Progress and Recent Strategies in the Synthesis and Catalytic Applications of Perovskites Based on Lanthanum and Aluminum
Abstract
:1. Introduction
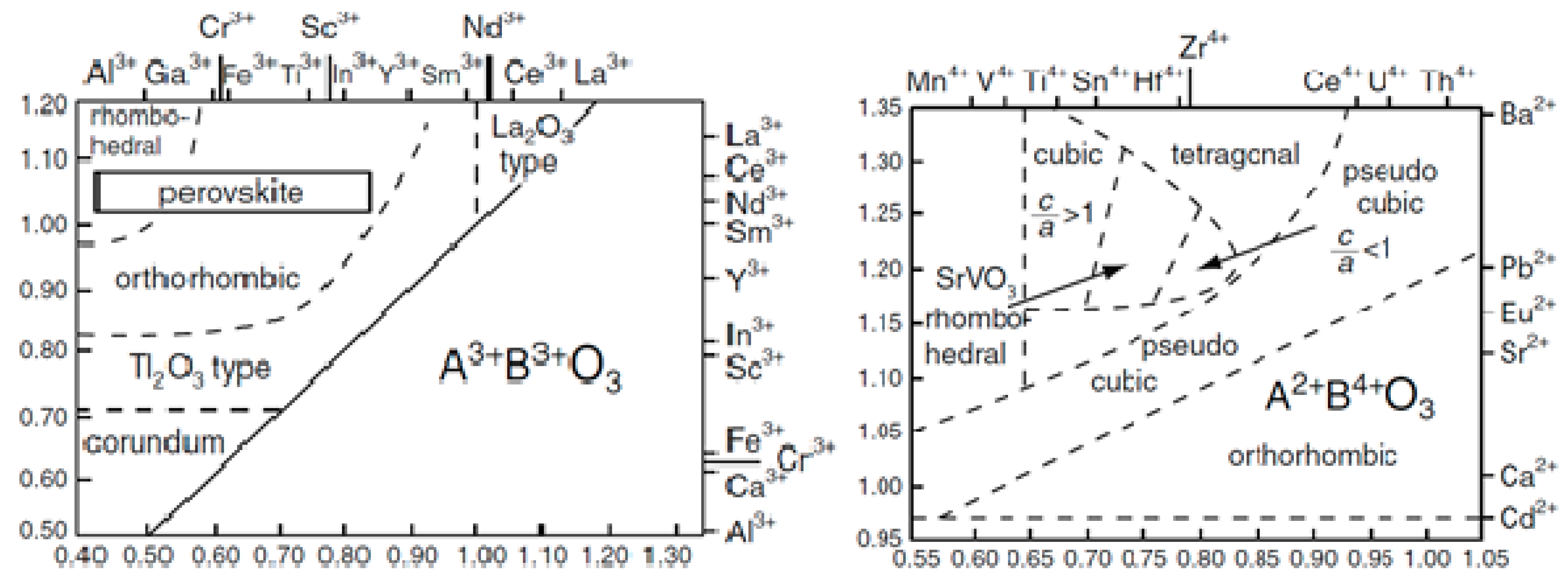
2. Structure of the Perovskite
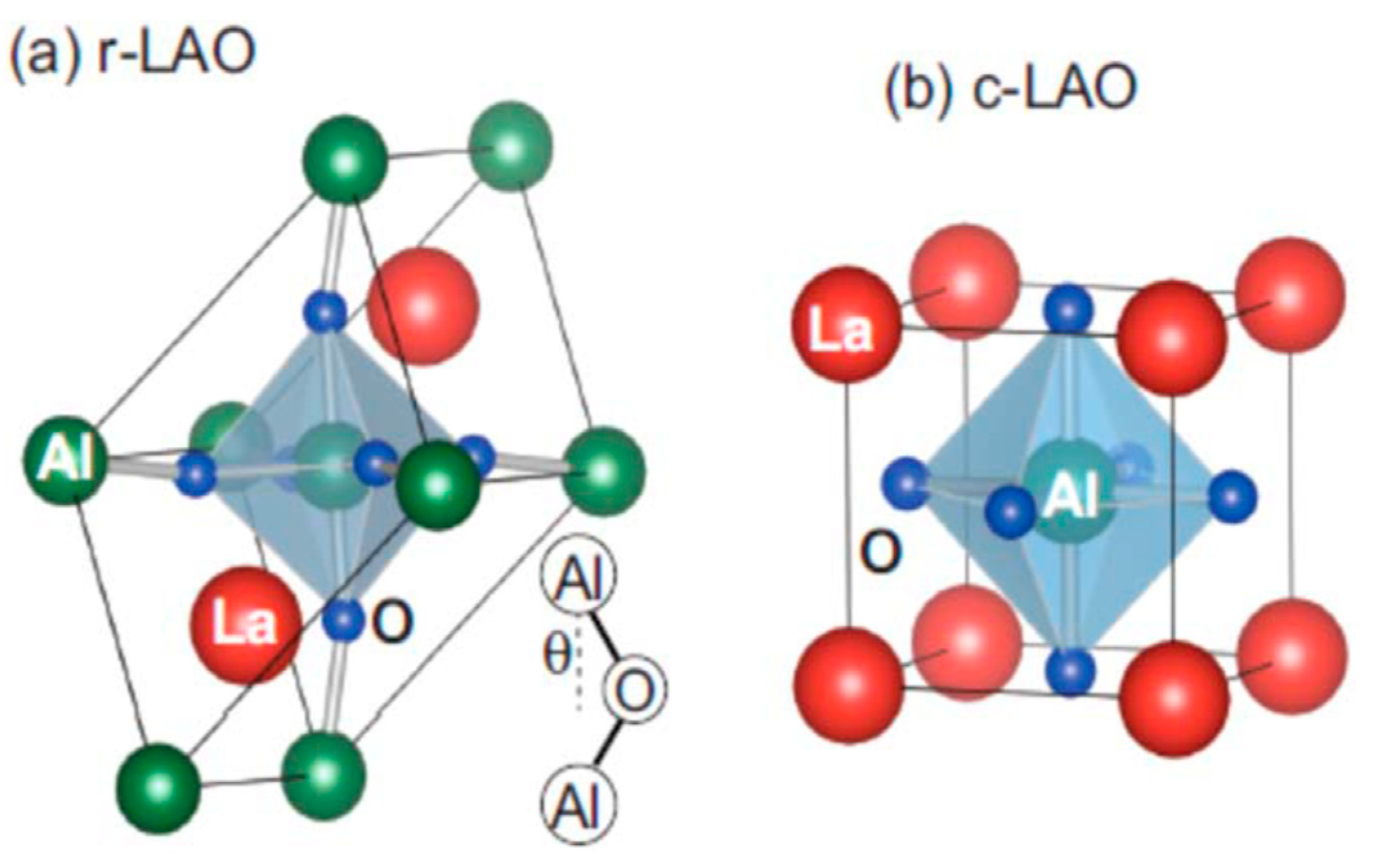
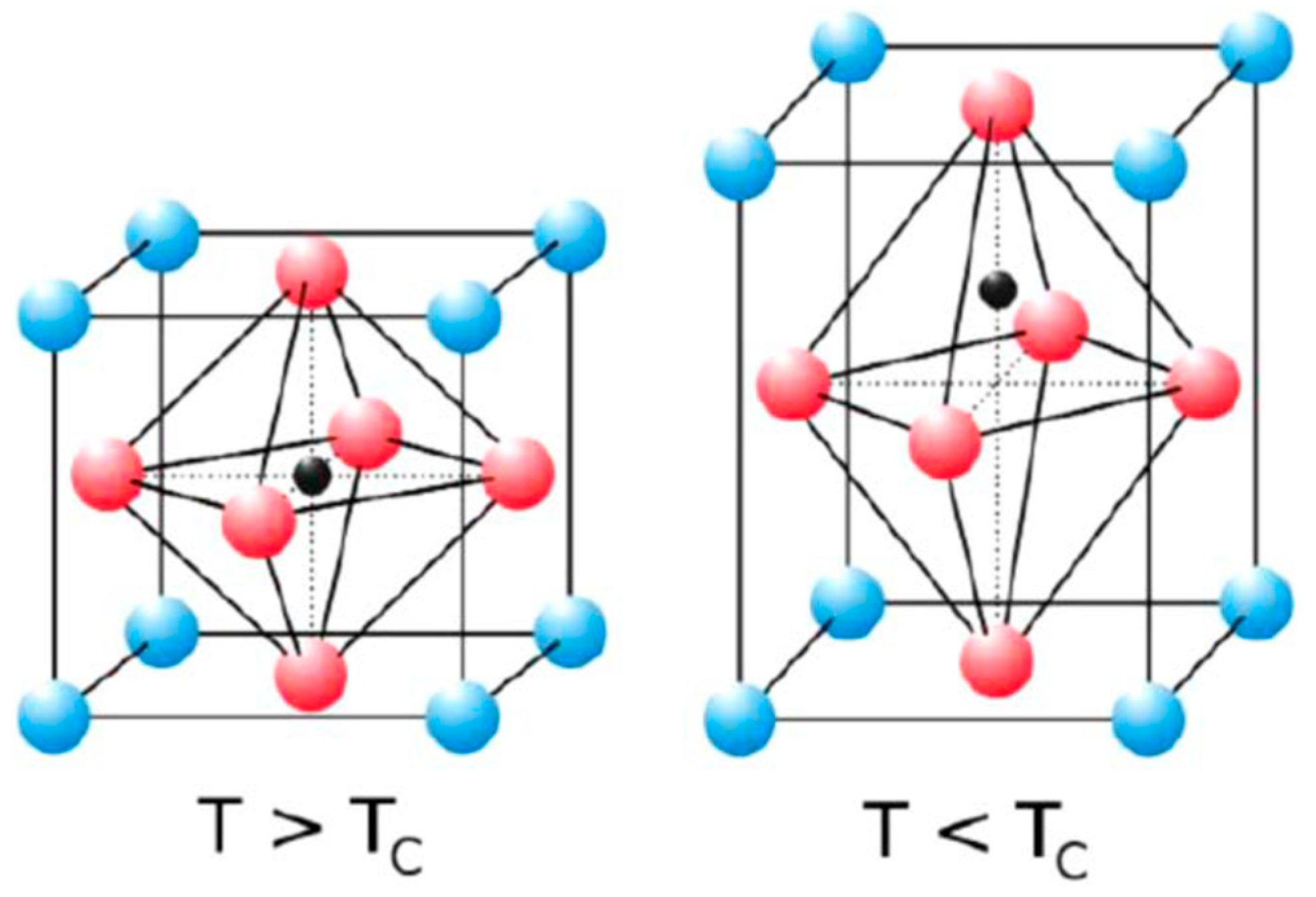
2.1. Structural Properties of LaAlO3 Perovskites
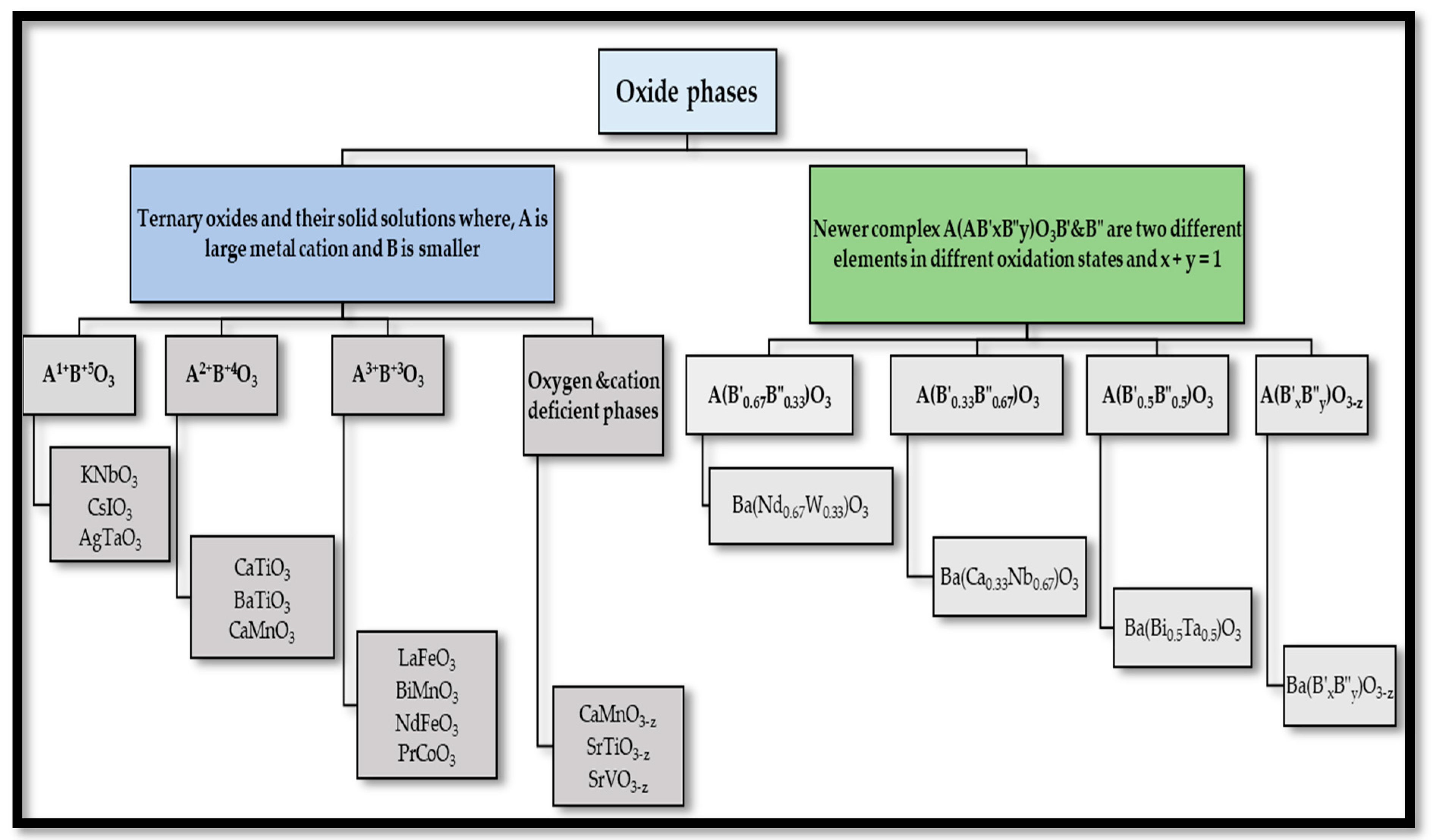
2.2. Classification of Perovskites
- Perovskites with oxygen deficient phases, namely A(B′xB″y)O3−z.
