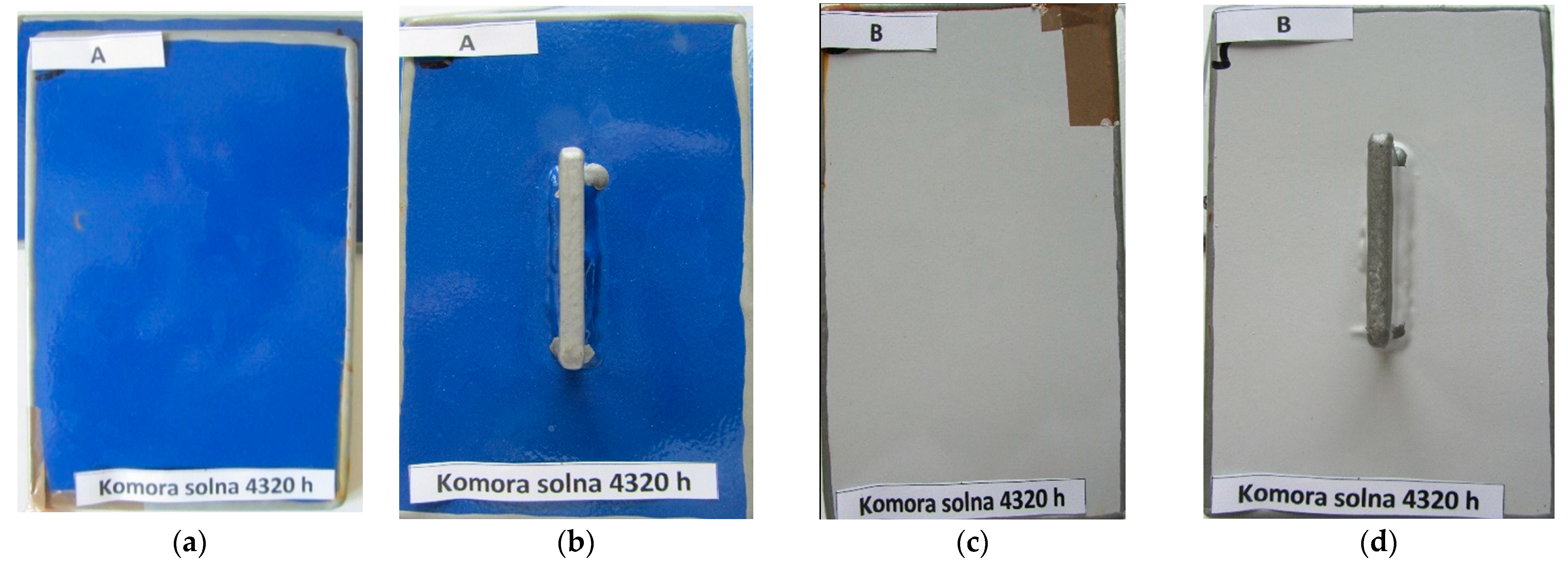

| Bridge Number/ Coating System According to Table 2 | System Service Life [Years] | Corrosivity Category 1 | Structure Appearance (Characteristic Photo) |
|---|---|---|---|
| Kośmin Bridge 1/A | 13 | C4 |  |
| Tryńcza Bridge 2/B | 10 | C4 |  |
| Góra Kalwaria Bridge 3/C1 | 16 | C5I |  |
| Gdański Bridge in Warsaw 4/C2 | 17 | C5I |  |
| Kazimierza Wielkiego Bridge 6/E | 16 | C4 |  |
| Fordon Bridge in Bydgoszcz 7/F | 15 | C5I |  |
| Praski Bridge in Warsaw 8/G | 15 | C4 |  |
| System | Coating Type | Resin/Curing Agent/Anticorrosive Pigment |
|---|---|---|
| A | Primer | EP (HS)/amine adduct/Al (2–4 wt.%) |
| Intermediate | EP (HS)/polyamine/Al | |
| Topcoat | PUR (acrylic)/HDI | |
| B | Primer | EP (HS)/polyaminoamide/Al (2 wt.%) |
| Intermediate | EP (HS)/polyaminoamide/Al (2 wt.%) | |
| Topcoat | PUR (acrylic)/HDI | |
| C | Primer | EP/polyamide/Zn (75 wt.% in a dry coating) |
| Intermediate | EP/polyamide/Al (1–2.5 wt.%) | |
| Topcoat | PUR (acrylic/polyester)/HDI | |
| D 1 | Primer | EP (HB)/polyamine/ion exchange pigment |
