Theoretical Prediction and Experimental Validation of the Glass-Forming Ability and Magnetic Properties of Fe-Si-B Metallic Glasses from Atomic Structures
Abstract
:1. Introduction
2. Simulation and Experimental Details
3. Results and Discussion
3.1. AIMD Simulations
3.2. Experimental Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suryanarayana, C.; Inoue, A. Iron-based bulk metallic glasses. Int. Mater. Rev. 2013, 58, 131–166. [Google Scholar] [CrossRef]
- Jiles, D. Recent advances and future directions in magnetic materials. Acta Mater. 2003, 51, 5907–5939. [Google Scholar] [CrossRef]
- Li, J.F.; Liu, X.; Zhao, S.F.; Ding, H.Y.; Yao, K.F. Fe-based bulk amorphous alloys with iron contents as high as 82 at%. J. Magn. Magn. Mater. 2015, 386, 107–110. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, C.; Men, H.; He, A.; Chang, C.; Wang, X.; Li, R.W. Fe-based amorphous alloys for wide ribbon production with high Bs and outstanding amorphous forming ability. J. Alloys Compd. 2015, 630, 209–213. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, C.; He, A.; Men, H.; Chang, C.; Wang, X. Composition design of high Bs Fe-based amorphous alloys with good amorphous-forming ability. J. Alloys Compd. 2016, 656, 729–734. [Google Scholar] [CrossRef]
- Li, X.; Sun, B.; Qu, C.; Ji, H.; Wang, L. The formation mechanism of novel Fe–P–B metallic glasses: A perspective from nearly free electron model. J. Alloys Compd. 2015, 620, 358–360. [Google Scholar] [CrossRef]
