Preparation of Organic-Inorganic Coupling Phase Change Materials with Enhanced Thermal Storage Performance via Emulsion Polymerization
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.1.1. Preparation of Sodium Sulfate Decahydrate/Diatomite Composite Material
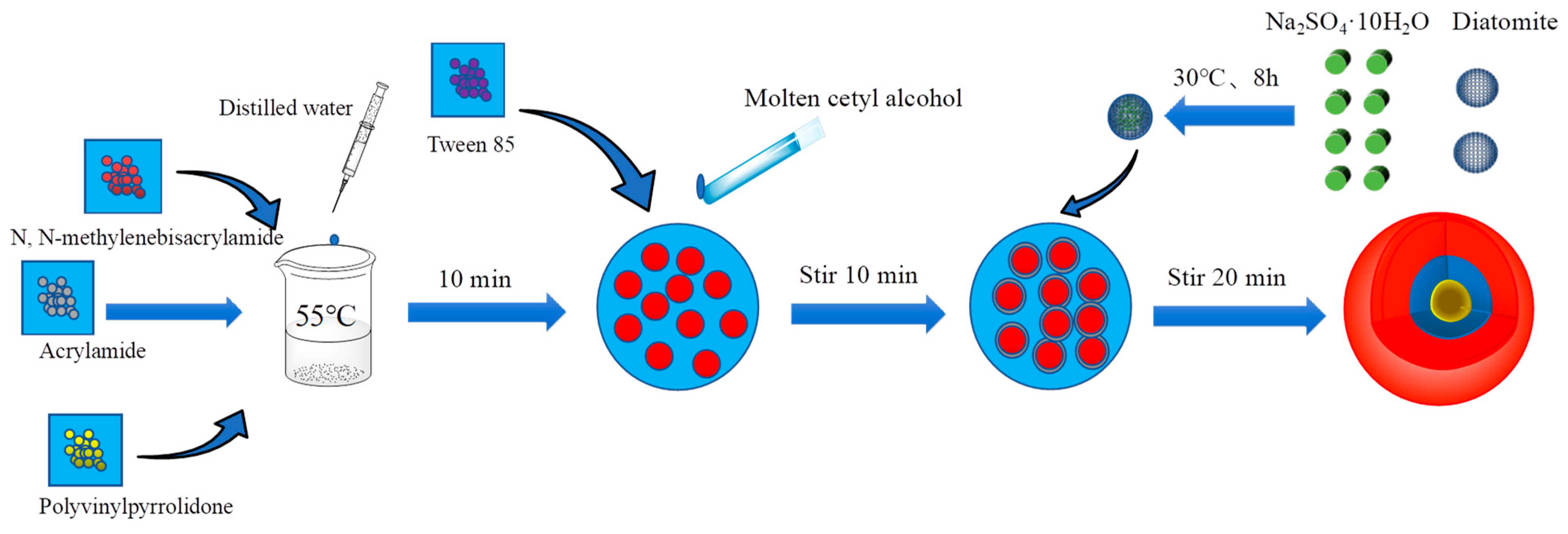
2.1.2. Preparation of Organic-Inorganic Coupling Phase Change Materials
2.2. Structural Characterization and Performance Testing
3. Results and Discussion
3.1. Optimization of the Preparation Conditions for Oic-PCM
3.2. Performance Characterization of Oic-PCM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Mater. 2020, 25, 251–295. [Google Scholar] [CrossRef]
- Umair, M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Barthwal, M.; Dhar, A.; Powar, S. Effect of nanomaterial inclusion in phase change materials for improving the thermal performance of heat storage: A review. ACS Appl. Energy Mater. 2021, 4, 7462–7480. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, D.; Shi, L.; Wang, L.; Jin, Y.; Tian, L.; Yan, Y. A review of phase change heat transfer in shape-stabilized phase change materials (ss-PCMs) based on porous supports for thermal energy storage. Renew. Sustain. Energy Rev. 2021, 135, 110127. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Li, A.; Atinafu, D.; Gao, H.; Dong, W.; Wang, G. Shape-stabilized phase change materials based on porous supports for thermal energy storage applications. Chem. Eng. J. 2019, 356, 641–661. [Google Scholar] [CrossRef]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on thermal conductivity enhancement, thermal properties, and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Lashgari, S.; Arabi, H.; Mahdavian, A.R.; Ambrogi, V. Thermal and morphological studies on novel PCM microcapsules containing n-hexadecane as the core in a flexible shell. Appl Energy 2017, 190, 612–622. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, C.; Yuan, M.; Ye, F.; Ju, X.; Du, X. Ca (NO3)2-NaNO3/expanded graphite composite as a novel shape-stable phase change material for mid-to high-temperature thermal energy storage. Energy Convers. Manag. 2018, 163, 50–58. [Google Scholar] [CrossRef]
- Yang, X.H.; Tan, S.C.; He, Z.Z.; Zhou, Y.X.; Liu, J. Evaluation and optimization of low melting point metal PCM heat sink against ultra-high thermal shock. Appl. Therm. Eng. 2017, 119, 34–41. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Zhuang, B.; Zhao, X.; Tang, Y.; Ding, X.; Chen, K. Preparation of novel copper-powder-sintered frame/paraffin form-stable phase change materials with extremely high thermal conductivity. Appl. Energy 2017, 206, 1147–1157. [Google Scholar] [CrossRef]
- Mo, B.Z.; Mo, S.P.; Jia, L.S.; Wang, Z.B.; Chen, Y. Microencapsulation of ethanol-soluble inorganic salts for high temperature thermal energy storage. Mater. Chem. Phys. 2022, 275, 125261. [Google Scholar] [CrossRef]
- Li, Y.; Tie, W.C.; Zhu, Q.Z.; Qiu, Z.Z. A study of LiNO3–NaCl/EG composite PCM for latent heat storage. Int. J. Thermophys. 2021, 42, 155. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Zhu, M.; Cojocaru-Mirédin, O.; Mio, A.M.; Keutgen, J.; Küpers, M.; Yu, Y.; Wuttig, M. Unique bond breaking in crystalline phase change materials and the quest for metavalent bonding. Adv. Mater. 2018, 30, 1706735. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, S.; Ma, Y.; Zhang, H.; Zeng, X.; Wu, F.; Guo, Z. Recent advances in organic/composite phase change materials for energy storage. ES Energy Environ. 2020, 9, 28–40. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Meng, R.; Zhang, M.F. Thermal performance of galactitol/mannitol eutectic mixture/expanded graphite composite as phase change material for thermal energy harvesting. J. Energy Storage 2021, 34, 101997. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, X.; Ji, J. Hygroscopic phase change composite material-a review. J. Energy Storage 2021, 36, 102395. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Smith, R.R.; Shah, U.V.; Parambil, J.V.; Burnett, D.J.; Thielmann, F.; Heng, J.Y. The effect of polymorphism on surface energetics of D-mannitol polymorphs. AAPS J. 2017, 19, 103–109. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Li, J.; Deng, Y.; Nian, H.; Jiang, H. Supercooling suppression and thermal conductivity enhancement of Na2HPO4·12H2O/expanded vermiculite form-stable composite phase change materials with alumina for heat storage. ACS Sustain. Chem. Eng. 2018, 6, 6792–6801. [Google Scholar] [CrossRef]
- Yin, D.; Ma, L.; Liu, J.; Zhang, Q. Pickering emulsion: A novel template for microencapsulated phase change materials with polymer–silica hybrid shell. Energy 2014, 64, 575–581. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, X.L.; Li, Y.Y.; Jotham, M.M.; Chen, Y.; Chen, Q.Y. Preparation and stability properties of sodium sulphate PCMs for thermal energy storage. Chem. Eng. J. 2018, 46, 13–15. [Google Scholar]
