In Situ Synthesis of Fe3O4 Nanoparticles and Wood Composite Properties of Three Tropical Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Situ Precipitation of Iron Oxide Nanoparticles within Wood
2.3. Experimental Techniques
2.4. Statistical Analysis
3. Results and Discussion
3.1. Weight Gain Percentage, Absorption and Density of Magnetic Wood an Ash and Ferron Content
3.2. SEM Observation
3.3. XRD Spectrum
3.4. FTIR Spectrum
3.5. Magnetic Properties of Wood
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Han, C.; Cao, W.-Q.; Cao, M.-S.; Yang, H.-J.; Yuan, J. A Nano-Micro engineering nanofiber for electromagnetic absorber, green shielding and sensor. Nano-Micro Lett. 2021, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Yu, H.; Wang, L.; Huang, Z.; Lin, T.; Huang, Y.; Yang, J.; Hong, Y.; Liu, J. State of the art and prospects in metal-organic framework-derived microwave absorption materials. Nano-Micro Lett. 2022, 14, 68. [Google Scholar] [CrossRef]
- Wang, L.; Li, N.; Zhao, T.; Li, B.; Ji, Y. Magnetic properties of FeNi3 nanoparticle modified Pinus radiata wood nanocomposites. Polymers 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Pineda, X.; Quintana, G.C.; Herrera, A.P.; Sánchez, J.H. Preparation and characterization of magnetic cellulose fibers modified with cobalt ferrite nanoparticles. Mater. Chem. Phys. 2021, 259, 122778. [Google Scholar] [CrossRef]
- Lykidis, C.; Bak, M.; Mantanis, G.; Németh, R. Biological resistance of pine wood treated with nano-sized zinc oxide and zinc borate against brown-rot fungi. Eur. J. Wood Wood Prod. 2016, 74, 909–911. [Google Scholar] [CrossRef]
- Ali, M.Z.; Javaid, A. Mechanical and rheological characterization of efficient and economical structural wood-plastic composite of wood and PVC. J. Chem. Soc. Pak. 2017, 39, 183–189. [Google Scholar]
