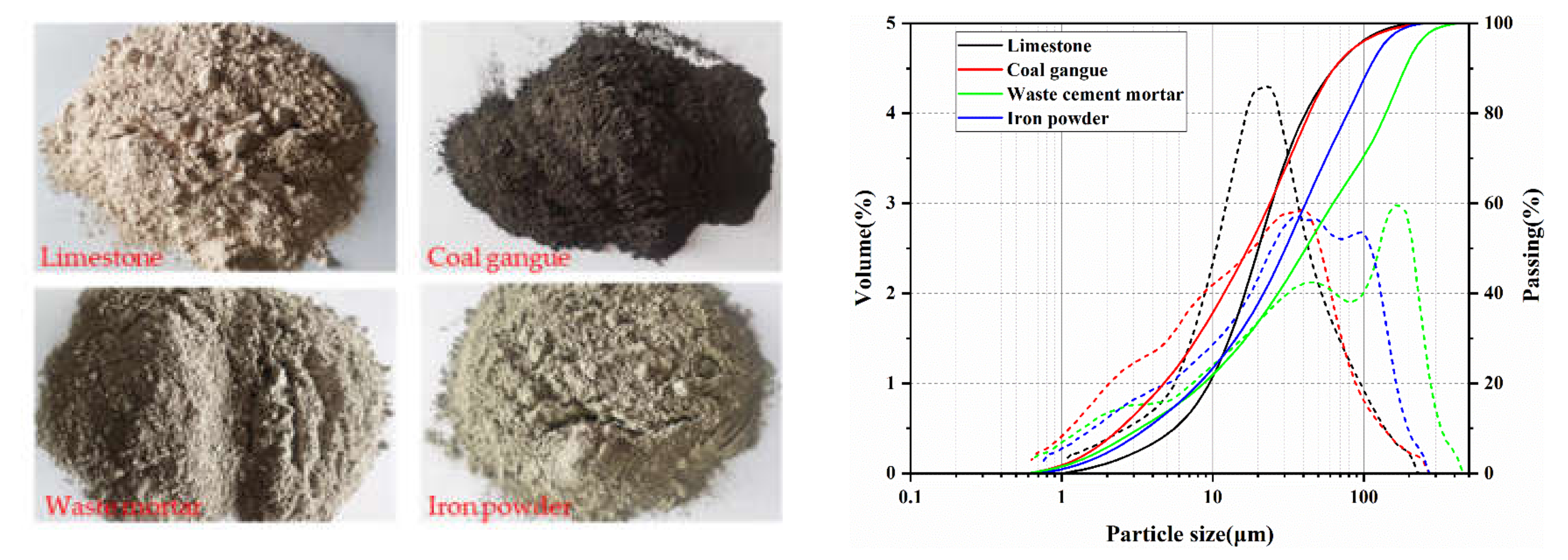
3.1. Composition of Clinkers
The TG curves of samples B4 and C4 are shown in
Figure 4. In general, the weight loss induced at temperatures between 30 and 200 °C resulted from the elimination of weakly bound water (dehydration). The slow reduction in weight between 200 and 700 °C resulted from the elimination of organic matter and the dehydroxylation of silicates. The weight loss between 700 and 870 °C was due to the decomposition of CaCO
3. According to the DSC analysis, once the CaCO
3 was completely decomposed following the heating process, the CaO began to react with the silicate and aluminosilicate in the temperature range of 897–1200 °C, and the reaction zone was the endothermic zone. There were no significant peaks in this range because these solid-state reactions were diffusion-controlled and exhibited flat and wide frequency bands. As the temperature continued to rise, exothermic spikes were seen at 1280 °C and 1290 °C due to the solidification of the liquid phase.
Endothermic peaks emerged at 1320 °C and 1334 °C due to the formation of the molten phase (clinkering). This is consistent with the results of Trezza [
36]. In addition, according to Staněk’s study [
37], the DSC curve of C4 with SO
3 emerged at 850~1200 °C, probably due to the decomposition of gypsum.
The XRD patterns of samples sintered at 1350 °C for 30 min are shown in
Figure 5, and the dotted line shows the diffraction peaks undergoing obvious change. The phase compositions are listed in
Table 6,
Table 7 and
Table 8. The contents of C
3S, C
2S, and C
4AF in the clinker are consistent with the mineral composition inferred from the XRF data. No diffraction peak of free-CaO could be clearly seen, while the diffraction peaks of C
3S and C
2S were present at 1350 °C, indicating calcination of the clinker at 1350 °C. The content of C
3S increased with the increase in KH, and the C
2S decreased accordingly. Because the SM was larger (about 2.7) and the IM was smaller (about 0.86), the amount of C
3A generated was lower, and the diffraction peaks were not obvious; this is because a certain amount of Al
2O
3 will be solubilized in C
2S and the content of C
3A will be reduced, while some C
3A may be solubilized in the glass phase [
37]. As
Figure 6 shows, with basically the same rate values, the amount of C
3S generated in group B (with more waste mortar) is much higher than in group A, because there is a certain content of hardened cement slurry in the waste mortar, and minerals with hydration properties, such as C
2S, C
12A
7, and C
4AF, can form after calcining [
38]. These act as crystal seeds, while the waste concrete in group B is greater than that in group A. Therefore, in the same period, the amount of C
3S in group B was higher than that in group A. This shows that the addition of waste mortar is beneficial to the calcination of cement clinker. The content of C
3S was lower, and that of C
2S higher, in the samples supplemented with SO
3. The addition of SO
3 obstructs the generation of C
3S, and stabilizes C
2S. Meanwhile, the content of C
4AF in the samples supplemented with SO
3 was also higher, which is consistent with Li’s [
39] research.
