Study on the Ignition Mechanism of Inert Fuel Tank Subjected to High-Velocity Impact of Fragments
Abstract
:1. Introduction
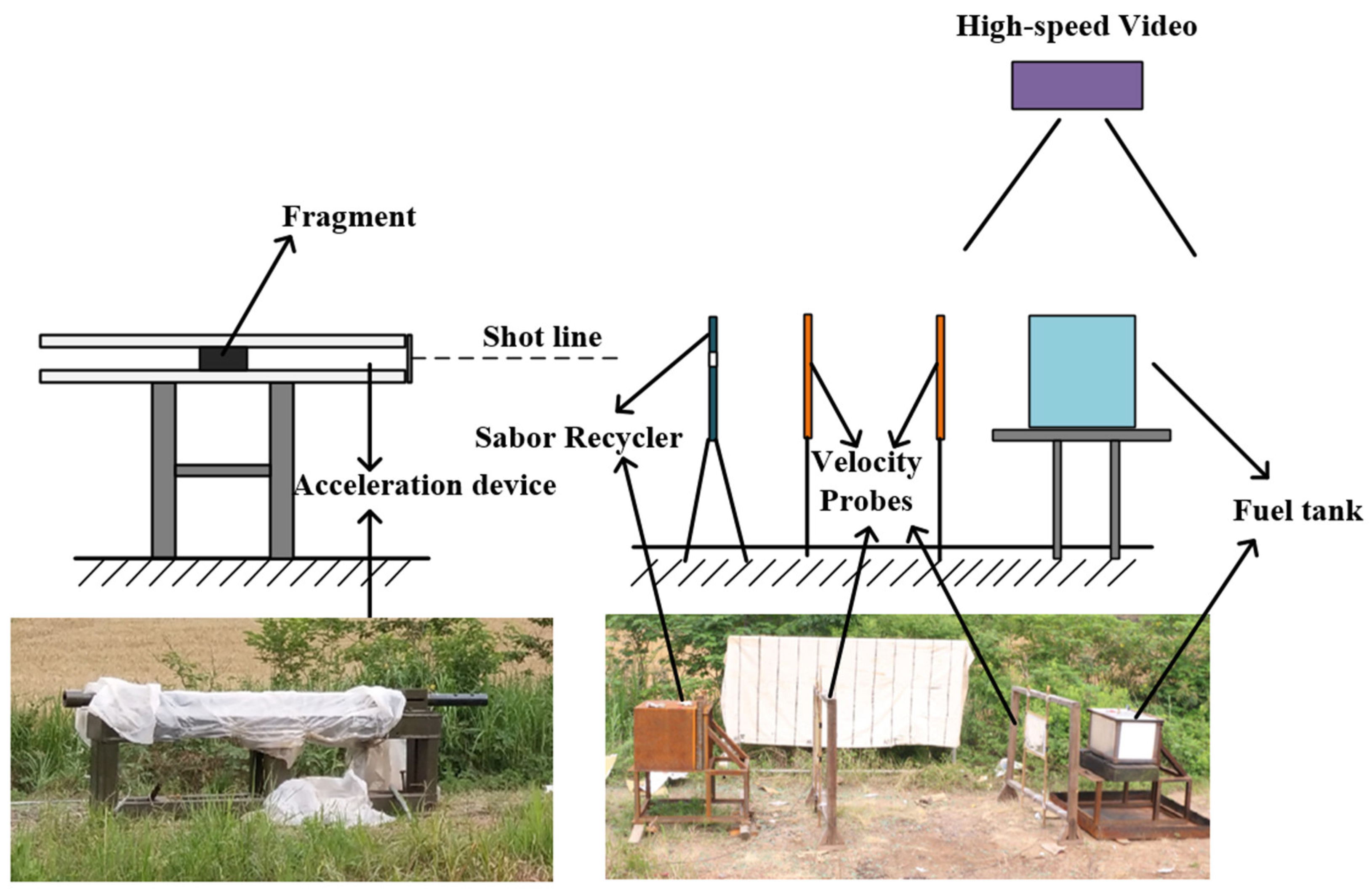
2. Experimental Setup
2.1. Equivalent Inert Fuel Tank
2.2. Fragments and Acceleration Devices
2.3. Experimental Principles
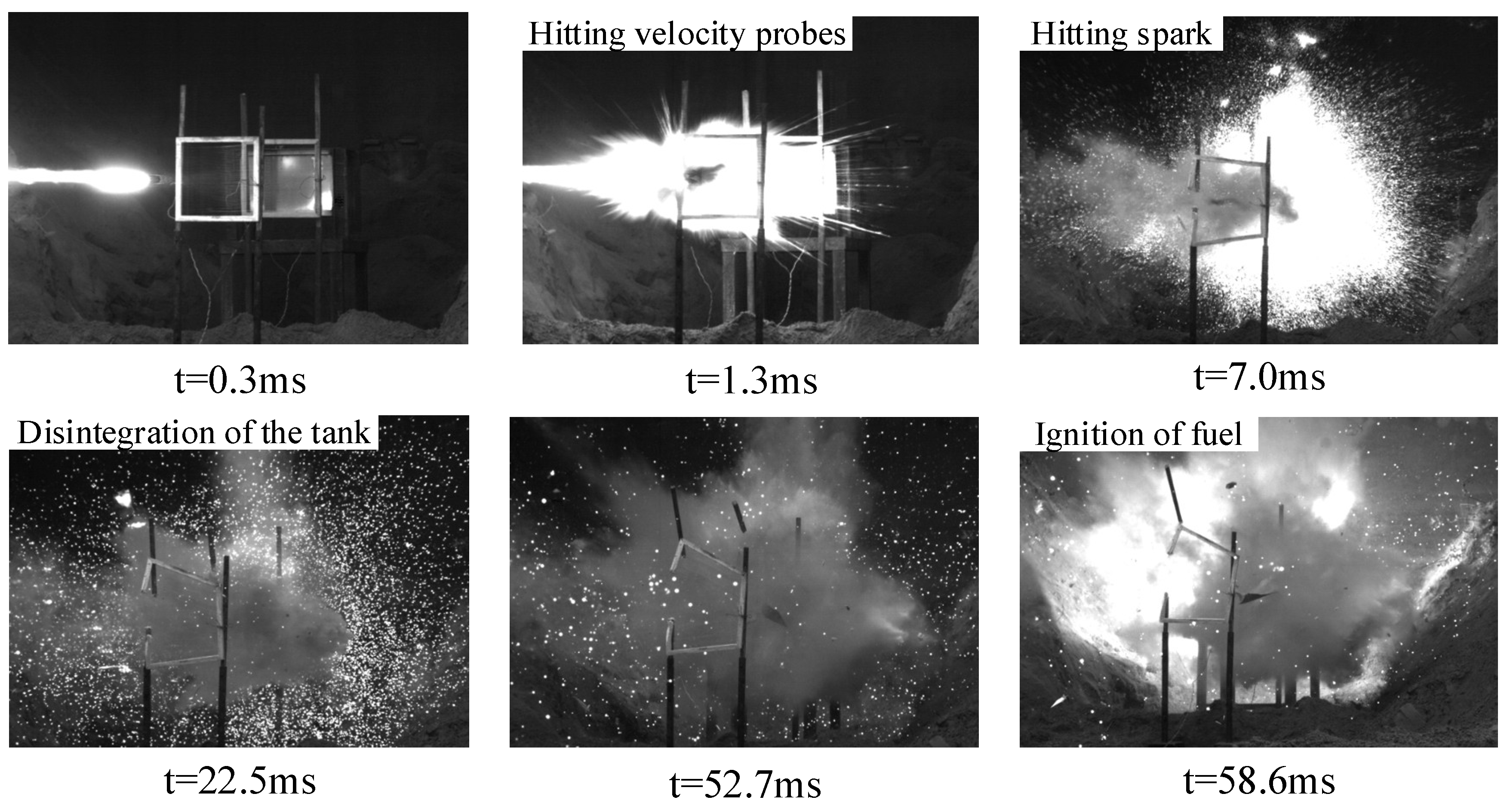
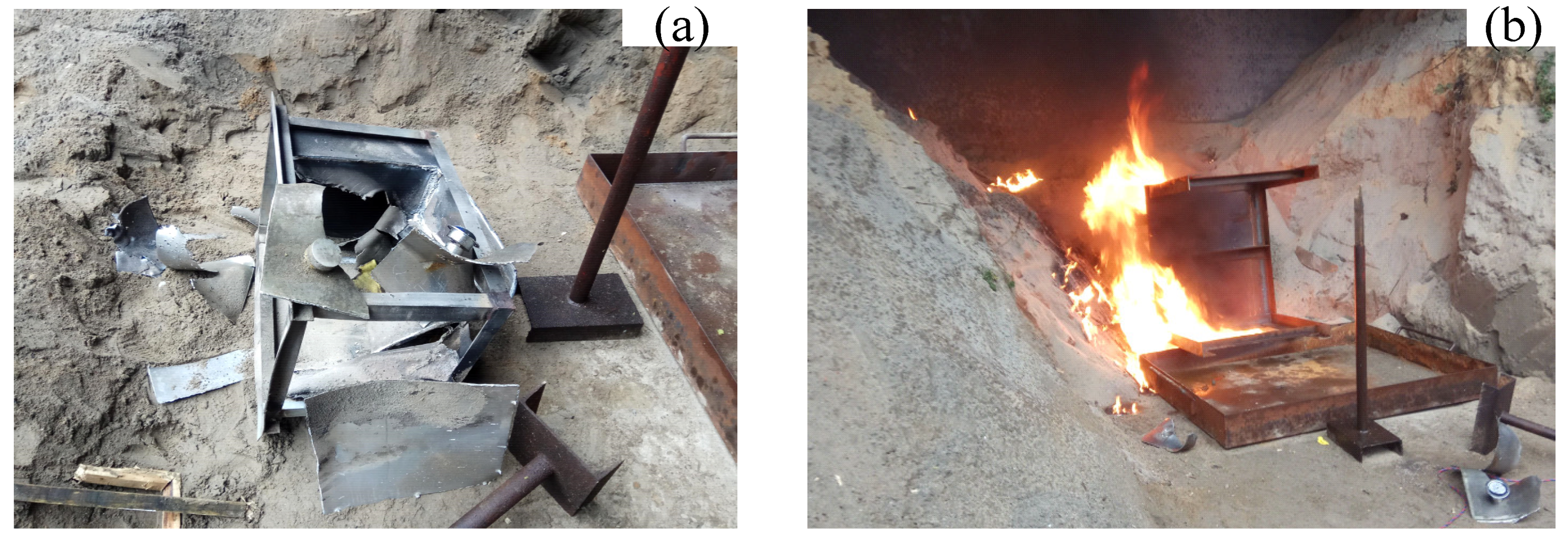
3. Experimental Results
4. Ignition Mechanisms
4.1. Gas Concentration Change
4.2. Ignition Criteria
4.3. Comparisons of Fuel Ignition Mechanism between Inert Fragments and Energetic Fragments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| residual velocity of the fragment | limit penetration velocity | ||
| resistance of the fragment | density of fuel | ||
| resistance coefficient | length of the fragment | ||
| energy flux | specific heat of the fragment | ||
| specific heat of fuel | boiling point of fuel | ||
| initial temperature of fuel | evaporation heat of fuel | ||
| heat transfer coefficient | conductivity coefficient of fuel | ||
| Prandtl constant | Reynolds number | ||
| kinematic viscosity coefficient of fuel | internal energy | ||
| conversion factor | mass of newly converted oil–gas | ||
| molar mass of RP-3 | delay time of ignition | ||
| activation energy of fuel | |||
| temperature of the fragment passing through the tank shell |
References
- Barnoon, P. Modeling of a high temperature heat exchanger to supply hydrogen required by fuel cells through reforming process. Energy Rep. 2021, 7, 5685–5699. [Google Scholar] [CrossRef]
