Sulfate Freeze–Thaw Resistance of Magnesium Potassium Phosphate Cement Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of the Mortar
2.3. Experimental Method
3. Results and Discussion
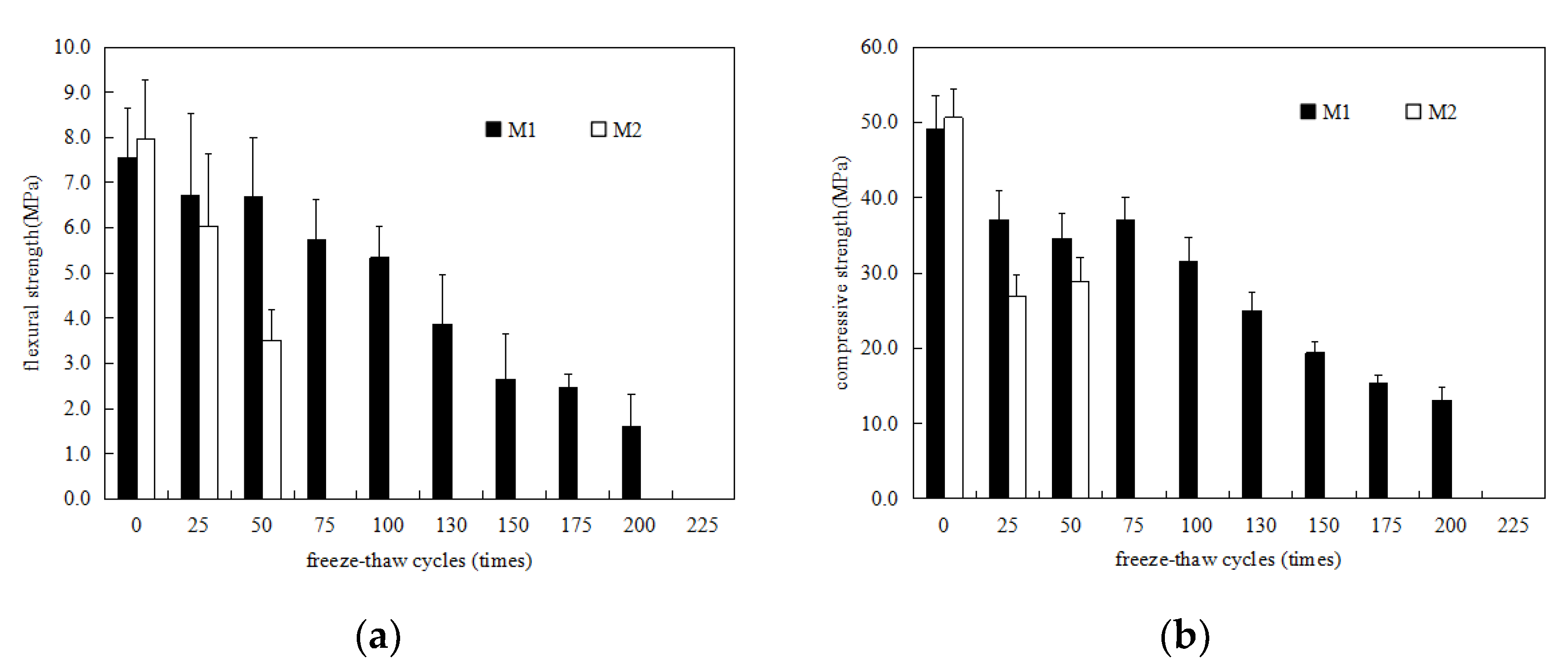