- Compounds containing equal amounts of the two B cations, namely A(B′0.5B″0.5)O3.
- Perovskites containing twice as much of the lower valence state element as the higher valence state element, namely A(B′0.33B″0.67)O3.
- Compounds containing twice as much of the higher valence state element as the lower valence state element, namely A(B′0.67B″0.33)O3.
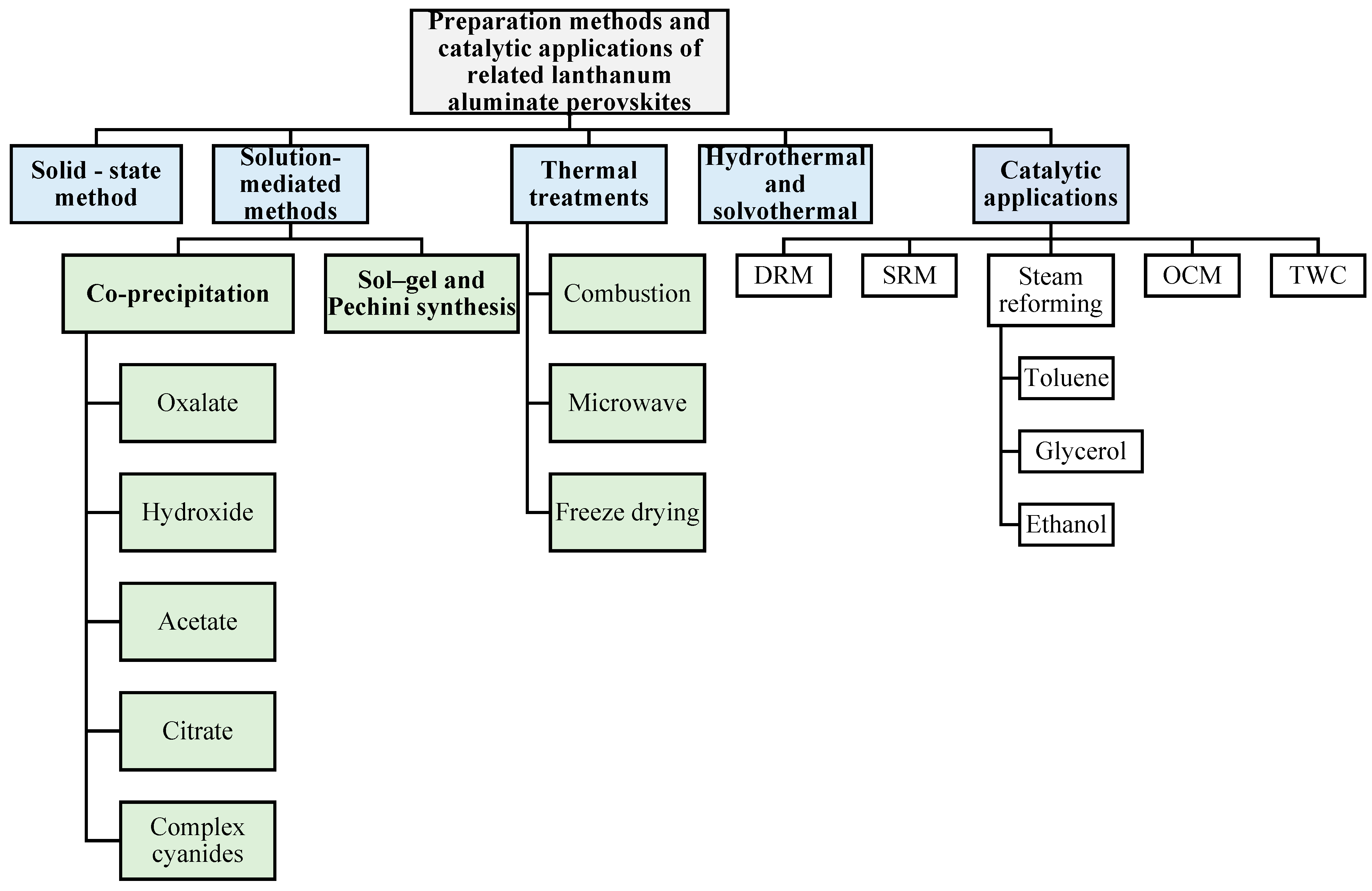
3. Synthesis Routes to LaAlO3 Perovskites
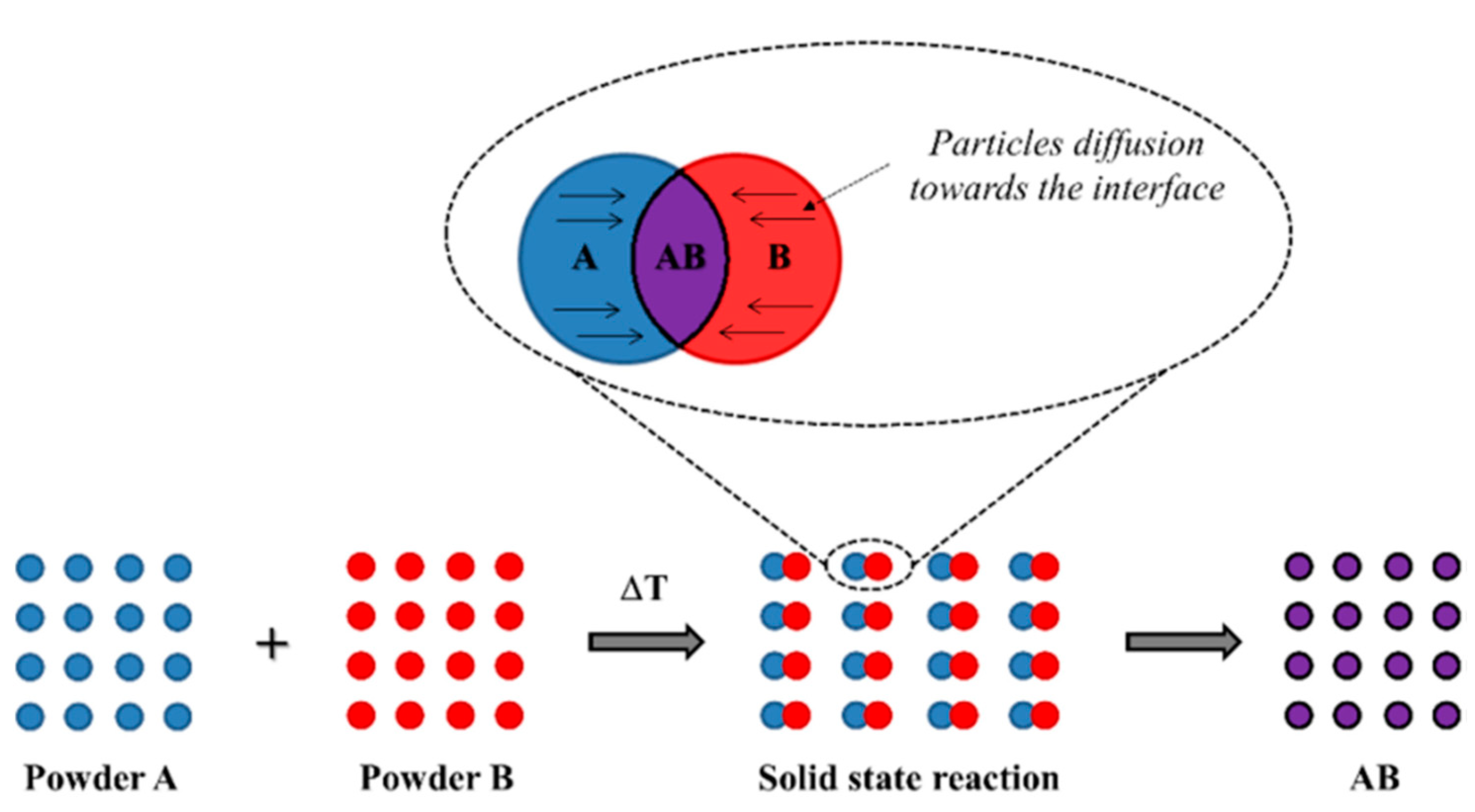
3.1. Solid-State Method
3.2. Solution-Mediated Methods
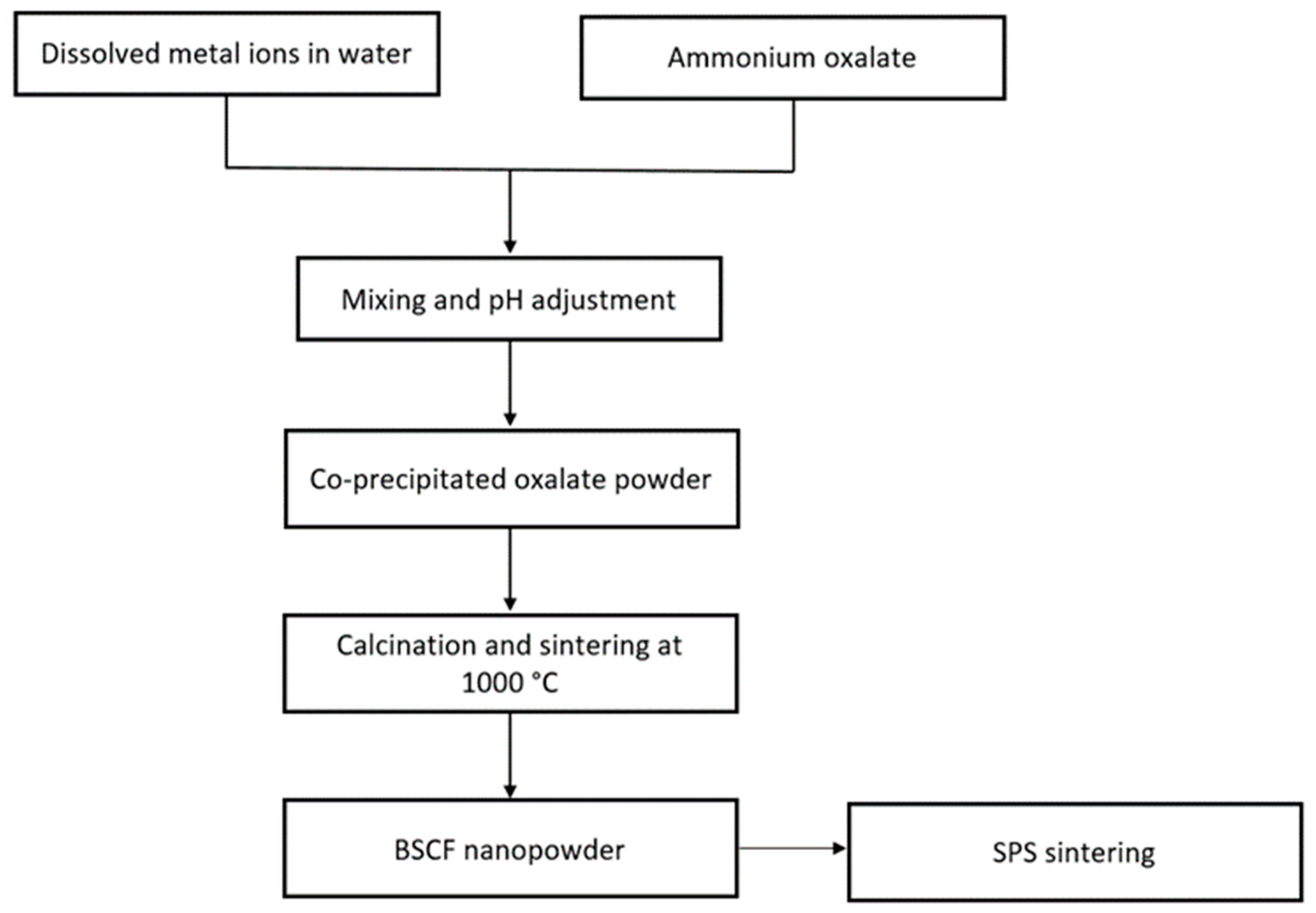
Co-Precipitation Method
| Perovskite | Preparation Method | Particle Size (nm) | Calcination Temperature (°C) | Refs. |
|---|---|---|---|---|
| La1−xCaxAlO3−δ | Mechanosynthesis | 11–34 | 1450 and 1700 | [46] |
| Ca2+-Mn2+ doped LaAlO3 | Solid-state reaction | * | 1550 | [47] |
| LaMO3 (M = Co, Al, Ga, Fe) | Solid solution method | * | 800–1500 | [48] |
| LaAlO3 | Solid-state reaction | * | 1000 | [44] |
| LaAlO3 | Co-precipitation and molten salt synthesis | 200–400 | 1000 | [54] |
| LaAlO3 | Thermal shock assisted solid-state method | 90 | 1300 | [13] |
| LaAl1−xNixO3−δ | Co-precipitation | * | 700 | [61] |
| LaAlO3 doped with Pr (III) and Yb (III) | Precipitation | 100 | 1300 | [62] |
| LaAlO3 | Co-precipitation | * | 950 | [63] |
| Eu3+-doped LaAlO3 | Co-precipitation | 20 | 700 to 900 | [64] |
| Mg-substituted LaAlO3 | Citrate sol–gel | 11.4–18.7 | 850 | [65] |
| LaAlO3 perovskite partially substituted with Ca or Ce | Citrate sol–gel | * | 800 | [66] |
| La1−xMxAlO3−δ(M = Sr, Ba, Ca) | Citrate sol–gel | * | 850 | [67] |
| LaAlO3, La0.7M0.3AlO3−δ (M = Sr, Ba, Mg, Ca), LaAl0.7M’0.3AlO3−δ (M′ = Fe, Co, Mn, Ti, Cr), and La1−xCaxAlO3−δ | Citrate sol–gel | * | 700 | [68] |
| LaAlO3 | Citrate sol–gel | * | 950 | [69] |
| LaAlO3 | Sol–gel and modified Pechini | 29–41 | 600–800 | [8] |
| LaAlO3:Bi3+, Tb3+ | Polyol mediated route | 21 | 700 | [70] |
| LaAlO3 with 25% molar Al substitution by Co, Cu or Ga | Citrate sol–gel | 30 | 700 | [71] |
| Cu-doped LaAlO3 | Pechini-type sol–gel process | 80–100 | 800 | [15] |
| Pd-substituted LaAlO3 | Citrate sol–gel | * | 700 | [72] |
| Alkali-addedLaAlO3 perovskite | Citrate sol–gel | * | 950 | [73] |
| (LaAlO3) and (RGO-LaAlO3) | Gel route and low temperature combustion method | * | 500 | [74] |
| Sr, Mn-doped LaAlO3 and Mn and Sr-codoped LaAlO3 | Pechini method | 10–20 | 900 | [21] |
| LaAlO3:Ln3+ (Ln = Eu3+ or Tb3+) | Pechini method | <60 | 900 | [75] |
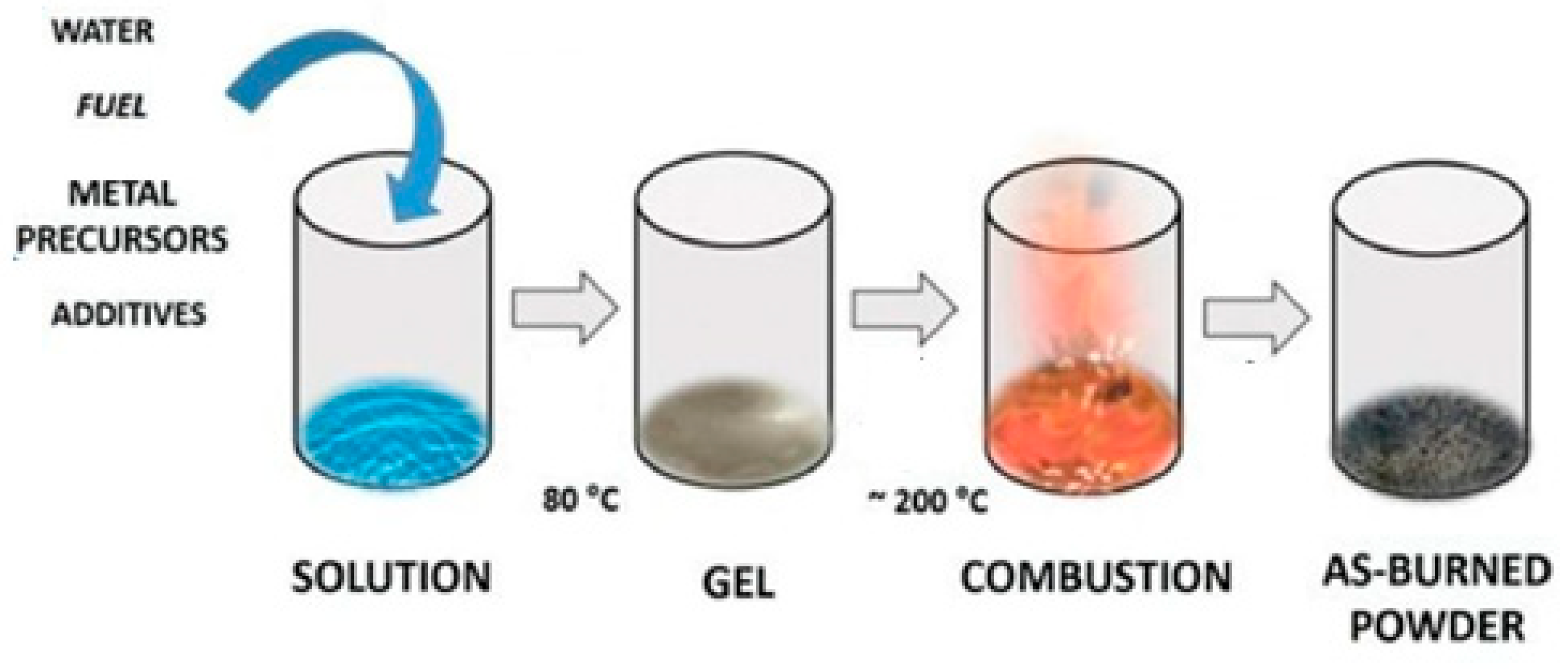
| LaAlO3 perovskite | Low-temperature solution combustion method | 45 | 500 | [14] |
| Ionic substitutions of Pd, Pt, and Ru in LaAlO3 perovskite | Combustion synthesis route | 31–48 | 700 | [18] |
| LaAlO3:Eu3+ | Combustion synthesis route | 70 | 600 | [76] |
| LaAlO3 perovskite | Combustion synthesis procedure | 36 | 1500 | [77] |
| Chromium-doped LaAlO3 | Combustion synthesis procedure | 51–71 | 450 | [77] |
| Ni/LaAlO3 | Microwave assisted combustion | ~41 | 900 | [19] |
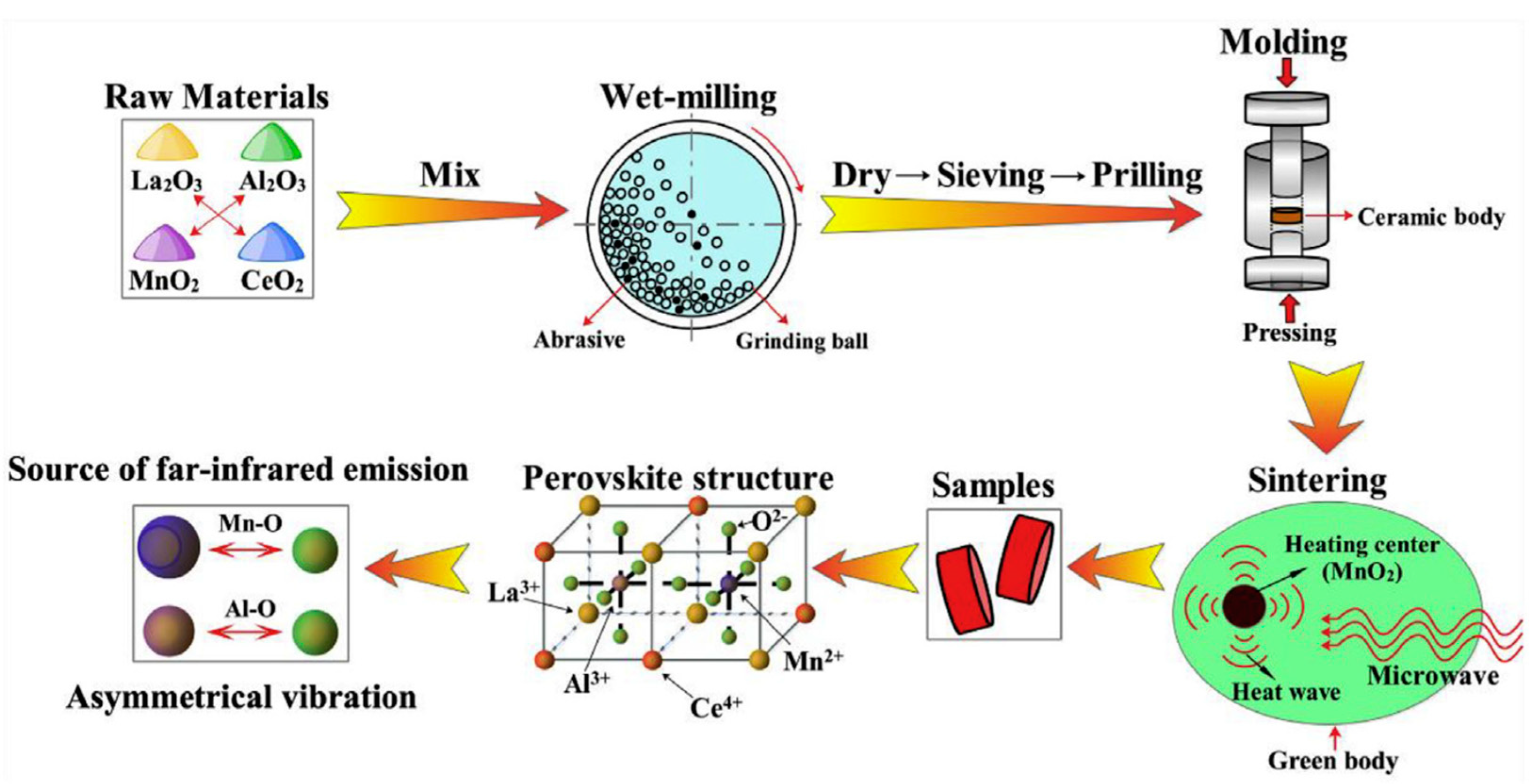
| Ce/Mn dual-doped LaAlO3 perovskites | Microwave sintering method | * | 1400 | [78] |
| LaNixAl1−xO3 | Hydrothermal method | * | 800 | [79] |
| Dy3+/Eu3+ co-doped LaAlO3 | Hydrothermal technique | 30 | 700 | [80] |
| RGO/LaAlO3 | Gel and hydrothermal methods | * | 500 | [81] |
| LaBO3 (B: Mn, Co, Fe, Al and Ni) | Supercritical hydrothermal method | * | 450 | [10] |
| Eu3+ co-doped LaAlO3 | Thermovaporous method | 100–700 | 400 | [82] |
| Eu3+ co-doped LaAlO3 | Solvothermal method | ~90 | 800 | [83] |
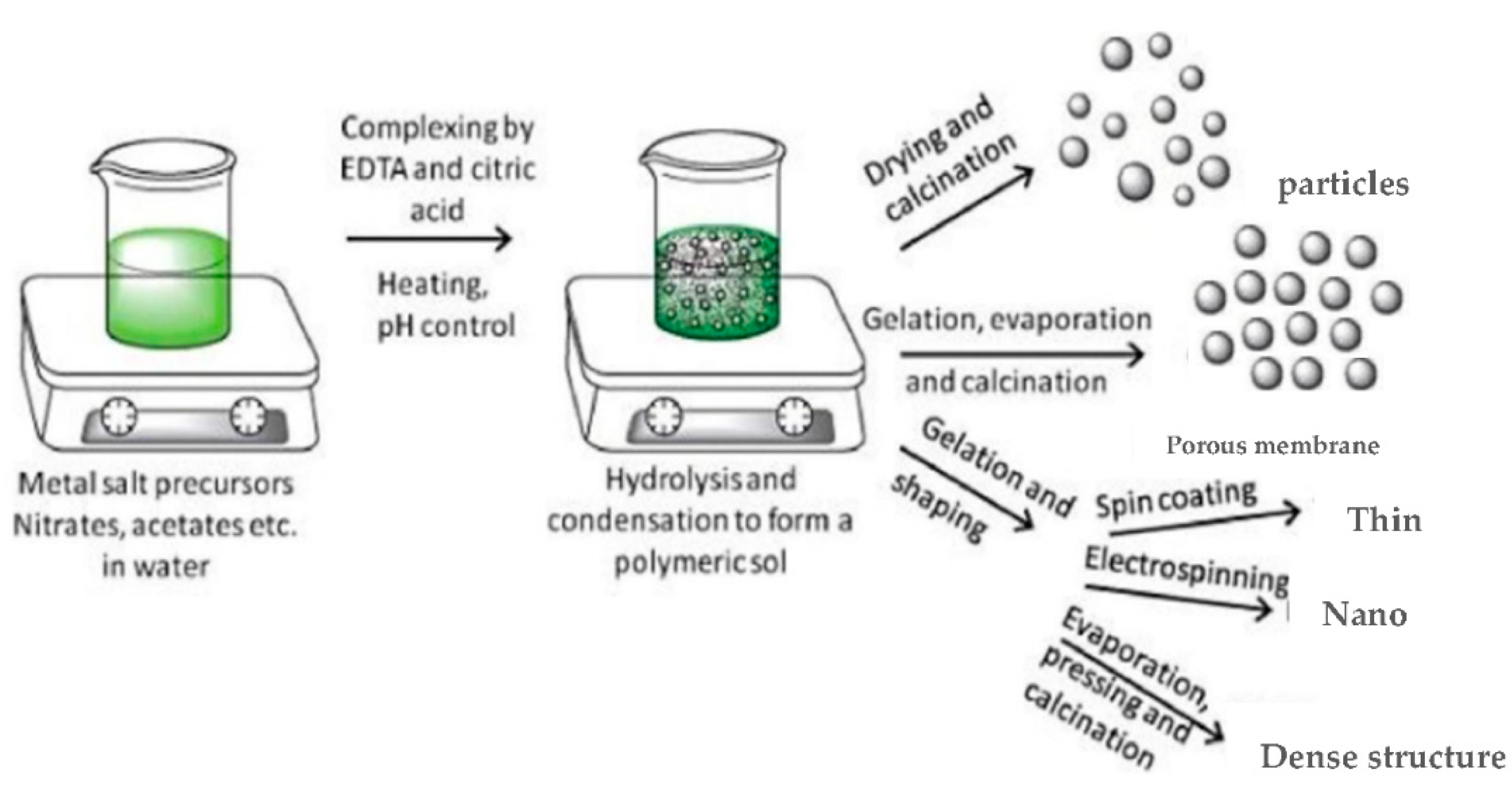
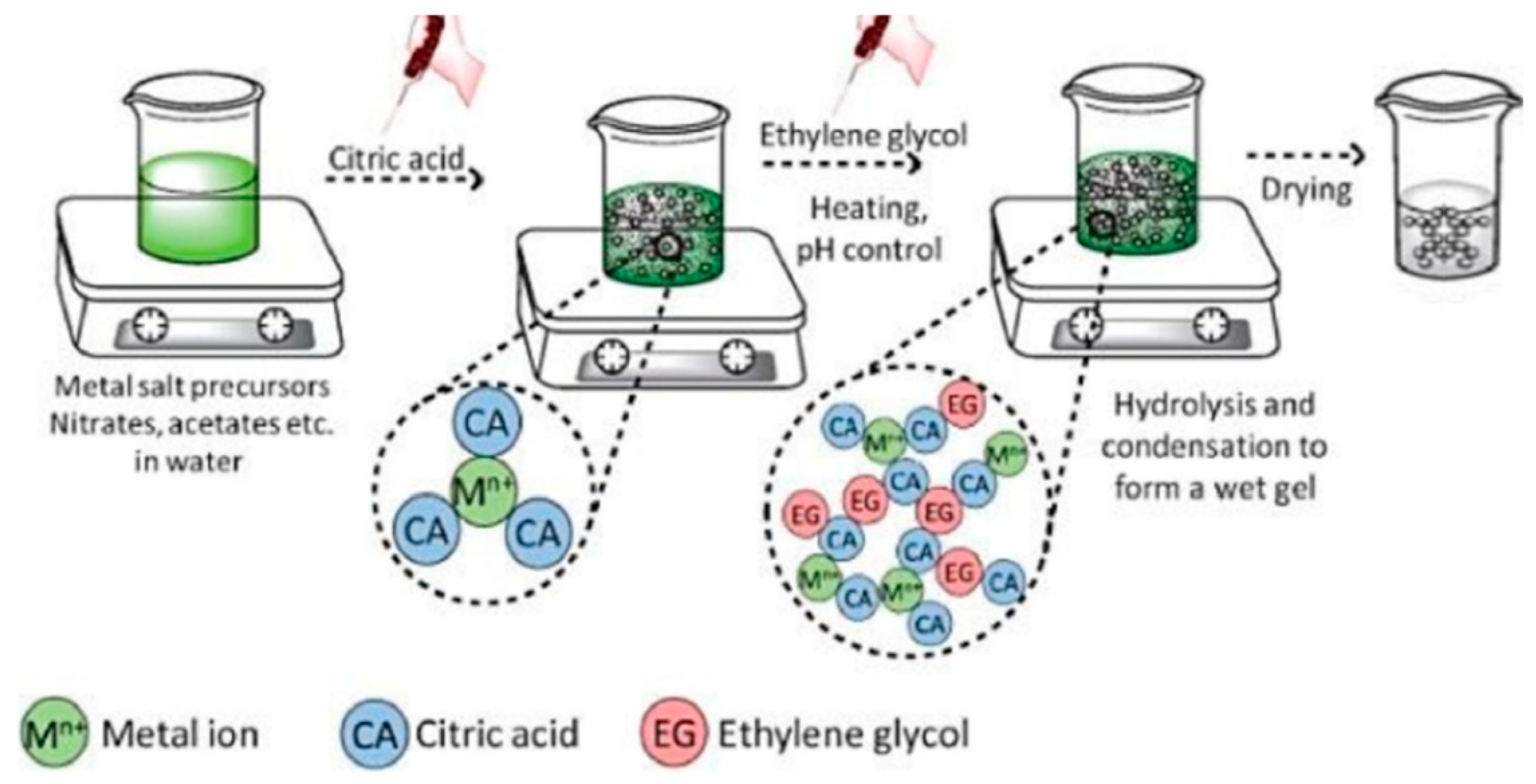
3.3. Sol–Gel Synthesis
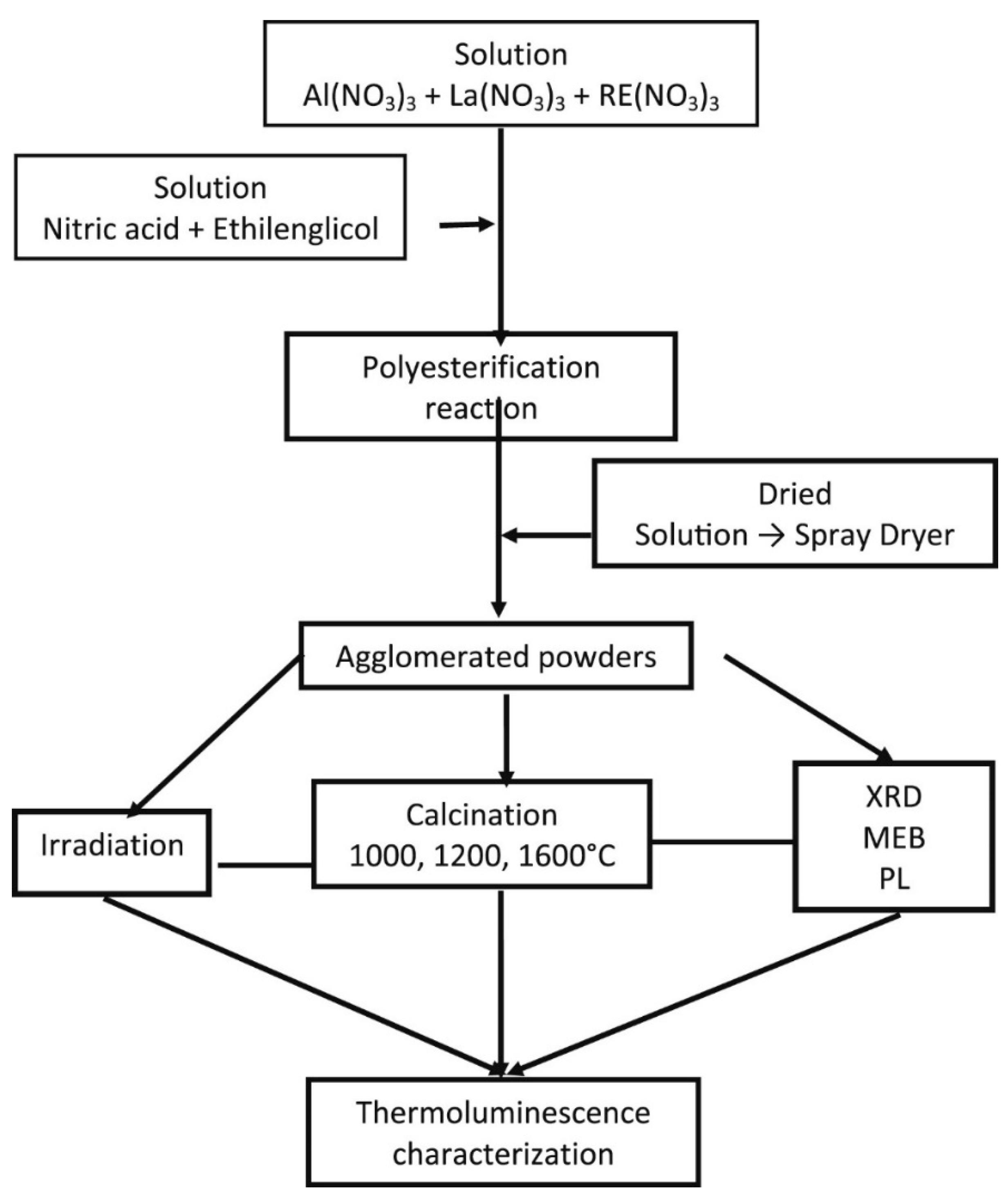
3.4. Thermal Treatments
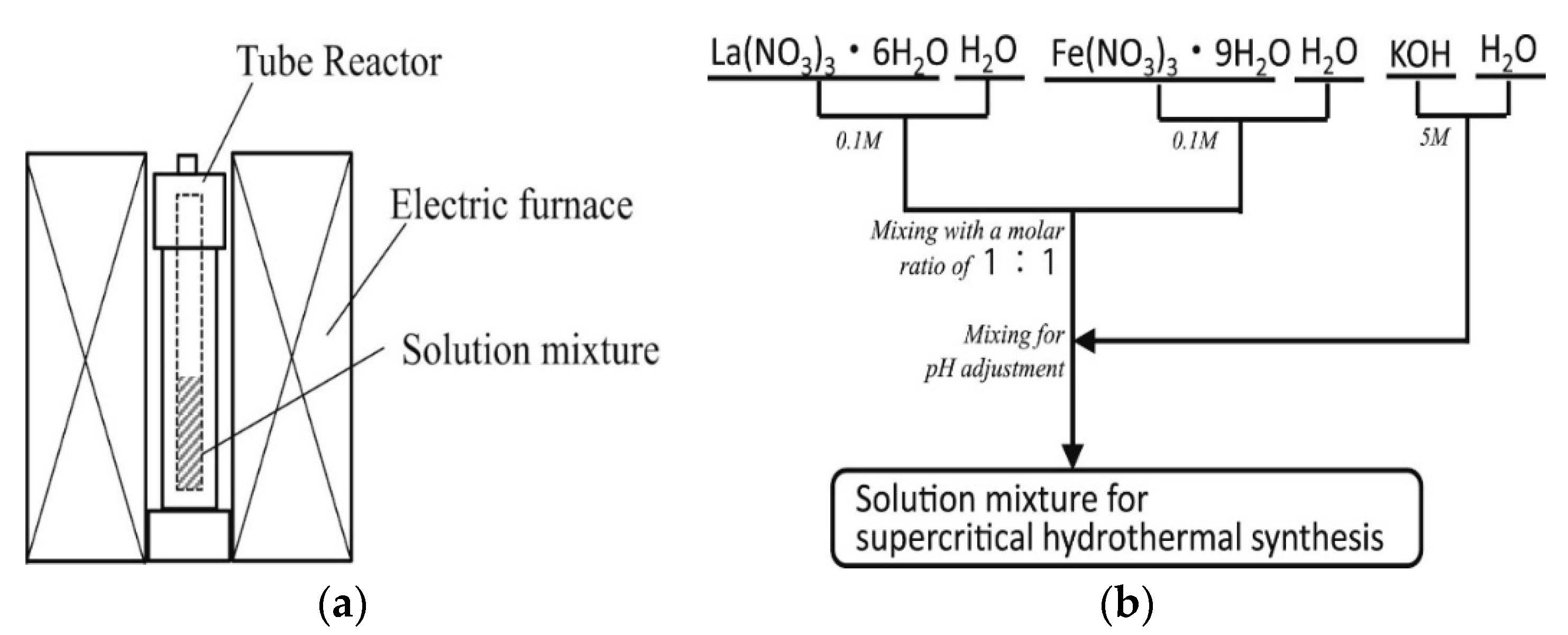
3.5. Hydrothermal/Solvothermal Synthesis
4. Catalytic Performances of LaAlO3 Perovskites
4.1. Dry and Steam Reforming of Methane and Steam Reforming of Toluene, Glycerol, and Ethanol
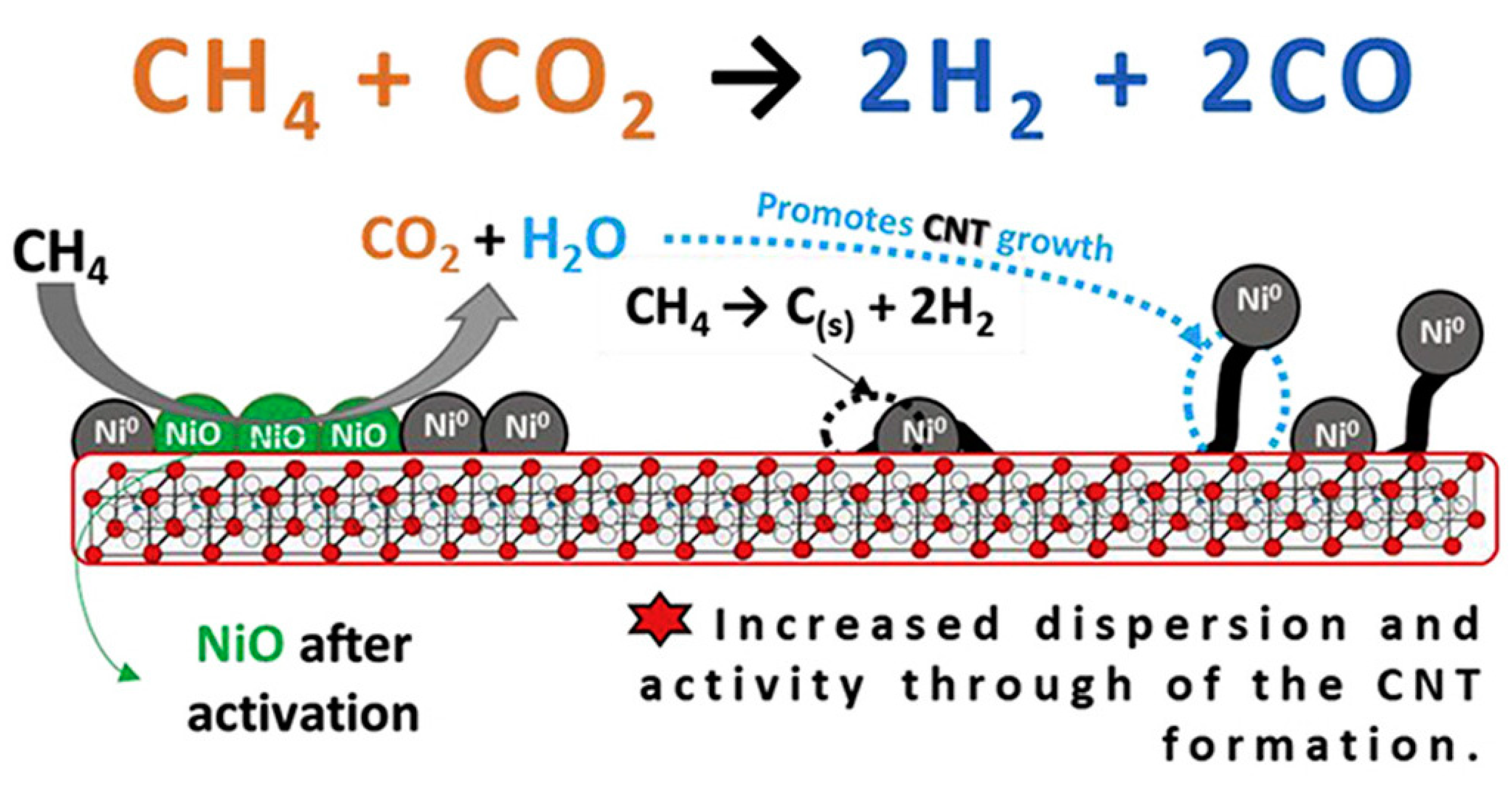
4.1.1. Dry Reforming of Methane to Syngas
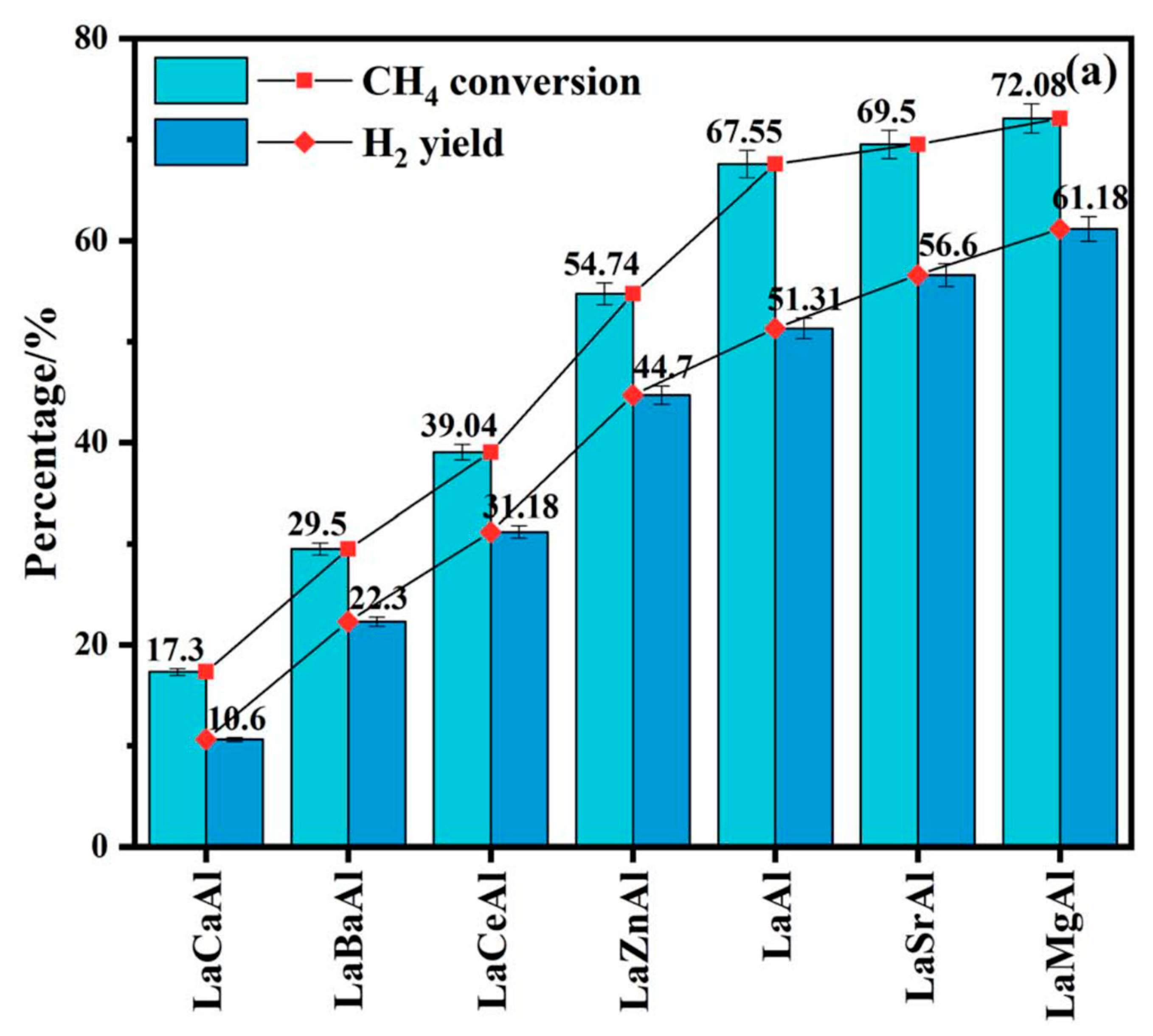
4.1.2. Steam Reforming of Methane to Generate H2
4.1.3. Steam Reforming of Toluene to Generate H2
4.1.4. Steam or Aqueous Phase Reforming of Glycerol to Generate H2
4.1.5. Ethanol Steam Reforming to Generate H2
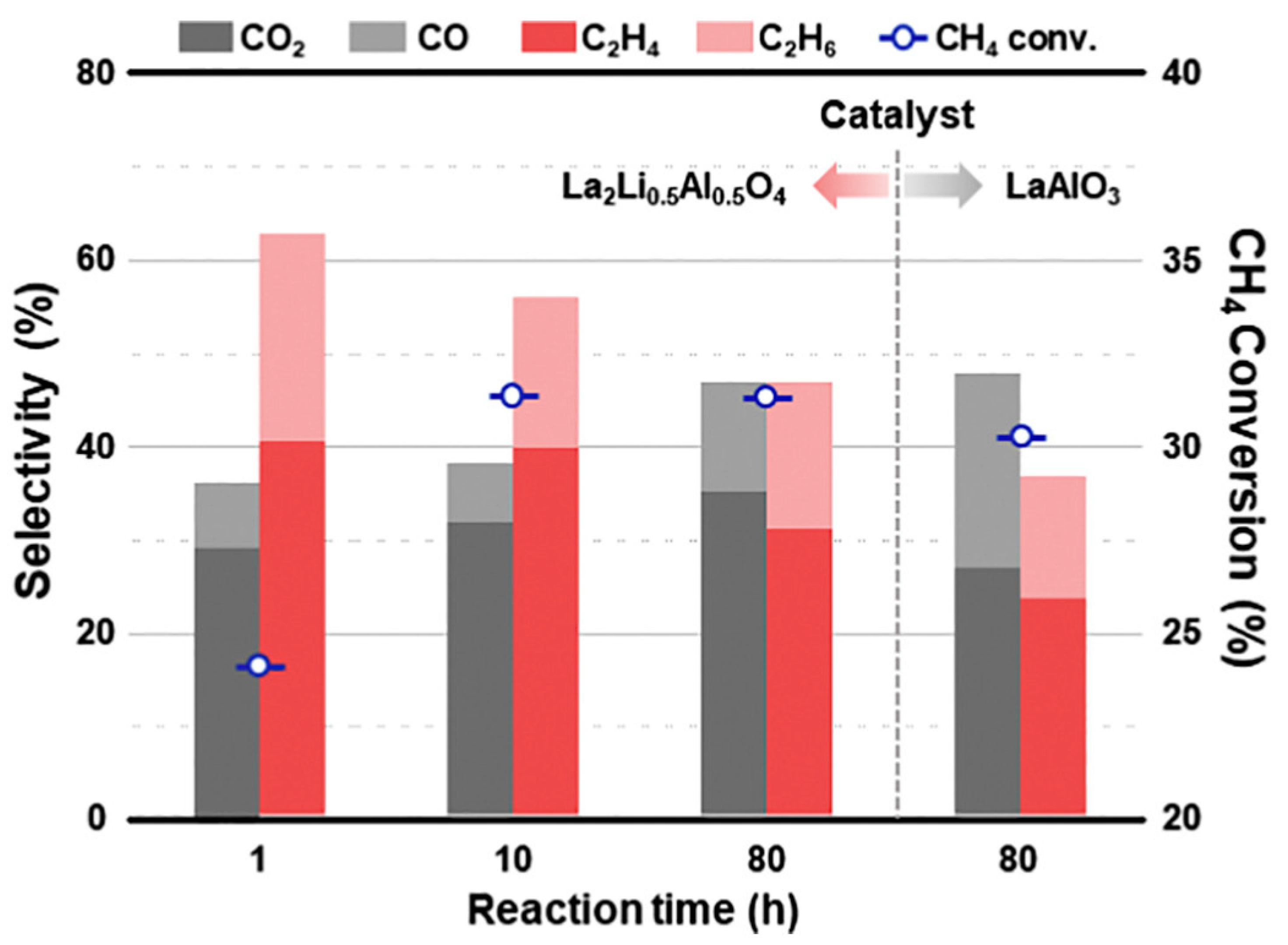
4.2. Oxidative Coupling of Methane
4.3. Three-Way Catalysts
5. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atta, N.F.; Galal, A.; El-Ads, E.H. Perovskite Materials Synthesis, Characterisation, Properties, and Applications; Tech: Rijeka, Croatia, 2016. [Google Scholar]
- Wolfram, T.; Ellialtioglu, S. Electronic and Optical Properties of d-Band Perovskites; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Ali, S. Synthesis of Nano-Particles Using Microwave Technique, the Study of Their Physical Properties and Some Applications. Ph.D. Dissertation, Cairo University, Cairo, Egypt, 2009. [Google Scholar]
