Influence of Solvent-Dependent Morphology on Molecular Doping and Charge Transport in Conductive Thiophene Polymer
Abstract
:1. Introduction
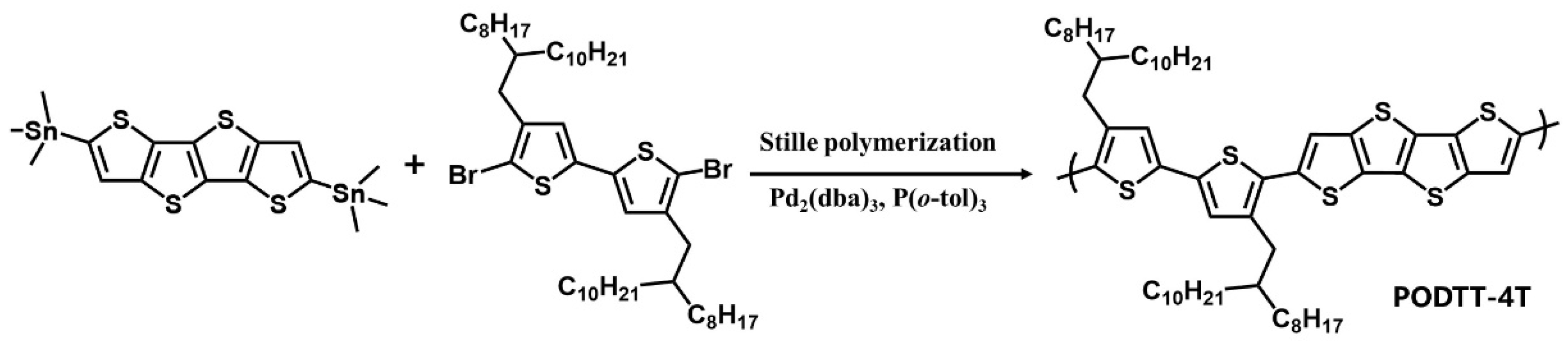
2. Synthesis and Characterization of Polymer
3. Results
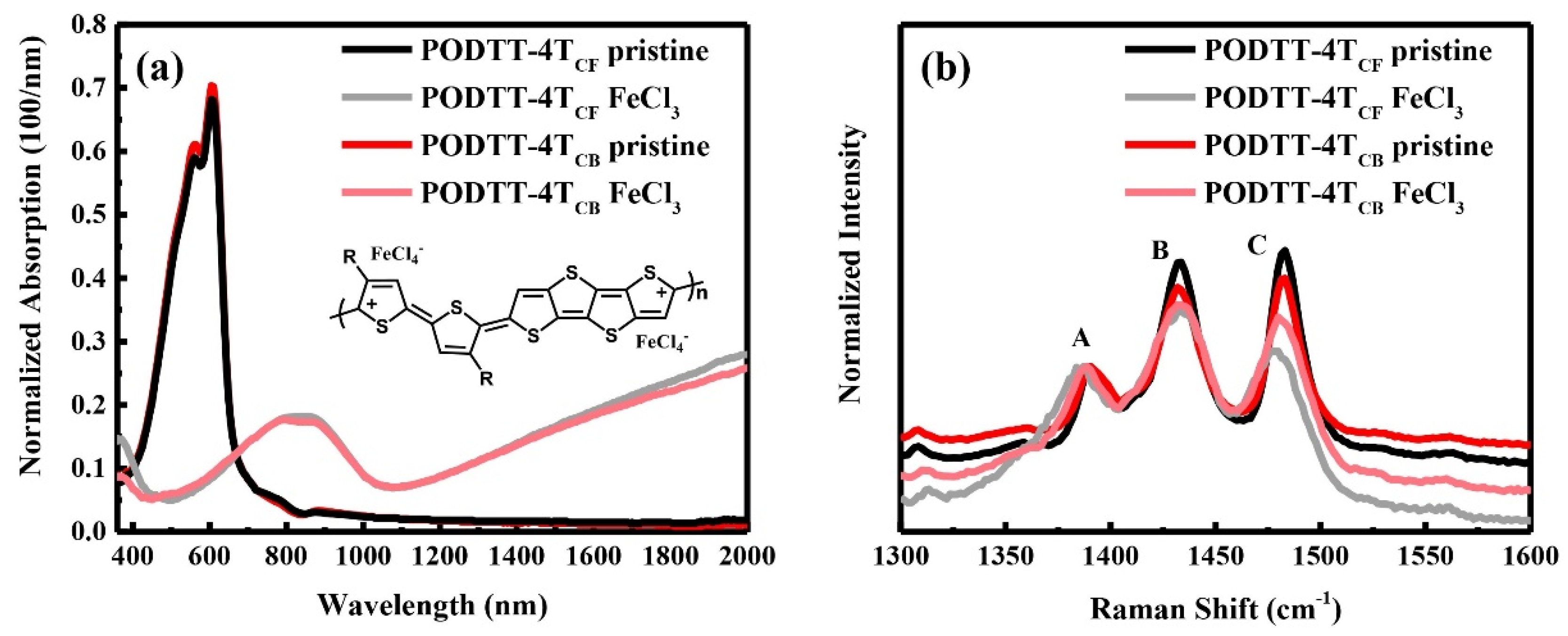
3.1. The Doping Level of Films Prepared from Two Solvents
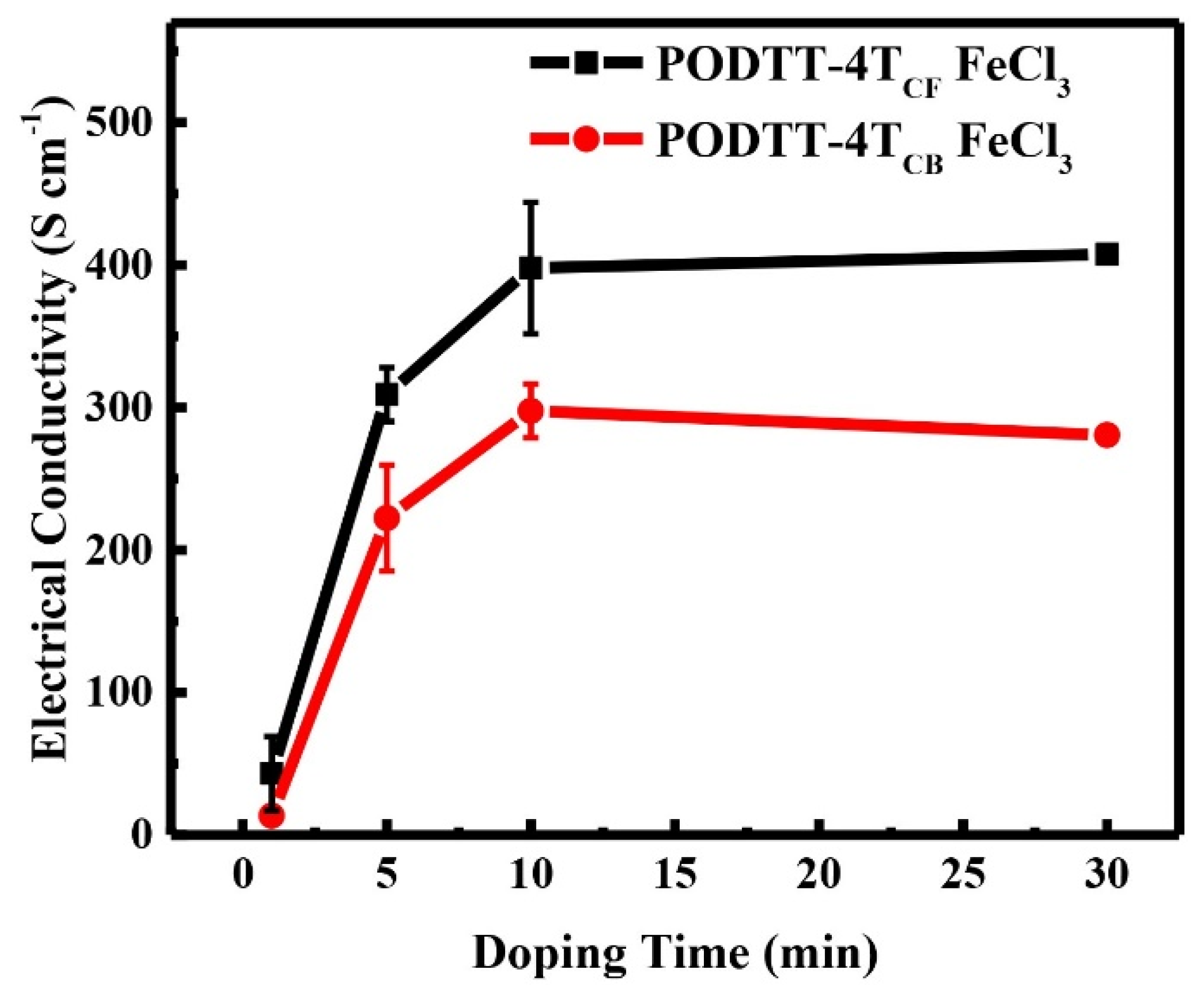
3.2. The Electrical Conductivity of Doped Films
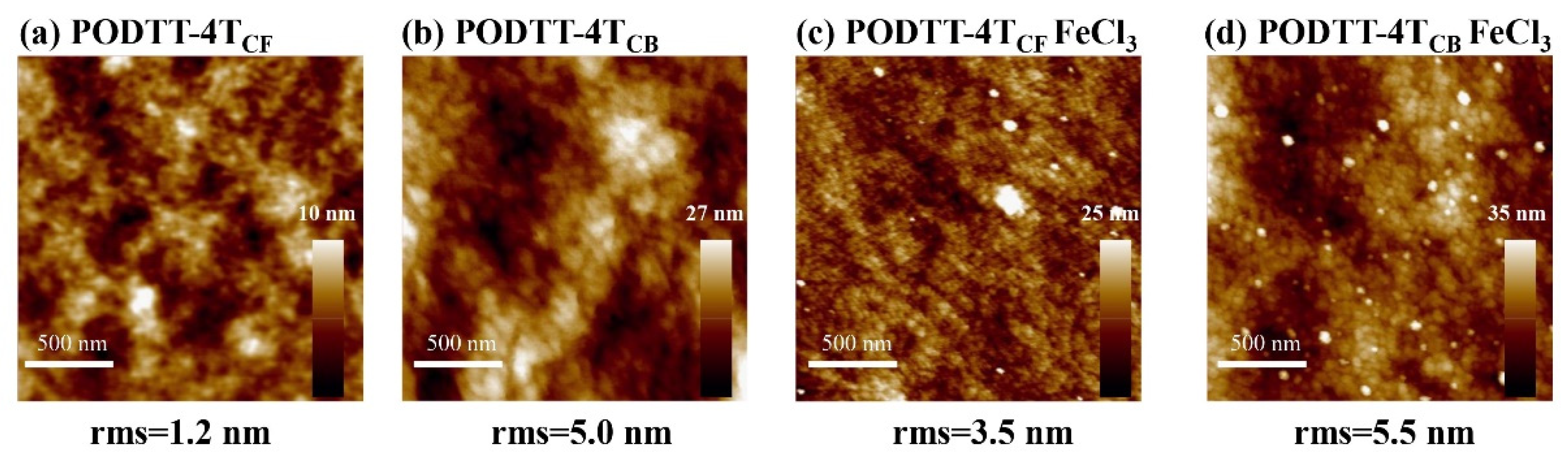
3.3. Microstructural Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heeger, A.J. Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials (Nobel Lecture). Angew. Chem. Int. Ed. 2001, 40, 2591–2611. [Google Scholar] [CrossRef]
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 16050. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Shi, X.L.; Shi, X.; Chen, L.; Dargusch, M.S.; Zou, J.; Chen, Z.G. Flexible Thermoelectric Materials and Generators: Challenges and Innovations. Adv. Mater. 2019, 31, e1807916. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. Organic thermoelectric materials: Emerging green energy materials converting heat to electricity directly and efficiently. Adv. Mater. 2014, 26, 6829–6851. [Google Scholar] [CrossRef]
- He, J.; Tritt, T.M. Advances in thermoelectric materials research: Looking back and moving forward. Science 2017, 357, 1369. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Ma, W. Molecular Doping Efficiency in Organic Semiconductors: Fundamental Principle and Promotion Strategy. Adv. Funct. Mater. 2021, 32, 2111351. [Google Scholar] [CrossRef]