- Mouhamad, M.; Elleau, C.; Mazaleyrat, F.; Guillaume, C.; Jarry, B. Physicochemical and accelerated aging tests of Metglas 2605SA1 and Metglas 2605HB1 amorphous ribbons for power applications. IEEE. Trans. Magn. 2011, 47, 3192–3195. [Google Scholar] [CrossRef] [Green Version]
- Yue, S.; Zhang, H.; Cheng, R.; Wang, A.; Dong, Y.; He, A.; Ni, H.; Liu, C.-T. Magnetic and thermal stabilities of FeSiB eutectic amorphous alloys: Compositional effects. J. Alloys Compd. 2019, 776, 833–838. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Qu, C.; Song, K.; Wang, L. Effects of Nb on the precipitation of α-Fe, glass forming ability and magnetic properties of Fe85B10P5 alloys. J. Alloys Compd. 2017, 694, 643–646. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, C.; Wang, A.; Shen, B. Development of quaternary Fe-based bulk metallic glasses with high saturation magnetization above 1.6 T. J. Non-Cryst. Solids 2012, 358, 1443–1446. [Google Scholar] [CrossRef]
- Kong, F.; Chang, C.; Inoue, A.; Shalaan, E.; Al-Marzouki, F. Fe-based amorphous soft magnetic alloys with high saturation magnetization and good bending ductility. J. Alloys Compd. 2014, 615, 163–166. [Google Scholar] [CrossRef]
- Han, Y.; Kong, F.; Chang, C.; Zhu, S.; Inoue, A.; Shalaan, E.-S.; Al-Marzouki, F. Syntheses and corrosion behaviors of Fe-based amorphous soft magnetic alloys with high-saturation magnetization near 1.7 T. J. Mater. Res. 2015, 30, 547–555. [Google Scholar] [CrossRef]
- Pang, L.L.; Inoue, A.; Zanaeva, E.N.; Wang, F.; Bazlov, A.I.; Han, Y.; Shull, R.B. Nanocrystallization, good soft magnetic properties and ultrahigh mechanical strength for Fe82–85B13–16Si1Cu1 amorphous alloys. J. Alloys Compd. 2019, 785, 25–37. [Google Scholar] [CrossRef]
