Tyrosinase-Based Biosensor—A New Tool for Chlorogenic Acid Detection in Nutraceutical Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Electrodes and Devices Used
2.3. Preparation of the GPH-MnPc/SPE Biosensor
2.4. Preparation of the GPH-MnPc-Tyr/SPE Biosensor
2.5. Methods of Analysis
2.5.1. Cyclic Voltammetry
2.5.2. Square-Wave Voltammetry
2.6. Real Samples and Preparation of the Solutions to Be Analysed
2.7. Antioxidant Activity (DPPH Method)
3. Results and Discussion
3.1. Preliminary Studies to Characterise the Electrode
3.2. Electrochemical Properties of Electrodes in CGA Solution
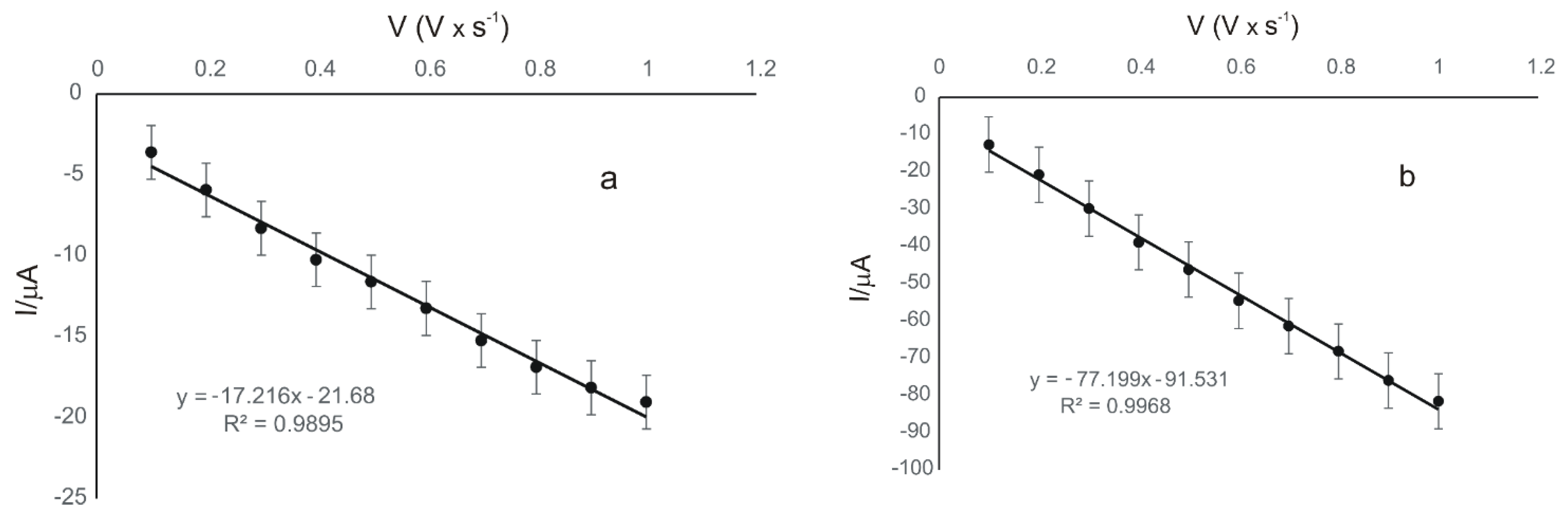
3.3. Influence of Scan Rate on the Voltammetric Response
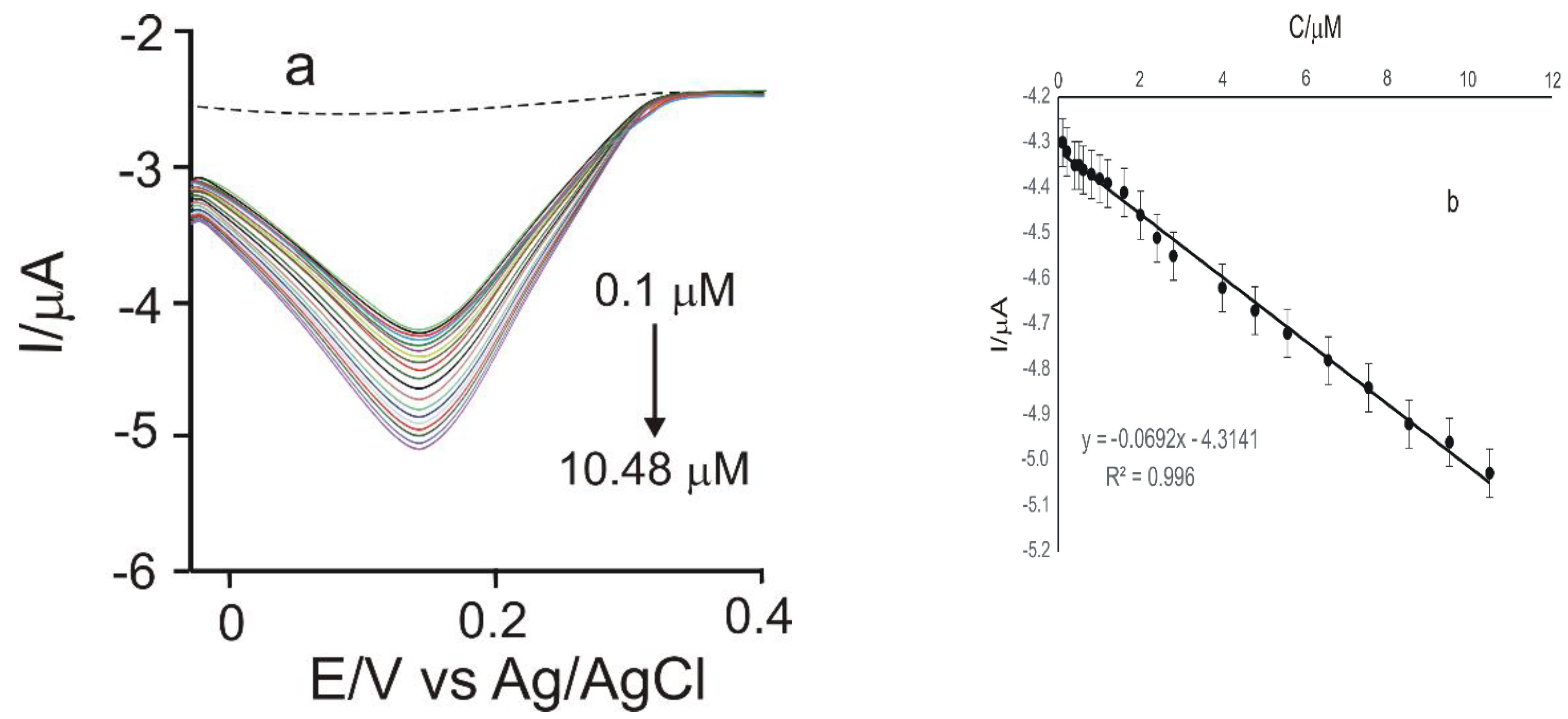
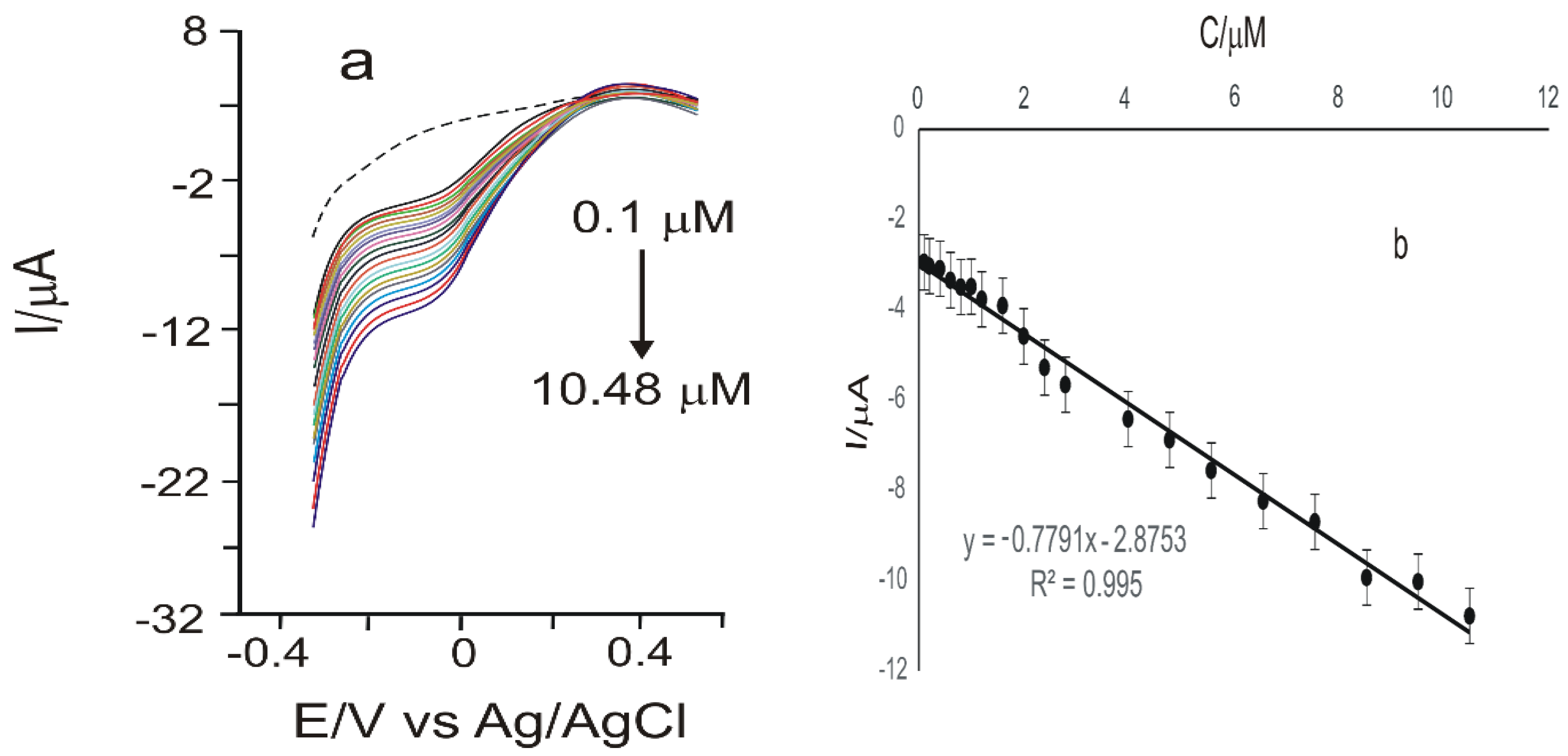
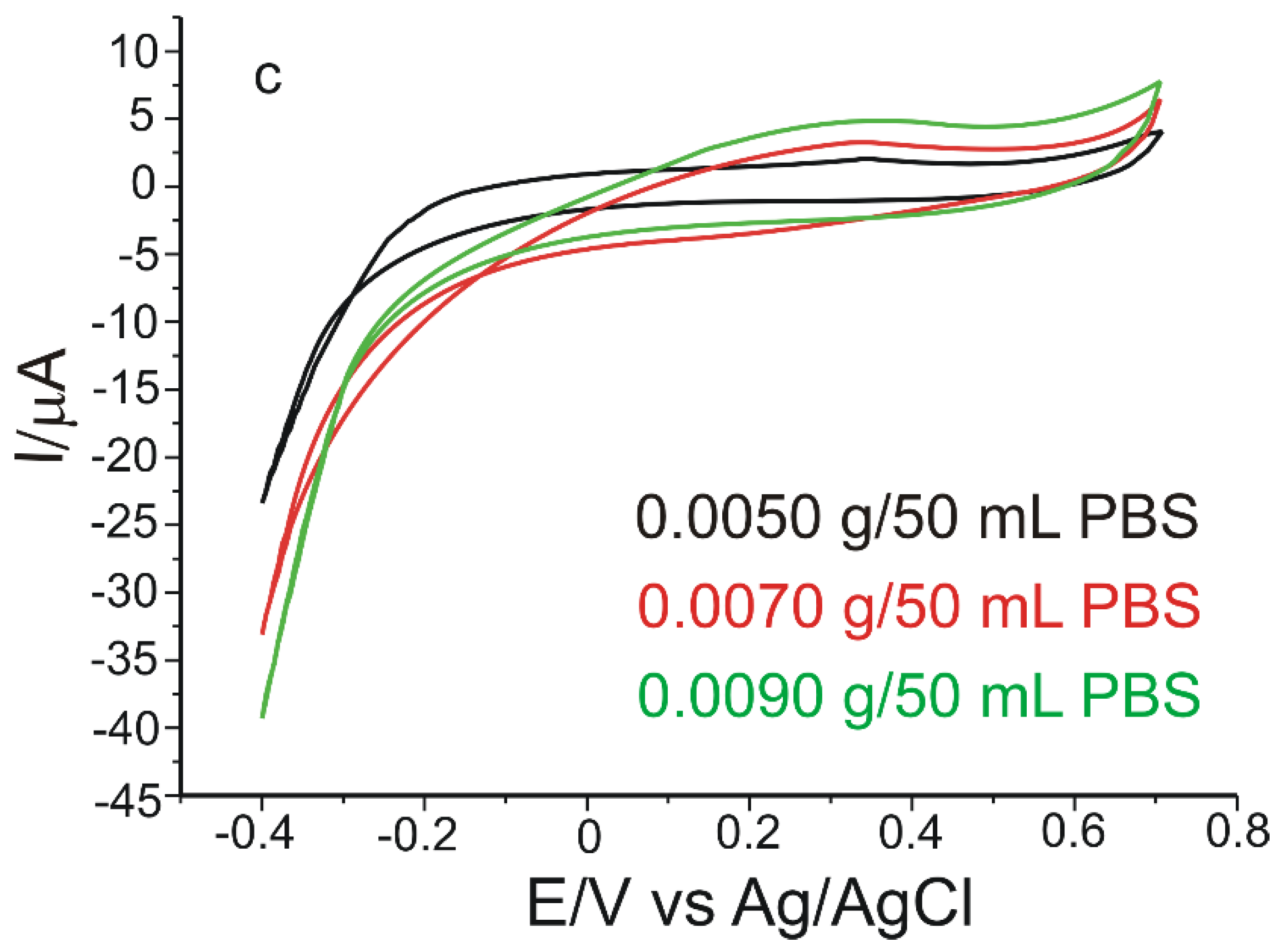
3.4. Calibration Curve and Limit of Detection
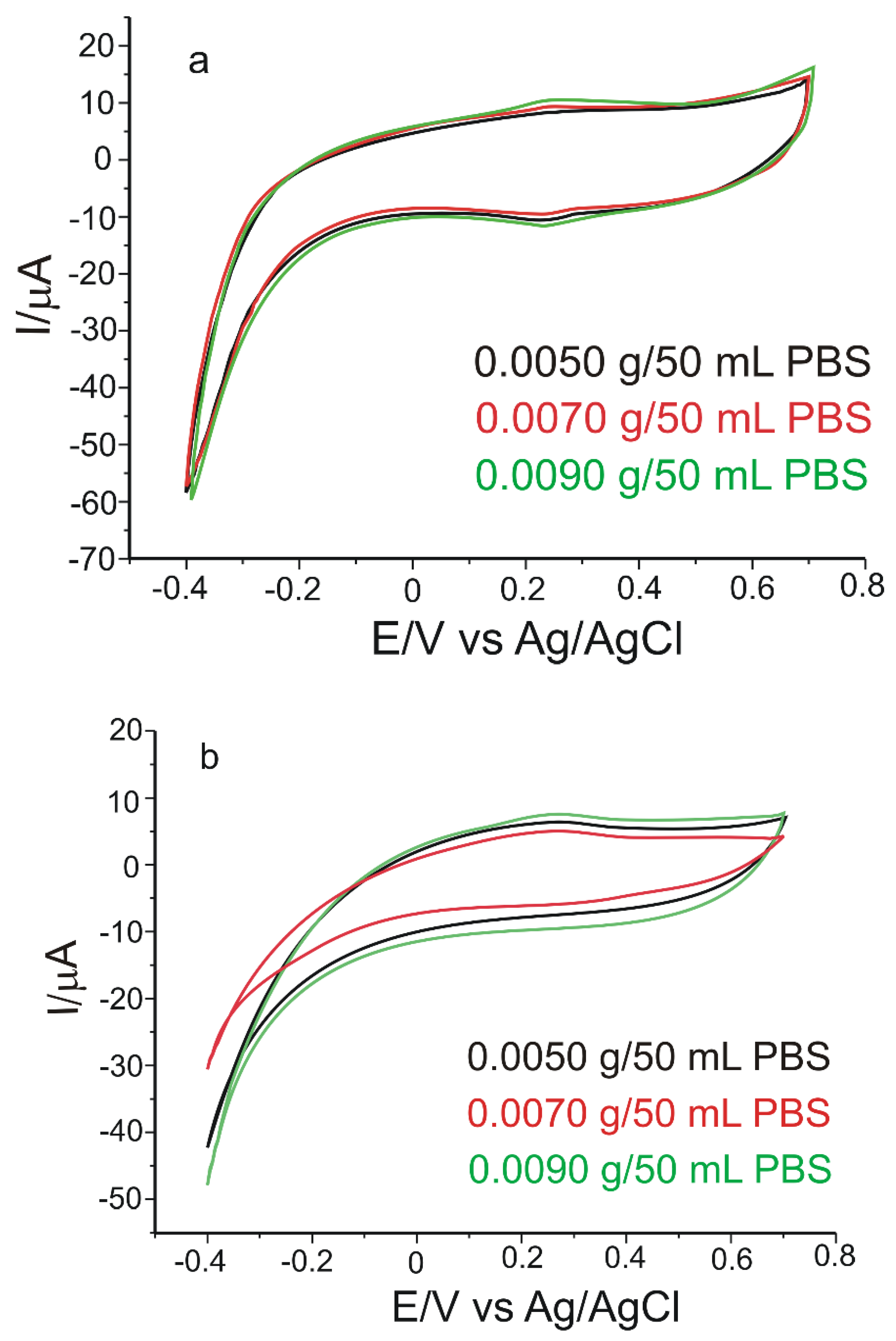
3.5. Stability, Reproducibility, and Repeatability of the Biosensor
3.6. Interference Studies
3.7. Quantitative Determination of CGA in Nutraceutical Formulations
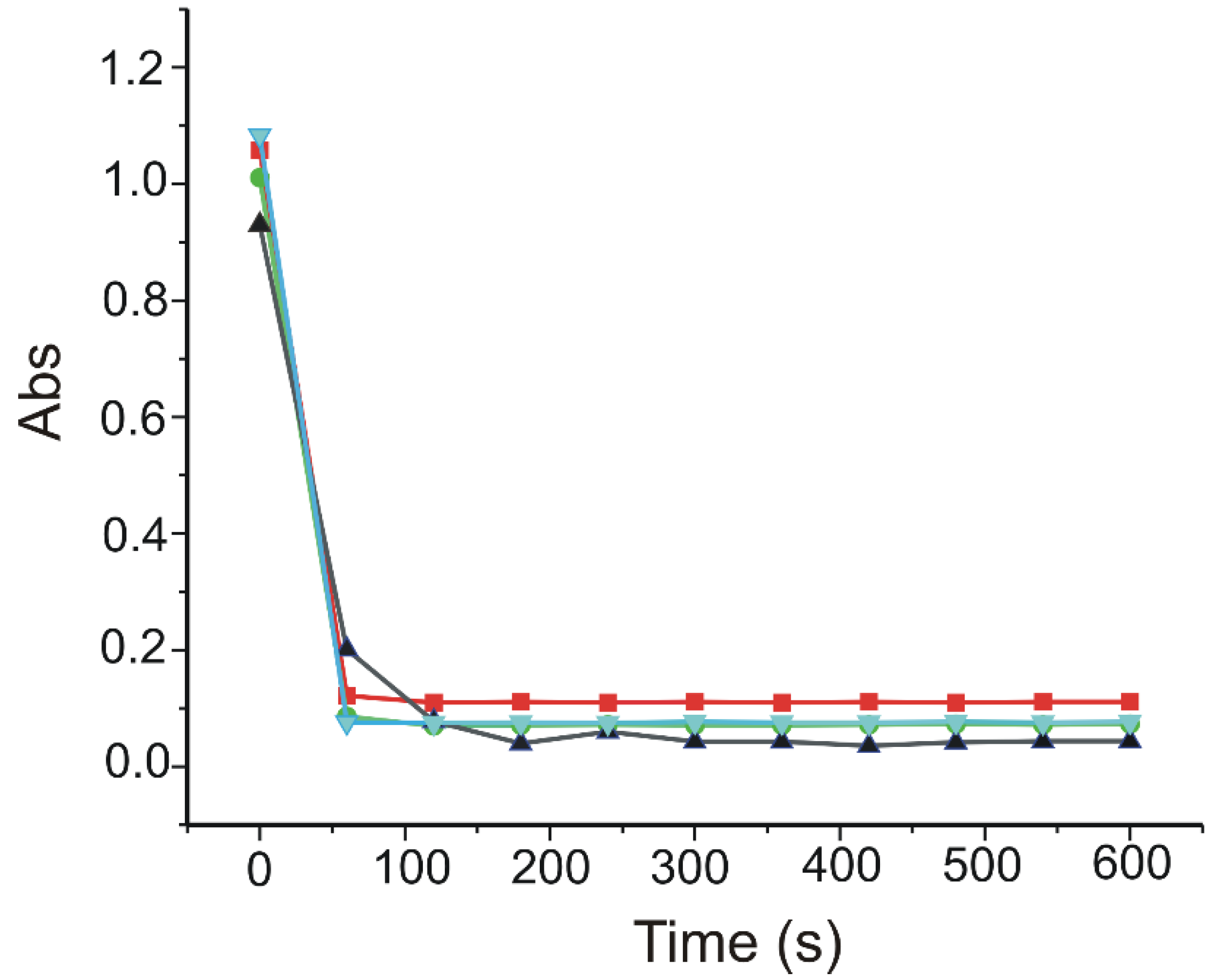
3.8. Determination of CGA Antioxidant Activity through DPPH Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stocker, R. Dietary and pharmacological antioxidants in atherosclerosis. Curr. Opin. Lipidol. 1999, 10, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. A review on electrochemical sensors and biosensors used in chlorogenic acid electroanalysis. Int. J. Mol. Sci. 2021, 22, 13138. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Mindaugas, L.; Viskelis, P.; Kviklys, D.; Raudonis, R.; Janulis, V. A comparative study of phenolic content in apple fruits. Int. J. Food Prop. 2014, 18, 945–953. [Google Scholar] [CrossRef]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Majhi, S.; Saha, B.P.; Mukherjee, P.K. Chlorogenic acid–phospholipid complex improve protection against UVA induced oxidative stress. J. Photochem. Photobiol. B Biol. 2014, 130, 293–298. [Google Scholar] [CrossRef]
- Karunanidhi, A.; Thomas, R.; Van Belkum, A.; Neela, V. In vitro antibacterial and antibiofilm activities of chlorogenic acid against clinical isolates of Stenotrophomonas maltophilia including the trimethoprim/sulfamethoxazole resistant strain. Biomed. Res. Int. 2013, 2013, 392058. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Wang, H.; Yang, B.; Tao, H. Identification of cyclodextrin inclusion complex of chlorogenic acid and its antimicrobial activity. Food Chem. 2010, 120, 1138–1142. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci. Rep. 2017, 7, 45723. [Google Scholar] [CrossRef] [Green Version]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.J.J.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W.; Lee, Y.G.; Cho, J.Y.; Kim, Y.C.; Lee, S.H.; Kim, W.S.; Moon, J.H. Establishment of a simple method for purification of high purity chlorogenic acid from immature fruit of pear (Pyrus pyrifolia Nakai). J. Korean Soc. Appl. Biol. Chem. 2015, 58, 335–341. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. Eur. Food Res. Technol. 2014, 238, 589–596. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Aseervatham, G.S.B.; Suryakala, U.; Doulethunisha; Sundaram, S.; Bose, P.C.; Sivasudha, T. Expression pattern of NMDA receptors reveals antiepileptic potential of apigenin 8-C-glucoside and chlorogenic acid in pilocarpine induced epileptic mice. Biomed. Pharmacother. 2016, 82, 54–64. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Zhou, C.-Y.; Qiu, C.-H.; Lu, X.-M.; Wang, Y.-T. Chlorogenic acid induced apoptosis and inhibition of proliferation in human acute promyelocytic leukemia HL-60 cells. Mol. Med. Rep. 2013, 8, 1106–1110. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Upadhyay, R.; Rao, L.J.M. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Hurtado-Fernández, E.; Gómez-Romero, M.; Carrasco-Pancorbo, A.; Fernández-Gutiérrez, A. Application and potential of capillary electroseparation methods to determine antioxidant phenolic compounds from plant food material. J. Pharm. Biomed. Anal. 2010, 53, 1130–1160. [Google Scholar] [CrossRef]
- Es’haghi, Z.; Golsefidi, M.A.; Saify, A.; Tanha, A.A.; Rezaeifar, Z.; Alian-Nezhadi, Z. Carbon nanotube reinforced hollow fiber solid/liquid phase microextraction: A novel extraction technique for the measurement of caffeic acid in Echinacea purpurea herbal extracts combined with high-performance liquid chromatography. J. Chromatogr. A 2010, 1217, 2768–2775. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Yang, N. Chemiluminescent determination of chlorogenic acid in fruits. Food Chem. 2007, 102, 422–426. [Google Scholar] [CrossRef]