- Jin, X.; Wu, F.; Xu, T.; Huang, G.; Wu, H.; Zhou, X.; Wang, D.; Liu, Y.; Lai, A.C.K. Experimental investigation of the novel melting point modified phase change material for heat pump latent heat thermal energy storage application. Energy 2021, 216, 119191. [Google Scholar] [CrossRef]
- Esquivel-Castro, T.A.; Ibarra-Alonso, M.C.; Oliva, J.; Martinez Luevanos, A. Porous aerogel and core/shell nanoparticles for controlled drug delivery: A review. Mater. Sci. Eng. 2019, 96, 915–940. [Google Scholar] [CrossRef] [PubMed]
- Atinafu, D.G.; Yun, B.Y.; Kang, Y.; Wi, S.; Kim, S. Three-dimensional hybrid carbon nanocomposite-based intelligent composite phase change material with leakage resistance, low electrical resistivity, and high latent heat. J. Ind. Eng. Chem. 2021, 98, 435–443. [Google Scholar] [CrossRef]
- Tao, W.; Kong, X.F.; Bao, A.Y.; Fan, C.G.; Zhang, Y. Preparation and phase change performance of graphene oxide and silica composite Na2SO4·10H2O phase change materials (PCMs) as thermal energy storage materials. Materials 2020, 13, 5186. [Google Scholar] [CrossRef]