- Wang, F.; Inoue, A.; Han, Y.; Zhu, S.; Kong, F.; Zanaeva, E.; Liu, G.; Shalaan, E.; Al-Marzouki, F.; Obaid, A. Soft magnetic Fe-Co-based amorphous alloys with extremely high saturation magnetization exceeding 1.9 T and low coercivity of 2 A/m. J. Alloys Compd. 2017, 723, 376–384. [Google Scholar] [CrossRef]
- Ogawa, Y.; Naoe, M.; Yoshizawa, Y.; Hasegawa, R. Magnetic properties of high Bs Fe-based amorphous material. J. Magn. Magn. Mater. 2006, 304, e675–e677. [Google Scholar] [CrossRef]
- Sheng, H.; Luo, W.; Alamgir, F.; Bai, J.; Ma, E. Atomic packing and short-to-medium-range order in metallic glasses. Nature 2006, 439, 419–425. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, X.; Lou, H.; Cao, Q.; Jiang, J. Atomic packing in Fe-based metallic glasses. Acta Mater. 2016, 102, 116–124. [Google Scholar] [CrossRef]
- Dong, B.; Zhou, S.; Li, D.; Qin, J.; Pan, S.; Wang, Y.; Li, Z. Effects of solute–solute avoidance on metallic glass formation. J. Non-Cryst. Solids 2012, 358, 2749–2752. [Google Scholar] [CrossRef]
- Vincze, I.; Boudreaux, D.; Tegze, M. Short-range order in Fe-B metallic glass alloys. Phys. Rev. B 1979, 19, 4896. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Q.; Duan, H. Study of the effects of metalloid elements (P, C, B) on Fe-based amorphous alloys by ab initio molecular dynamics simulations. J. Appl. Phys. 2015, 117, 104901. [Google Scholar] [CrossRef]