- Johnsson, M.; Lemmens, P. Crystallography and Chemistry of Perovskites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Assirey, E.A.R. Perovskite synthesis, properties and their related biochemical and industrial application. Saudi Pharm. J. 2019, 27, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Ono, L.K.; Juarez-Perez, E.J.; Qi, Y. Progress on perovskite materials and solar cells with mixed cations and halide anions. ACS Appl. Mater. Interfaces 2017, 9, 30197–30246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejuca, L.G.; Fierro, J.L. Properties and Applications of Perovskite-Type Oxides; CRC Press: New York, NY, USA, 1992. [Google Scholar]
- Silveira, I.S.; Ferreira, N.S.; Souza, D.N. Structural, morphological and vibrational properties of LaAlO3 nanocrystals produced by four different methods. Ceram. Int. 2021, 47, 27748–27758. [Google Scholar] [CrossRef]
- Girish, H.N.; Vijaya Kumar, M.S.; Byrappa, K.; Basavalingu, B. Hydrothermal synthesis of some of Lanthanide aluminium perovskites-LnAlO3 (Ln = La, Sm and Gd). Mater. Res. Innov. 2015, 19, 270–274. [Google Scholar] [CrossRef]
- Abe, Y.; Satou, I.; Aida, T.; Adschiri, T. Formation of La-based perovskite compounds in supercritical water. Ceram. Int. 2018, 44, 12996–13003. [Google Scholar] [CrossRef]
- Morales-Hernández, A.; Zarate-Medina, J.; Contreras-García, M.E.; Azorín-Nieto, J.; Rivera-Montalvo, T. Synthesis and thermoluminescence of LaAlO3:Pr3+ to UVC radiation dosimetry. Appl. Radiat. Isot. 2016, 118, 12–17. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, W.; Bian, X.; Wu, Y. Low temperature green synthesis of LaAlO3 using microcrystalline LaOCl and amorphous Al2O3 precursors derived from spray pyrolysis. Green Process. Synth. 2017, 6, 49–54. [Google Scholar] [CrossRef]
- Beheshti, M.; Malekfar, R. A novel approach for the synthesis of lanthanum aluminate nanoparticles using thermal shock assisted solid-state method as a microwave absorber layer. Mater. Chem. Phys. 2021, 270, 124848. [Google Scholar] [CrossRef]
- Manjunatha, C.R.; Nagabhushana, B.M.; Raghu, M.S.; Pratibha, S.; Dhananjaya, N.; Narayana, A. Perovskite lanthanum aluminate nanoparticles applications in antimicrobial activity, adsorptive removal of Direct Blue 53 dye and fluoride. Mater. Sci. Eng. 2019, 101, 674–685. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Hu, C.; Wang, X.; Lyu, L.; Sheng, G. Enhanced degradation of organic pollutants over Cu-doped LaAlO3 perovskite through heterogeneous Fenton-like reactions. Chem. Eng. J. 2018, 332, 572–581. [Google Scholar] [CrossRef]
- Kumar, A.; Husale, S.; Dogra, A.; Gupta, A.; Aloysius, R.P. Superconducting properties of Al wires deposited on SrTiO3 and LaAlO3/SrTiO3 substrates. Mater. Today Proc. 2020, 28, 88–91. [Google Scholar] [CrossRef]
- Chen, H.; Yang, C.; Zhang, J.; He, W.; Liao, Y.; Zhang, Q.; Zheng, S.; Lei, G. High performance distributed CPW phase shifters with etched BST thin films on φ 3″ LaAlO3 substrates. Solid State Sci. 2012, 14, 117–120. [Google Scholar] [CrossRef]
- Anil, C.; Modak, J.M.; Madras, G. Syngas production via CO2 reforming of methane over noble metal (Ru, Pt, and Pd) doped LaAlO3 perovskite catalyst. Mol. Catal. 2020, 484, 110805. [Google Scholar] [CrossRef]
- Figueredo, G.P.; Medeiros, R.L.B.A.; Macedo, H.P.; de Oliveira, Â.A.S.; Braga, R.M.; Mercury, J.M.R.; Melo, M.A.F.; Melo, D.M.A. A comparative study of dry reforming of methane over nickel catalysts supported on perovskite-type LaAlO3 and commercial α-Al2O3. Int. J. Hydrogen Energy 2018, 43, 11022–11037. [Google Scholar] [CrossRef]
- Tran, Q.N.; Martinovic, F.; Ceretti, M.; Esposito, S.; Bonelli, B.; Paulus, W.; Di Renzo, F.; Deorsola, F.A.; Bensaid, S.; Pirone, R. Co-doped LaAlO3 perovskite oxide for NOx-assisted soot oxidation. Appl. Catal. A Gen. 2020, 589, 117304. [Google Scholar] [CrossRef]
- Da Silva, C.A.; De Miranda, P.E.V. Synthesis of LaAlO3 based materials for potential use as methane-fueled solid oxide fuel cell anodes. Int. J. Hydrogen Energy 2015, 40, 10002–10015. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as substitutes of noble metals for heterogeneous catalysis: Dream or reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- García-Espejo, G. Synthesis and Characterization of Nanostructured Materials: Hybrid and Double Perovskites and Diacetylenes. Ph.D. Dissertation, Cordoba University, Cordoba, Spain, 2020. [Google Scholar]
- Peña, M.A.; Fierro, J.L.G. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef]
- Ishihara, T. Perovskite Oxide for Solid Oxide Fuel Cells; Springer Science & Business Media: New York, NY, USA, 2009. [Google Scholar]
- Kawamura, Y.; Mashiyama, H.; Hasebe, K. Structural Study on Cubic-Tetragonal Transition of CH3NH3PbI3. J. Phys. Soc. Jpn. 2002, 71, 1694–1697. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Jayatissa, A.H. Perovskites-based solar cells: A review of recent progress, materials and processing methods. Materials 2018, 11, 729. [Google Scholar] [CrossRef] [Green Version]