- Mei, B.; Barnoon, P.; Toghraie, D.; Su, C.H.; Nguyen, H.C.; Khan, A. Energy, exergy, environmental and economic analyzes (4E) and multi-objective optimization of a PEM fuel cell equipped with coolant channels. Renew. Sustain. Energy Rev. 2022, 157, 112021. [Google Scholar] [CrossRef]
- Jia, Y.; Zeng, M.; Barnoon, P.; Toghraie, D. CFD simulation of time-dependent oxygen production in a manifold electrolyzer using a two-phase model. Int. Commun. Heat Mass Transf. 2021, 126, 105446. [Google Scholar] [CrossRef]
- Wang, H.F.; Xie, J.W.; Ge, C.; Guo, H.G.; Zheng, Y.F. Experimental investigation on enhanced damage to fuel tanks by reactive projectiles impact. Def. Technol. 2021, 17, 599–608. [Google Scholar] [CrossRef]
- Liu, S.B.; Yuan, Y.; Zheng, Y.F.; Ge, C.; Wang, H.F. Enhanced ignition behavior of reactive material projectiles impacting fuel-filled tank. Def. Technol. 2019, 15, 533–540. [Google Scholar] [CrossRef]
- Ball, R.E. Vulnerability (PK|H and PK|F). In The Fundamentals of Aircraft Combat Survivability Analysis and Design, 2nd ed.; American Institute of Aeronautics and Astronautics Inc.: New York, NY, USA, 2003; pp. 603–738. [Google Scholar]
- Ball, R.E. Appendix B: Probability Theory and Its Application to Survivability Assessment. In The Fundamentals of Aircraft Combat Survivability Analysis and Design, 2nd ed.; American Institute of Aeronautics and Astronautics Inc.: New York, NY, USA, 2003; pp. 799–864. [Google Scholar]
- Mcconnell, P.M.; Da Lan, G.A.; Anderson, C.L. Vulnerability Methodology and Protective Measures for Aircraft Fire and Explosion Hazards. Volume 3. On-Board Inert Gas Generator System (OBIGGS) Studies. Part 3. Aircraft OBIGGS Designs; Defense Technical Information Center: Fort Belvoir, VA, USA, 1986.