- Kaban, I.; Jovari, P.; Waske, A.; Stoica, M.; Bednarčik, J.; Beuneu, B.; Mattern, N.; Eckert, J. Atomic structure and magnetic properties of Fe–Nb–B metallic glasses. J. Alloys Compd. 2014, 586, S189–S193. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Dong, B.; Zhou, S.; Li, X.; Qin, J. Structural, magnetic, and electronic properties of Fe82Si4B10P4 metallic glass. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Y.; Huang, L.; Li, J. Influential electronic and magnetic properties of the gallium sulfide monolayer by substitutional doping. J. Phys. Chem. C 2015, 119, 29148–29156. [Google Scholar] [CrossRef]
- Shan, G.; Zhang, J.L.; Li, J.; Zhang, S.; Jiang, Z.; Huang, Y.; Shek, C.-H. Atomic-level structures and physical properties of magnetic CoSiB metallic glasses. J. Magn. Magn. Mater. 2014, 352, 49–55. [Google Scholar] [CrossRef]
- Lizárraga, R. Structural and magnetic properties of the Gd-based bulk metallic glasses GdFe2, GdCo2, and GdNi2 from first principles. Phys. Rev. B 2016, 94, 174201. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Qin, J.; Dong, B. The relationship between atomic structure and magnetic property of amorphous Fe78Si9B13 alloy at different pressures. J. Magn. Magn. Mater. 2017, 443, 216–221. [Google Scholar] [CrossRef]
- Wang, Y.; Takeuchi, A.; Makino, A.; Liang, Y.; Kawazoe, Y. Atomic packing and diffusion in Fe85Si2B9P4 amorphous alloy analyzed by ab initio molecular dynamics simulation. J. Appl. Phys. 2015, 117, 17B705. [Google Scholar] [CrossRef]
- Miracle, D.B.; Egami, T.; Flores, K.M.; Kelton, K.F. Structural aspects of metallic glasses. MRS Bull. 2007, 32, 629–634. [Google Scholar] [CrossRef]