- Whitfield, P.S.; Herron, N.; Guise, W.E.; Page, K.; Cheng, Y.Q.; Milas, I.; Crawford, M.K. Structures, phase transitions and tricritical tehavior of the hybrid perovskite methyl ammonium lead iodide. Sci. Rep. 2016, 6, 35685. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Gul, S.; Iqbal, T.; Mushtaq, U.; Farooq, M.H.; Farman, M.; Bibi, R.; Ijaz, M. A review on perovskite lanthanum aluminate (LaAlO3), its properties and applications. Mater. Res. Express 2019, 6, 112001. [Google Scholar] [CrossRef]
- Hayward, S.A.; Morrison, F.D.; Redfern, S.A.T.; Salje, E.K.H.; Scott, J.F.; Knight, K.S.; Tarantino, S.; Glazer, A.M.; Shuvaeva, V.; Daniel, P.; et al. Transformation processes in LaAlO3: Neutron diffraction, dielectric, thermal, optical, and raman studies. Phys. Rev. B. 2005, 72, 054110. [Google Scholar] [CrossRef]
- Luo, X.; Wang, B. Structural and elastic properties of from first-principles calculations. J. Appl. Phys. 2008, 104, 073518. [Google Scholar] [CrossRef]
- Steele, E.A. Surface Characterization of Lanthanum Aluminate (LaAlO3). Ph.D. Dissertation, Northwestern University, Evanston, IL, USA, 2016. [Google Scholar]
- Kuzmanovski, I.; Dimitrovska-lazova, S.; Aleksovska, S. Classification of perovskites with supervised self-organizing maps. Anal. Chim. Acta. 2007, 595, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Aleksovska, S.; Dimitrovska, S.; Kuzmanovski, I. Prediction of the unit cell edge length of cubic A2+2BB′O6 perovskites by multiple linear regression and artificial neural networks. Cent. Eur. J. Chem. 2005, 3, 198–215. [Google Scholar]
- Galasso, F.S. Structure, Properties and Preparation of Perovskite-Type Compounds: International Series of Monographs in Solid State Physics; Elsevier: Oxford, UK, 2013. [Google Scholar]
- Bhalla, A.S.; Guo, R.; Roy, R. The perovskite structure–A review of its role in ceramic science and technology. Mater. Res. Innov. 2000, 4, 3–26. [Google Scholar] [CrossRef]
- Modeshia, D.R.; Walton, R.I. Solvothermal synthesis of perovskites and pyrochlores: Crystallisation of functional oxides under mild conditions. Chem. Soc. Rev. 2010, 39, 4303–4325. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Remeika, J.P.; Trimble, L.E. Defect chemistry and catalysis in oxidation and reduction over perovskite-type oxides. Ann. N. Y. Acad. Sci. 1976, 272, 3–21. [Google Scholar] [CrossRef]
- Teraoka, Y.; Zhang, H.-M.; Furukawa, S.; Yamazoe, N. Oxygen permeation through perovskite-type oxides. Chem. Lett. 1985, 14, 1743–1746. [Google Scholar] [CrossRef]
- Royer, S.; Bérubé, F.; Kaliaguine, S. Effect of the synthesis conditions on the redox and catalytic properties in oxidation reactions of LaCo1−xFexO3. Appl. Catal. A Gen. 2005, 282, 273–284. [Google Scholar] [CrossRef]
- Smart, L.E.; Moore, E.A. Solid State Chemistry: An Introduction; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Athayde, D.D.; Souza, D.F.; Silva, A.M.A.; Vasconcelos, D.; Nunes, E.H.M.; Da Costa, J.C.D.; Vasconcelos, W.L. Review of perovskite ceramic synthesis and membrane preparation methods. Ceram. Int. 2016, 42, 6555–6571. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Ran, R.; Zhong, Y.; Zhou, W.; Ni, M.; Shao, Z. Advances in porous perovskites: Synthesis and electrocatalytic performance in fuel cells and metal–air batteries. Energy Environ. Mater. 2020, 3, 121–145. [Google Scholar] [CrossRef]
- Lee, M.H.; Jung, W.S. Facile synthesis of LaAlO3 and Eu(II)-doped LaAlO3 powders by a solid-state reaction. Ceram. Int. 2015, 41, 5561–5567. [Google Scholar] [CrossRef]
- Zhang, Q.; Saito, F. Mechanochemical synthesis of lanthanum aluminate by grinding lanthanum oxide with transition alumina. J. Am. Ceram. Soc. 2000, 83, 439–441. [Google Scholar] [CrossRef]
- Fabián, M.; Arias-Serrano, B.I.; Yaremchenko, A.A.; Kolev, H.; Kaňuchová, M.; Briančin, J. Ionic and electronic transport in calcium-substituted LaAlO3 perovskites prepared via mechanochemical route. J. Eur. Ceram. Soc. 2019, 39, 5298–5308. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Ji, H.; Zhao, Z.; Zhang, P. Effect of Ca2+ and Mn2+ ions on the radiation properties of LaAlO3. Ceram. Int. 2018, 44, 20427–20431. [Google Scholar] [CrossRef]
- Araki, W.; Takeda, K.; Arai, Y. Mechanical behaviour of ferroelastic lanthanum metal oxides LaMO3 (M = Co, Al, Ga, Fe). J. Eur. Ceram. Soc. 2016, 36, 4089–4094. [Google Scholar] [CrossRef]
- Dhahri, A.; Horchani-Naifer, K.; Benedetti, A.; Enrichi, F.; Ferid, M. Combustion synthesis and photoluminescence of Eu3+ doped LaAlO3 nanophosphors. Opt. Mater. (Amst). 2012, 34, 1742–1746. [Google Scholar] [CrossRef]
- Brylewski, T.; Bućko, M.M. Low-temperature synthesis of lanthanum monoaluminate powders using the co-precipitation-calcination technique. Ceram. Int. 2013, 39, 5667–5674. [Google Scholar] [CrossRef]
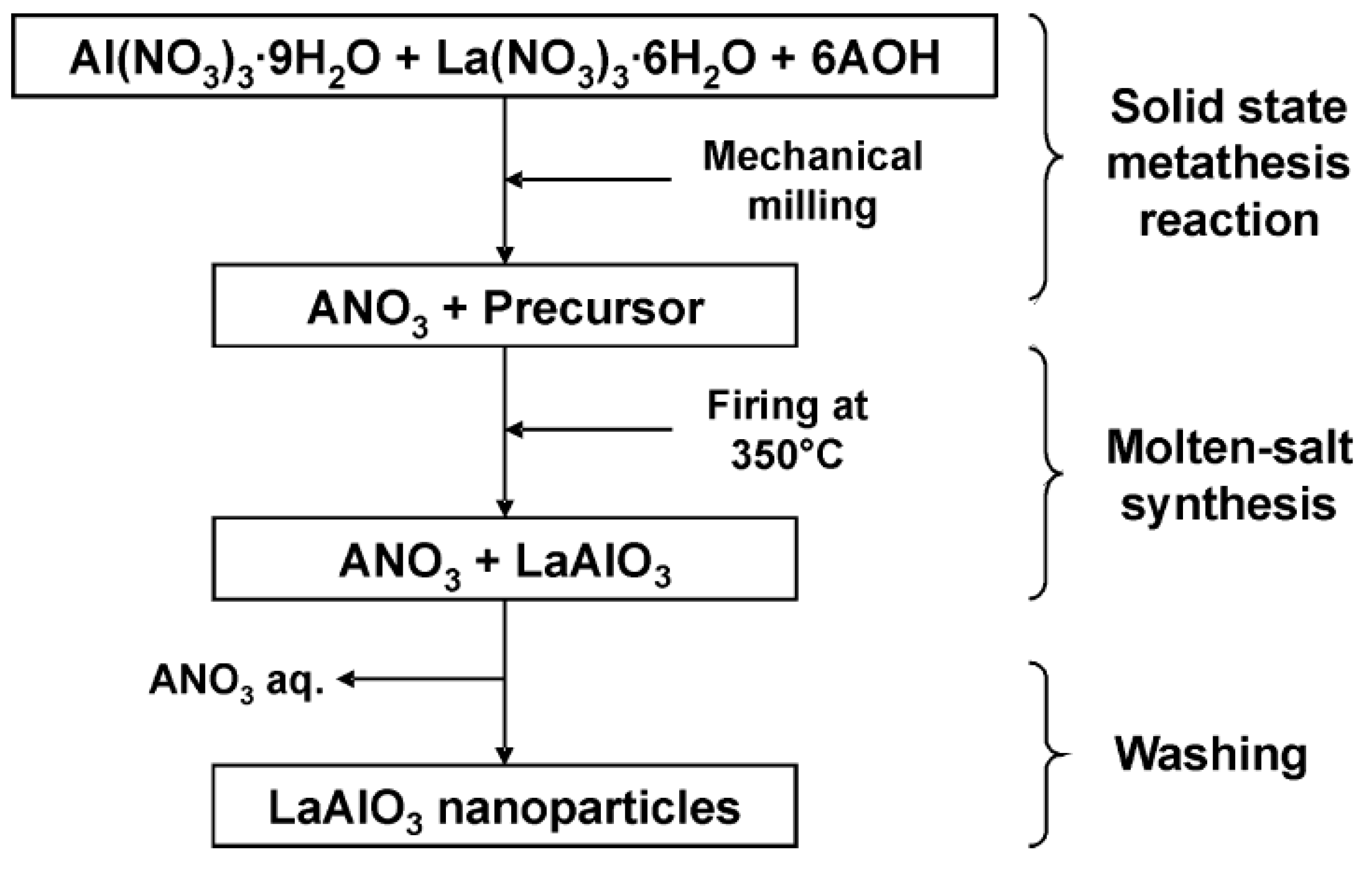
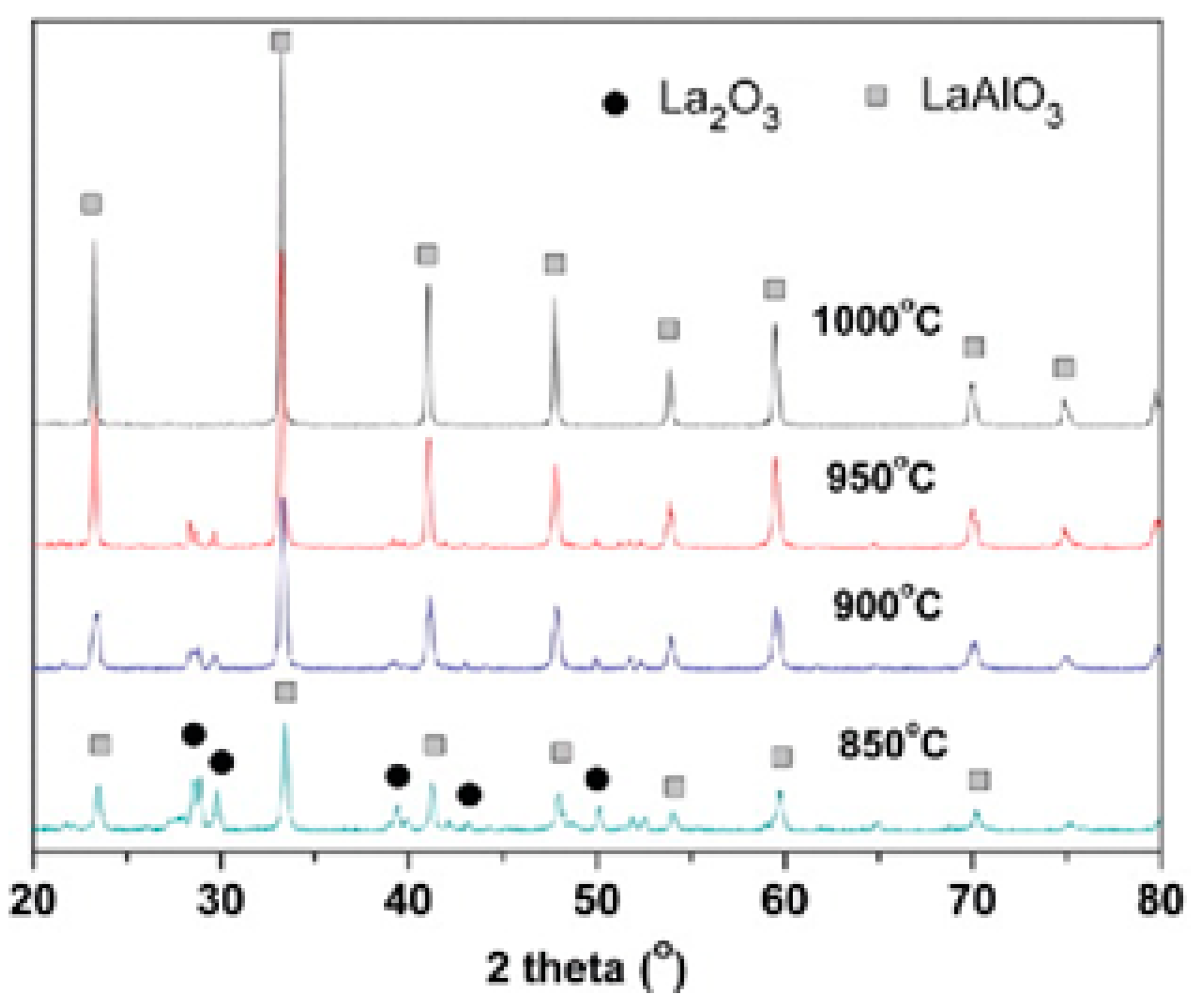
- Mendoza-Mendoza, E.; Montemayor, S.M.; Escalante-García, J.I.; Fuentes, A.F. A “Green chemistry” approach to the synthesis of rare-earth aluminates: Perovskite-type LaAlO3 nanoparticles in molten nitrates. J. Am. Ceram. Soc. 2012, 95, 1276–1283. [Google Scholar] [CrossRef]
- Li, S.; Bergman, B.; Zhao, Z. Synthesis and characterization of lanthanum aluminate powders via a polymer complexing plus combustion route. Mater. Chem. Phys. 2012, 132, 309–315. [Google Scholar] [CrossRef]