- Sun, N.; Xiao, Z. Synthesis and performances of phase change materials microcapsules with a polymer/BN/TiO2 hybrid shell for thermal energy storage. Energy Fuels 2017, 31, 10186–10195. [Google Scholar] [CrossRef]
- Li, C.C.; Wang, M.F.; Xie, B.S.; Ma, H.; Chen, J. Enhanced properties of diatomite-based composite phase change materials for thermal energy storage. Renew. Energy 2020, 147, 265–274. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Wu, B.; Wang, J.; Lei, J. Preparation and thermal properties of stearic acid/diatomite composites as form-stable phase change materials for thermal energy storage via direct impregnation method. J. Therm. Anal. Calorim. 2016, 123, 1173–1181. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Xie, B.; Zhao, X.; Chen, J. Tailored phase change behavior of Na2SO4·10H2O /expanded graphite composite for thermal energy storage. Energy Convers. Manag. 2020, 208, 112586. [Google Scholar] [CrossRef]
- Kahwaji, S.; White, M.A. Organic phase change materials for thermal energy storage: Influence of molecular structure on properties. Molecules 2021, 26, 6635. [Google Scholar]
- Liu, H.; Qian, Z.Q.; Wang, Q.W.; Wu, D.Z.; Wang, X.D. Development of renewable biomass-derived carbonaceous aerogel/mannitol phase-change composites for high thermal-energy-release efficiency and shape stabilization. ACS Appl. Energy Mater. 2021, 4, 1714–1730. [Google Scholar] [CrossRef]
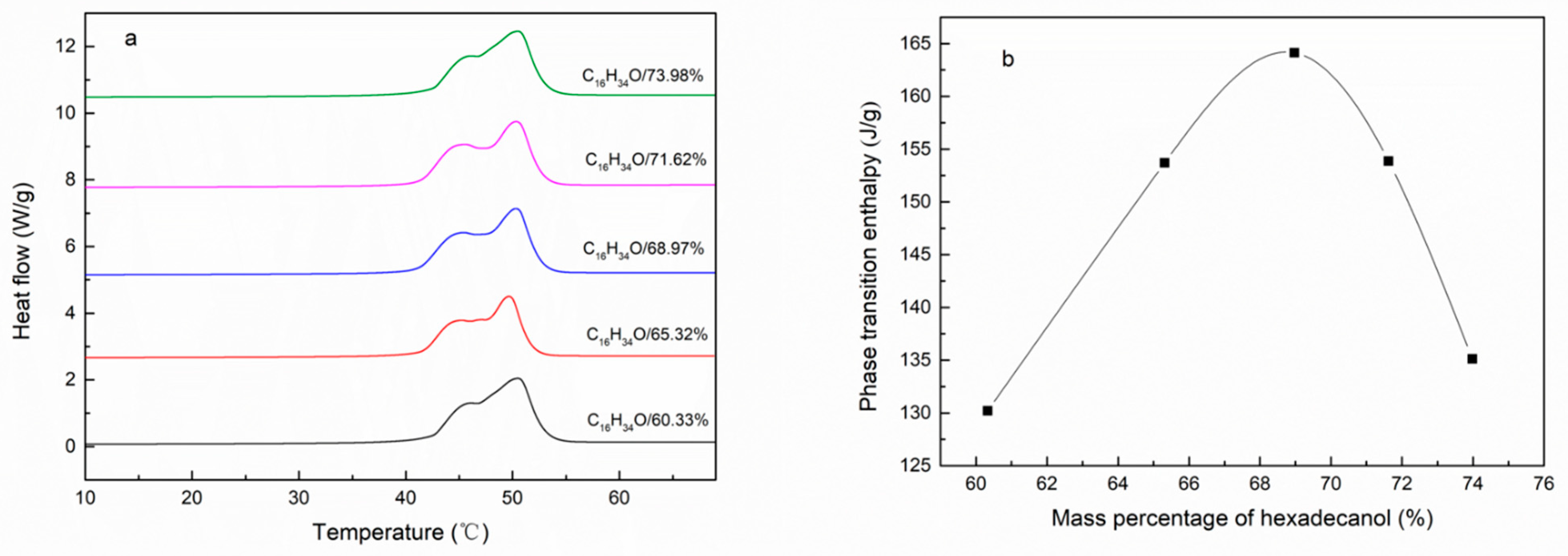
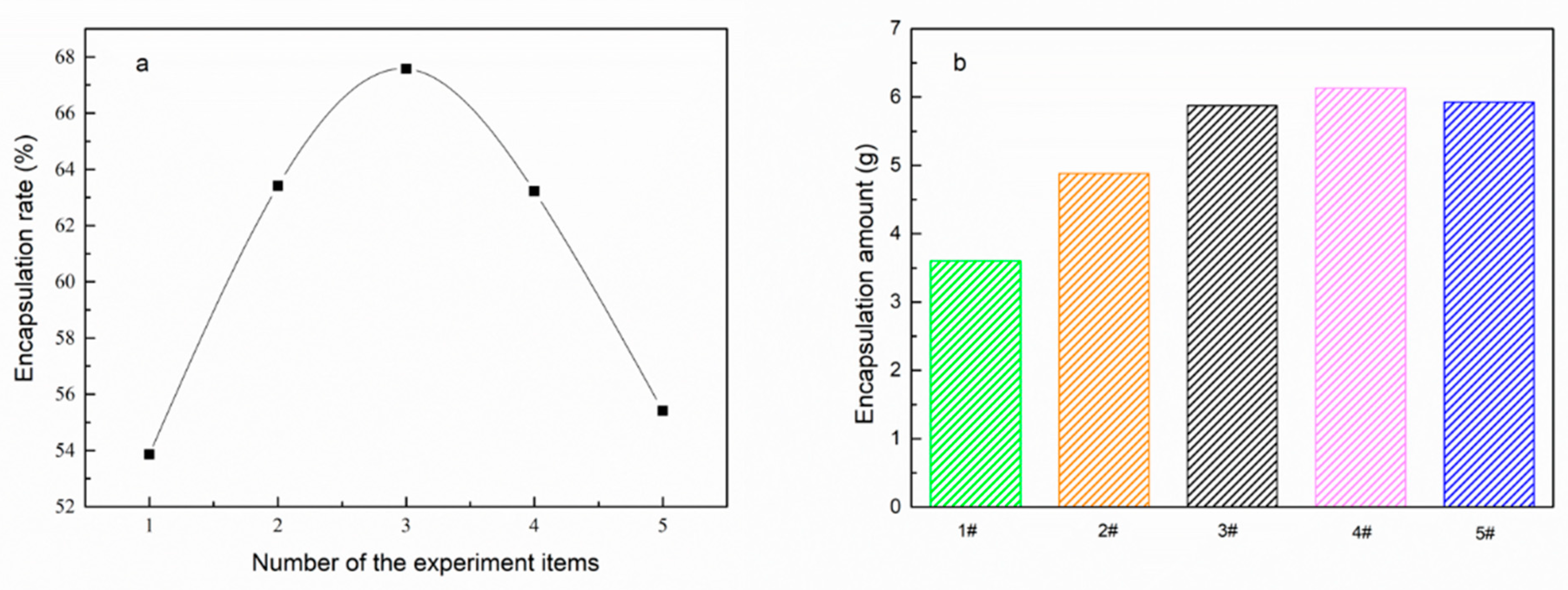
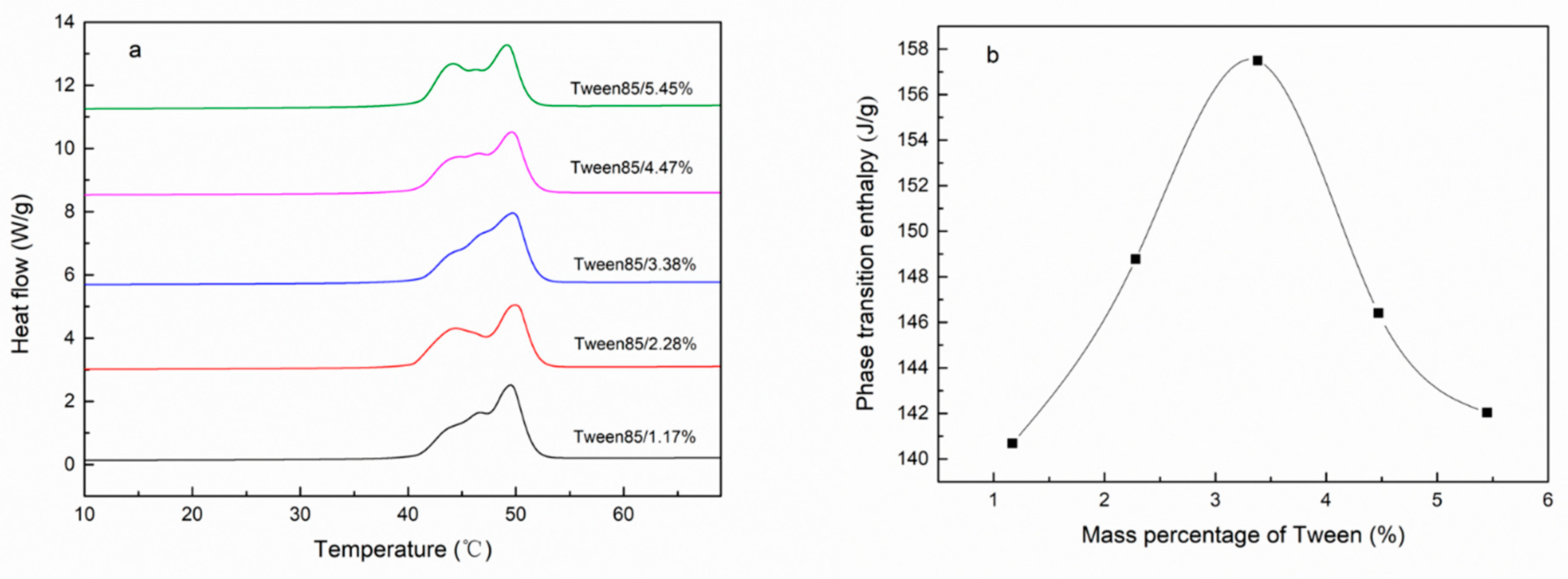
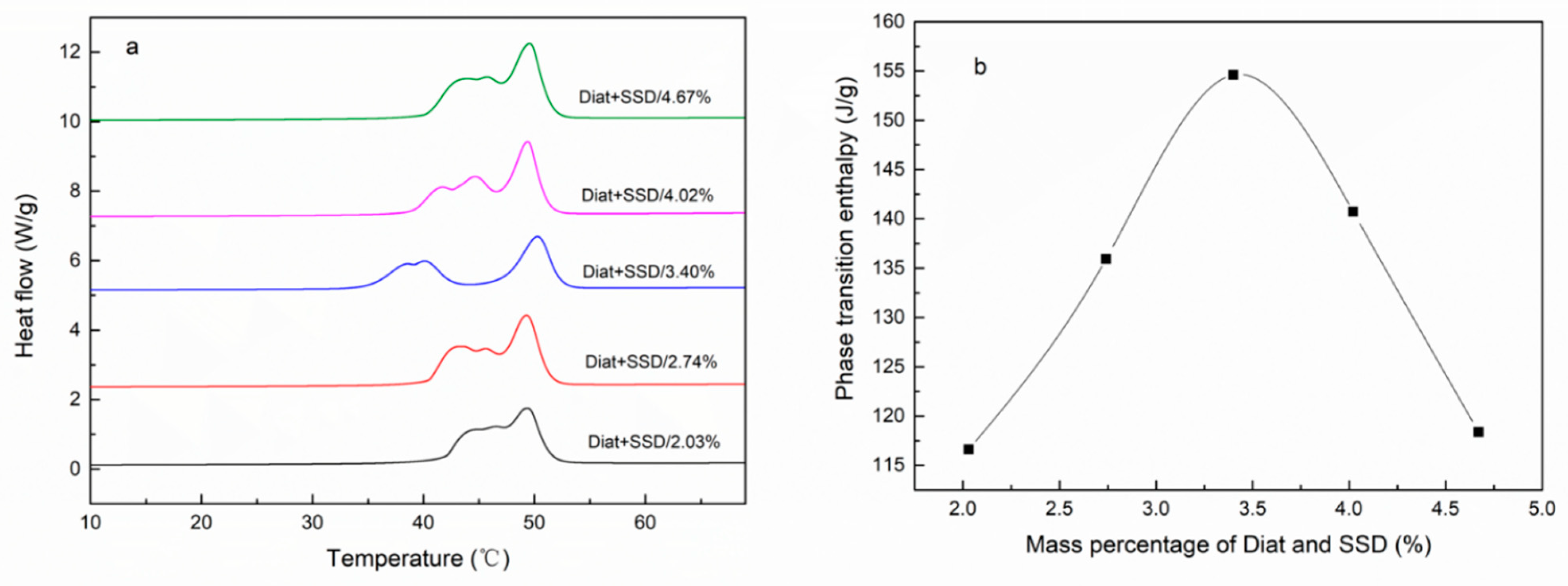
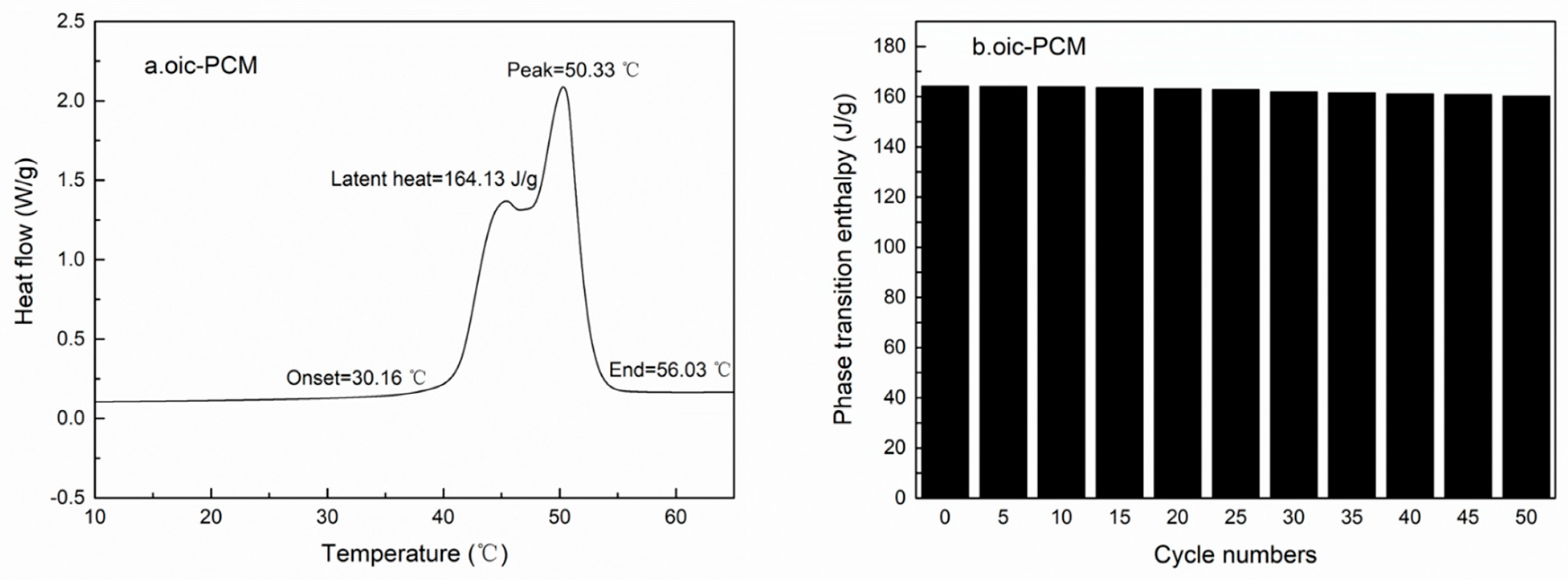

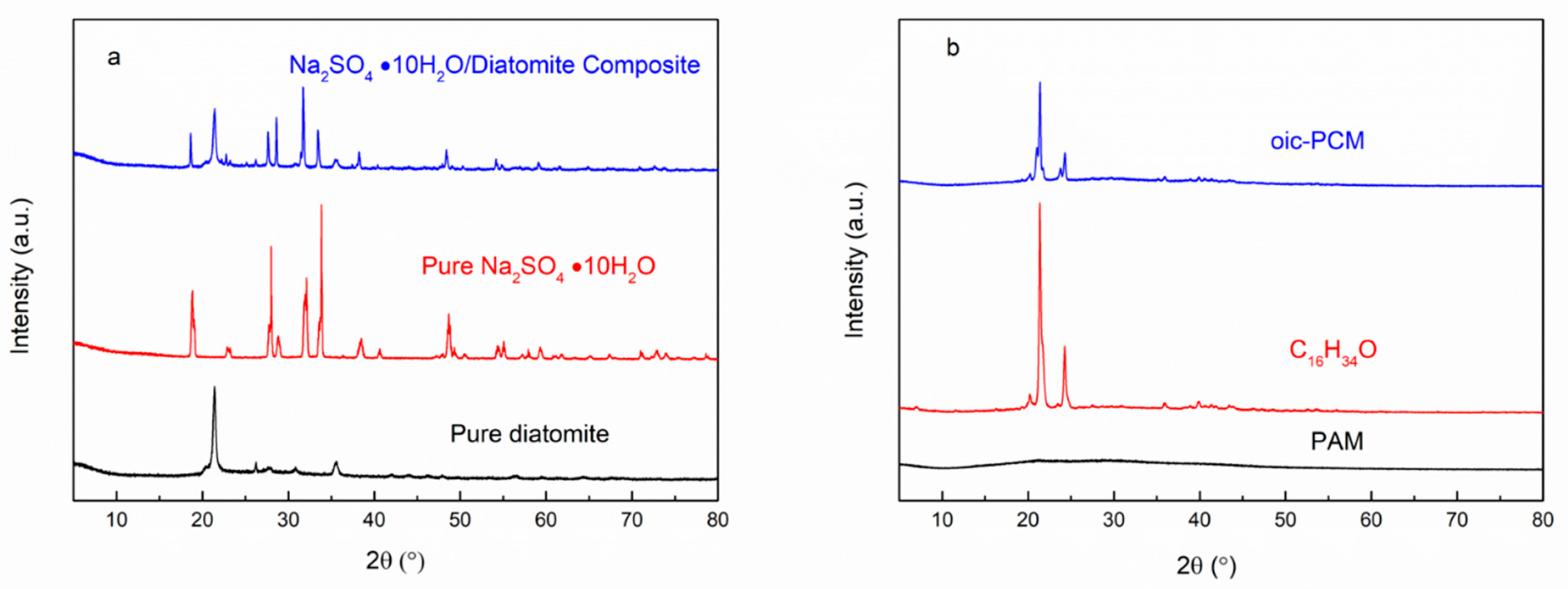


| Emulsifier % | Cetyl Alcohol % | SSD/Diatomite % | Tm °C | ΔH J/g | The Coating Rate % |
|---|---|---|---|---|---|
| 2.86% | 73.98% | 2.86% | 50.48 | 135.12 | 55.41% |
| 3.12% | 71.62% | 3.12% | 50.45 | 153.86 | 63.22% |
| 3.41% | 68.97% | 3.41% | 50.33 | 164.13 | 67.58% |
| 3.81% | 65.32% | 3.81% | 49.74 | 153.71 | 63.41% |
| 4.36% | 60.33% | 4.36% | 50.48 | 130.20 | 53.86% |
| 5.45% | 67.30% | 3.36% | 49.27 | 142.03 | 58.48% |
| 4.47% | 67.99% | 3.40% | 49.74 | 146.40 | 60.28% |
| 3.38% | 68.77% | 3.44% | 49.88 | 157.50 | 64.85% |
| 2.28% | 69.55% | 3.48% | 49.95 | 148.77 | 61.26% |
| 1.17% | 70.34% | 3.52% | 49.45 | 140.69 | 57.93% |
| 3.39% | 67.85% | 4.67% | 49.51 | 118.36 | 48.73% |
| 3.42% | 68.31% | 4.02% | 49.31 | 140.72 | 57.94% |
| 3.44% | 68.75% | 3.40% | 50.22 | 154.60 | 63.66% |