- Ma, X.; Yang, H.; Xiong, H.; Li, X.; Gao, J.; Gao, Y. Electrochemical behavior and determination of chlorogenic acid based on multi-walled carbon nanotubes modified screen-printed electrode. Sensors 2016, 16, 1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bounegru, A.V.; Apetrei, C. Development of a novel electrochemical biosensor based on carbon nanofibers–cobalt phthalocyanine–laccase for the detection of p-coumaric acid in phytoproducts. Int. J. Mol. Sci. 2021, 22, 9302. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. A modified nanostructured graphene-gold nanoparticle carbon screen-printed electrode for the sensitive voltammetric detection of rutin. Measurement 2018, 114, 37–43. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Voltammetric determination of melatonin using a graphene-based sensor in pharmaceutical products. Int. J. Nanomed. 2016, 11, 1859–1866. [Google Scholar] [CrossRef] [Green Version]
- Munteanu, I.-G.; Apetrei, C. Electrochemical determination of chlorogenic acid in nutraceuticals using voltammetric sensors based on screen-printed carbon electrode modified with graphene and gold nanoparticles. Int. J. Mol. Sci. 2021, 22, 8897. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Hu, X.; Yu, H. Highly stable and ultrasensitive chlorogenic acid sensor based on metal-organic frameworks/titanium dioxide nanocomposites. Analyst 2016, 141, 4647–4653. [Google Scholar] [CrossRef]
- Mohammadi, N.; Najafi, M.; Adeh, N.B. Highly defective mesoporous carbon-ionic liquid paste electrode as sensitive voltammetric sensor for determination of chlorogenic acid in herbal extracts. Sens. Actuators B Chem. 2017, 243, 838–846. [Google Scholar] [CrossRef]
- Chokkareddy, R.; Redhi, G.G.; Karthick, T. A lignin polymer nanocomposite based electrochemical sensor for the sensitive detection of chlorogenic acid in coffee samples. Heliyon 2019, 5, e01457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baig, N.; Sajid, M.; Saleh, T.A. Recent trends in nanomaterial-modified electrodes for electroanalytical applications. Trends Anal. Chem. 2019, 111, 47–61. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 96, 2563–2606. [Google Scholar] [CrossRef]
- Matemadombo, F.; Apetrei, C.; Nyokong, T.; Rodríguez-Méndez, M.; De Saja, J.A. Comparison of carbon screen-printed and disk electrodes in the detection of antioxidants using CoPc derivatives. Sens. Actuators B Chem. 2012, 166, 457–466. [Google Scholar] [CrossRef]