- Zhao, W.; Ding, J.; Zou, Y.; Di, C.A.; Zhu, D. Chemical doping of organic semiconductors for thermoelectric applications. Chem. Soc. Rev. 2020, 49, 7210–7228. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tsurumi, J.; Ohno, M.; Fujimoto, R.; Kumagai, S.; Kurosawa, T.; Okamoto, T.; Takeya, J.; Watanabe, S. Efficient molecular doping of polymeric semiconductors driven by anion exchange. Nature 2019, 572, 634–638. [Google Scholar] [CrossRef]
- Deng, L.; Chen, G. Recent progress in tuning polymer oriented microstructures for enhanced thermoelectric performance. Nano Energy 2021, 80, 105448. [Google Scholar] [CrossRef]
- Patel, S.N.; Glaudell, A.M.; Peterson, K.A.; Thomas, E.M.; O’Hara, K.A.; Lim, E.; Chabinyc, M.L. Morphology controls the thermoelectric power factor of a doped semiconducting polymer. Sci. Adv. 2017, 3, e1700434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hynynen, J.; Kiefer, D.; Müller, C. Influence of crystallinity on the thermoelectric power factor of P3HT vapour-doped with F4TCNQ. RSC Adv. 2018, 8, 1593–1599. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Li, H.; Chai, H.; Xu, Q.; Chen, Y.; Chen, L. Anion-Dependent Molecular Doping and Charge Transport in Ferric Salt-Doped P3HT for Thermoelectric Application. ACS Appl. Electron. Mater. 2021, 3, 1252–1259. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. What To Expect from Conducting Polymers on the Playground of Thermoelectricity: Lessons Learned from Four High-Mobility Polymeric Semiconductors. Macromolecules 2014, 47, 609–615. [Google Scholar] [CrossRef]