| Intermediate | EP (HB)/polyamine/– | |
| Topcoat | PUR (acrylic)/HDI | |
| E | Primer | EP/polyamidoamine/Zn (94 wt.% in a dry coating) |
| Intermediate | EP/polyaminoamide/MIOX (58 wt.%) | |
| Topcoat | PUR (acrylic)/HDI/MIOX (47 wt.%) | |
| F | Primer | EP/polyaminoamide/Al (10 wt.%) |
| Intermediate | EP/polyamine/MIOX (12 wt.%), Al (10 wt.%), Zn phosphate (5 wt.%) | |
| Topcoat | PUR (acrylic)/HDI | |
| G | Primer | EP/polyaminoamide/Zn phosphate (10.6 wt.%) |
| Intermediate | EP/polyaminoamide/MIOX (36.5 wt.%) | |
| Topcoat | PUR (acrylic)/HDI |
| Bridge/ Coating System According to Table 2 | EIS Measurement Sites |
|---|---|
| Kośmin Bridge 1/A |  |
| Tryńcza Bridge 2/B |  |
| Góra Kalwaria Bridge 3/C1 | 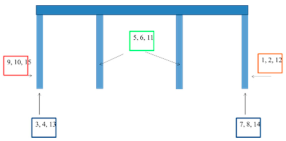 |
| Gdański Bridge in Warsaw 4/C2 | 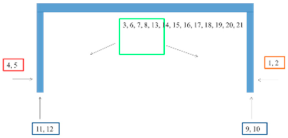 |
| Kazimierza Wielkiego Bridge 6/E |  |
| Fordon Bridge in Bydgoszcz 7/F |  |
| Praski Bridge in Warsaw 8/G | 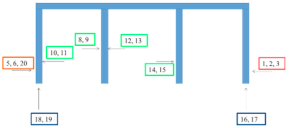 |
| System | Average Thickness, µm | Adhesion Degree | Degradation Degree, General Comments |