- Lee, M.H.; Jung, W.S. Luminescence spectra of Eu (III/II)-doped LaAlO3 powders prepared by a solid-state reaction of Eu (III)-doped LaCO3OH and Al2O3. Ceram. Int. 2014, 40, 13419–13425. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, L.; Luo, H.; Fan, X.; Jin, L.; Liu, B.; Li, D.; Qiu, Z.; Gan, Y. Preparation of Eu3+ doped LaAlO3 phosphors by coprecipitation-molten salt synthesis. Integr. Ferroelectr. 2018, 188, 1–11. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Recent progress in structural development and band engineering of perovskites materials for photocatalytic solar hydrogen production: A review. Int. J. Hydrogen Energy 2020, 45, 19078–19111. [Google Scholar] [CrossRef]
- Shao, X.; Dong, D.; Parkinson, G.; Li, C.Z. Improvement of oxygen permeation through microchanneled ceramic membranes. J. Memb. Sci. 2014, 454, 444–450. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, G.; Wang, G.; Irvine, J.T.S. Synthesis and applications of nanoporous perovskite metal oxides. Chem. Sci. 2018, 9, 3623–3637. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ahmad, M.; Sun, H. Three-dimensional ZnO hierarchical nanostructures: Solution phase synthesis and applications. Materials 2017, 10, 1304. [Google Scholar] [CrossRef] [Green Version]
- Carrier, X.; Royer, S.; Marceau, E. Synthesis of metal oxide catalysts. In Metal Oxides in Heterogeneous Catalysis, 1st ed.; Vedrine, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 43–103. [Google Scholar]
- George, G.; Ede, S.R.; Luo, Z. Fundamentals of Perovskite Oxides: Synthesis, Structure, Properties and Applications; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Djoudi, L.; Omari, M. Synthesis and characterization of perovskite oxides LaAl1−xNixO3−δ (0 ≤ x ≤ 0.6) via co-precipitation method. J. Inorg. Organomet. Polym. Mater. 2015, 25, 796–803. [Google Scholar] [CrossRef]
- Lemański, K.; Dereń, P. Luminescent properties of LaAlO3 nanocrystals, doped with Pr3+ and Yb3+ ions. J. Lumin 2014, 146, 239–242. [Google Scholar] [CrossRef]
- Sim, Y.; Yoo, J.; Ha, J.M.; Jung, J.C. Oxidative coupling of methane over LaAlO3 perovskite catalysts prepared by a co-precipitation method: Effect of co-precipitation pH value. J. Energy Chem. 2019, 35, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Deng, H.; Ding, N.; Yan, H.; Yang, P.; Chu, J. Structure characterisation and optical properties of Eu3+-doped LaAlO3. Mater. Sci. Forum 2013, 745–746, 136–141. [Google Scholar] [CrossRef]
- Bai, X.; Xie, G.; Guo, Y.; Tian, L.; El-Hosainy, H.M.; Awadallah, A.E.; Ji, S.; Wang, Z.J. A highly active Ni catalyst supported on Mg-substituted LaAlO3 for carbon dioxide reforming of methane. Catal. Today 2021, 368, 78–85. [Google Scholar] [CrossRef]
- Martínez, A.H.; Lopez, E.; Cadús, L.E.; Agüero, F.N. Elucidation of the role of support in Rh/perovskite catalysts used in ethanol steam reforming reaction. Catal. Today 2021, 372, 59–69. [Google Scholar] [CrossRef]
- Sato, A.; Ogo, S.; Takeno, Y.; Takise, K.; Seo, J.G.; Sekine, Y. Electric field and mobile oxygen promote low-temperature oxidative coupling of methane over La1−xCaxAlO3−δ perovskite catalysts. ACS Omega 2019, 4, 10438–10443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabe, T.; Kamite, Y.; Sugiura, K.; Ogo, S.; Sekine, Y. Low-temperature oxidative coupling of methane in an electric field using carbon dioxide over Ca-doped LaAlO3 perovskite oxide catalysts. J. CO2 Util. 2017, 20, 156–162. [Google Scholar] [CrossRef]
- Lee, G.; Kim, I.; Yang, I.; Ha, J.M.; Na, H.B.; Jung, J.C. Effects of the preparation method on the crystallinity and catalytic activity of LaAlO3 perovskites for oxidative coupling of methane. Appl. Surf. Sci. 2018, 429, 55–61. [Google Scholar] [CrossRef]
- Shaik, E.B.; Pindiprolu, S.K.S.S.; Phanikumar, C.S.; Samuel, T.; Kumar, B.V.N.; Santhoshi, P.M.; Reddy, P.V.S.S.S.N.; Kumar B, P.; Ramachandra, R.K. Optical emissions of chitosan modified LaAlO3: Bi3+, Tb3+ nanoparticles for bio labeling and drug delivery to breast cancer cells. Opt. Mater. 2020, 107, 110162. [Google Scholar] [CrossRef]
- Tran, Q.N.; Gimello, O.; Tanchoux, N.; Ceretti, M.; Albonetti, S.; Paulus, W.; Bonelli, B.; Renzo, F.D. Transition metal B-Site substitutions in LaAlO3 perovskites reorient bio-ethanol conversion reactions. Catalysts 2021, 11, 344. [Google Scholar] [CrossRef]
- Yoon, D.Y.; Kim, Y.J.; Lim, J.H.; Cho, B.K.; Hong, S.B.; Nam, I.S.; Choung, J.W. Thermal stability of Pd-containing LaAlO3 perovskite as a modern TWC. J. Catal. 2015, 330, 71–83. [Google Scholar] [CrossRef]
- An, S.; Cho, J.; Kwon, D.; Jung, J.C. Alkali-added catalysts based on LaAlO3 perovskite for the oxidative coupling of methane. Chem. Eng. 2021, 5, 14. [Google Scholar] [CrossRef]
- Alrobei, H.; Prashanth, M.K.; Manjunatha, C.R.; Kumar, C.B.P.; Chitrabanu, C.P.; Shivaramu, P.D.; Kumar, K.Y.; Raghu, M.S. Adsorption of anionic dye on eco-friendly synthesised reduced graphene oxide anchored with lanthanum aluminate: Isotherms, kinetics and statistical error analysis. Ceram. Int. 2021, 47, 10322–10331. [Google Scholar] [CrossRef]