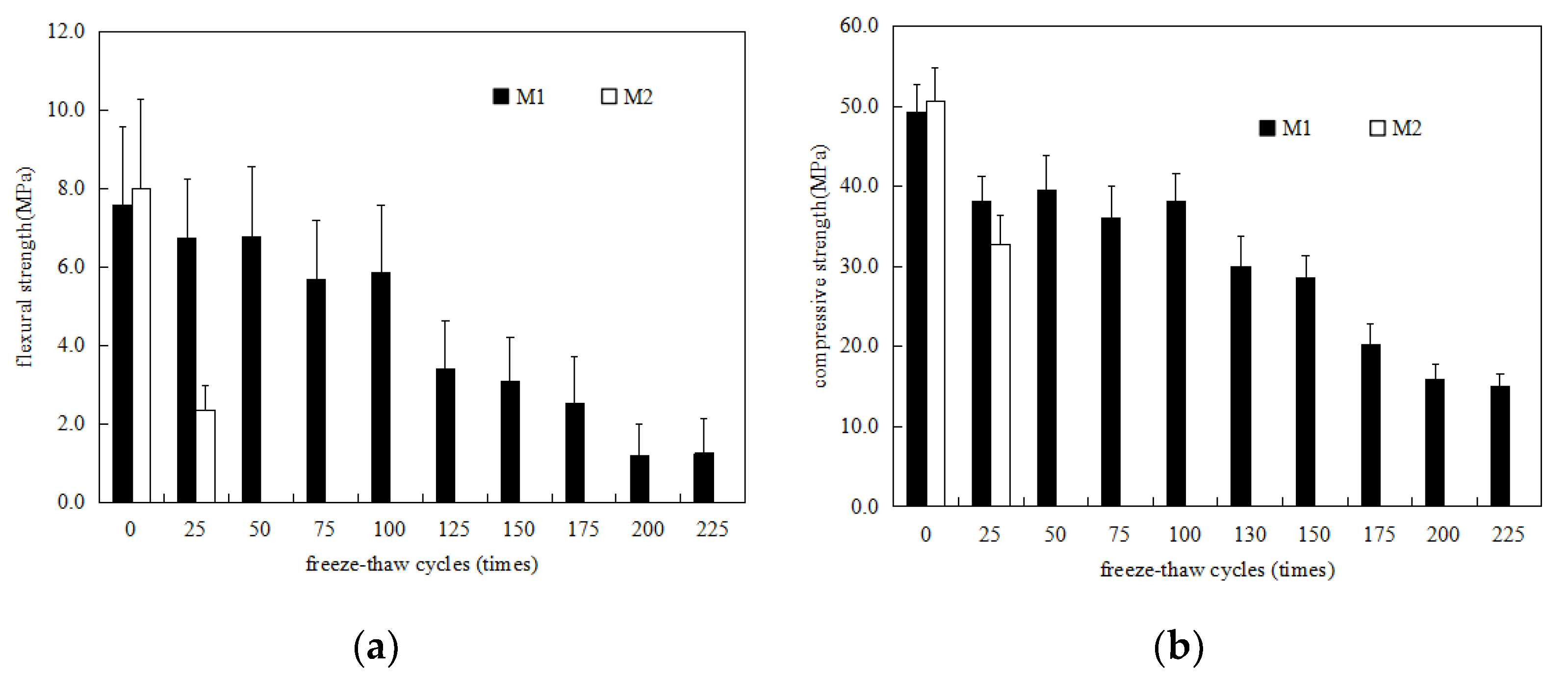
3.1. Strength Development
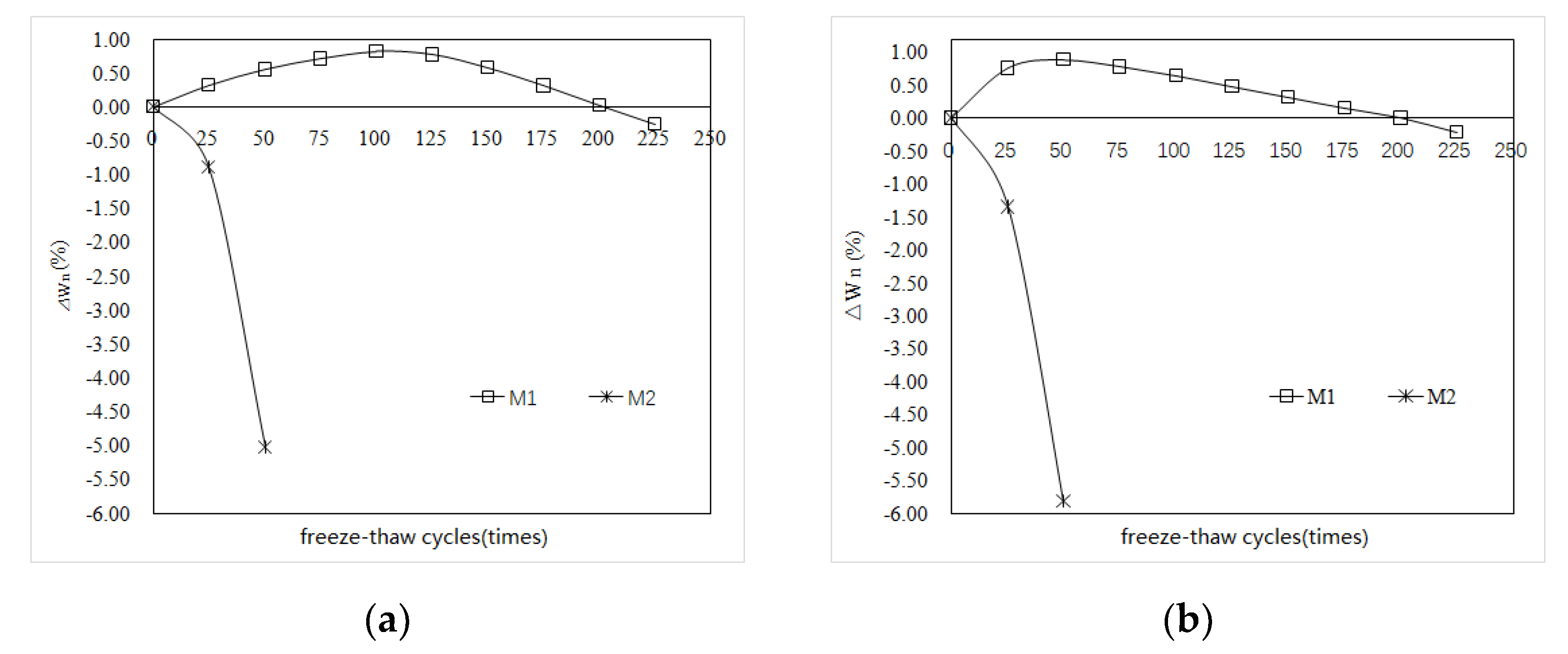
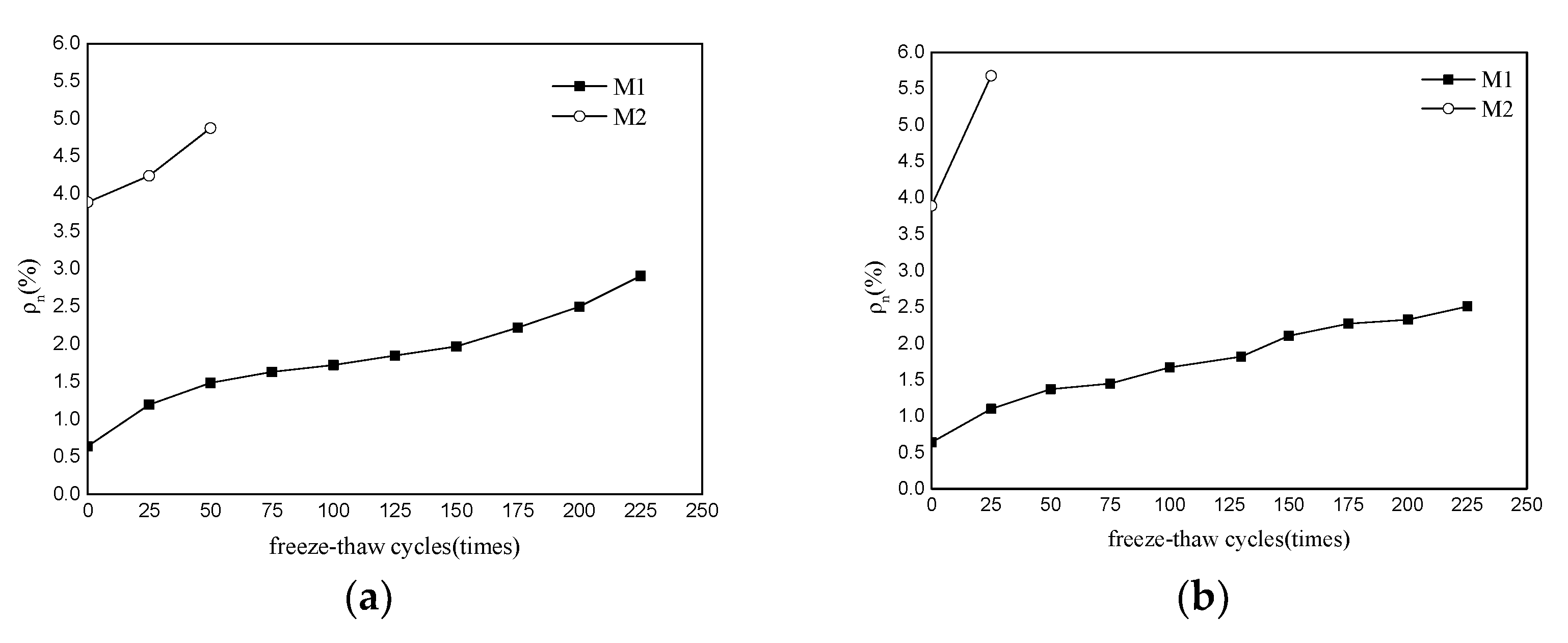
3.2. Weight Loss
3.3. Water Absorption
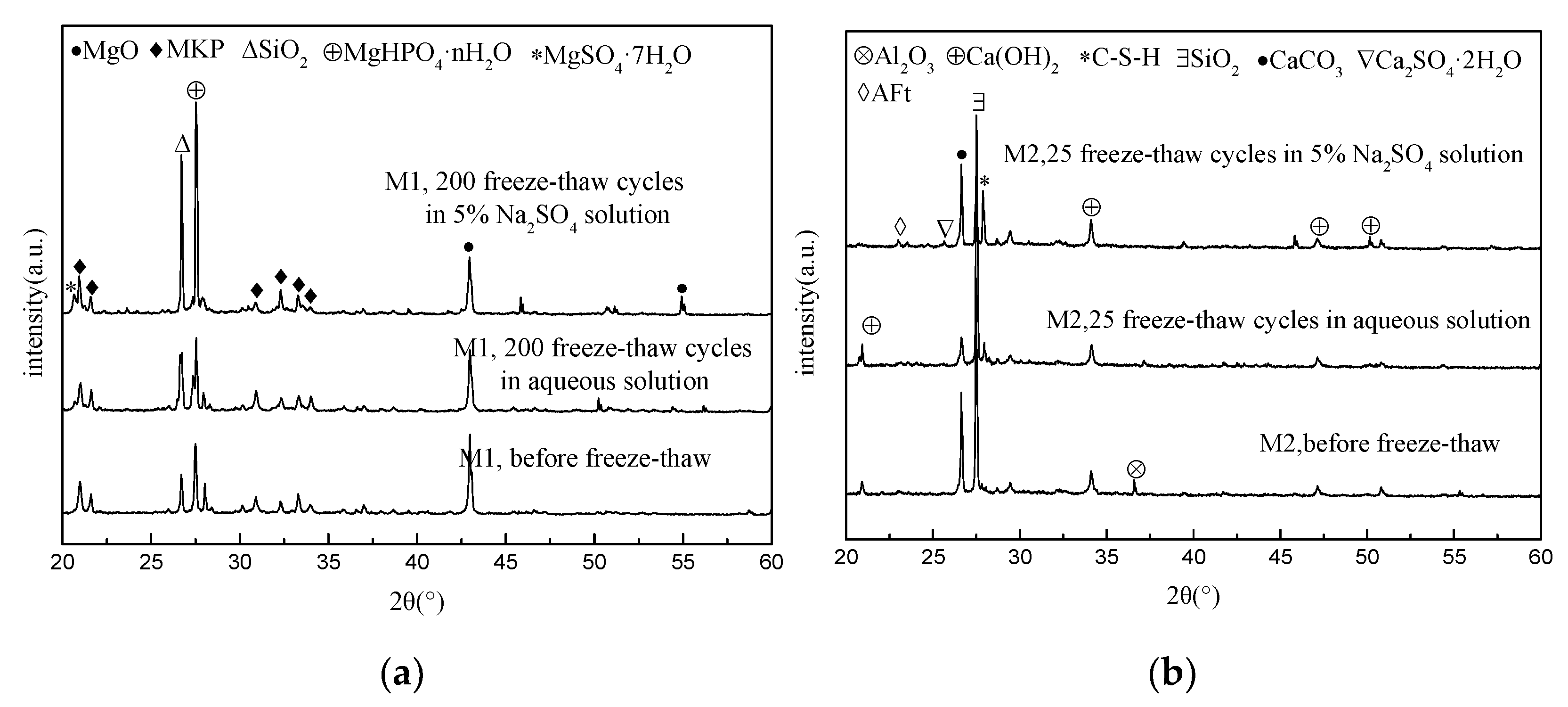