|---|---|---|---|
| A | 207 ± 11.8 | 0 | no degradation |
| B | 447 ± 14.5 | 0–2 | chalking 2, rust degree Ri1, crevice corrosion |
| C1 | 252 ± 11.2 | 2 | chalking 2 |
| C2 | 410 ± 16.2 | 2–3 | chalking 1, on the lower flange of the girder chalking 2, rust degree Ri1 |
| E | 281 ± 5.1 | 2 | chalking 3 |
| F | 365 ± 8.0 | 0 | chalking 1, crevice corrosion |
| G | 188 ± 5.2 | 1 | chalking 1, corrosion on sheet packages and on the surface of the lower girder flange |
| Measurement Site | System | ||||||
|---|---|---|---|---|---|---|---|
| A | B | C1 | C2 | E | F | G | |
| Log|Z| at 0.1 Hz Values | |||||||
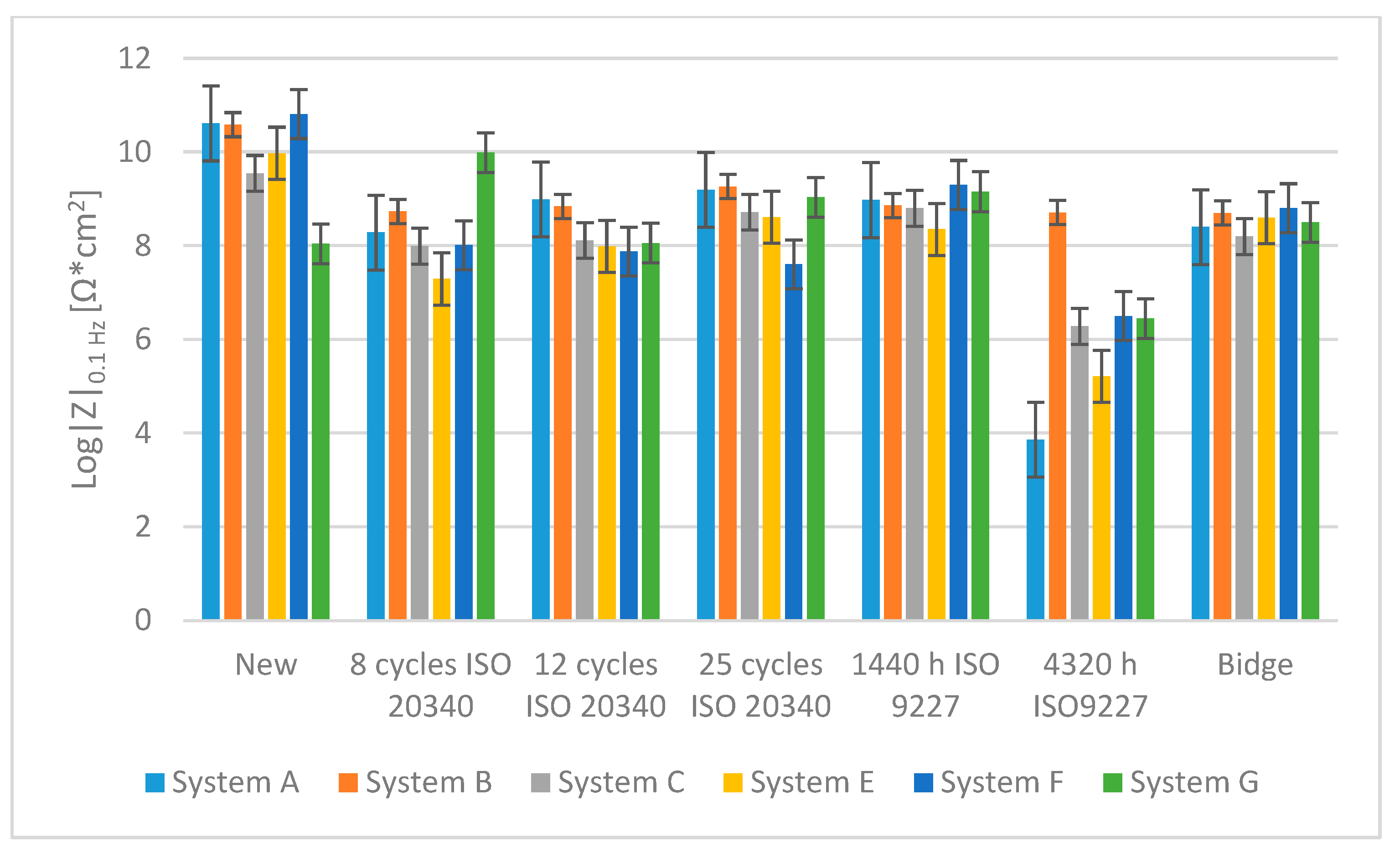
| 1 | 9.1 | 8.6 | 8.1 | 6.6 | 9.4 | 9.6 | 7.7 |
| 2 | 8.7 | 10.8 | 8.2 | 6.5 | 6.7 | 10.2 | 7.8 |
| 3 | 8.6 | 8.9 | 8.7 | 9.7 | 8.8 | 9.4 | 8.1 |
| 4 | 9.0 | 7.5 | 6.0 | 6.4 | 8.6 | 8.3 | 7.1 |
| 5 | 8.8 | 9.2 | 5.7 | 7.2 | 9.7 | 8.6 | 9.7 |
| 6 | 8.5 | 10.0 | 8.3 | 6.7 | 9.6 | 5.6 | 9.4 |
| 7 | 8.3 | 6.5 | 9.0 | 9.9 | 7.6 | 9.1 | 10.3 |
| 8 | 8.3 | 8.5 | 8.0 | 9.7 | 8.8 | 9.0 | 6.5 |
| 9 | 8.3 | 8.6 | 8.4 | 8.9 | 7.6 | 10.1 | 8.8 |