- Jacobs, I.E.; Aasen, E.W.; Oliveira, J.L.; Fonseca, T.N.; Roehling, J.D.; Li, J.; Zhang, G.; Augustine, M.P.; Mascal, M.; Moulé, A.J. Comparison of solution-mixed and sequentially processed P3HT:F4TCNQ films: Effect of doping-induced aggregation on film morphology. J. Mater. Chem. C 2016, 4, 3454–3466. [Google Scholar] [CrossRef] [Green Version]
- Scholes, D.T.; Yee, P.Y.; McKeown, G.R.; Li, S.; Kang, H.; Lindemuth, J.R.; Xia, X.; King, S.C.; Seferos, D.S.; Tolbert, S.H.; et al. Designing Conjugated Polymers for Molecular Doping: The Roles of Crystallinity, Swelling, and Conductivity in Sequentially-Doped Selenophene-Based Copolymers. Chem. Mater. 2018, 31, 73–82. [Google Scholar] [CrossRef]
- Nam, G.-H.; Sun, C.; Chung, D.S.; Kim, Y.-H. Enhancing Doping Efficiency of Diketopyrrolopyrrole-Copolymers by Introducing Sparse Intramolecular Alkyl Chain Spacing. Macromolecules 2021, 54, 7870–7879. [Google Scholar] [CrossRef]
- Liu, J.; Ye, G.; Potgieser, H.G.O.; Koopmans, M.; Sami, S.; Nugraha, M.I.; Villalva, D.R.; Sun, H.; Dong, J.; Yang, X.; et al. Amphipathic Side Chain of a Conjugated Polymer Optimizes Dopant Location toward Efficient N-Type Organic Thermoelectrics. Adv. Mater. 2021, 33, e2006694. [Google Scholar] [CrossRef]
- Li, H.; DeCoster, M.E.; Ming, C.; Wang, M.; Chen, Y.; Hopkins, P.E.; Chen, L.; Katz, H.E. Enhanced Molecular Doping for High Conductivity in Polymers with Volume Freed for Dopants. Macromolecules 2019, 52, 9804–9812. [Google Scholar] [CrossRef]
- Kroon, R.; Kiefer, D.; Stegerer, D.; Yu, L.; Sommer, M.; Muller, C. Polar Side Chains Enhance Processability, Electrical Conductivity, and Thermal Stability of a Molecularly p-Doped Polythiophene. Adv. Mater. 2017, 29, 1700930. [Google Scholar] [CrossRef]