- Liu, J.; Declercq, N.F. Acoustic Wood anomaly in transmitted diffraction field. J. Appl. Phys. 2017, 121, 114902. [Google Scholar] [CrossRef]
- Sohn, J.; Cha, S. Effect of Chemical modification on mechanical properties of wood-plastic composite injection-molded parts. Polymers 2018, 10, 1391. [Google Scholar] [CrossRef]
- Oka, H.; Kataoka, Y.; Osada, H.; Aruga, Y.; Izumida, F. Experimental study on electromagnetic wave absorbing control of coating-type magnetic wood using a grooving process. J. Magn. Magn. Mater. 2007, 310, e1028–e1029. [Google Scholar] [CrossRef]
- Oka, H.; Uchidate, S.; Sekino, N.; Namizaki, Y.; Kubota, K.; Osada, H.; Dawson, F.P.; Lavers, J.D. Electromagnetic wave absorption characteristics of half carbonized powder-type magnetic wood. IEEE Trans. Magn. 2011, 47, 3078–3080. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, Y.; Zhang, Y.; Zhang, S.; Li, J. Combined treatment for conversion of fast-growing poplar wood to magnetic wood with high dimensional stability. Wood Sci. Technol. 2016, 50, 503–517. [Google Scholar] [CrossRef]
- Oka, H.; Tanaka, K.; Osada, H.; Kubota, K.; Dawson, F.P. Study of electromagnetic wave absorption characteristics and component parameters of laminated-type magnetic wood with stainless steel and ferrite powder for use as building materials. J. Appl. Phys. 2009, 105, 07E701. [Google Scholar] [CrossRef]
- Gan, W.; Gao, L.; Xiao, S.; Zhang, W.; Zhan, X.; Li, J. Transparent magnetic wood composites based on immobilizing Fe3O4 nanoparticles into a delignified wood template. J. Mater. Sci. 2017, 52, 3321–3329. [Google Scholar] [CrossRef]
- Gan, W.; Gao, L.; Liu, Y.; Zhan, X.; Li, J. The Magnetic, Mechanical, Thermal Properties and Uv Resistance of COFe2O4/SiO2-Coated Film on Wood. J. Wood Chem. Technol. 2016, 36, 94–104. [Google Scholar] [CrossRef]