- Luo, W.; Sheng, H.; Ma, E. Pair correlation functions and structural building schemes in amorphous alloys. Appl. Phys. Lett. 2006, 89, 131927. [Google Scholar] [CrossRef]
- Moroni, E.; Kresse, G.; Hafner, J.; Furthmüller. Ultrasoft pseudopotentials applied to magnetic Fe, Co, and Ni: From atoms to solids. Phys. Rev. B 1997, 56, 15629. [Google Scholar] [CrossRef]
- Sheng, H.; Cheng, Y.; Lee, P.; Shastri, S.; Ma, E. Atomic packing in multicomponent aluminum-based metallic glasses. Acta Mater. 2008, 56, 6264–6272. [Google Scholar] [CrossRef]
- Sha, Z.; Feng, Y.; Li, Y. Statistical composition-structure-property correlation and glass-forming ability based on the full icosahedra in Cu–Zr metallic glasses. Appl. Phys. Lett. 2010, 96, 061903. [Google Scholar] [CrossRef]
- Li, H.; Lu, Z.; Wang, S.; Wu, Y.; Lu, Z. Fe-based bulk metallic glasses: Glass formation, fabrication, properties and applications. Prog. Mater. Sci. 2019, 103, 235–318. [Google Scholar] [CrossRef]
- Jiao, Z.; Li, H.; Gao, J.; Wu, Y.; Lu, Z. Effects of alloying elements on glass formation, mechanical and soft-magnetic properties of Fe-based metallic glasses. Intermetallics 2011, 19, 1502–1508. [Google Scholar] [CrossRef]
- Heisenberg, W. Zur Theorie des Ferromagnetismus. Z. Phys. 1928, 49, 619. [Google Scholar] [CrossRef]
- Dong, C.; Inoue, A.; Wang, X.; Kong, F.; Zanaeva, E.; Wang, F.; Bazlov, A.; Zhu, S.; Li, Q. Soft magnetic properties of Fe82–83B14–15Si2C0.5–1 amorphous alloys with high saturation magnetization above 1.7 T. J. Non-Cryst. Solids 2018, 500, 173–180. [Google Scholar] [CrossRef]