- Kiefer, D.; Kroon, R.; Hofmann, A.I.; Sun, H.; Liu, X.; Giovannitti, A.; Stegerer, D.; Cano, A.; Hynynen, J.; Yu, L.; et al. Double doping of conjugated polymers with monomer molecular dopants. Nat. Mater. 2019, 18, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, T.K.; Kang, I.; Yun, H.J.; Cha, H.; Hwang, J.; Park, S.; Kim, J.; Kim, Y.J.; Chung, D.S.; Kwon, S.K.; et al. Solvent Additive to Achieve Highly Ordered Nanostructural Semicrystalline DPP Copolymers: Toward a High Charge Carrier Mobility. Adv. Mater. 2013, 25, 7003–7009. [Google Scholar] [CrossRef] [PubMed]
- Di, B.C.; Lu, K.; Zhang, L.; Liu, Y.; Guo, Y.; Sun, X.; Wen, Y.; Yu, G.; Zhu, D. Solvent-Assisted Re-annealing of Polymer Films for Solution-Processable Organic Field-Effect Transistors. Adv. Mater. 2010, 22, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, J.; Xiao, J.; Wu, L.; Katz, H.E.; Chen, L. Synergistically Improved Molecular Doping and Carrier Mobility by Copolymerization of Donor–Acceptor and Donor–Donor Building Blocks for Thermoelectric Application. Adv. Funct. Mater. 2020, 30, 2004378. [Google Scholar] [CrossRef]
- Liu, Q.; Kumagai, S.; Manzhos, S.; Chen, Y.; Angunawela, I.; Nahid, M.M.; Feron, K.; Bottle, S.E.; Bell, J.; Ade, H.; et al. Synergistic Use of Pyridine and Selenophene in a Diketopyrrolopyrrole-Based Conjugated Polymer Enhances the Electron Mobility in Organic Transistors. Adv. Funct. Mater. 2020, 30, 2000489. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Z.; Zhao, W.; Jin, W.; Xiang, L.; Wang, Z.; Zeng, Y.; Zou, Y.; Zhang, F.; Yi, Y.; et al. Selenium-Substituted Diketopyrrolopyrrole Polymer for High-Performance p-Type Organic Thermoelectric Materials. Angew. Chem. Int. Ed. 2019, 58, 18994–18999. [Google Scholar] [CrossRef]