3.4. XRD Analysis
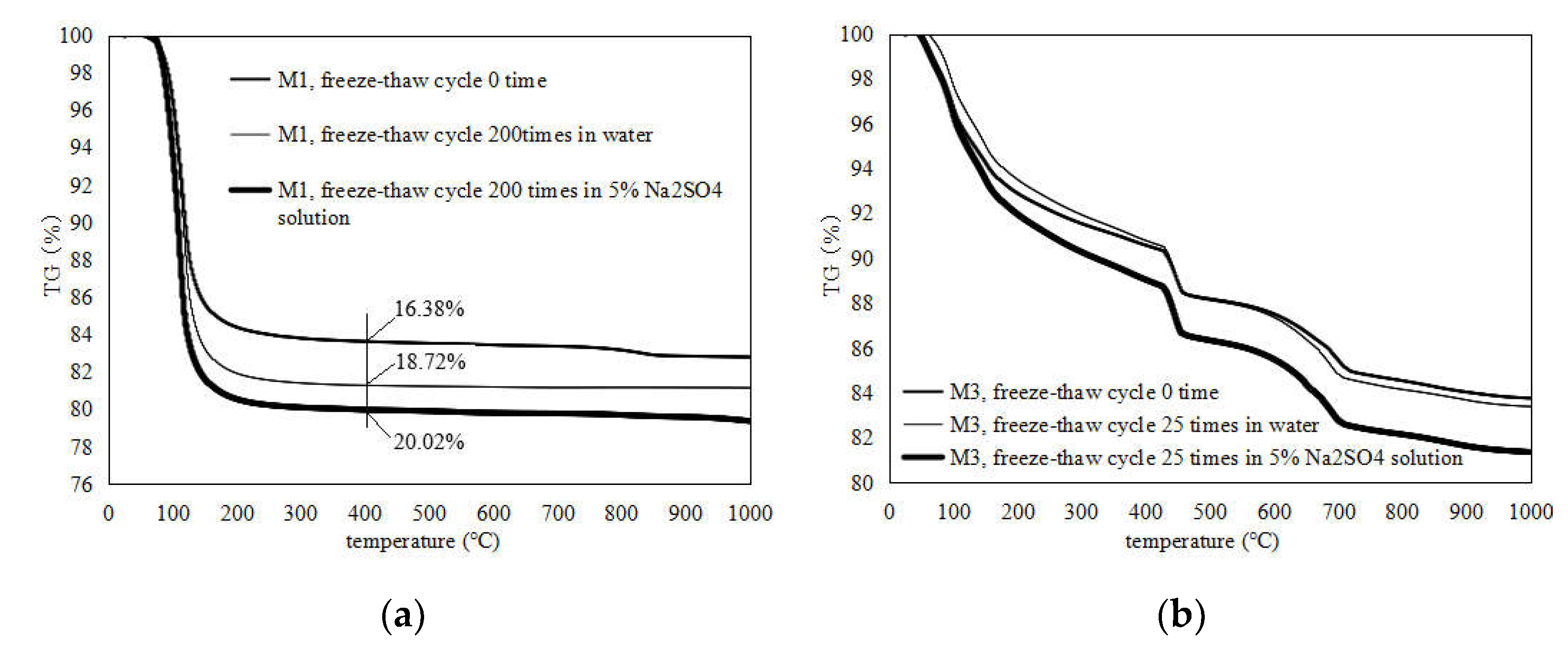
3.5. TG Measurements
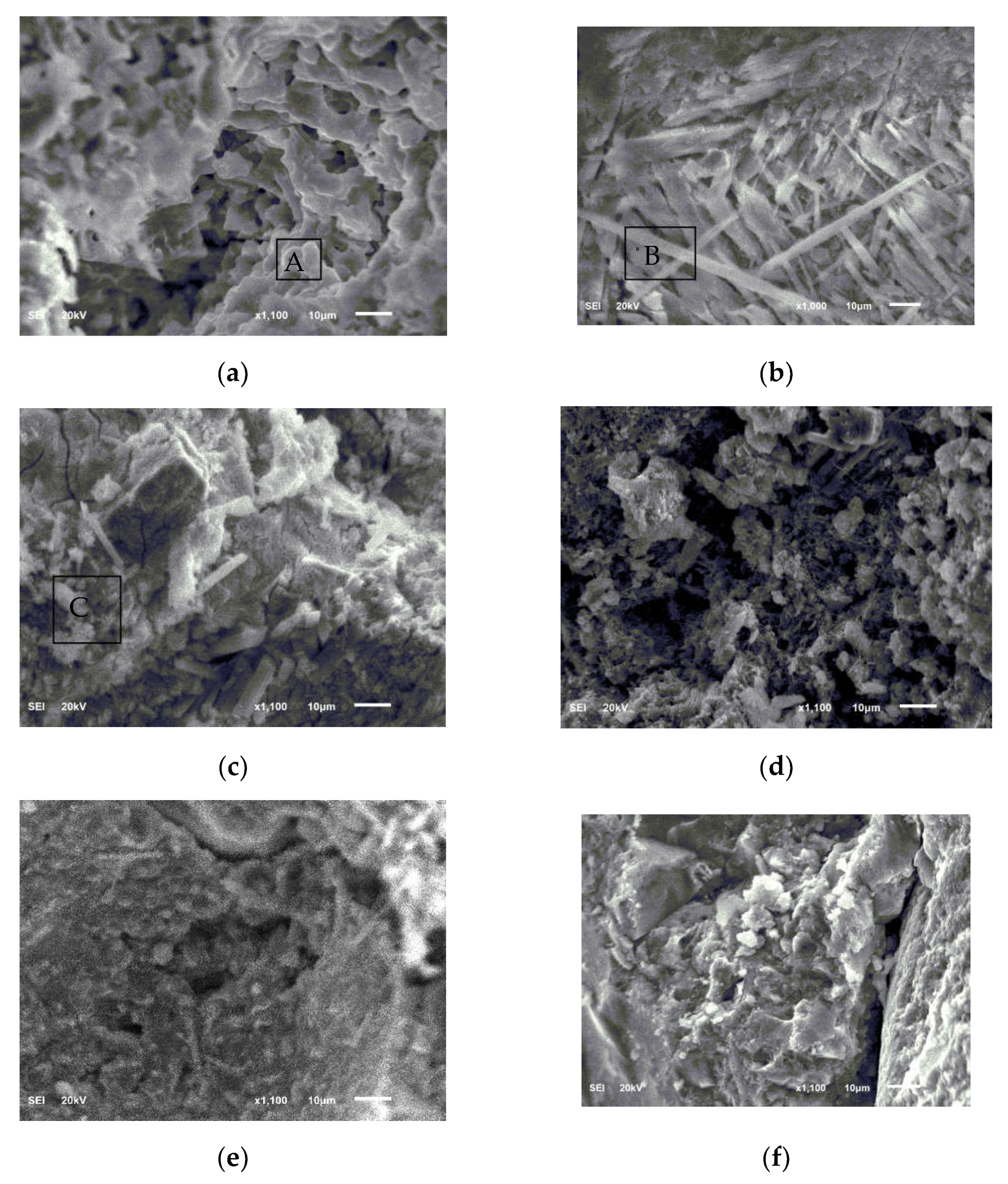
3.6. SEM-EDS
4. Conclusions
- (1)
- The P.O mortar specimen (M2) completely lost its strength after 75 freeze–thaw cycles in water and 50 freeze–thaw cycles in 5% Na2SO4 solution. The MKPC mortar specimen (M1) with the same strength grade was frozen and thawed in water 75 times and 5% Na2SO4 solution 100 times, where the residual strength rate was higher than 75%. After 50 freeze–thaw cycles in water and 5% Na2SO4 solution, the mass loss of the M2 specimens exceeded the 5% damage standard, while the mass loss of M1 was much lower than 5%. These results show that the water freeze–thaw resistance and sulfate solution freeze–thaw resistance of MKPC mortar specimens is much higher than that of Portland cement mortar specimens, and the salt freeze–thaw resistance is better than the water freeze–thaw resistance.