- Bai, L.T. Research on the Design of Aircraft Explosion Suppression System. Technol. Entrep. 2014, 7, 140. [Google Scholar]
- Wang, Z. Explosion-proof and anti-explosion materials application technology for aircraft fuel tanks. Aeronaut. Sci. Technol. 2002, 3, 33–35. [Google Scholar]
- Huang, Y. The burning performance of a fuel tank filled with anti-explosion materials. Energ. Mater. 2015, 23, 490–495. [Google Scholar]
- Reynolds, T.L.; Eklund, T.I.; Haack, G.A. Onboard Inert Gas Generation System/Onboard Oxygen Gas Generation System (OBIGGS/OBOGS) Study. Part 1: Aircraft System Requirements; National Aeronautics and Space Administration: Washington, DC, USA, 2001.
- Wang, H.F.; Zheng, Y.F.; Yu, Q.P. Experimental Study on the Ignition of Aviation Kerosene by Active Fragments. Acta Armamentarius 2012, 33, 1148–1152. [Google Scholar]
- Li, X.; Liang, Z.F.; Liu, Y.; Chen, J.; Chen, Y.J. Research progress on ignition characteristics of air target fuel tank. Fly. Missile 2019, 6, 69–74. [Google Scholar]
- Wang, C.L.; Huang, G.Y.; Feng, S.S. Study on the ignition effect of reaction fragments on the dense protective fuel tank. Acta Armamentarius 2018, 39 (Suppl. 1), 23–28. [Google Scholar]
- Moussa, N.A.; Whale, M.D.; Groszmann, D.E.; Zhang, X.J. The Potential for Fuel Tank Fire and Hydrodynamic Ram from Uncontained Aircraft Engine Debris; U.S. Department of Transportation Federal Aviation Administration: Washington, DC, USA, 1997.