- Wang, H.; Yao, Q.; Wang, C.; Ma, Z.; Sun, Q.; Fan, B.; Jin, C.; Chen, Y. Hydrothermal synthesis of nanooctahedra mnfe2o4 onto the wood surface with soft magnetism, fire resistance and electromagnetic wave absorption. Nanomaterials 2017, 7, 118. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, Y.; Wang, K.; Li, J.; Zhang, S.; Xia, C.; Shi, S.Q.; Cai, L. Improvement of water resistance, dimensional stability, and mechanical properties of poplar wood by rosin impregnation. Eur. J. Wood Wood Prod. 2016, 74, 177–184. [Google Scholar] [CrossRef]
- Lou, Z.; Han, H.; Zhou, M.; Han, J.; Cai, J.; Huang, C.; Zou, J.; Zhou, X.; Zhou, H.; Sun, Z. Synthesis of magnetic wood with excellent and tunable electromagnetic wave-absorbing properties by a facile vacuum/pressure impregnation method. ACS Sustain. Chem. Eng. 2018, 6, 1000–1008. [Google Scholar] [CrossRef]
- Mashkour, M.; Ranjbar, Y. Superparamagnetic Fe3O4@ wood flour/polypropylene nanocomposites: Physical and mechanical properties. Ind. Crops Prod. 2018, 111, 47–54. [Google Scholar] [CrossRef]
- Oka, H.; Terui, M.; Osada, H.; Sekino, N.; Namizaki, Y.; Oka, H.; Dawson, F.P. Electromagnetic wave absorption characteristics adjustment method of recycled powder-type magnetic wood for use as a building Material. IEEE Trans. Magn. 2012, 48, 3498–3500. [Google Scholar] [CrossRef]
- Trey, S.; Olsson, R.T.; Ström, V.; Berglund, L.; Johansson, M. Controlled deposition of magnetic particles within the 3-D template of wood: Making use of the natural hierarchical structure of wood. RSC Adv. 2014, 4, 35678–35685. [Google Scholar] [CrossRef]