- (2)
- Before the freeze–thaw test, the open porosity of the MKPC mortar hardened body was significantly lower than that of the Portland cement mortar, and environmental water could not easily infiltrate. In a supercooled water environment, there will still be incompletely reacted acid–base components in the MKPC hardened body to continue the hydration process, and the hydrolysis loss of the generated MKP in the low-temperature water environment will be alleviated, and the newly generated MKP will fill the pores of the hardened body. The MKPC hardened body tends to be dense; the aforementioned effects make the MKPC mortar’s anti-water freeze–thaw and sulfate freeze–thaw properties significantly better than those of P.O mortar.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, L.; Liu, C. Durability of Concrete and Its Protection and Repair; China Construction Industry Press: Beijing, China, 1990. [Google Scholar]
- Liang, Y.; Yuan, Y. The mechanism of concrete corrosion damage in sodium sulfate and magnesium sulfate solution. J. Silic. 2007, 35, 504–508. [Google Scholar]
- Chen, J.; Jiang, M.; Zhu, J. Damage evolution in cement mortar due to erosion of sulphate. Corros. Sci. 2008, 50, 2478–2483. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.K. Phosphate cement-based fast setting binders. Ceram. Bull. 1990, 69, 234–238. [Google Scholar]
- Seehra, S.S.; Gupta, S.; Kumar, S. Rapid setting magnesium phosphate cement for quick repair of concrete pavements-characterisation and durability aspects. Cem. Concr. Res. 1993, 23, 254–266. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, X. Factors influencing properties of phosphate cement-based binder for rapid repair of concrete. Cem. Concr. Res. 1999, 29, 389–396. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, B.; Wu, X. Characteristics and durability test of magnesium phosphate cement-based material for rapid repair of concrete. Mater. Struct. 2000, 33, 229–234. [Google Scholar] [CrossRef]
- Jiang, H.; Liang, B.; Zhang, U. Research on MPB ultra-early strength concrete repair materials. J. Build. Mater. 2001, 4, 196–198. [Google Scholar]
- Yang, Q.; Zhang, S.; Wu, X. Deicer-scaling resistance of phosphate cement-based binder for rapid repair of concrete. Cem. Concr. Res. 2002, 32, 165–168. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Phosphate Ceramics: 21st Century Materials with Diverse Applications; Elsevier Science: London, UK, 2004; p. 12. [Google Scholar]
- Wagh, A.S. Recent Progress in Chemically Bonded Phosphate Ceramics; Hindawi Publishing Corporation: London, UK, 2013. [Google Scholar]
- Zhu, D.; Li, Z.; Xing, F. Chemical durability investigation of magnesium phosphosilicate cement. Key Eng. Mater. 2006, 302–303, 275–281. [Google Scholar]
- Li, Y.; Shi, T.; Li, J. Effects of fly ash and quartz sand on water-resistance and salt-resistance of magnesium phosphate cement. Constr. Build. Mater. 2006, 105, 384–390. [Google Scholar] [CrossRef]
- Yang, J.; Tan, Q.; Wu, Q.; Li, X.; Sun, Z. The effect of seawater curing on properties of magnesium potassium phosphate cement. Constr. Build. Mater. 2017, 141, 470–478. [Google Scholar] [CrossRef]