- Artero-Guerrero, J.A.; Pernas-Sanchez, J.; Lopez-Puente, J.; Varas, D. On the influence of filling level in CFRP aircraft fuel tank subjected to high velocity impacts. Compos. Struct. 2014, 107, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Moussa, N.A. The Only Protection Against Hydrodynamic Ram and Ullage Explosion. Available online: http://www.blazetech.com/hard_inst/fuelshield.html (accessed on 22 December 2021).
- Hill, R.; Johnson, G.R. Investigation of Aircraft Fuel Tank Explosions and Nitrogen Inerting Requirements during Ground Fires; US Government Science and Technology Report; National Technical Information Service: Springfield, VA, USA, 1975.
- Anderson, C.L. Test and Evaluation of Halon 1301 and Nitrogen Inerting against 23 mm HEI Projectiles; AFFDL-TR-78-66; Air Force Flight Dynamics Laboratory: Dayton, OH, USA, 1978. [Google Scholar]
- Tyson, J.H.; Barnes, J.F. The Effectiveness of Ullage Nitrogen Inerting System against 30 mm High Explosive Incendiary Projectiles; NWC TP-7129; Naval Weapons Center: Ridgecrest, CA, USA, 1991. [Google Scholar]
- Shi, B.; Pei, Y. Method for analyzing the effect of projectile impact on aircraft fuel tank inerting for survivability design. Proc. Inst. Mech. Eng. Part G. J. Aerosp. Eng. 2016, 230, 2345–2355. [Google Scholar]