- Garcia, A.B.S.; Bispo-Jr, A.G.; Lima, S.A.M.; Pires, A.M. Effects of the Pechini’s modified synthetic route on structural and photophysical properties of Eu3+ or Tb3+-doped LaAlO3. Mater. Res. Bull. 2021, 143, 111462. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, B. Solution combustion synthesis, photoluminescence and X-ray luminescence of Eu3+-doped LaAlO3 nanophosphors. Ceram. Int. 2016, 42, 2357–2363. [Google Scholar] [CrossRef]
- Ianoş, R.; Lazǎu, R.; Borcǎnescu, S.; Bǎbuèǎ, R. Single-step combustion synthesis of LaAlO3 powders and their sintering behavior. Ceram. Int. 2014, 40, 7561–7565. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, K.; Yang, Y.; Shi, X.; Yang, L.; Yang, W.; Wang, Y.; Chen, Z.G. Ce/Mn dual-doped LaAlO3 ceramics with enhanced far-infrared emission capability synthesized via a facile microwave sintering method. J. Alloys Compd. 2019, 774, 434–442. [Google Scholar] [CrossRef]
- Sagar, T.V.; Padmakar, D.; Lingaiah, N.; Rama rao, K.S.; Reddy, I.A.K.; Sai Prasad, P.S. Syngas production by CO2 reforming of methane on LaNixAl1−xO3 perovskite catalysts: Influence of method of preparation. J. Chem. Sci. 2017, 129, 1787–1794. [Google Scholar] [CrossRef]
- Ankoji, P.; Hemalatha Rudramadevi, B. Tunable white light emission from Dy3+/Eu3+ doped LaAlO3 nanophosphors via hydrothermal method. Mater. Sci. Eng. B 2021, 263, 114883. [Google Scholar] [CrossRef]
- Vinuth Raj, T.N.; Hoskeri, P.A.; Muralidhara, H.B.; Manjunatha, C.R.; Yogesh Kumar, K.; Raghu, M.S. Facile synthesis of perovskite lanthanum aluminate and its green reduced graphene oxide composite for high performance supercapacitors. J. Electroanal. Chem. 2020, 858, 113830. [Google Scholar] [CrossRef]
- Kreisberg, V.A.; Ivakin, Y.D.; Danchevskaya, M.N. Mechanism of water and gas release during heat treatment of europium-doped lanthanum aluminate crystals. Ceram. Int. 2021, 47, 18838–18847. [Google Scholar] [CrossRef]
- Lee, S.H.; Du, P.; Bharat, L.K.; Yu, J.S. Ultraviolet radiation excited strong red-emitting LaAlO3:Eu3+ nanophosphors: Synthesis and luminescent properties. Ceram. Int. 2017, 43, 4599–4605. [Google Scholar] [CrossRef]
- Bělina, P.; Sádovská, G.; Krejčíková, V.; Dohnalová, Ž.; Šulcová, P. Preparation of LaNiO3 perovskite by oxalate and carbonate precursor method for utilization as catalyst for high-temperature decomposition of N2O. J. Therm. Anal. Calorim. 2019, 138, 4197–4202. [Google Scholar] [CrossRef]
- Toprak, M.S.; Darab, M.; Syvertsen, G.E.; Muhammed, M. Synthesis of nanostructured BSCF by oxalate co-precipitation--As potential cathode material for solid oxide fuels cells. Int. J. Hydrogen Energy 2010, 35, 9448–9454. [Google Scholar] [CrossRef]
- Varandili, S.B.; Babaei, A.; Ataie, A. Characterization of B site codoped LaFeO3 nanoparticles prepared via co-precipitation route. Rare Met. 2018, 37, 181–190. [Google Scholar] [CrossRef]
- Zhang, H.M.; Shimizu, Y.; Teraoka, Y.; Miura, N.; Yamazoe, N. Oxygen sorption and catalytic properties of La1−xSrxCo1−yFeyO3 perovskite-type oxides. J. Catal. 1990, 121, 432–440. [Google Scholar] [CrossRef]
- Asamoto, M.; Iwasaki, Y.; Yamaguchi, S.; Yahiro, H. Synthesis of perovsite-type oxide catalysts, Ln(Fe, Co)O3 (Ln = La, Pr, Sm, Gd, Dy, Ho, Er, and Yb), from the thermal decomposition of the corresponding cyano complexes. Catal. Today 2012, 185, 230–235. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, D.; Wada, H.; Yamaguchi, S.; Farjas, J.; Yahiro, H. Synthesis of LaFeO3 perovskite-type oxide via solid-state combustion of a cyano complex precursor: The effect of oxygen diffusion. Ceram. Int. 2017, 43, 3156–3165. [Google Scholar] [CrossRef] [Green Version]
- Andrei, F.; Zavoianu, R.; Marcu, I.C. Complex catalytic materials based on the perovskite-type structure for energy and environmental applications. Materials 2020, 13, 5555. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wu, Z. Magnetic and optical properties of multiferroic bismuth ferrite nanoparticles by tartaric acid-assisted sol-gel strategy. Mater. Lett. 2010, 64, 486–488. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Y. Synthesis of nanocrystalline LaFeO3 powders via glucose sol-gel route. Mater. Chem. Phys. 2011, 129, 1047–1050. [Google Scholar] [CrossRef]
- Ghosh, S.; Dasgupta, S.; Sen, A.; Maiti, H.S. Low temperature synthesis of bismuth ferrite nanoparticles by a ferrioxalate precursor method. Mater. Res. Bull. 2005, 40, 2073–2079. [Google Scholar] [CrossRef]
- Sunde, T.O.L.; Grande, T.; Einarsrud, M.A. Modified pechini synthesis of oxide powders and thin films. In Handbook of Sol-Gel Science and Technology; Springer: Cham, Switzerland, 2016; Volume 373, pp. 1–30. [Google Scholar] [CrossRef] [Green Version]
- Shu, J.; Kaliaguine, S. Well-dispersed perovskite-type oxidation catalysts. Appl. Catal. B Environ. 1998, 16, 303. [Google Scholar] [CrossRef]
- Rida, K.; Benabbas, A.; Bouremmad, F.; Peña, M.A.; Sastre, E.; Martínez-Arias, A. Effect of calcination temperature on the structural characteristics and catalytic activity for propene combustion of sol-gel derived lanthanum chromite perovskite. Appl. Catal. A Gen. 2007, 327, 173–179. [Google Scholar] [CrossRef]