| 3.46% | 69.22% | 2.74% | 49.27 | 135.93 | 55.97% |
| 3.49% | 69.73% | 2.03% | 49.29 | 116.61 | 48.01% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Shen, X.; Zhang, L.; Wang, Y.; Wang, F. Preparation of Organic-Inorganic Coupling Phase Change Materials with Enhanced Thermal Storage Performance via Emulsion Polymerization. Materials 2022, 15, 3373. https://doi.org/10.3390/ma15093373
Lv X, Shen X, Zhang L, Wang Y, Wang F. Preparation of Organic-Inorganic Coupling Phase Change Materials with Enhanced Thermal Storage Performance via Emulsion Polymerization. Materials. 2022; 15(9):3373. https://doi.org/10.3390/ma15093373
Chicago/Turabian StyleLv, Xifeng, Xuehua Shen, Luxiang Zhang, Yazhou Wang, and Fang Wang. 2022. "Preparation of Organic-Inorganic Coupling Phase Change Materials with Enhanced Thermal Storage Performance via Emulsion Polymerization" Materials 15, no. 9: 3373. https://doi.org/10.3390/ma15093373
APA StyleLv, X., Shen, X., Zhang, L., Wang, Y., & Wang, F. (2022). Preparation of Organic-Inorganic Coupling Phase Change Materials with Enhanced Thermal Storage Performance via Emulsion Polymerization. Materials, 15(9), 3373. https://doi.org/10.3390/ma15093373