| 10 | 8.9 | 8.4 | 8.4 | 7.7 | 7.4 | 9.3 | 9.7 |
| 11 | 7.6 | 8.9 | 8.4 | 7.0 | 8.0 | 7.4 | 6.7 |
| 12 | 8.6 | 8.8 | 8.9 | 6.9 | 9.8 | 7.5 | 9.8 |
| 13 | 8.6 | 9.0 | 8.9 | 7.3 | 9.9 | 7.9 | 6.2 |
| 14 | 7.5 | 7.9 | 9.0 | 10.9 | 8.2 | 9.9 | 9.8 |
| 15 | 8.0 | 8.8 | 8.6 | 6.7 | 7.8 | 9.8 | 8.5 |
| 16 | 8.2 | 8.8 | - | 10.7 | 8.3 | 9.0 | 9.0 |
| 17 | 8.4 | - | - | 10.5 | 9.6 | 9.2 | 9.2 |
| 18 | - | - | - | 10.4 | 9.6 | 10.5 | 8.0 |
| 19 | - | - | - | 7.5 | 9.3 | 10.5 | 7.9 |
| 20 | - | - | - | 8.5 | - | 7.8 | 12.5 |
| Average | 8.4 ± 0.2 | 8.7 ± 0.5 | 8.2 ± 0.5 | 8.2 ± 0.7 | 8.6 ± 0.4 | 8.8 ± 0.6 | 8.5 ± 0.7 |
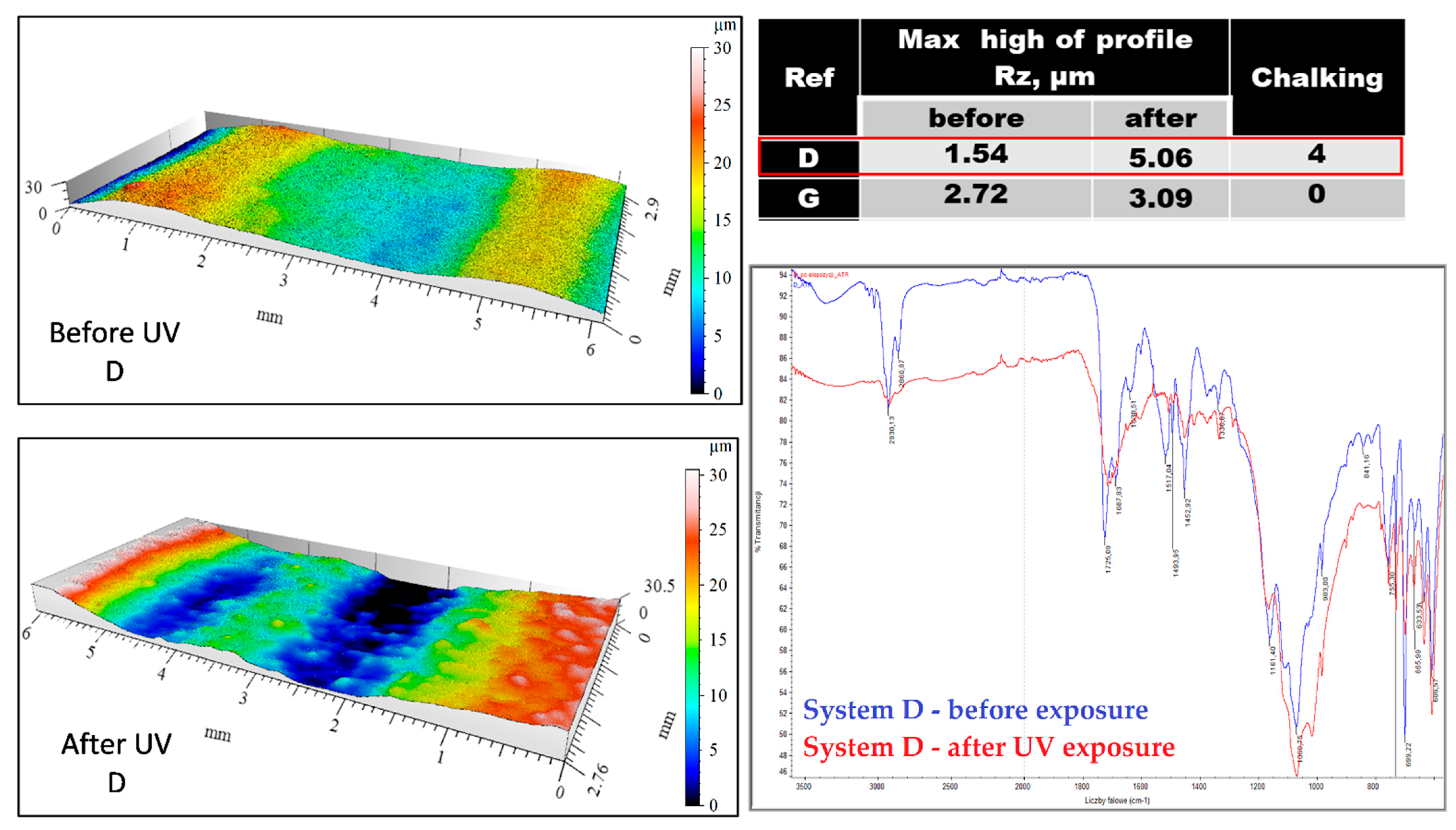
| System | Chalking Degree after UV Chamber | Chalking Degree on the Bridge |
|---|---|---|
| A | 0 | 0 |
| B | 0 | 2 |
| C | 1 | 1–2 |
| D | 4 | – |
| E | 0 | 3 |
| F | 0 | 1 |
| G | 0 | 1 |
| System | Average Adhesion of the Coatings without Scratches, MPa | ||
|---|---|---|---|
| New | After Salt Chamber Test | After 25 Cycles | |
| A | 12.5 | 10.0 | 8.8 |
| B | 12.1 | 11.0 | 12.1 |
| C | 7.8 | 7.5 | 6.2 |
| D | 8.1 | 8.2 | 8.0 |