- Zuo, X.; Zhang, H.; Li, N. An electrochemical biosensor for determination of ascorbic acid by cobalt (II) phthalocyanine–multi-walled carbon nanotubes modified glassy carbon electrode. Sens. Actuators B Chem. 2012, 161, 1074–1079. [Google Scholar] [CrossRef]
- Gunache, R.O.; Apetrei, C. Estimation of active compounds quantity from pharmaceuticals based on ginkgo biloba. Chemosensors 2020, 8, 110. [Google Scholar] [CrossRef]
- Apetrei, I.; Apetrei, C. Development of a novel biosensor based on tyrosinase/platinum nanoparticles/chitosan/graphene nanostructured layer with applicability in bioanalysis. Materials 2019, 12, 1009. [Google Scholar] [CrossRef] [Green Version]
- Sanz, V.; Mena, M.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J. Development of a tyrosinase biosensor based on gold nanoparticles-modified glassy carbon electrodes: Application to the measurement of a bioelectrochemical polyphenols index in wines. Anal. Chim. Acta 2005, 528, 1–8. [Google Scholar] [CrossRef]
- Arteaga, J.F.; Ruiz-Montoya, M.; Palma, A.; Alonso-Garrido, G.; Pintado, S.; Rodríguez-Mellado, J.M. Comparison of the simple cyclic voltammetry (CV) and DPPH assays for the determination of antioxidant capacity of active principles. Molecules 2012, 17, 5126–5138. [Google Scholar] [CrossRef] [PubMed]
- Leite, K.C.S.; Garcia, L.F.; Lobón, G.S.; Thomaz, D.V.; Moreno, E.K.G.; De Carvalho, M.F.; Rocha, M.L.; Santos, W.T.P.D.; Gil, E.D. Antioxidant activity evaluation of dried herbal extracts: An electroanalytical approach. Rev. Bras. Farmacogn. 2018, 28, 325–332. [Google Scholar] [CrossRef]
- Piovesan, J.V.; Jost, C.L.; Spinelli, A. Electroanalytical determination of total phenolic compounds by square-wave voltammetry using a poly (vinylpyrrolidone)-modified carbon-paste electrode. Sens. Actuators B Chem. 2015, 216, 192–197. [Google Scholar] [CrossRef]
- Newair, E.F.; Abdel-Hamid, R.; Kilmartin, P.A. Electrochemical determination of the antioxidant activity in echinacea purpurea roots using square wave voltammetry. Electroanalysis 2017, 29, 1131–1140. [Google Scholar] [CrossRef]
- González, M.; Guzman, B.; Rudyk, R.; Romano, E.; Molina, M.A.A. Spectrophotometric determination of phenolic compounds in propolis. Acta Farm. Bonaer. 2003, 22, 243–248. [Google Scholar]