- Wang, Y.; Moo, Y.X.; Chen, C.; Gunawan, P.; Xu, R. Fast precipitation of uniform CaCO3 nanospheres and their transformation to hollow hydroxyapatite nanospheres. J. Colloid Interface Sci. 2010, 352, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Liu, Y.; Gao, L.; Zhan, X. Magnetic property, thermal stability, UV-resistance, and moisture absorption behavior of magnetic wood composites. Polym. Compos. 2017, 38, 1646–1654. [Google Scholar] [CrossRef]
- Tenorio, C.; Moya, R.; Salas, C.; Berrocal, A. Evaluation of wood properties from six native species of forest plantations in Costa Rica. Bosque (Valdivia) 2016, 37, 71–84. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monocultures in plantation forestry: Development, benefits, ecosystem services and perspectives for the future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Tenorio, C.; Moya, R. Evaluation of wood properties of four ages of Cedrela odorata trees growing in agroforestry systems with Theobroma cacao in Costa Rica. Agrofor. Syst. 2018, 93, 973–988. [Google Scholar] [CrossRef]
- Moya, R.; Gaitan-Alvarez, J.; Berrocal, A.; Araya, F. Effect of CaCO3 in the wood properties of tropical hardwood species from fast-grown plantation in Costa Rica. BioResources 2020, 15, 4802–4822. [Google Scholar] [CrossRef]
- Gaitán-Álvarez, J.; Moya, R.; Berrocal, A.; Araya, F. In-situ mineralization of calcium carbonate of tropical hardwood species from fast-grown plantations in Costa Rica. Fresenius Environ. Bull. 2020, 29, 9184–9194. [Google Scholar]
- Gaitán-Alvarez, J.; Moya, R.; Mantanis, G.I.; Berrocal, A. Furfurylation of tropical wood species with and without silver nanoparticles: Part I: Analysis with confocal laser scanning microscopy and FTIR spectroscopy. Wood Mater. Sci. Eng. 2021, 1–10. [Google Scholar] [CrossRef]
- Adebawo, F.G.; Naithani, V.; Sadeghifar, H.; Tilotta, D.; Lucia, L.A.; Jameel, H.; Ogunsanwo, O.Y. Morphological and interfacial properties of chemically-modified tropical hardwood. RSC Adv. 2016, 6, 6571–6576. [Google Scholar] [CrossRef]