- Müller, L.; Nanova, D.; Glaser, T.; Beck, S.; Pucci, A.; Kast, A.K.; Schröder, R.R.; Mankel, E.; Pingel, P.; Neher, D.; et al. Charge-Transfer–Solvent Interaction Predefines Doping Efficiency in p-Doped P3HT Films. Chem. Mater. 2016, 28, 4432–4439. [Google Scholar] [CrossRef]
- Francis, C.; Fazzi, D.; Grimm, S.B.; Paulus, F.; Beck, S.; Hillebrandt, S.; Pucci, A.; Zaumseil, J. Raman spectroscopy and microscopy of electrochemically and chemically doped high-mobility semiconducting polymers. J. Mater. Chem. C 2017, 5, 6176–6184. [Google Scholar] [CrossRef]
- Paternò, G.M.; Robbiano, V.; Fraser, K.J.; Frost, C.; García Sakai, V.; Cacialli, F. Neutron Radiation Tolerance of Two Benchmark Thiophene-Based Conjugated Polymers: The Importance of Crystallinity for Organic Avionics. Sci. Rep. 2017, 7, 41013. [Google Scholar] [CrossRef]
- Li, Y.; Singh, S.P.; Sonar, P. A high mobility P-type DPP-thieno[3,2-b]thiophene copolymer for organic thin-film transistors. Adv. Mater. 2010, 22, 4862–4866. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Song, J.; Chai, H.; Wu, L.; Chen, L. Single-Solution Doping Enabling Dominant Integer Charge Transfer for Synergistically Improved Carrier Concentration and Mobility in Donor–Acceptor Polymers. Adv. Funct. Mater. 2021, 32, 2110047. [Google Scholar] [CrossRef]
- Rivnay, J.; Mannsfeld, S.C.; Miller, C.E.; Salleo, A.; Toney, M.F. Quantitative determination of organic semiconductor microstructure from the molecular to device scale. Chem. Rev. 2012, 112, 5488–5519. [Google Scholar] [CrossRef] [PubMed]