- Summer, S. Limiting Oxygen Concentration Required to Inert Jet Fuel Vapors Existing at Reduced Fuel Tank Pressures-Final Phase; U.S. Department of Transportation Federal Aviation Administration: Washington, DC, USA, 2004.
- Irimia, M.A. Estimation of LOC (limiting oxygen concentration) of fuel–air–inert mixtures at elevated temperatures by means of adiabatic flame temperatures. Chem. Eng. Process. Process Intensif. 2006, 45, 193–197. [Google Scholar]
- Summer, S.M.; Cavage, W.M. A Comparison of Flammability Characteristics of Composite and Aluminum Wing Fuel Tanks; U.S. Department of Transportation Federal Aviation Administration: Washington, DC, USA, 2011.
- Bless, S.J. Fuel Tank Survivability for Hydrodynamic Ram Induced by High Velocity Fragments: Part I. Experimental Results and Design Summary; University of Dayton Research Institute: Dayton, OH, USA, 1979. [Google Scholar]
- Clow, D.J.; Cooper, F.L. Gunfire Tests of F-15 No. 1 Fuel Tank Explosion Suppression Foam Configuration; Aeronautical Systems Division, Air Force Systems Command, Wright-Patterson Air Force Base: Greene County, OH, USA, 1974. [Google Scholar]
- Summer, S.M. Fuel Tank Flammability Assessment Method (Monte Carlo Model); Version 10; U.S. Department of Transportation Federal Aviation Administration: Washington, DC, USA, 2008.
- Xu, H.Z.; Li, X.D. The Igniting Damage Effect of Energetic Fragments on Diesel Oil Box. J. Proj. Rocket. Missiles Guid. 2012, 32, 85–88. [Google Scholar]