- Ahmad, N.; Fazal, H.; Abbasi, B.; Farooq, S. Efficient free radical scavenging activity of Ginkgo biloba, Stevia rebaudiana and Parthenium hysterophorous leaves through DPPH (2, 2-diphenyl-1-picrylhydrazyl). Int. J. Phytomedicine 2010, 2, 231–239. [Google Scholar] [CrossRef]
- Council of Europe, European Pharmacopoeia Commission, European Directorate for the Quality of Medicines & Healthcare. European Pharmacopoeia; Council of Europe: Strasbourg, France, 2010. [Google Scholar]
- Wu, L.; Lu, X.; Niu, K.; Ma, D.; Chen, J. Tyrosinase nanocapsule based nano-biosensor for ultrasensitive and rapid detection of bisphenol A with excellent stability in different application scenarios. Biosens. Bioelectron. 2020, 165, 112407. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.; Liu, S.; Korzeniewski, C.L.; Wang, S.; Fan, Z. Comparing graphene-TiO2 nanowire and graphene-TiO2 nanoparticle composite photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 3944–3950. [Google Scholar] [CrossRef]
- Yilmaz, I. In situ monitoring of metallation of metal-free phthalocyanine via UV-Vis and steady-state fluorescence techniques. Thin-layer UV-Vis and fluorescence spectroelectrochemistry of a new non-aggregating and electrochromic manganese (3+) phthalocyanine. New J. Chem. 2008, 32, 37–46. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Biyiklioglu, Z.; Bacaksiz, E.; Polat, I.; Stathopoulos, V.N. Synthesis, characterization, and photocatalytic evaluation of manganese (III) phthalocyanine sensitized ZnWO4 (ZnWO4MnPc) for bisphenol a degradation under UV irradiation. Nanomaterials 2020, 10, 2139. [Google Scholar] [CrossRef] [PubMed]
- Bounegru, A.V.; Apetrei, C. Development of a novel electrochemical biosensor based on carbon nanofibers-gold nanoparticles-tyrosinase for the detection of ferulic acid in cosmetics. Sensors 2020, 20, 6724. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, I.M.; Rodríguez-Méndez, M.; Apetrei, C.; De Saja, J.A. Enzyme sensor based on carbon nanotubes/cobalt (II) phthalocyanine and tyrosinase used in pharmaceutical analysis. Sens. Actuators B Chem. 2013, 177, 138–144. [Google Scholar] [CrossRef]
- Pan, S.; Zhuang, X.; Wang, B.; Tang, L.; Liu, L.; Qi, J. Preparation and properties of carbon coated manganese dioxide electrode materials. Chin. J. Mater. Res. 2019, 33, 530–536. [Google Scholar] [CrossRef]