| E | 4.2 | 5.2 | 5.1 |
| F | 8.2 | 9.1 | 8.1 |
| G | 12.5 | 10.0 | 8.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Królikowska, A.; Komorowski, L.; Langer, E.; Zubielewicz, M. Promising Results of the Comparison of Coatings on Aged Bridges and of Same Coatings in Laboratory. Materials 2022, 15, 3064. https://doi.org/10.3390/ma15093064
Królikowska A, Komorowski L, Langer E, Zubielewicz M. Promising Results of the Comparison of Coatings on Aged Bridges and of Same Coatings in Laboratory. Materials. 2022; 15(9):3064. https://doi.org/10.3390/ma15093064
Chicago/Turabian StyleKrólikowska, Agnieszka, Leszek Komorowski, Ewa Langer, and Małgorzata Zubielewicz. 2022. "Promising Results of the Comparison of Coatings on Aged Bridges and of Same Coatings in Laboratory" Materials 15, no. 9: 3064. https://doi.org/10.3390/ma15093064
APA StyleKrólikowska, A., Komorowski, L., Langer, E., & Zubielewicz, M. (2022). Promising Results of the Comparison of Coatings on Aged Bridges and of Same Coatings in Laboratory. Materials, 15(9), 3064. https://doi.org/10.3390/ma15093064