- Lundin, S.J.; Mueller, R.B. Advanced Aircraft Materials, Engine Debris Penetration Testing; U.S. Department of Transportation Federal Aviation Administration: Washington, DC, USA, 2005.
- Nemat-Nasser, K.S. Determination of temperature rise during high strain rate deformation. Mech. Mater. 1998, 27, 1–12. [Google Scholar]
- Mason, J.J.; Rosakis, A.J.; Ravichandran, G. On the strain and strain rate dependence of the fraction of plastic work converted to heat: An experimental study using high speed infrared detectors and the Kolsky bar. Mech. Mater. 1994, 17, 135–145. [Google Scholar] [CrossRef]

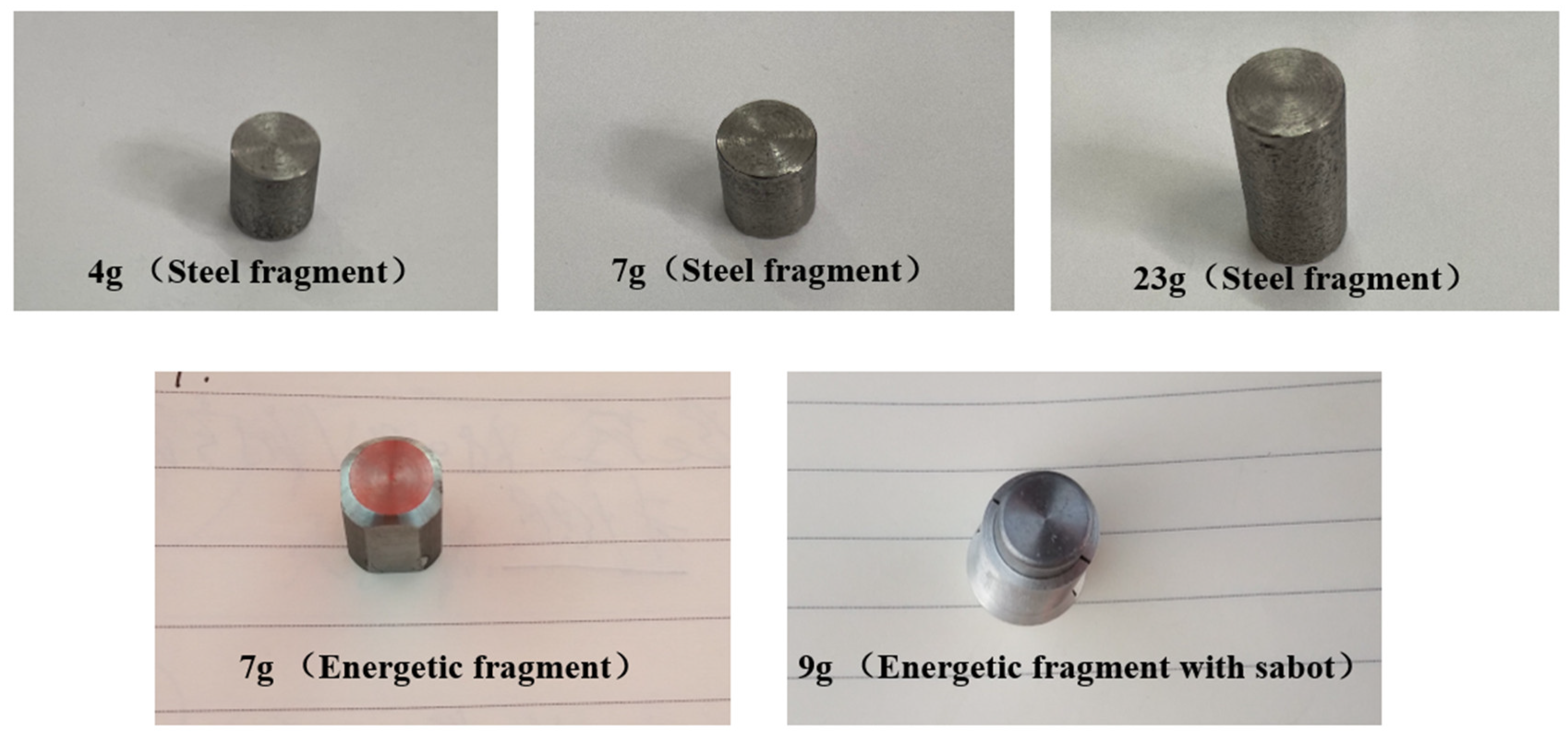



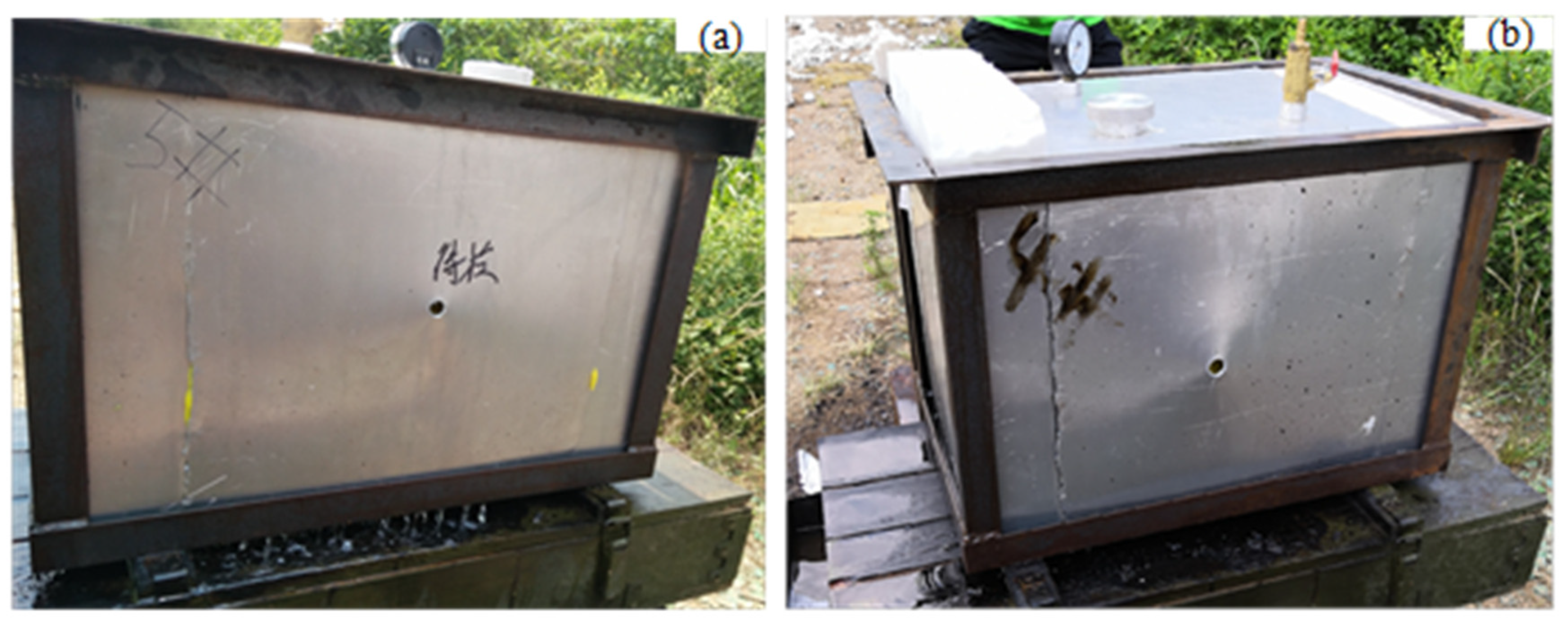

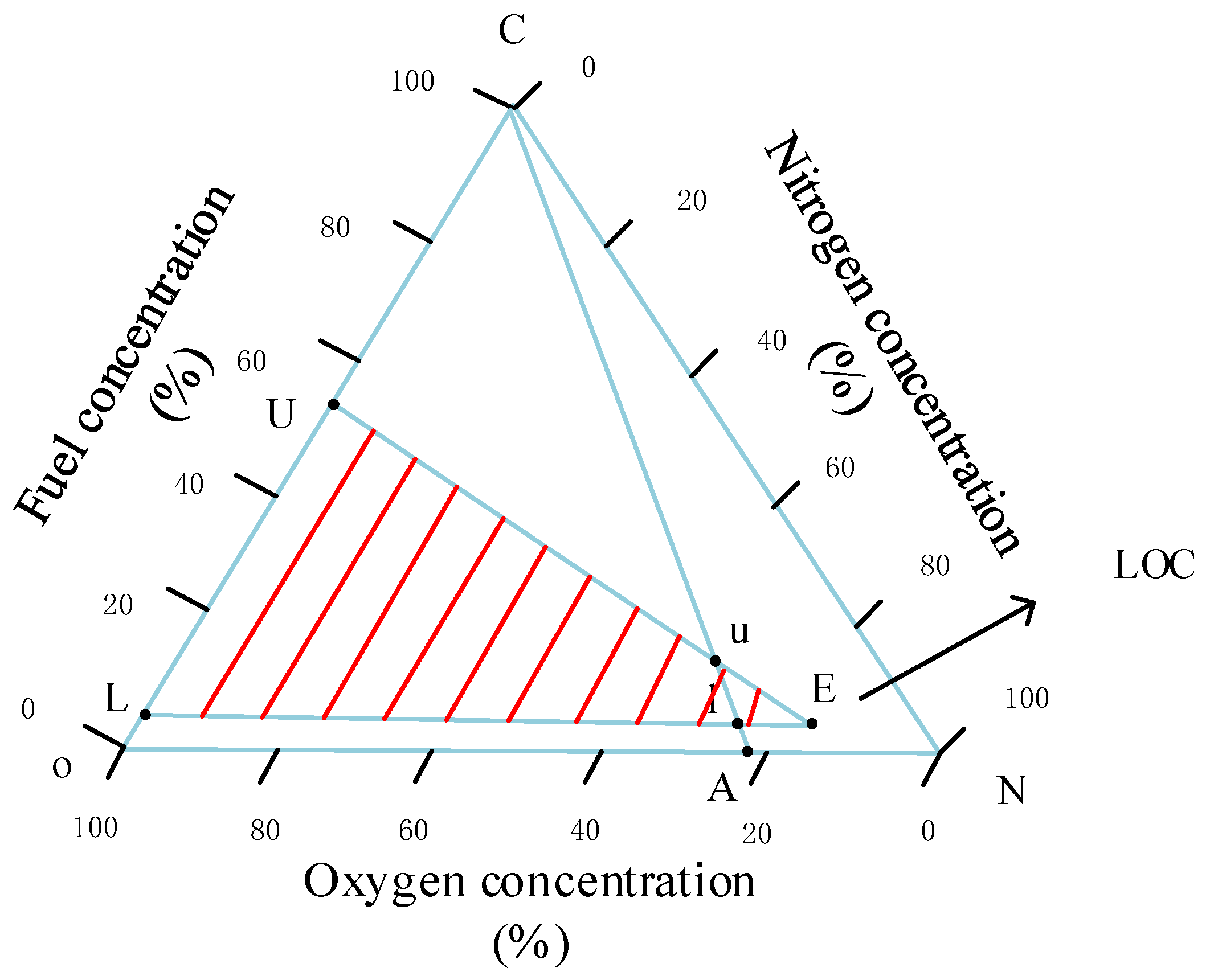
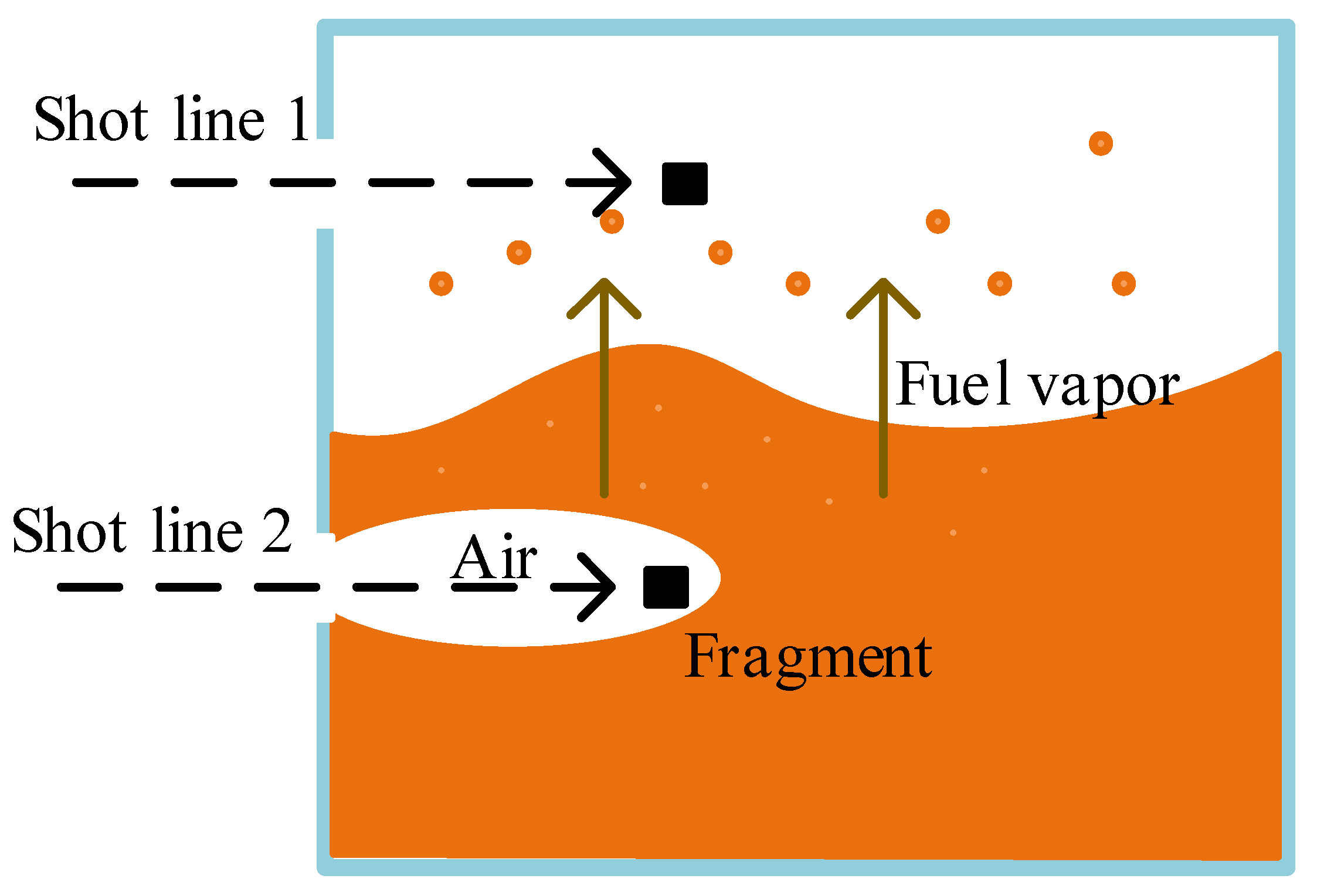


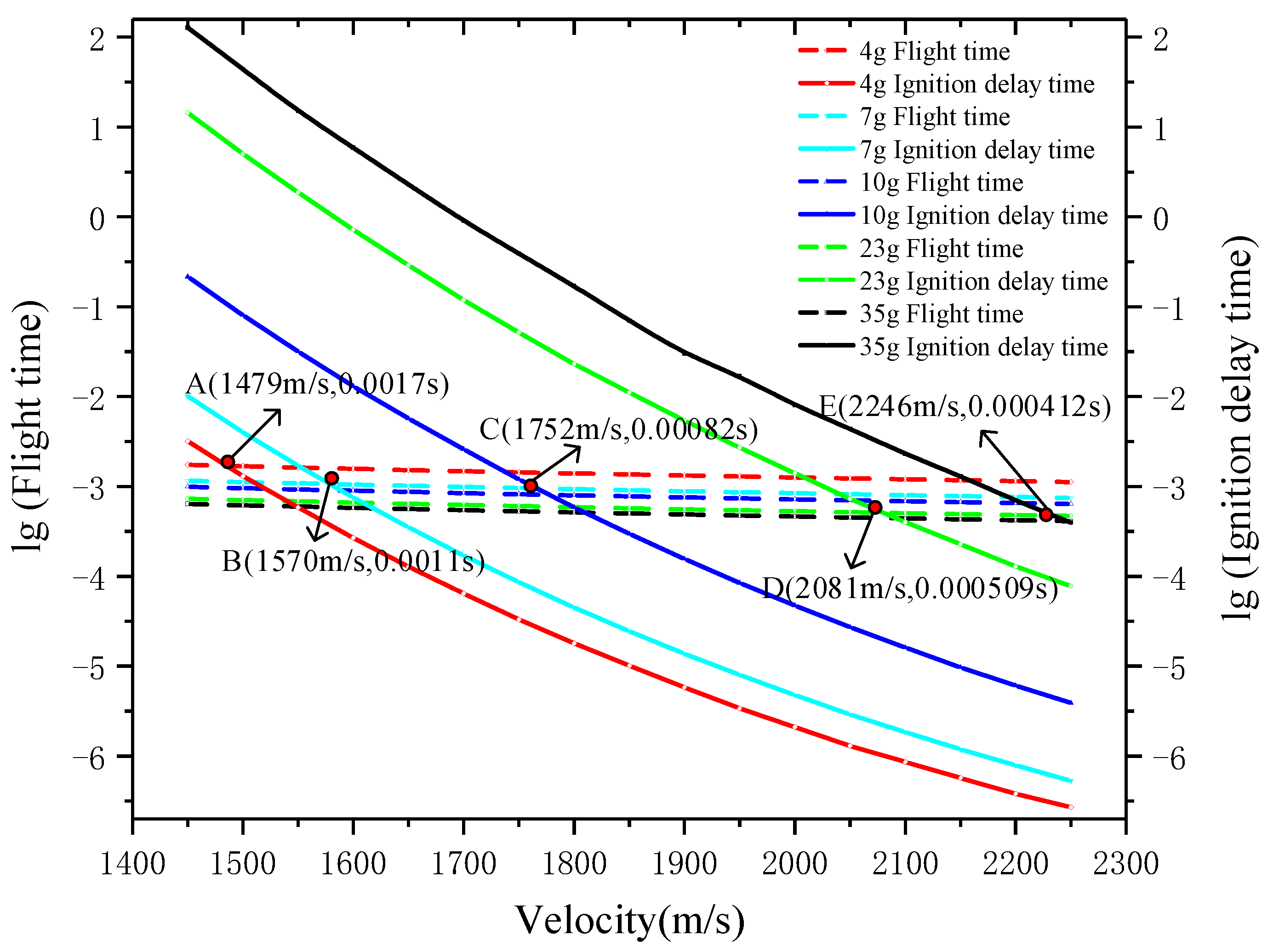
| Physicochemical Properties | RP-3 | JP-8 |
|---|---|---|
| Density (g/cm3) | 0.78 | 0.78 |
| Saturated vapor pressure at 25 °C (kPa) | 4.25 | 4.21 |
| Flash point (K) | 311 | 311 |
| Freezing point (K) | 226 | 226 |
| Viscosity at 20 °C (mm2/s) | 1.25 | 1.25 |
| Heat of evaporation (KJ/kg) | 345 | 291 |
| Net heat of combustion (MJ/kg) | 42.8 | 42.8 |
| Combustion concentration limit | 0.83–5.68% | 0.6–4.7% |
| Critical oxygen concentration | 12% | 12% |