- Mpeta, L.S.; Sen, P.; Nyokong, T. Development of manganese phthalocyanine decorated with silver nanoparticles nanocomposite for improved electrocatalytic oxidation of hydrazine. J. Electroanal. Chem. 2020, 866, 114173. [Google Scholar] [CrossRef]
- Nyokong, T. Electronic spectral and electrochemical behavior of near infrared absorbing metallophthalocyanines. In Functional Phthalocyanine Molecular Materials; Jiang, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 45–87. [Google Scholar] [CrossRef]
- Leznoff, C.C.; Black, L.S.; Hiebert, A.; Causey, P.W.; Christendat, D.; Lever, A.B.P. Red manganese phthalocyanines from highly hindered hexadecaalkoxyphthalocyanines. Inorg. Chim. Acta 2006, 9, 2690–2699. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; He, D.; Xie, A.; Qu, W.; Tang, Y.; Zhou, L.; Zhu, R. The electrochemical oxidation of hydroquinone and catechol through a novel poly-geminal dicationic ionic liquid (PGDIL)–TiO2 composite film electrode. Polymers 2019, 11, 1907. [Google Scholar] [CrossRef] [Green Version]
- Centeno, D.A.; Solano, X.H.; Castillo, J.J. A new peroxidase from leaves of guinea grass (Panicum maximum): A potential biocatalyst to build amperometric biosensors. Bioelectrochemistry 2017, 116, 33–38. [Google Scholar] [CrossRef]
- Wee, Y.; Park, S.; Kwon, Y.H.; Ju, Y.; Yeon, K.-M.; Kim, J. Tyrosinase-immobilized CNT based biosensor for highly-sensitive detection of phenolic compounds. Biosens. Bioelectron. 2019, 132, 279–285. [Google Scholar] [CrossRef]
- Apetrei, C.; De Saja, J.A.; Zurro, J.; Rodríguez-Méndez, M.L. Advantages of the biomimetic nanostructured films as an immobilization method vs. the carbon paste classical method. Catalysts 2012, 2, 517–531. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Guan, L.; Gu, Z.; Zhuang, Q. Determination of phenolic compounds based on the tyrosinase- single walled carbon nanotubes sensor. Electroanalysis 2005, 17, 85–88. [Google Scholar] [CrossRef]
- Streffer, K.; Vijgenboom, E.; Tepper, A.W.; Makower, A.; Scheller, F.W.; Canters, G.W.; Wollenberger, U. Determination of phenolic compounds using recombinant tyrosinase from Streptomyces antibioticus. Anal. Chim. Acta 2001, 427, 201–210. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. 1), S49–S52. [Google Scholar] [PubMed]
- Zhang, T.; Chen, Y.; Huang, W.; Wang, Y.; Hu, X. A novel AuNPs-doped COFs composite as electrochemical probe for chlorogenic acid detection with enhanced sensitivity and stability. Sens. Actuators B Chem. 2018, 276, 362–369. [Google Scholar] [CrossRef]