In order to study the influence of calcination temperature and holding time on the synthesis of clinker using waste concrete as the raw material, four groups of raw materials with similar rates of A5, B4, C2, and C4 were specifically selected. As all the samples contained the same raw materials but in different proportions, the KH, SM, and IM of the A5, B4, C2, and C4 samples were sufficiently close to show the regularity of this clinker. These samples were heated to 1320 °C, 1350 °C, and 1380 °C, respectively, and held for 0, 15, 30, 45, 60, 75, and 90 min. The sintered samples were analyzed by XRD and Rietveld refinement.
Figure 7 shows the XRD results of calcination. At 1320 °C, a large number of γ-C
2S and CaO diffraction peaks emerged. The reaction between CaO and C
2S in the clinker was not complete, and the content of belite in the clinker was too high, meaning the belite could not undergo rapid crystal transformation during rapid cooling from β-C
2S (with hydration properties) to γ-C
2S (without hydration properties). Under the conditions, volume expansion will occur and lead to clinker pulverization [
40]. However, at 1350 °C and 1380 °C, no obvious γ-C
2S diffraction peaks emerged. This was because the increase in temperature led to an increase in C
3S production and a decrease in belite activity [
41]. The XRD results show that the sintering range of the high belite cement prepared from waste mortar was wide.
The sintered samples were analyzed by XRD and Rietveld refinement.
Figure 8 shows the contours of C
2S and C
3S contents in different clinkers, which varied with calcination time and temperature. Since γ-C
2S, with no hydration activity, was present in groups A5 and B4 at low temperatures, the contour diagram of β-C
2S with hydration properties exhibits a ring profile at 1320 °C. Furthermore, the content of β-C
2S decreased first and then increased with the increase in holding time. With the increase in calcination temperature and sintering time, the content of C
3S increased gradually, and the content of C
2S in each sample showed a trend of first increasing and then decreasing. The high belite cement showed a wide sintering range and could calcinate clinker with less free CaO at 1320~1380 °C. The addition of waste concrete does not lead to the generation of other mineral phases, but increases the content of C
3S because it acts as a seed crystal.
The composition of the high belite cement clinker mixed with SO
3 was the same as that of group B without SO
3, showing the same trend, and there was no characteristic peak of calcium sulfoaluminate, which may be due to the lower IM and alumina contents in the set ratio. There was no γ-C
2S when the calcination temperature and holding time were lower because the β-C
2S was stabilized by the addition of SO
3. At the same calcination temperature and sintering time, the rate of C
3S production in group C was significantly lower than that in groups A and B, while more C
2S was produced in this group than in groups A and B. This is because the increase in SO
3 prevents the generation of C
3S and stabilizes C
2S. Andrade’s study [
42] also showed the shrinkage of the C
3S stability field, and the preferential uptake of sulfur by β-C
2S, which thus stabilizes β-C
2S and prevents the formation of γ-C
2S. The higher the SO
3 content, the higher the calcium–silicon ratio, and the higher the required Ca
2+ content, the lower the C
2S content. In general, the content of C
2S decreases with increases in calcination temperature and holding time, and the content of C
3S increases with increases in calcination temperature and holding time. The sintering range of belite cement is relatively wide. When used as a raw material, waste mortar acts as part of the seed crystal, which aids in the sintering of cement clinker, while the addition of SO
3 slows down the generation rate of C
3S and increases the content of C
2S.
Figure 9 shows BSE images of the high belite cement clinker. The alite shown in
Figure 9a has euhedral crystal outlines, while the crystal size of alite is small, which indicates that alite is not over-burned in the formation of large crystals, resulting in reductions in the hydration activity and strength of the cement clinker [
43]. The belite shown in
Figure 9b is round and elliptical, with a smooth surface and a high content. In the intermediate phase of c, there is an even distribution between alite and belite, showing the lightest and smoothest color. In
Figure 9b, we see belite inlaid in the middle of the alite, indicating that the synthesis of alite is dominated by belite here, whereby CaO gathers around the belite and gradually forms alite.