- Jianming, Y.; Jie, Z.; Shucon, Z. Experimental research on seawater erosion resistance of magnesium potassium phosphate cement pastes. Constr. Build. Mater. 2018, 183, 534–543. [Google Scholar] [CrossRef]
- Jianming, Y.; Luming, W.; Cheng, J.; Dong, S. Effect of fly ash on the corrosion resistance of magnesium potassium phosphate cement paste in sulfate solution. Constr. Build. Mater. 2020, 237, 117639. [Google Scholar] [CrossRef]
- Yang, J.; Shi, C.; Chang, Y. Hydration and hardening properties of potassium magnesium phosphate cement paste mixed with composite retarder. J. Build. Mater. 2013, 16, 43–49. [Google Scholar]
- Zhao, X.; Wei, J.; Huang, Y. The comparison of evaluation indexes for durability degradation of concrete in freezing-thawing cycles. J. Huazhong Univ. Sci. Tech. (Nat. Sci. Ed.) 2003, 31, 103–106. [Google Scholar]
- Cao, J.; Li, J. Research on Frost Resistance of High Strength Concrete. J. Build. Mater. 1992, 2, 292–297. [Google Scholar]
- Yang, Q. Salt-freezing failure mechanism of concrete (I)—Capillary water saturation and freezing pressure. J. Build. Mater. 2007, 50, 522–527. [Google Scholar]
- Yang, Q. Salt-freezing failure mechanism of concrete (II)—Freeze–thaw saturation and freezing pressure. J. Build. Mater. 2012, 15, 741–745. [Google Scholar]
- Zhang, S. Dehydration characteristics of struvite-K pertaining to magnesium potassium phosphate cement system in non-isothermal condition. J. Therm. Anal. Calorim. 2013, 111, 35–40. [Google Scholar] [CrossRef]
- Chong, L.; Yang, J.; Xu, Z.; Xu, X. Freezing and thawing resistance of MKPC paste under different corrosion solutions. Constr. Build. Mater. 2019, 212, 663–674. [Google Scholar] [CrossRef]
| Oxide Composition | MgO | SiO2 | CaO | Fe2O3 | Al2O3 | Na2O | TiO2 | Others |
|---|---|---|---|---|---|---|---|---|
| Content/% | 91.85 | 3.68 | 3.14 | 0.865 | 0.17 | - | - | 0.285 |
| Code | P.O 42.5 | MKPC | WS/ Wcement | WW/ Wcement | Flexural Strength/MPa | Compressive Strength/MPa | ||
|---|---|---|---|---|---|---|---|---|
| 3 d | 28 d | 3 d | 28 d | |||||
| M1 | - | 1.00 | 1.50 | 0.20 | 6.16 | 7.96 | 35.1 | 51.8 |
| M2 | 1.00 | - | 3.0 | 0.5 | 6.03 | 8.15 | 30.7 | 53.3 |
| Element | Area | O | Mg | S | P | Cl | K | Na |
|---|---|---|---|---|---|---|---|---|
| Atomic % | A | 77.73 | 9.74 | - | 6.65 | - | 5.88 | - |
| B | 70.21 | 11.93 | - | 8.78 | - | 9.08 | - | |
| C | 69.37 | 7.22 | 1.45 | 9.69 | 0.27 | 9.60 | 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Ji, R.-J.; Lan, Q.; Yang, J.-M.; Xu, J. Sulfate Freeze–Thaw Resistance of Magnesium Potassium Phosphate Cement Mortar. Materials 2022, 15, 3342. https://doi.org/10.3390/ma15093342
Yang B, Ji R-J, Lan Q, Yang J-M, Xu J. Sulfate Freeze–Thaw Resistance of Magnesium Potassium Phosphate Cement Mortar. Materials. 2022; 15(9):3342. https://doi.org/10.3390/ma15093342
Chicago/Turabian StyleYang, Bin, Rong-Jian Ji, Qian Lan, Jian-Ming Yang, and Jun Xu. 2022. "Sulfate Freeze–Thaw Resistance of Magnesium Potassium Phosphate Cement Mortar" Materials 15, no. 9: 3342. https://doi.org/10.3390/ma15093342
APA StyleYang, B., Ji, R.-J., Lan, Q., Yang, J.-M., & Xu, J. (2022). Sulfate Freeze–Thaw Resistance of Magnesium Potassium Phosphate Cement Mortar. Materials, 15(9), 3342. https://doi.org/10.3390/ma15093342