- Kojima, M.; Yamamoto, H.; Marsoem, S.N.; Okuyama, T.; Yoshida, M.; Nakai, T.; Yamashita, S.; Saegusa, K.; Matsune, K.; Nakamura, K.; et al. Effects of the lateral growth rate on wood quality of Gmelina arborea from 3.5-, 7- and 12-year-old plantations. Ann. For. Sci. 2009, 66, 507. [Google Scholar] [CrossRef]
- Musah, M.; Wang, X.Ñ.; Dickinson, Y.; Ross, R.J.; Rudnicki, M.; Xie, X. Durability of the adhesive bond in cross-laminated northern hardwoods and softwoods. Constr. Build. Mater. 2021, 307, 124267. [Google Scholar] [CrossRef]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Gibson, L.J. The hierarchical structure and mechanics of plant materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S. Evolution of Water Transport and Xylem Structure. Int. J. Plant Sci. 2003, 164, S115–S127. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Chun, S.K.; Miller, R.B.; Chong, S.H.; Kim, A.J. Liquid penetration in different cells of two hardwood species. J. Wood Sci. 2011, 57, 179–188. [Google Scholar] [CrossRef]
- Gaitán-Alvarez, J.; Berrocal, A.; Mantanis, G.I.; Moya, R.; Araya, F. Acetylation of tropical hardwood species from forest plantations in Costa Rica: An FTIR spectroscopic analysis. J. Wood Sci. 2020, 66, 49. [Google Scholar] [CrossRef]
- ASTM D1102-84; Standard Test Method for Ash in Wood. ASTM International: West Conshohocken, PA, USA, 2013; pp. 1–2.
- ASTM D6357-19; Standard test method for Determination of Trace Elements in Coal, Coke, and Combustion Residues from Coal Utilization Processes by Inductively Coupled Plasma Atomic Emission Spectrometry, Inductively Coupled Plasma Mass Spectrometry. ASTM International: West Conshohocken, PA, USA, 2019.