- Salleo, A.; Kline, R.J.; DeLongchamp, D.M.; Chabinyc, M.L. Microstructural characterization and charge transport in thin films of conjugated polymers. Adv. Mater. 2010, 22, 3812–3838. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Diest, K.; Wong, M.H.; Hsieh, B.R.; Dunlap, D.H.; Malliaras, G.G. Charge transport in doped organic semiconductors. Phys. Rev. B 2003, 68, 081204. [Google Scholar] [CrossRef] [Green Version]
- Un, H.; Gregory, S.A.; Mohapatra, S.K.; Xiong, M.; Longhi, E.; Lu, Y.; Rigin, S.; Jhulki, S.; Yang, C.; Timofeeva, T.V.; et al. Understanding the Effects of Molecular Dopant on n-Type Organic Thermoelectric Properties. Adv. Energy Mater. 2019, 9, 1900817. [Google Scholar] [CrossRef]
- Gregory, S.A.; Menon, A.K.; Ye, S.; Seferos, D.S.; Reynolds, J.R.; Yee, S.K. Effect of Heteroatom and Doping on the Thermoelectric Properties of Poly(3-alkylchalcogenophenes). Adv. Energy Mater. 2018, 8, 1802419. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, H.; Li, H.; Zhong, F.; Xu, Z.; Bai, S.; Chen, L. Influence of Solvent-Dependent Morphology on Molecular Doping and Charge Transport in Conductive Thiophene Polymer. Materials 2022, 15, 3293. https://doi.org/10.3390/ma15093293
Chai H, Li H, Zhong F, Xu Z, Bai S, Chen L. Influence of Solvent-Dependent Morphology on Molecular Doping and Charge Transport in Conductive Thiophene Polymer. Materials. 2022; 15(9):3293. https://doi.org/10.3390/ma15093293
Chicago/Turabian StyleChai, Haoyu, Hui Li, Fei Zhong, Zhen Xu, Shengqiang Bai, and Lidong Chen. 2022. "Influence of Solvent-Dependent Morphology on Molecular Doping and Charge Transport in Conductive Thiophene Polymer" Materials 15, no. 9: 3293. https://doi.org/10.3390/ma15093293