- Santos, W.D.R.; Santhiago, M.; Yoshida, I.V.P.; Kubota, L.T. Novel electrochemical sensor for the selective recognition of chlorogenic acid. Anal. Chim. Acta 2011, 695, 44–50. [Google Scholar] [CrossRef]
- Chao, M.; Ma, X. Voltammetric determination of chlorogenic acid in pharmaceutical products using poly (aminosulfonic acid) modified glassy carbon electrode. J. Food Drug Anal. 2014, 22, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.C.; Moccelini, S.K.; Scheeren, C.W.; Migowski, P.; Dupont, J.; Heller, M.; Micke, G.A.; Vieira, I.C. Biosensor for chlorogenic acid based on an ionic liquid containing iridium nanoparticles and polyphenol oxidase. Talanta 2009, 79, 222–228. [Google Scholar] [CrossRef]
- Moccelini, S.K.; Spinelli, A.; Vieira, I.C. Biosensors based on bean sprout homogenate immobilized in chitosan microspheres and silica for determination of chlorogenic acid. Enzym. Microb. Technol. 2008, 43, 381–387. [Google Scholar] [CrossRef]
- Danilewicz, J. The folin-ciocalteu, FRAP, and DPPH * assays for measuring polyphenol concentration in white wine. Am. J. Enol. Vitic. 2015, 66, 463–471. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Alam, A.H.M.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef] [Green Version]

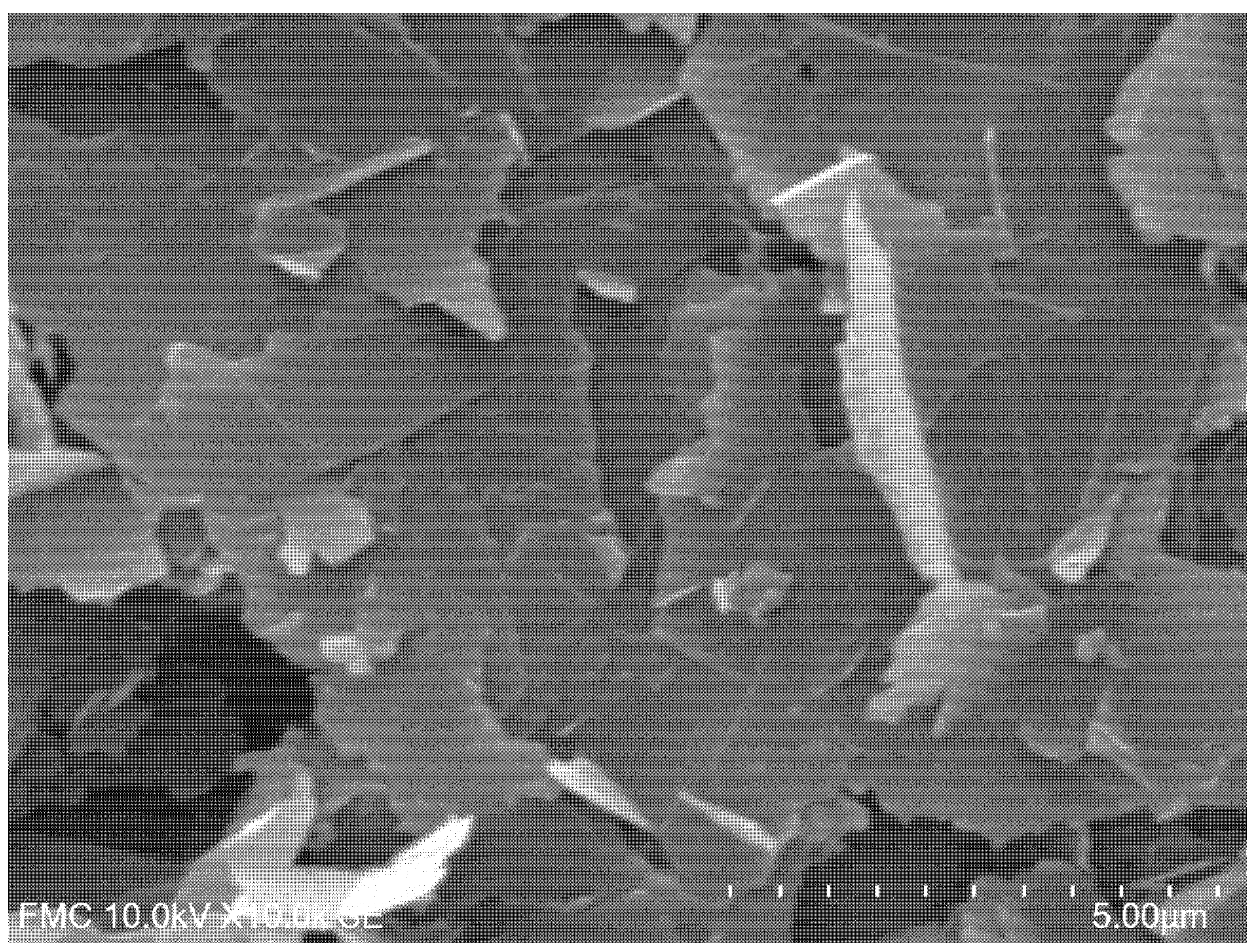
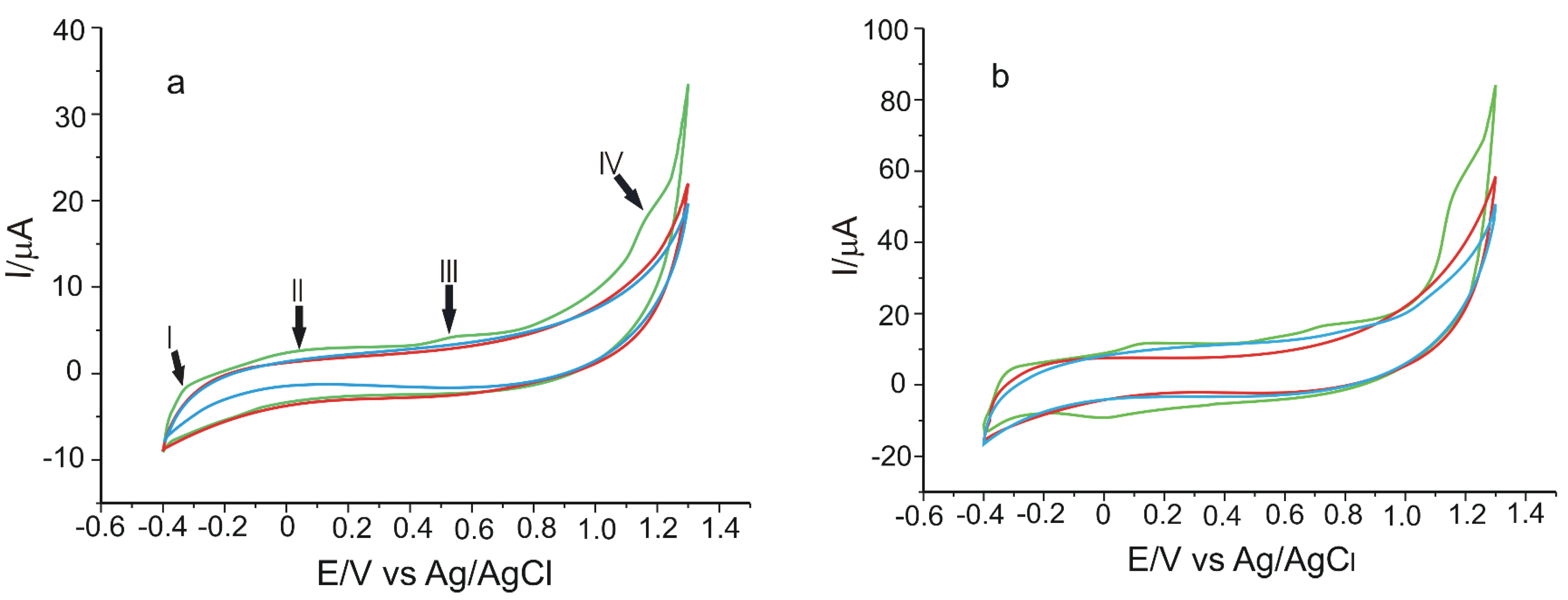

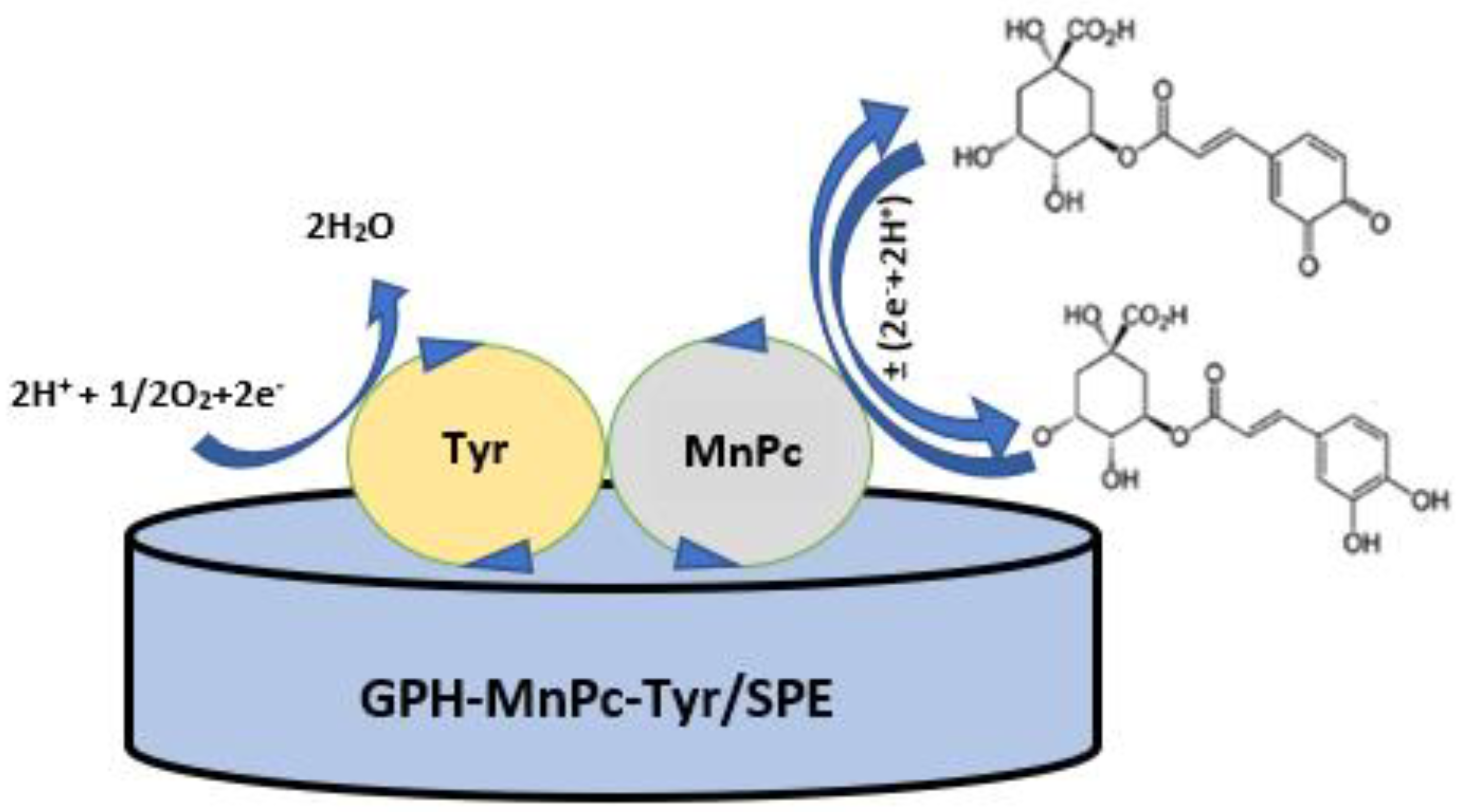
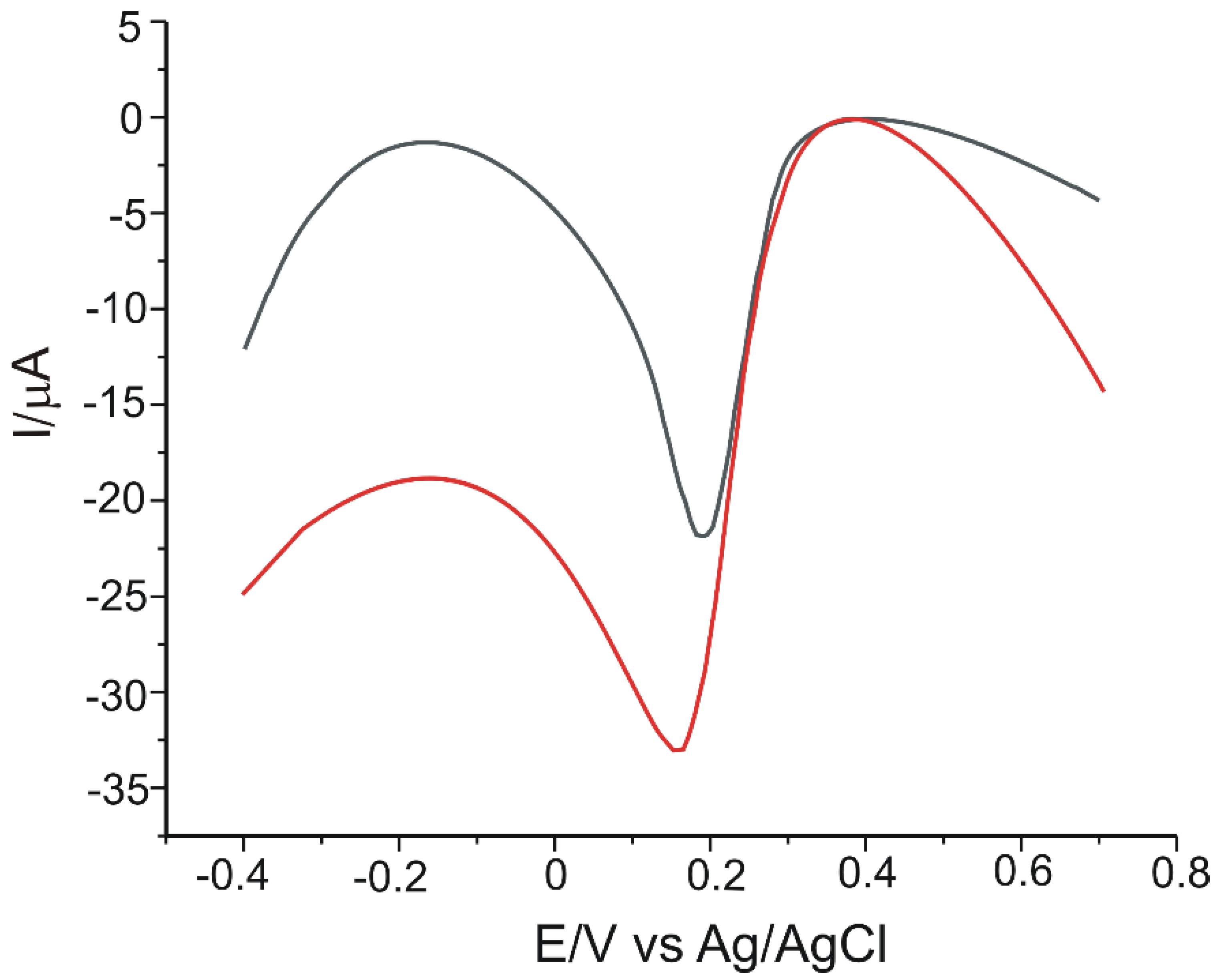

| Electrode | Ipa vs. v1/2 | R2 | Active Area (cm2) | Geometrical Area (cm2) | Roughness Factor |
|---|---|---|---|---|---|
| GPH/SPE | Ipa (A) = 2.46 × 10−4 v1/2 (V·s−1)1/2 − 2.87 × 10−5 | 0.9949 | 0.1240 | 0.1256 | 0.9872 |
| GPH-MnPc/SPE | Ipa (A) = 1.29 × 10−4 v1/2 (V·s−1)1/2 − 1.36 × 10−5 | 0.9953 | 0.1244 | 0.9904 | |
| GPH-MnPc-Tyr/SPE | Ipa (A) = 1.75 × 10−4 v1/2 (V·s−1)1/2 − 3.50 × 10−5 | 0.9956 | 0.1688 | 1.3439 |
| Sensor | Epa 1(V) | Ipa 2(µA) | Epc 3(V) | Ipc 4(µA) | Ipc/Ipa | ΔE 5(V) | E1/2 6 |
|---|---|---|---|---|---|---|---|
| GPH-MnPc/SPE | 0.190 | 4.30 | 0.117 | −3.22 | 0.74 | 0.073 | 0.153 |
| GPH-Tyr/SPE | 0.283 | 7.65 | 0.095 | −10.20 | 1.33 | 0.188 | 0.189 |
| GPH-MnPc-Tyr/SPE | 0.214 | 10.81 | 0.017 | −14.14 | 1.31 | 0.197 | 0.115 |
| Electrode | Equation | R2 | Г (mol × cm−2) |
|---|---|---|---|
| GPH-MnPc/SPE | Ipc = 17.216 × 10−5 v (V·s−1) − 21.68 × 10−5 | 0.9895 | 3.34 × 10−11 |