- Gao, X.; Dong, Y.; Wang, K.; Chen, Z.; Yan, Y.; Li, J.; Zhang, S. Improving dimensional and thermal stability of poplar wood via aluminum-based sol-gel and furfurylation combination treatment. BioResources 2017, 12, 3277–3288. [Google Scholar] [CrossRef]
- Gao, H.L.; Wu, G.Y.; Guan, H.T.; Zhang, G.L. In situ preparation and magnetic properties of Fe3O4/wood composite. Mater. Technol. 2012, 27, 101–103. [Google Scholar] [CrossRef]
- Hamaya, T.; Kono, T.; Aono, R.; Nishimoto, K. Research on the properties of magnetic wood. I. Preparation of magnetic wood by means of enzyme dependent reaction. Wood Preserv. 1996, 22, 287–293. [Google Scholar] [CrossRef][Green Version]
- Lin, C.-C.; Ho, J.-M. Structural analysis and catalytic activity of Fe3O4 nanoparticles prepared by a facile co-precipitation method in a rotating packed bed. Ceram. Int. 2014, 40, 10275–10282. [Google Scholar] [CrossRef]
- Yang, L.; Lou, Z.; Han, X.; Liu, J.; Wang, Z.; Zhang, Y.; Wu, X.; Yuan, C.; Li, Y. Fabrication of a novel magnetic reconstituted bamboo with mildew resistance properties. Mater. Today Commun. 2020, 23, 101086. [Google Scholar] [CrossRef]
- Garskaite, E.; Stoll, S.L.; Forsberg, F.; Lycksam, H.; Stankeviciute, Z.; Kareiva, A.; Quintana, A.; Jensen, C.J.; Liu, K.; Sandberg, D. The Accessibility of the cell wall in scots pine (Pinus sylvestris L.) sapwood to colloidal Fe3O4 Nanoparticles. ACS Omega 2021, 6, 21719–21729. [Google Scholar] [CrossRef] [PubMed]
- Barauna, E.E.P.; Lima, J.T.; Monteiro, T.C.; dos Santos, V.B.; dos Santos, J.H. Permeability of Parkia gigantocarpa as affected by wood anatomy. BioResources 2021, 16, 4924–4933. [Google Scholar] [CrossRef]