- Rivera-Montalvo, T.; Morales-Hernandez, A.; Barrera-Angeles, A.A.; Alvarez-Romero, R.; Falcony, C.; Zarate-Medina, J. Modified Pechini’s method to prepare LaAlO3:RE thermoluminescent materials. Radiat. Phys. Chem. 2017, 140, 68–73. [Google Scholar] [CrossRef]
- Ianoş, R.; Lazău, R.; Băbuță, R.; Muntean, E.; Moacă, E.A.; Păcurariu, C. Solution combustion synthesis: A straightforward route for the preparation of chromium-doped lanthanum aluminate, LaAl1−xCrxO3, pink red pigments. Dyes Pigments 2018, 155, 218–224. [Google Scholar] [CrossRef]
- Kasala, S.; Sudandara Doss, M.V. Microwave Assisted Synthesis and Powder Flowability Characteristics of Rare-Earth Aluminate (ReAlO3, Re = La, Gd, Nd, Y) Powders. Trans. Indian Ceram. Soc. 2019, 78, 13–19. [Google Scholar] [CrossRef]
- Prado-Gonjal, J.; Arévalo-López, Á.M.; Morán, E. Microwave-assisted synthesis: A fast and efficient route to produce LaMO3 (M = Al, Cr, Mn, Fe, Co) perovskite materials. Mater. Res. Bull. 2011, 46, 222–230. [Google Scholar] [CrossRef]
- Shandilya, M.; Kaur, G.A. Low temperature crystal growth of lead-free complex perovskite nano-structure by using sol-gel hydrothermal process. J. Solid State Chem. 2019, 280, 120988. [Google Scholar] [CrossRef]
- Teh, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent progress in ceria-based catalysts for the dry reforming of methane: A review. Chem. Eng. Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Ou, Z.; Zhang, Z.; Qin, C.; Xia, H.; Deng, T.; Niu, J.; Ran, J.; Wu, C. Highly active and stable Ni/perovskite catalysts in steam methane reforming for hydrogen production. Sustain. Energy Fuels 2021, 5, 1845–1856. [Google Scholar] [CrossRef]
- Arandiyan, H.; Li, J. Catalytic CO2 reforming of methane over perovskite noble metals. Adv. Mater. Res. 2012, 356–360, 1070–1074. [Google Scholar] [CrossRef]
- Urasaki, K.; Sekine, Y.; Kawabe, S.; Kikuchi, E.; Matsukata, M. Catalytic activities and coking resistance of Ni/perovskites in steam reforming of methane. Appl. Catal. A Gen. 2005, 286, 23–29. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Zhang, R.; Liu, T.; Xia, L.; Chen, Z.; Fang, X.; Xu, X.; Xu, J.; Wang, X. Ni/LaBO3 (B = Al, Cr, Fe) Catalysts for Steam Reforming of Methane (SRM): On the Interaction Between Ni and LaBO3 Perovskites with Differed Fine Structures. Catal. Surv. Asia 2021, 25, 424–436. [Google Scholar] [CrossRef]
- Mukai, D.; Izutsu, Y.; Sekine, Y. Highly and stably dispersed Pt catalysts supported over La1−xSrxAlO3−0.5x perovskite for oxidative methane activation and their structures. Appl. Catal. A Gen. 2013, 458, 71–81. [Google Scholar] [CrossRef]
- Higo, T.; Saito, H.; Ogo, S.; Sugiura, Y.; Sekine, Y. Promotive effect of Ba addition on the catalytic performance of Ni/LaAlO3 catalysts for steam reforming of toluene. Appl. Catal. A Gen. 2017, 530, 125–131. [Google Scholar] [CrossRef]
- Oemar, U.; Ang, M.L.; Chin, Y.C.; Hidajat, K.; Kawi, S. Role of lattice oxygen in oxidative steam reforming of toluene as a tar model compound over Ni/La0.8Sr0.2AlO3 catalyst. Catal. Sci. Technol. 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Mukai, D.; Tochiya, S.; Murai, Y.; Imori, M.; Hashimoto, T.; Sugiura, Y.; Sekine, Y. Role of support lattice oxygen on steam reforming of toluene for hydrogen production over Ni/La0.7Sr0.3AlO3−δ catalyst. Appl. Catal. A Gen. 2013, 453, 60–70. [Google Scholar] [CrossRef]
- Mukai, D.; Tochiya, S.; Murai, Y.; Imori, M.; Sugiura, Y.; Sekine, Y. Structure and activity of Ni/La0.7Sr0.3AlO3−δ catalyst for hydrogen production by steam reforming of toluene. Appl. Catal. A Gen. 2013, 464–465, 78–86. [Google Scholar] [CrossRef]
- Mukai, D.; Murai, Y.; Higo, T.; Ogo, S.; Sugiura, Y.; Sekine, Y. Effect of Pt addition to Ni/La0.7Sr0.3AlO3−δ catalyst on steam reforming of toluene for hydrogen production. Appl. Catal. A Gen. 2014, 471, 157–164. [Google Scholar] [CrossRef]
- Kim, S.H.; Go, Y.J.; Park, N.C.; Kim, J.H.; Kim, Y.C.; Moon, D.J. Steam reforming of glycerol over nano size Ni-Ce/LaAlO3 catalysts. J. Nanosci. Nanotechnol. 2015, 15, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, J.Y.; Moon, D.J.; Park, N.C.; Kim, Y.C. Effect of LaAlO3-supported modified Ni-based catalysts on aqueous phase reforming of glycerol. Res. Chem. Intermed. 2015, 41, 9603–9614. [Google Scholar] [CrossRef]
- Lee, H.J.; Shin, G.S.; Kim, Y.C. Characterization of supported Ni catalysts for aqueous-phase reforming of glycerol. Korean J. Chem. Eng. 2015, 32, 1267–1272. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.H.; Moon, D.J.; Kim, J.H.; Park, N.C.; Kim, Y.C. Aqueous phase reforming of glycerol over nanosize Cu-Ni catalysts. J. Nanosci. Nanotechnol. 2013, 13, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Ochibe, S.; Wachi, H.; Hirai, S.; Arai, T.; Sakamoto, N.; Suzuki, H.; Matsuda, T. Preparation of metal catalyst component doped perovskite catalyst particle for steam reforming process by chemical solution deposition with partial reduction. Adv. Powder Technol. 2018, 29, 584–589. [Google Scholar] [CrossRef]