- Jin, Y.; Fan, X.; Men, H.; Liu, X.; Shen, B. FePCCu nanocrystalline alloys with excellent soft magnetic properties. Sci. China Technol. Sci. 2012, 55, 3419–3424. [Google Scholar] [CrossRef]
- Turnbull, D. Under what conditions can a glass be formed? Contemp. Phys 1969, 10, 473–488. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, C. Glass formation criterion for various glass-forming systems. Phys. Rev. Lett. 2003, 91, 115505. [Google Scholar] [CrossRef] [PubMed]
- Masood, A.; Biswas, A.; Ström, V.; Belova, L.; Ågren, J.; Rao, K. The effect of Ni-substitution on physical properties of Fe72−xB24Nb4Nix bulk metallic glassy alloys. MRS Online Proc. Libr. (OPL) 2011, 1300, 607. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Song, K.; Wu, Y.; Ji, H.; Wang, L. The mismatch entropy for bulk metallic glasses: A thermodynamic approach. Mater. Lett. 2013, 107, 17–19. [Google Scholar] [CrossRef]
- Luborsky, F.; Becker, J.; Walter, J.; Liebermann, H. Formation and magnetic properties of Fe-B-Si amorphous alloys. IEEE. Trans. Magn. 1979, 15, 1146–1149. [Google Scholar] [CrossRef]
- Carow-Watamura, U.; Louzguine, D.; Takeuchi, A. B-Fe-Si (144). Landolt Börnstein 2011, 37, 200. [Google Scholar] [CrossRef]
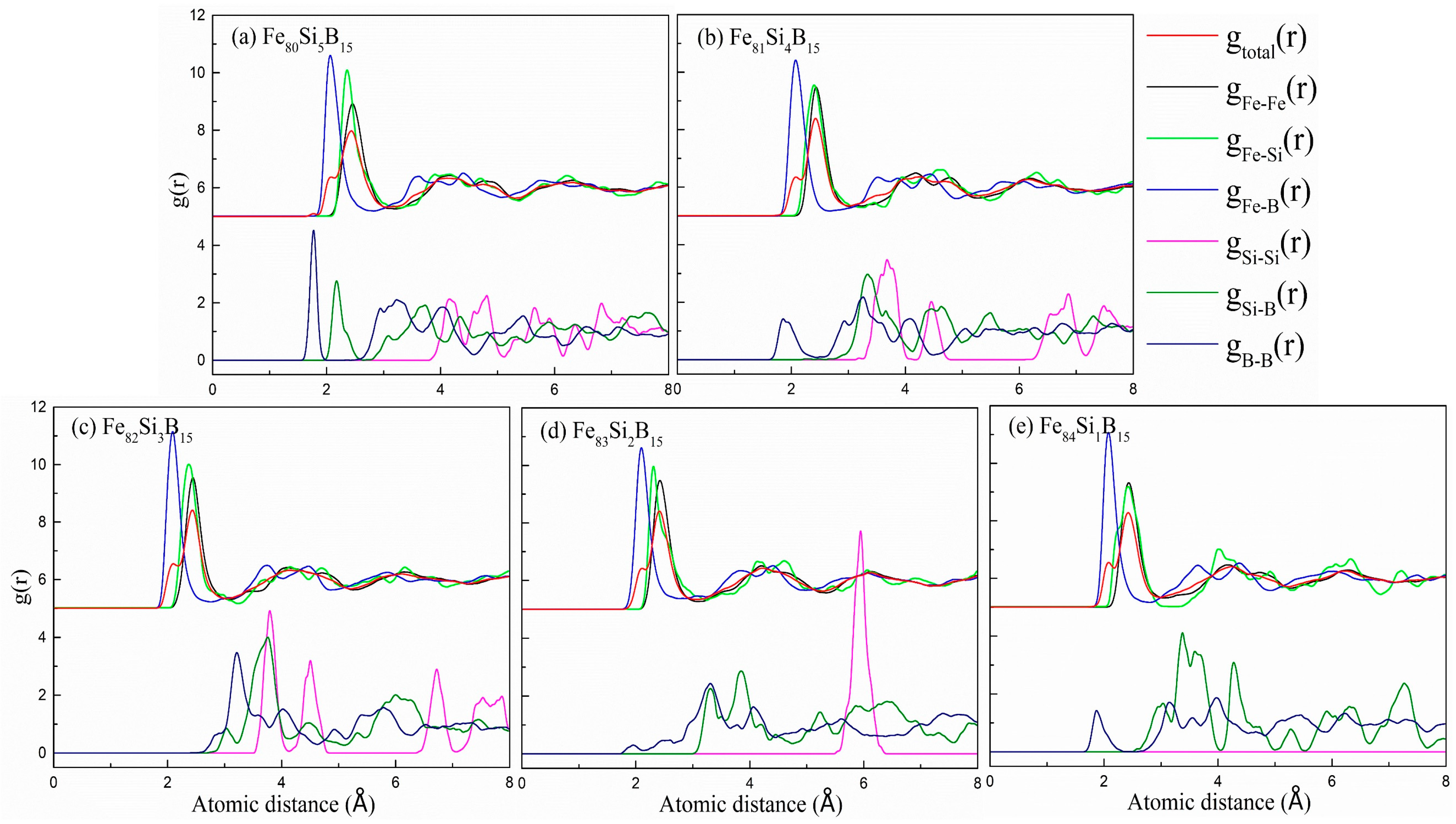

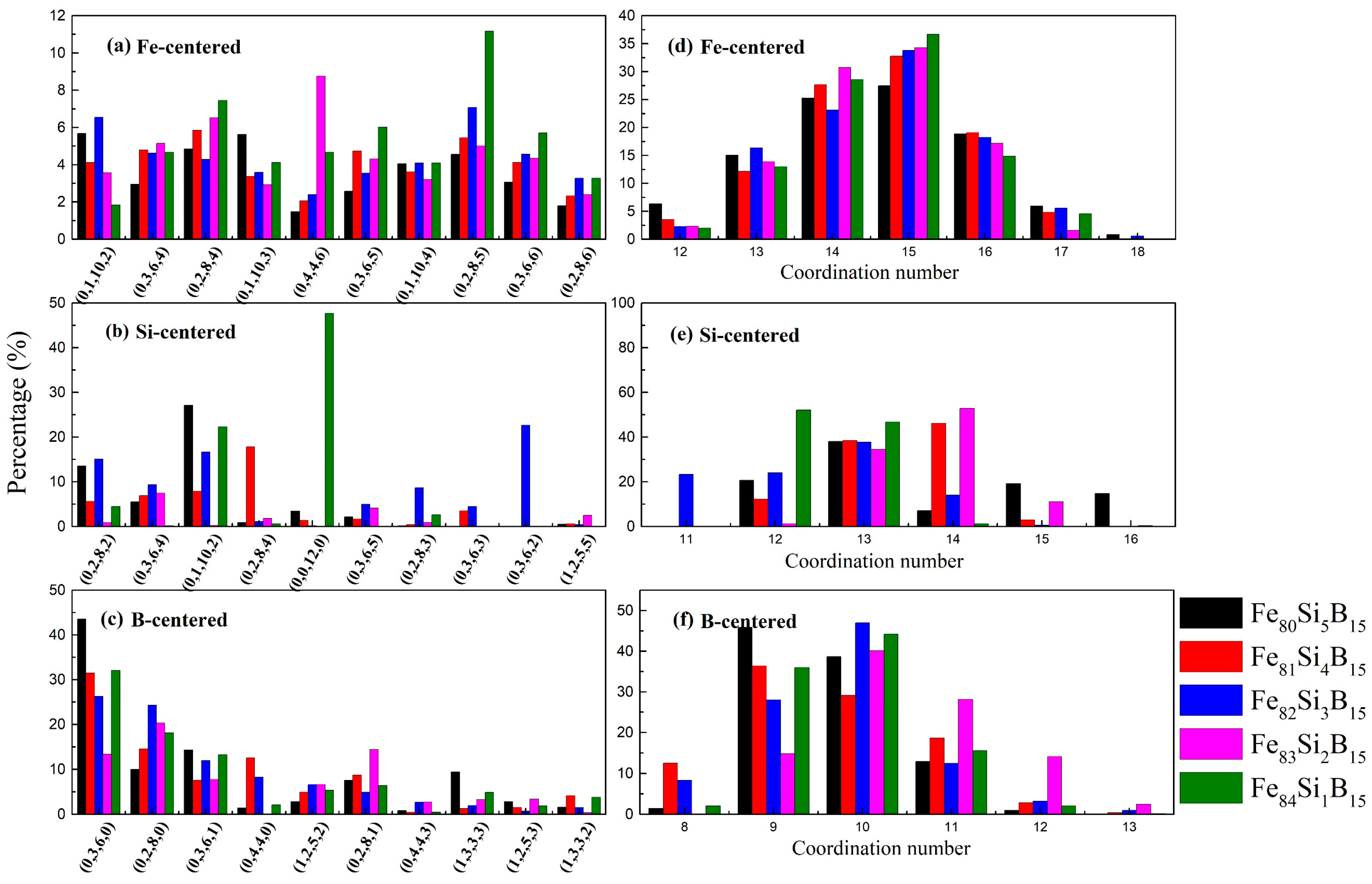
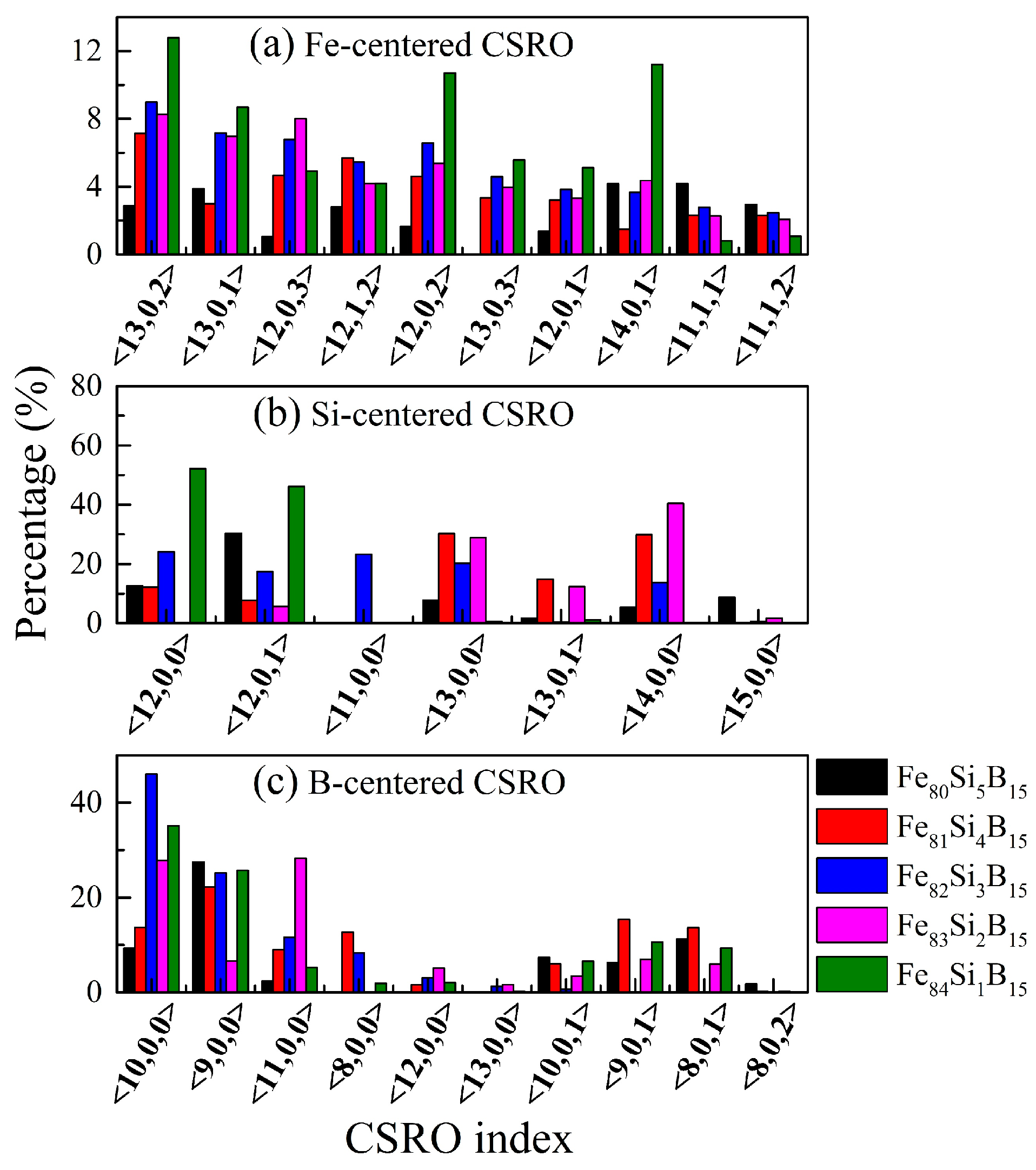
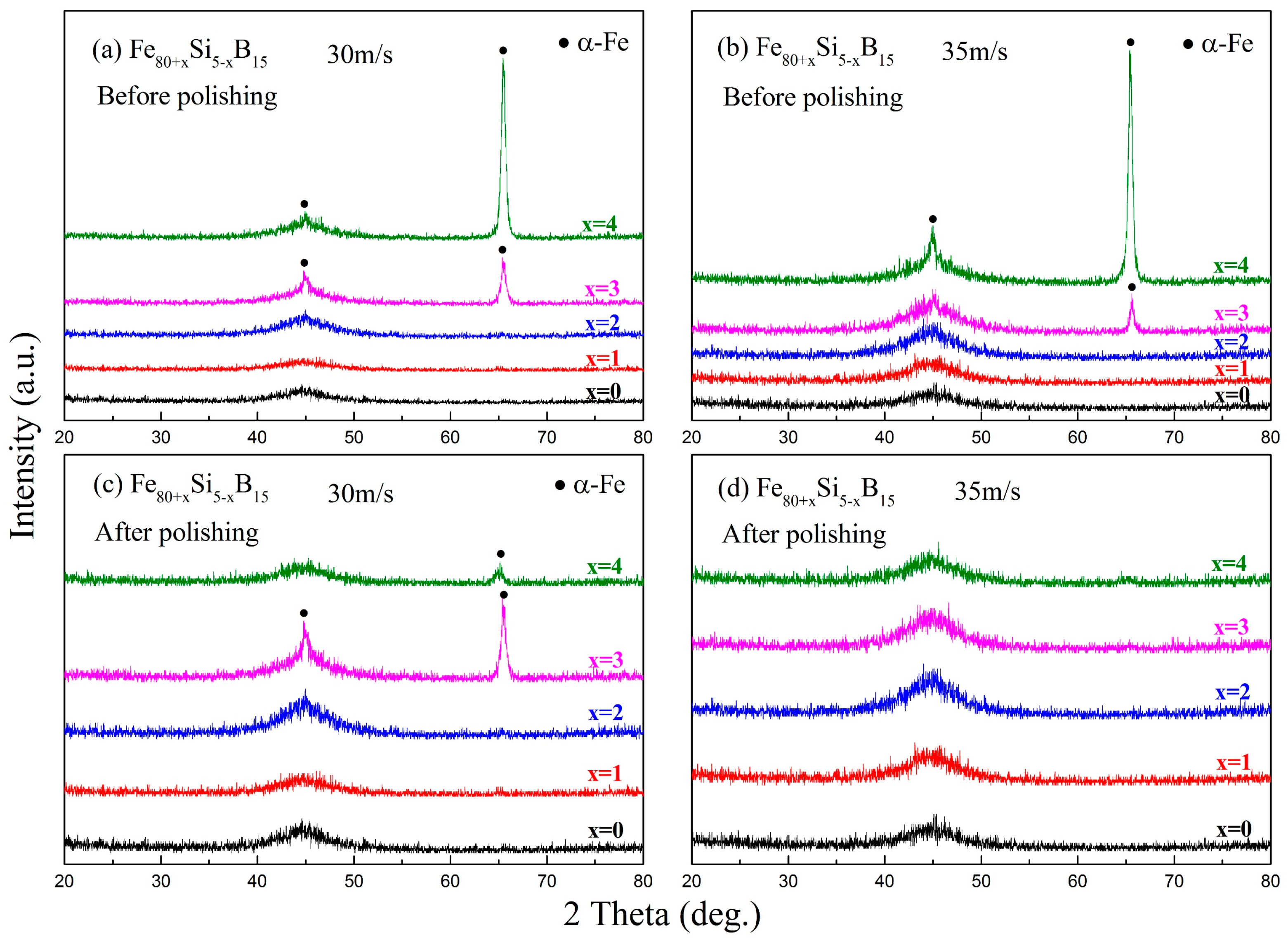
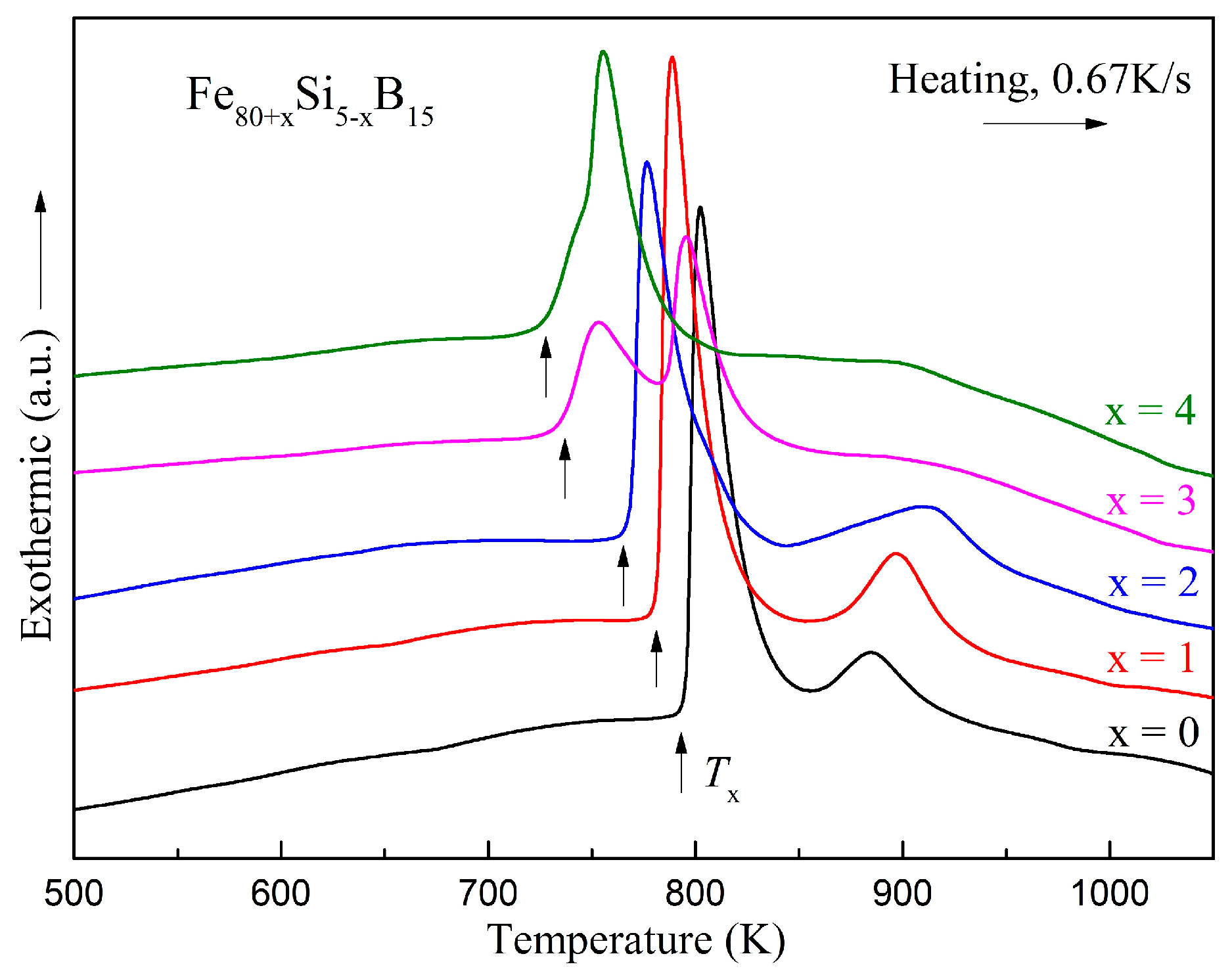
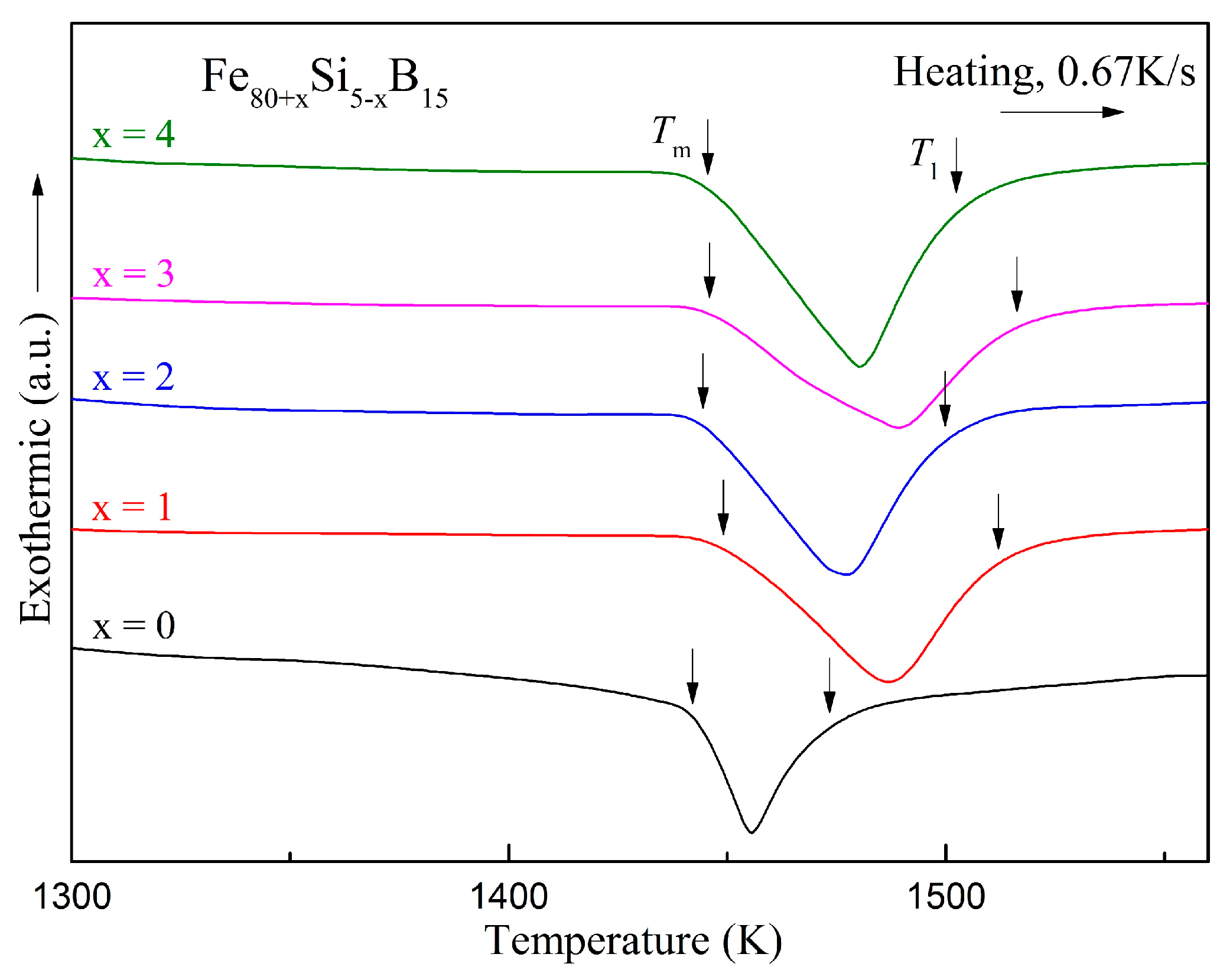

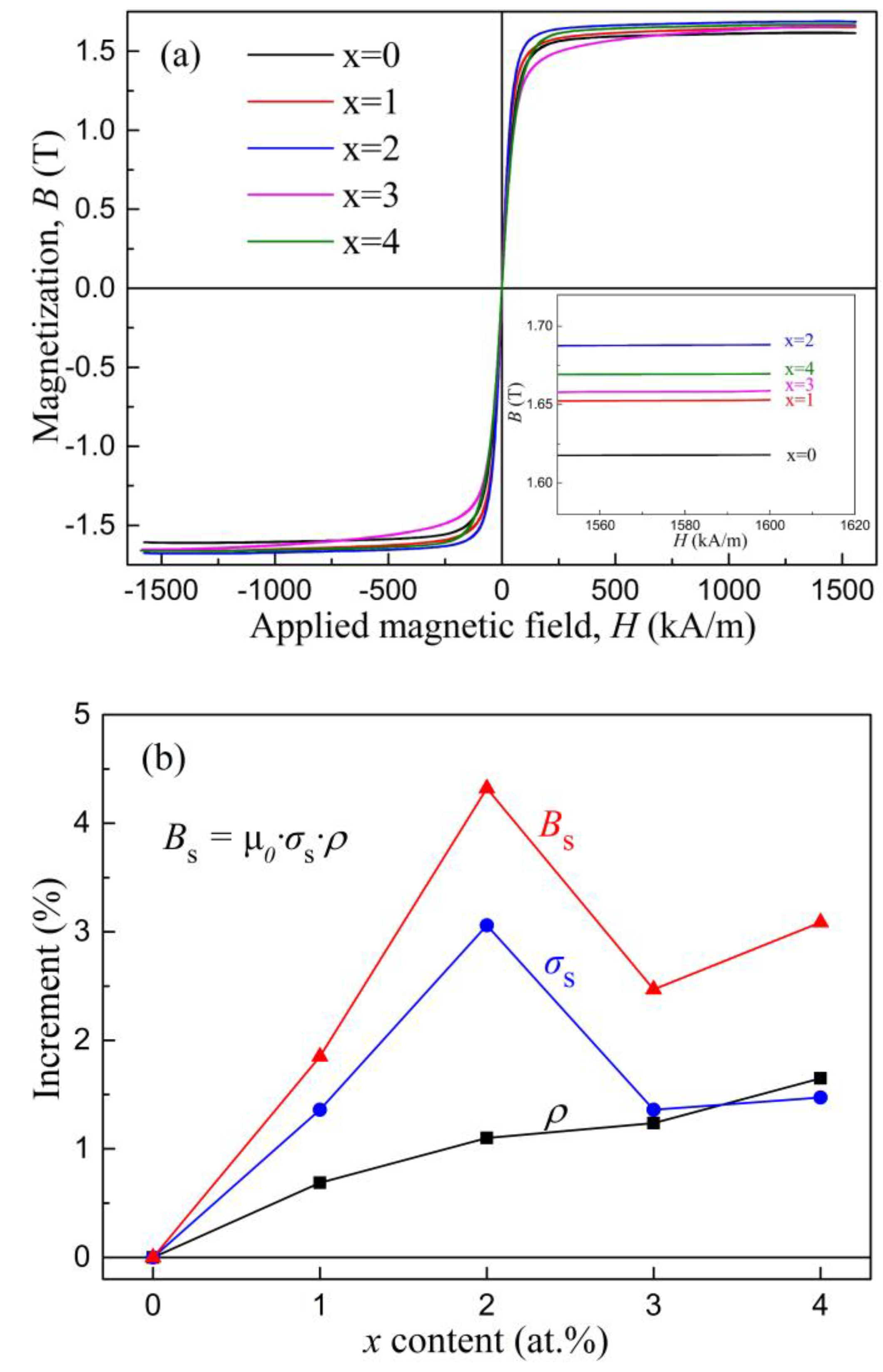