- França, F.J.; Shmulsky, R.; Ratcliff, T.; Farber, B.; Senalik, C.A.; Ross, R.; Seale, R.D. Interrelationships of specific gravity, stiffness, and strength of yellow pine across five decades. BioResources 2021, 16, 3815–3826. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Merk, V.; Chanana, M.; Gierlinger, N.; Hirt, A.M.; Burgert, I. Hybrid wood materials with magnetic anisotropy dictated by the hierarchical cell structure. ACS Appl. Mater. Interfaces 2014, 6, 9760–9767. [Google Scholar] [CrossRef]
- Segmehl, J.S.; Laromaine, A.; Keplinger, T.; May-Masnou, A.; Burgert, I.; Roig, A. Magnetic wood by in situ synthesis of iron oxide nanoparticles via a microwave-assisted route. J. Mater. Chem. C 2018, 6, 3395–3402. [Google Scholar] [CrossRef]
- Lou, Z.; Zhang, Y.; Zhou, M.; Han, H.; Cai, J.; Yang, L.; Yuan, C.; Li, Y. Synthesis of magnetic wood fiber board and corresponding multi-layer magnetic composite board, with electromagnetic wave absorbing properties. Nanomaterials 2018, 8, 441. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, W.; Yuan, C.; Zhang, Y.; Li, Y.; Yang, L. Fabrication of Fe/C composites as effective electromagnetic wave absorber by carbonization of pre-magnetized natural wood fibers. J. Bioresour. Bioprod. 2019, 4, 43–50. [Google Scholar] [CrossRef]
- Fabián-Plesníková, I.; Sáenz-Romero, C.; Cruz-De-León, J.; Martínez-Trujillo, M.; Sánchez-Vargas, N.M.; Terrazas, T. Heritability and characteristics of resin ducts in Pinus oocarpa stems in Michoacán, Mexico. IAWA J. 2021, 42, 258–278. [Google Scholar] [CrossRef]
- Leggate, W.; Redman, A.; Wood, J.; Bailleres, H.; Lee, D.J. Radial permeability of the hybrid pine (Pinus elliottii × Pinus caribaea) in Australia. BioResources 2019, 14, 4358–4372. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhu, J.Y.; Ragauskas, A.J.; Hu, L. In situ wood delignification toward sustainable applications. Acc. Mater. Res. 2021, 2, 606–620. [Google Scholar] [CrossRef]