The chemistry of the belite grains was determined by electron microanalysis, which also shows the total charge of the cations and skeleton elements. The results are given in
Table 9, where it is assumed that all Mg is located at the Ca site and all Fe is trivalent, containing the Si site. The calculations of the atomic ratios of elements filling the Ca site. (i.e., Ca, Mg, Na, K) versus those filling the Si site (Si, S, P, Al, Fe
3+, Ti) show that they are close to stoichiometric 2:1, while the Ca/Si ratio of the belite supplemented with SO
3 is 2.3~2.6. The belite Ca/Si ratio found during calcination experiments performed on sulfur-rich raw materials by Herfort et al. ranged from 2.37 to 2.42 [
44]. The average atomic ratio values of the belite components shown in
Table 10 indicate a statistical correlation between the various elements (α = 0.05). The significant negative correlations of Si with Al and S (Pearson’s r = −0.897 and −0.929), and the significant positive correlation between Al and S (Pearson’s r = 0.77), indicate that the higher the content of Al and S in belite, the lower the content of Si, and the Al and S increase synchronously, indicating the occurrence of Si ↔ Al + S substitution [
45,
46]. Staněk et al. [
37] reached the same conclusion.
Figure 10 illustrates the microscopic regression analysis of belite when replacing SiO
4 groups with AlO
4 and SO
4; here, the atomic ratio of Al/S is close to 1.5:1 (average 1.411). In the study of Bonafous [
47], 3[AlO
4]
5− and 2[SO
4]
2− replaced 5[SiO
4]
4−. The equation is
The solution of S6+ will promote the solution of Al3+, and the solution of S6+ and Al3+ will change the crystal structure of C2S when in 4-fold coordination (r(Si4+) = 0.26 nm, r(Al3+) = 0.39 nm). When Al3+ replaces Si4+ and enters the silicon-oxygen tetrahedron, the ionic radius of Al3+ will be larger than that of Si4+, which will expand the space occupied by the silicon–oxygen tetrahedron. The adjacent Ca–O octahedron is indirectly deformed, and the space becomes smaller. Our study has shown that the sulfur-rich belite clinker had higher Ca/Si, leading to more Al entering belite, which leads to a reduction in C3A and C3S.
Figure 10.
Relationship between the average content of sulfur and aluminum in belite.
Figure 10.
Relationship between the average content of sulfur and aluminum in belite.
3.2. Cement Properties
Figure 11 shows the hydration heat release of the belite clinker prepared from waste cement mortar. The cumulative heat release of all samples was less than 180 J/g, which satisfies the requirements of low-heat cement.
In
Figure 8, the high belite cement shows two exothermic peaks; the first, between 0.8 h and 1.4 h, mainly concerns the rapid hydration of C
3A, the initial precipitation of hydrates, and the wetting of the system; the second, between 10 and roughly 20 h, mainly concerns the hydration of C
3S, C
4AF, and active C
2S. The sample with high alite content showed a higher hydration heat release rate and cumulative heat release. Among the different samples, the hydration heat release rate and cumulative heat release rate of group B were higher than those of group A, with the same rate value. This is because there was more waste mortar in group B, resulting in more alite. This shows that the addition of waste mortar is beneficial to the strength of high belite cement. Group C, doped with SO
3, showed the largest hydration heat release rate and cumulative heat release. Although the addition of SO
3 had an inhibitory effect on the generation of C
3S, according to Xuerun L’s [
39] research, SO
3 can not only activate belite, but it also stabilizes the M1-type alite with a higher hydration activity, meaning samples doped with SO
3 themselves show a higher hydration activity.
Figure 12 shows the strengths of the A3, A5, B2, B4, and C1–C4 cement clinkers at 3, 7, 28, 56, and 90 d. The strength of the sample prepared from waste mortar without SO
3 was 13–18 MPa at 3 d, and 48–57 MPa at 28 d. At the same hydration age, the early-phase strength of group B was higher than that of group A, because the mortar content of group B was higher, such that the seed crystal effect was more obvious, and more alite was produced. This indicates that the addition of waste mortar was conducive to the increase in the early-phase strength of belite cement. The 3 d strength of the sample prepared from SO
3-mixed waste mortar was 17–26 Mpa, and at 28 d it reached 51–63 MPa. The early-phase strength of the sulfur-rich belite clinker was significantly higher than that of the SO
3-free clinker, indicating that the higher the calcium–silicon ratio, the faster the hydration of belite. At the same time, using more Al solution will also increase the activity of the belite. The addition of SO
3 improves the early-phase strength of the belite clinker. Compared to pure C
2S, the clinker prepared from a waste mortar instead of silicon source showed greater early-phase strength growth when it contained 20% to 30% alite. SO
3 can stabilize monoclinic M1-type alite, and alite formation also improves the parameters of burning and grindability. After 56 days, the SO
3-doped belite clinker tends to show the same characteristics as that without SO
3, and the strength of the 90-day net slurry can be above 100 Mpa, showing an upward trend. The cement clearly has good developmental benefits. In contrast, this kind of belite clinker requires more limestone and has a higher limestone saturation coefficient, but this is still about 10% lower than in ordinary Portland cement, which ensures its reaction with water and the formation of large amounts of silicate (Ca(OH)
2). This increases the overall alkalinity, which speeds up the hydration process. In addition, the presence of 6% dihydrate gypsum had a positive effect on the development of the cement’s strength [
48].