| Alloys | Fe80Si5B15 | Fe81Si4B15 | Fe82Si3B15 | Fe83Si2B15 | Fe84Si1B15 |
|---|---|---|---|---|---|
| rFe-Fe | 2.45 | 2.43 | 2.45 | 2.42 | 2.43 |
| Alloys | Fe-Fe | Fe-Si | Fe-B | Total CN |
|---|---|---|---|---|
| Fe80Si5B15 | 12.10 | 0.81 | 1.68 | 14.59 |
| Fe81Si4B15 | 12.32 | 0.65 | 1.70 | 14.67 |
| Fe82Si3B15 | 12.45 | 0.45 | 1.80 | 14.70 |
| Fe83Si2B15 | 12.40 | 0.32 | 1.82 | 14.55 |
| Fe84Si1B15 | 12.80 | 0.14 | 1.70 | 14.64 |
| Alloys | Si-Fe | Si-Si | Si-B | Total CN |
|---|---|---|---|---|
| Fe80Si5B15 | 12.93 | 0 | 0.76 | 13.69 |
| Fe81Si4B15 | 13.09 | 0.04 | 0.27 | 13.40 |
| Fe82Si3B15 | 12.26 | 0 | 0.18 | 12.44 |
| Fe83Si2B15 | 13.46 | 0 | 0.29 | 13.75 |
| Fe84Si1B15 | 12.02 | 0 | 0.47 | 12.49 |
| Alloys | B-Fe | B-Si | B-B | Total CN |
|---|---|---|---|---|
| Fe80Si5B15 | 8.97 | 0.25 | 0.44 | 9.66 |
| Fe81Si4B15 | 9.17 | 0.07 | 0.40 | 9.64 |
| Fe82Si3B15 | 9.74 | 0.03 | 0 | 9.77 |
| Fe83Si2B15 | 10.08 | 0.04 | 0.37 | 10.49 |
| Fe84Si1B15 | 9.50 | 0.03 | 0.27 | 9.80 |
| Alloys | CSRO Type | Fe | Si | B |
|---|---|---|---|---|
| Fe80Si5B15 | P-type | 2.5% | 34.6% | 39.2% |
| S-type | 100.0% | 0.0% | 35.5% | |
| Fe81Si4B15 | P-type | 5.8% | 72.3% | 59.8% |
| S-type | 100.0% | 4.4% | 40.0% | |
| Fe82Si3B15 | P-type | 4.9% | 82.4% | 95.6% |
| S-type | 100.0% | 0.0% | 0.6% | |
| Fe83Si2B15 | P-type | 5.3% | 71.2% | 60.5% |
| S-type | 100.0% | 0.0% | 35.6% | |
| Fe84Si1B15 | P-type | 8.6% | 52.7% | 70.1% |
| S-type | 100.0% | 0.0% | 26.7% |
| Compositions | Thermal Properties | Magnetic Properties | ||||