| GPH-MnPc-Tyr/SPE | Ipc = 77.199 × 10−5 v (V·s−1) − 91.531 × 10−5 | 0.9968 | 1.50 × 10−10 |
| Electrode | Equation | R2 | LOD (M) | LOQ (M) |
|---|---|---|---|---|
| GPH-MnPc-Tyr/SPE | Ipc = −0.0692 × −4.3141 | 0.996 | 1.40 × 10−6 | 4.69 × 10−6 |
| Sensitive Material | Detection Technique | LOD (µmol × L−1) | References |
|---|---|---|---|
| TAPB-DMTP-COFs/AuNPs 1 | CV, DPV | 9.5 × 10−3 | [66] |
| LGN-MWCNTs-CuONPs-GCE 2 | CV, DPV | 1.25 × 10−2 | [31] |
| MWCNTs/SPE 3 | DPV | 3.4 × 10−1 | [24] |
| MIS/Au electrode 4 | DPV | 1.48 × 10−1 | [67] |
| PASA/GCE 5 | CV | 0.4 × 10−1 | [68] |
| Ir-BMI.PF6-PPO 6 | SWV | 9.1 × 10−1 | [69] |
| IL/DMC/PE | SWV | 1 × 10−2 | [30] |
| Biosensor I and biosensor II 7 | SWV | 8 × 10−1 and 8.5 × 10−1 | [70] |
| GPH-SPE 8 and GPH-GNP-SPE 9 | CV | 7.3 × 10−1 and 6.2 × 10−1 | [28] |
| GPH-MnPc-Tyr/SPE | SWV | 2.32 × 10−1 | This study |
| Interfering Compound | Concentration of the Interferents | Ratio | Recovery % | RSD % |
|---|---|---|---|---|
| - | - | - | 100 | 0.50 |
| L-ascorbic acid | 10−5 M | 1:1 | 97.45 | 1.82 |
| Vanillic acid | 10−5 M | 1:1 | 99.15 | 0.60 |
| Ferulic acid | 10−5 M | 1:1 | 97.24 | 1.97 |
| p-Coumaric acid | 10−5 M | 1:1 | 96.81 | 2.28 |
| Food Supplement | Voltammetric Method C % CGA | Spectrophotometric Method C % CGA |
|---|---|---|
| Green Coffee Bean Complex (Adams Vision) | 83.72 | 87.39 |
| Green Coffee (Biotech USA) | 79.77 | 80.21 |
| Green Coffee Bean 400 mg Jarrow Formulas | 79.72 | 80.11 |
| Nutraceutical Product | Green Coffee Bean Complex Adams Vision | Green Coffee Biotech USA | Green Coffee Bean 400 mg Jarrow Formulas |
|---|---|---|---|
| % DPPH Reduction | 91.66 | 91.05 | 93.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, I.G.; Apetrei, C. Tyrosinase-Based Biosensor—A New Tool for Chlorogenic Acid Detection in Nutraceutical Formulations. Materials 2022, 15, 3221. https://doi.org/10.3390/ma15093221
Munteanu IG, Apetrei C. Tyrosinase-Based Biosensor—A New Tool for Chlorogenic Acid Detection in Nutraceutical Formulations. Materials. 2022; 15(9):3221. https://doi.org/10.3390/ma15093221
Chicago/Turabian StyleMunteanu, Irina Georgiana, and Constantin Apetrei. 2022. "Tyrosinase-Based Biosensor—A New Tool for Chlorogenic Acid Detection in Nutraceutical Formulations" Materials 15, no. 9: 3221. https://doi.org/10.3390/ma15093221