| Fragments | Number | Masses (g) | Speeds (m/s) | Kinetic Energy (J) | Results |
|---|---|---|---|---|---|
| Inert fragments (steel) | 1 | 4 | 1461 | 4269 | The fuel tank was perforated, the perforation was slightly deformed, and the fuel was not ignited. |
| 2 | 4 | 1497 | 4482 | ||
| 3 | 10 | 1455 | 10,585 | The fuel tank was perforated and obviously deformed, the weld was cracked greatly, and the fuel was not ignited. | |
| 4 | 10 | 1454 | 10,570 | ||
| 5 | 7 | 1762 | 10,866 | ||
| 6 | 7 | 1868 | 12,212 | ||
| 7 | 7 | 2611 | 23,860 | The fuel tank was perforated, bulging and deformed, the bottom weld was serious cracked and fell off, and the fuel was not ignited. | |
| 8 | 23 | 1885 | 40,862 | The penetrated side of the fuel tank was bulging and deformed, cracking the weld, the upper cover was lifted, and the fuel was not ignited. | |
| 9 | 35 | 2298 | 92,414 | The fuel tank was completely destroyed and disassembled, the oil–gas was quickly mixed with air, and the fuel was ignited. | |
| Energetic fragments | 10 | 3.3 | 1812 | 5418 | The penetrated side of the fuel tank was broken, and the weld was cracked due to bulging; the tank was relatively intact, the fuel was not ignited. |
| 11 | 5 | 1783 | 7948 | ||