- Ortiz-Bravo, C.A.; Chagas, C.A.; Toniolo, F.S. Oxidative coupling of methane (OCM): An overview of the challenges and opportunities for developing new technologies. J. Nat. Gas Sci. Eng. 2021, 96, 104254. [Google Scholar] [CrossRef]
- Murthy, P.R.; Liu, Y.; Wu, G.; Diao, Y.; Shi, C. Oxidative coupling of methane: Perspective for high-value C2 chemicals. Crystals 2021, 11, 1011. [Google Scholar] [CrossRef]
- Arinaga, A.M.; Ziegelski, M.C.; Marks, T.J. Alternative Oxidants for the Catalytic Oxidative Coupling of Methane. Angew. Chem.-Int. Ed. 2021, 60, 10502–10515. [Google Scholar] [CrossRef]
- Kim, I.; Lee, G.; Na, H.B.; Ha, J.M.; Jung, J.C. Selective oxygen species for the oxidative coupling of methane. Mol. Catal. 2017, 435, 13–23. [Google Scholar] [CrossRef]
- Kwon, D.; Yang, I.; Sim, Y.; Ha, J.M.; Jung, J.C. A K2NiF4-type La2Li0.5Al0.5O4 catalyst for the oxidative coupling of methane (OCM). Catal. Commun. 2019, 128, 105702. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Wei, Y.; Liu, J.; Zhao, Z. Research advances of rare earth catalysts for catalytic purification of vehicle exhausts−Commemorating the 100th anniversary of the birth of Academician Guangxian Xu. J. Rare Earths 2021, 39, 1151–1180. [Google Scholar] [CrossRef]
- Higo, T.; Ueno, K.; Omori, Y.; Tsuchiya, H.; Ogo, S.; Hirose, S.; Mikami, H.; Sekine, Y. Perovskite lattice oxygen contributes to low-temperature catalysis for exhaust gas cleaning. RSC Adv. 2019, 9, 22721–22728. [Google Scholar] [CrossRef] [Green Version]









| Dodecahedral A-Site | Octahedral B-Site | ||||
|---|---|---|---|---|---|
| Ion | Radius(Å)a | Radius(Å)b | Ion | Radius(Å)a | Radius(Å)b |
| Na+ | 1.06 | 1.32(IX) | Li+ | 0.68 | 0.74 |
| K+ | 1.45 | 1.60 | Cu2+ | 0.72 | 0.73 |
| Rb+ | 1.61 | 1.73 | Mg2+ | 0.66 | 0.72 |
| Ag+ | 1.40 | 1.30(VIII) | Zn2+ | 0.74 | 0.75 |
| Ca2+ | 1.08 | 1.35 | Ti3+ | 0.76 | 0.67 |
| Sr2+ | 1.23 | 1.44 | V3+ | 0.74 | 0.64 |
| Ba2+ | 1.46 | 1.60 | Cr3+ | 0.70 | 0.62 |
| Pb2+ | 1.29 | 1.49 | Mn3+ | 0.66 | 0.65 |
| La3+ | 1.22 | 1.32 | Fe3+ | 0.64 | 0.64 |
| Pr3+ | 1.10 | 1.14(VIII) | Co3+(LS) | - | 0.52 |
| Nd2+ | 1.09 | 1.12(VIII) | Co3+(HS) | 0.63 | 0.61 |
| Bi3+ | 1.07 | 1.11(VIII) | Ni3+(LS) | - | 0.56 |
| Ce4+ | 1.02 | 0.97(VIII) | Ni3+(HS) | 0.62 | 0.60 |
| Th4+ | 1.09 | 1.04(VIII) | Rh3+ | 0.68 | 0.66 |
| Ti4+ | 0.68 | 0.60 | |||
| Mn4+ | 0.56 | 0.54 | |||
| Ru4+ | 0.67 | 0.62 | |||
| Pt4+ | 0.65 | 0.63 | |||
| Nb5+ | 0.69 | 0.64 | |||
| Ta5+ | 0.69 | 0.64 | |||
| Mo6+ | 0.62 | 0.60 | |||
| W6+ | 0.62 | 0.60 | |||
| Synthesis Method | Surface Area (m2/g) | Particle Size and Extent of Agglomeration | Purity | Temperature of Crystallization (°C) | Advantages | Limitations |
|---|---|---|---|---|---|---|
| Solid-state reaction | <2.5 | >1000 nm with moderate agglomeration | Very low | 1100–1400 | Cost effective, conventional, simplest, and operational simplicity. | Gives broad particle distribution as well as secondary phase formation. |
| Co-precipitation | 5.5–20 | >10 nm with high agglomeration | High | 800 | Control of size and shape of perovskites, simple and environmental friendly. | Lacks overall optimization, which could be attributed to the required controls during the washing step. Deficiency of metal cations. |
| Sol–gel and Pechini process | 5–20 | >10 nm with moderate agglomeration | Excellent | 800–1000 | High homogeneity and purity Accurate control of the composition of the final product | High temperature and long periods of time. |
| Combustion | >10 nm with low agglomeration | High | 600–800 | Highly pure, homogeneity and crystallinity | Production of large amount carbon in end product. | |
| Microwave assisted method | 1–36 | >100 nm with low agglomeration | Excellent | 600–800 | Highly pure and avoiding particle coarsening. Time and energy saving. | Hard for scale-up and expensive equipment |
| Hydrothermal and solvothermal routes | ~50 | >100 nm with low agglomeration | Very high | No calcination | Can easily control morphology particle size, and crystallinity | Require high pressures (up to 15 MPa) inside autoclave |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, H.J.; Korili, S.A.; Gil, A. Progress and Recent Strategies in the Synthesis and Catalytic Applications of Perovskites Based on Lanthanum and Aluminum. Materials 2022, 15, 3288. https://doi.org/10.3390/ma15093288
Muñoz HJ, Korili SA, Gil A. Progress and Recent Strategies in the Synthesis and Catalytic Applications of Perovskites Based on Lanthanum and Aluminum. Materials. 2022; 15(9):3288. https://doi.org/10.3390/ma15093288
Chicago/Turabian StyleMuñoz, Helir Joseph, Sophia A. Korili, and Antonio Gil. 2022. "Progress and Recent Strategies in the Synthesis and Catalytic Applications of Perovskites Based on Lanthanum and Aluminum" Materials 15, no. 9: 3288. https://doi.org/10.3390/ma15093288
APA StyleMuñoz, H. J., Korili, S. A., & Gil, A. (2022). Progress and Recent Strategies in the Synthesis and Catalytic Applications of Perovskites Based on Lanthanum and Aluminum. Materials, 15(9), 3288. https://doi.org/10.3390/ma15093288