|---|---|---|---|---|---|---|
| Tx (K) | Tm (K) | Tl (K) | Trx | Bs (T) | Hc (A/m) | |
| Fe80Si5B15 | 793 | 1441 | 1473 | 0.538 | 1.62 | 5.9 |
| Fe81Si4B15 | 781 | 1449 | 1514 | 0.516 | 1.65 | 6.4 |
| Fe82Si3B15 | 765 | 1444 | 1500 | 0.510 | 1.69 | 6.5 |
| Fe83Si2B15 | 735 | 1443 | 1517 | 0.485 | 1.66 | 8.6 |
| Fe84Si1B15 | 728 | 1445 | 1505 | 0.484 | 1.67 | 9.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Jia, S.; Chen, S.; Li, X.; Wang, L.; Han, X. Theoretical Prediction and Experimental Validation of the Glass-Forming Ability and Magnetic Properties of Fe-Si-B Metallic Glasses from Atomic Structures. Materials 2022, 15, 3149. https://doi.org/10.3390/ma15093149
Jiang Y, Jia S, Chen S, Li X, Wang L, Han X. Theoretical Prediction and Experimental Validation of the Glass-Forming Ability and Magnetic Properties of Fe-Si-B Metallic Glasses from Atomic Structures. Materials. 2022; 15(9):3149. https://doi.org/10.3390/ma15093149
Chicago/Turabian StyleJiang, Yuhang, Shangke Jia, Shunwei Chen, Xuelian Li, Li Wang, and Xiujun Han. 2022. "Theoretical Prediction and Experimental Validation of the Glass-Forming Ability and Magnetic Properties of Fe-Si-B Metallic Glasses from Atomic Structures" Materials 15, no. 9: 3149. https://doi.org/10.3390/ma15093149
APA StyleJiang, Y., Jia, S., Chen, S., Li, X., Wang, L., & Han, X. (2022). Theoretical Prediction and Experimental Validation of the Glass-Forming Ability and Magnetic Properties of Fe-Si-B Metallic Glasses from Atomic Structures. Materials, 15(9), 3149. https://doi.org/10.3390/ma15093149