| 12 | 7 | 1857 | 12,070 | The energetic fragment hit the gas phase space of the tank. There was a circular hole on the penetrating surface. The fuel tank was not obviously deformed. Additionally, there was also a hole in the back. | |
| 13 | 7 | 1762 | 10,866 | The upper cover was lifted directly, the surrounding area of the penetrated side cracked and fell off, there were large cracks in the bottom weld, and the fuel was ignited. | |
| 14 | 7 | 1875 | 12,304 | ||
| 15 | 9 | 1781 | 14,273 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; An, F.; Wu, C.; Zhang, L.; Zhang, Y.; Li, Y. Study on the Ignition Mechanism of Inert Fuel Tank Subjected to High-Velocity Impact of Fragments. Materials 2022, 15, 3360. https://doi.org/10.3390/ma15093360
Liu J, An F, Wu C, Zhang L, Zhang Y, Li Y. Study on the Ignition Mechanism of Inert Fuel Tank Subjected to High-Velocity Impact of Fragments. Materials. 2022; 15(9):3360. https://doi.org/10.3390/ma15093360
Chicago/Turabian StyleLiu, Jian, Fengjiang An, Cheng Wu, Longhui Zhang, Yanxi Zhang, and Yipeng Li. 2022. "Study on the Ignition Mechanism of Inert Fuel Tank Subjected to High-Velocity Impact of Fragments" Materials 15, no. 9: 3360. https://doi.org/10.3390/ma15093360
APA StyleLiu, J., An, F., Wu, C., Zhang, L., Zhang, Y., & Li, Y. (2022). Study on the Ignition Mechanism of Inert Fuel Tank Subjected to High-Velocity Impact of Fragments. Materials, 15(9), 3360. https://doi.org/10.3390/ma15093360