- Kojima, E.; Yamasaki, M.; Imaeda, K.; Lee, C.G.; Sugimoto, T.; Sasaki, Y. XRD investigation of mechanical properties of cellulose microfibrils in S1 and S3 layers of thermally modified wood under tensile loading. Wood Sci. Technol. 2021, 55, 955–969. [Google Scholar] [CrossRef]
- Li, C.; Weng, Q.; Chen, J.-B.; Li, M.; Zhou, C.; Chen, S.; Zhou, W.; Guo, D.; Lu, C.; Chen, J.-C.; et al. Genetic parameters for growth and wood mechanical properties in Eucalyptus cloeziana F. Muell. New For. 2017, 48, 33–49. [Google Scholar] [CrossRef]
- Lou, Z.; Yuan, C.; Zhang, Y.; Li, Y.; Cai, J.; Yang, L.; Wang, W.; Han, H.; Zou, J. Synthesis of porous carbon matrix with inlaid Fe3C/Fe3O4 micro-particles as an effective electromagnetic wave absorber from natural wood shavings. J. Alloys Compd. 2019, 775, 800–809. [Google Scholar] [CrossRef]
- Kania, A.; Berent, K.; Mazur, T.; Sikora, M. 3D printed composites with uniform distribution of Fe3O4 nanoparticles and magnetic shape anisotropy. Addit. Manuf. 2021, 46, 102149. [Google Scholar] [CrossRef]
- Echeverría, J.C.; Moriones, P.; Garrido, J.J.; Ugarte, M.D.; Cervera, L.; Garaio, E.; Gómez-Polo, C.; Pérez-Landazábal, J.I. Steering the synthesis of Fe3O4 nanoparticles under sonication by using a fractional factorial design. Mater. Chem. Phys. 2021, 270, 124760. [Google Scholar] [CrossRef]
- Barabaszová, K.Č.; Holešová, S.; Hundáková, M.; Mohyla, V. Mechanically treated vermiculite particles in PCL/vermiculite thin films. Mater. Today Proc. 2022, 52, 239–247. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
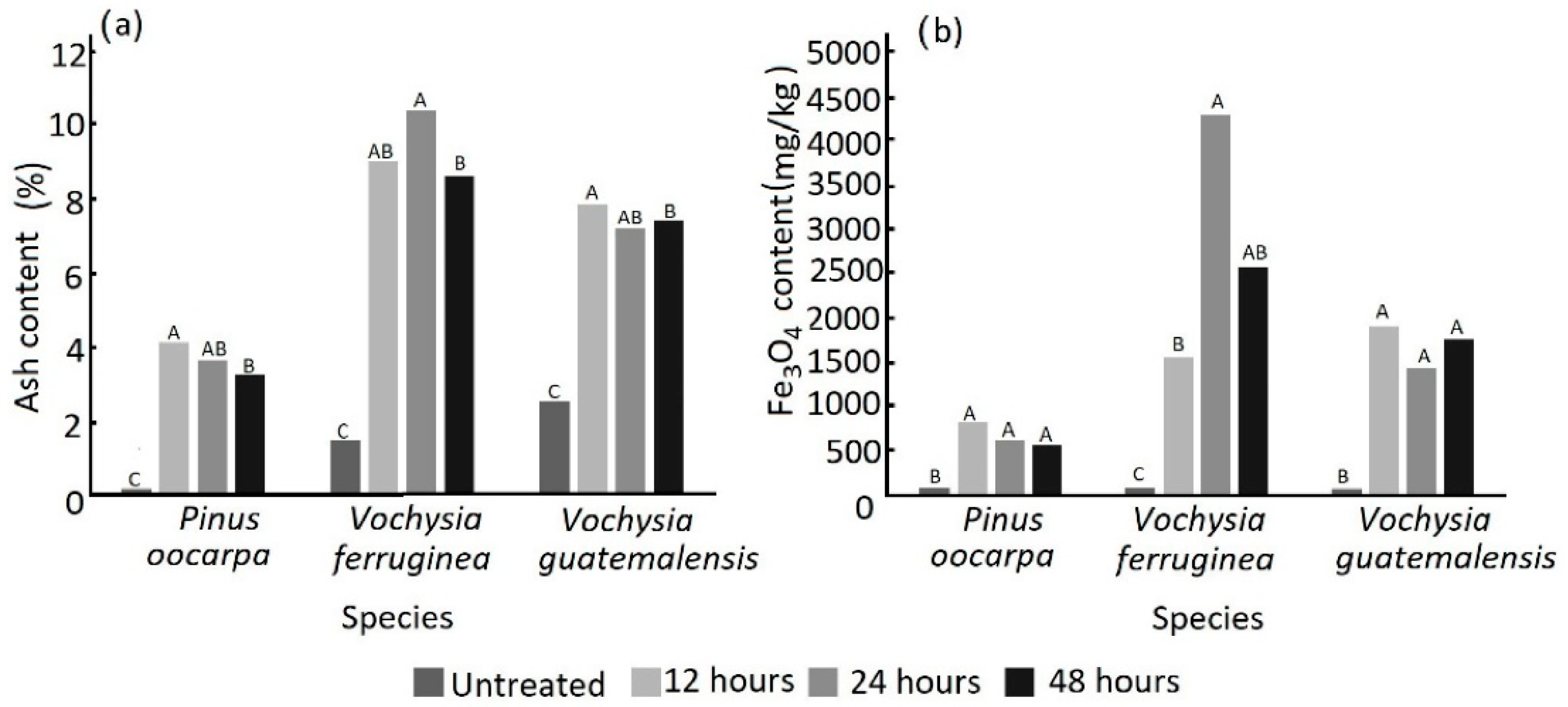
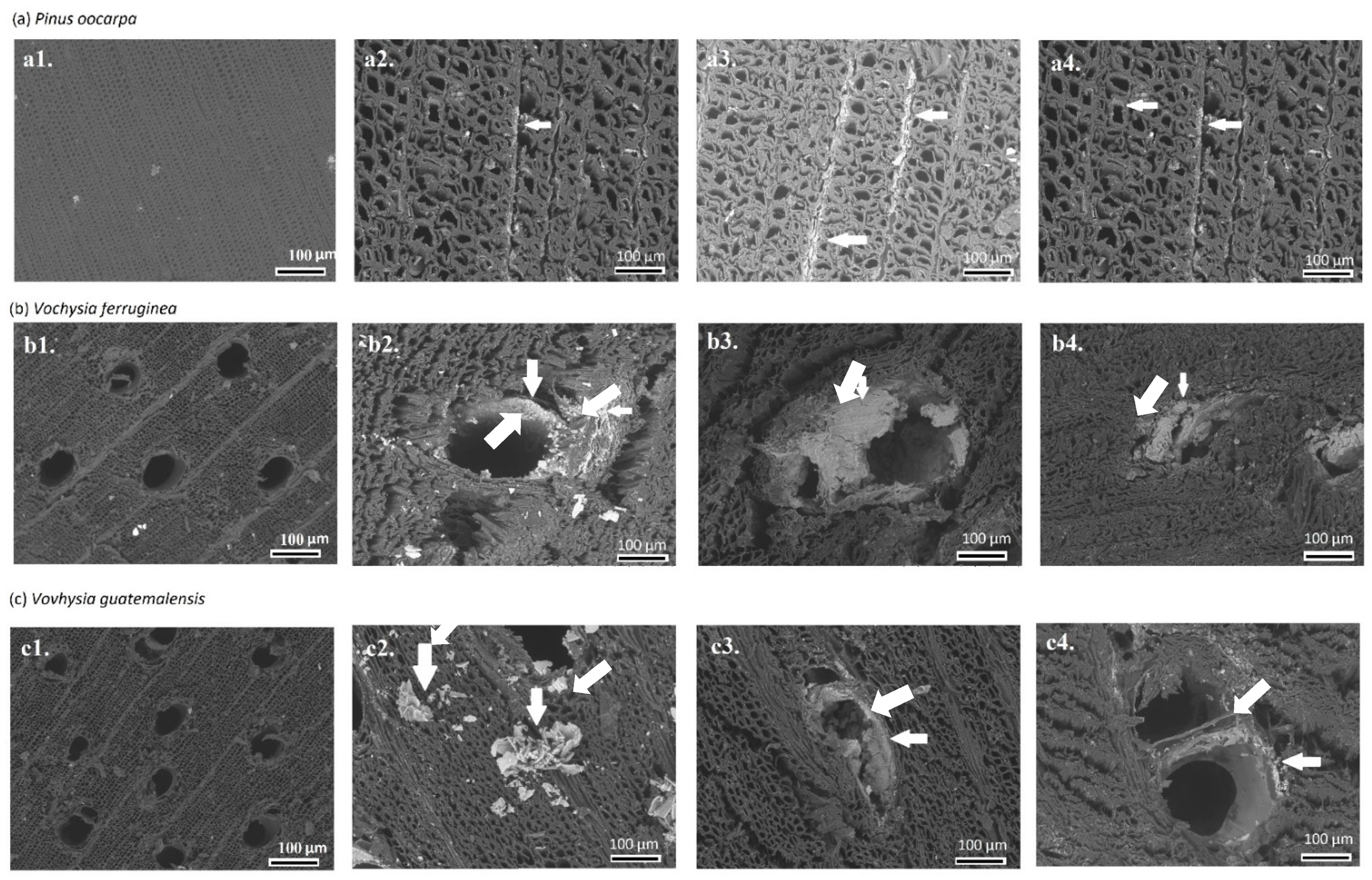
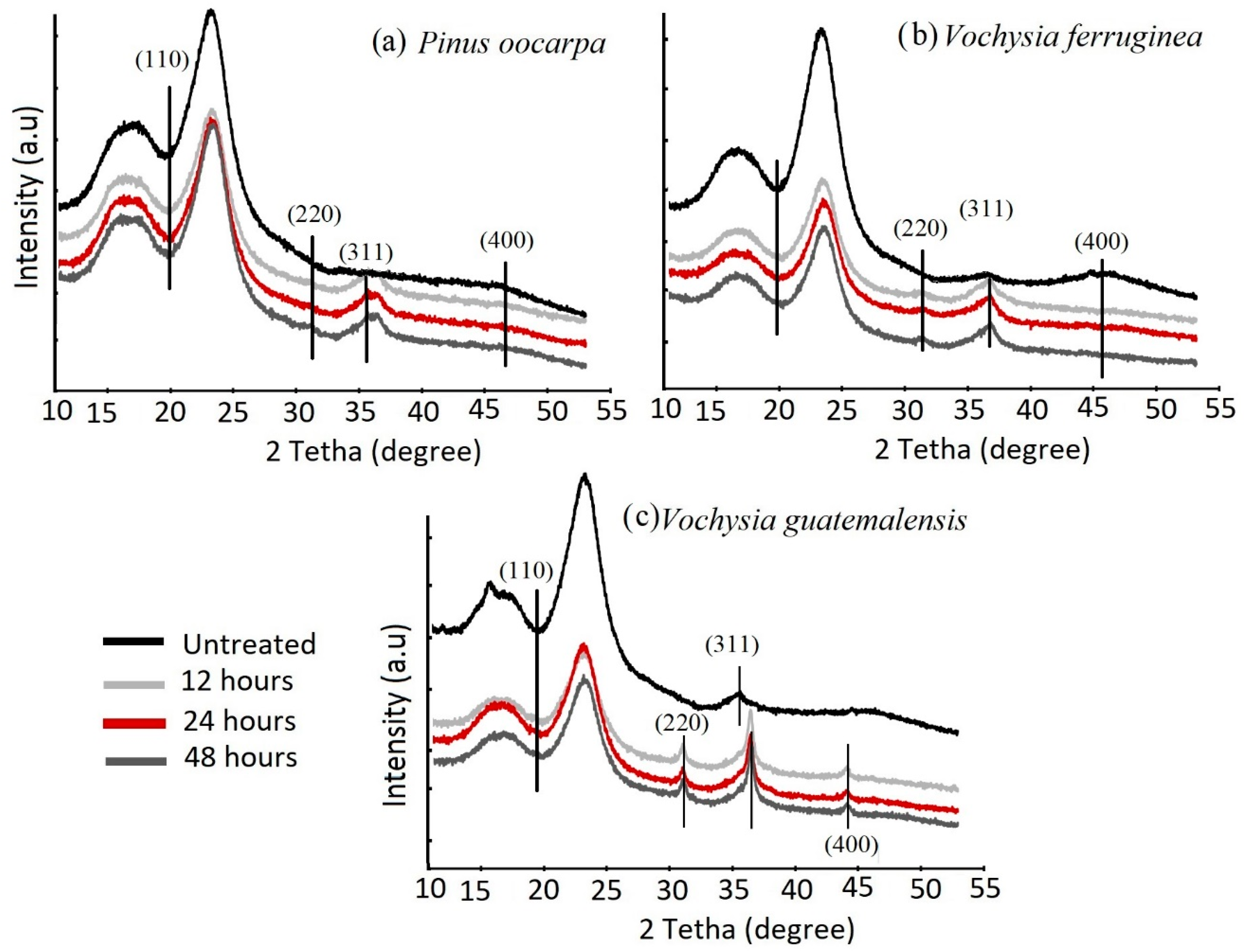

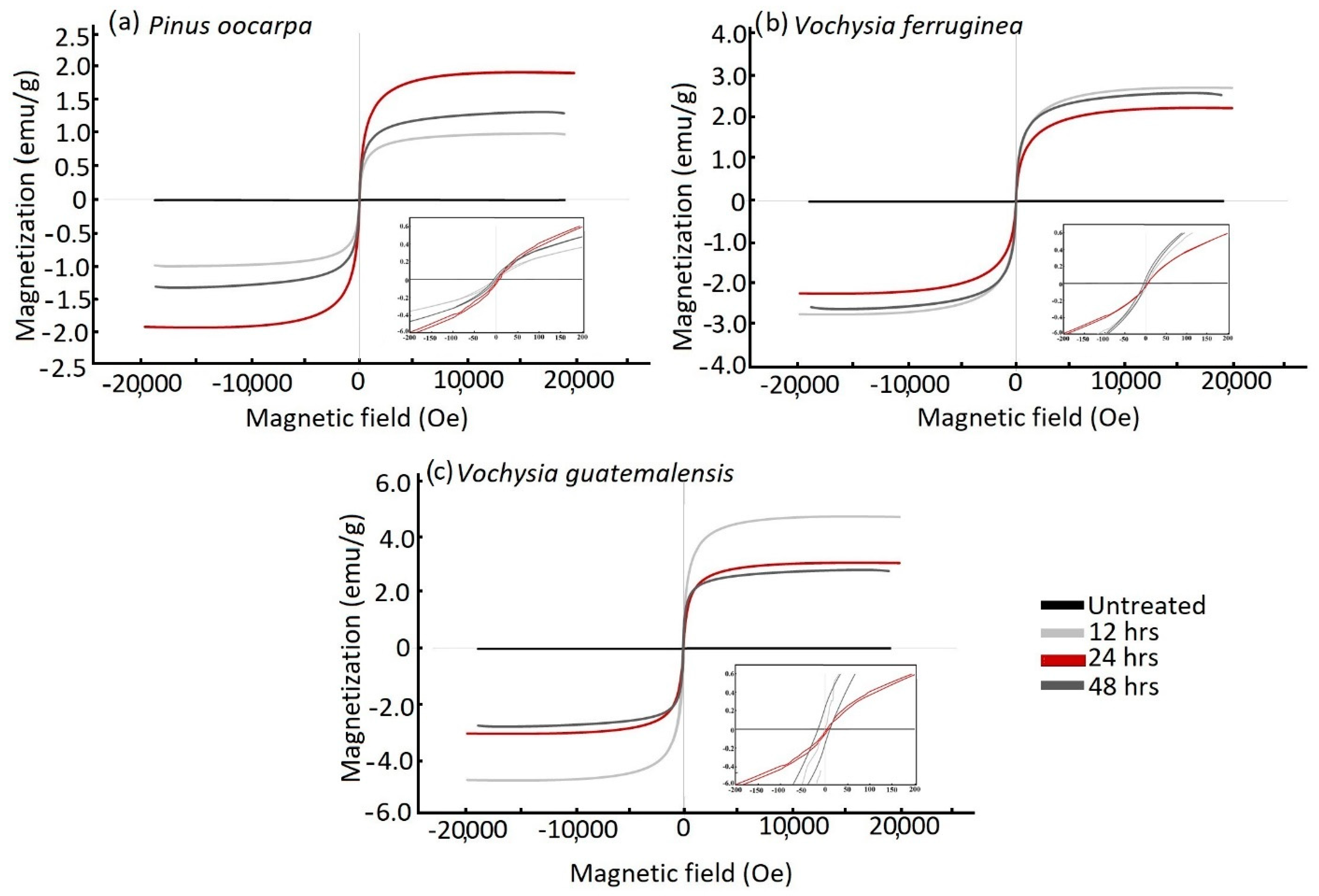

| Species | Treatment (h) | Weight Gain (%) | Absorption (L/m3) | Density (g/cm3) |
|---|---|---|---|---|
| Pinus oocarpa | Untreated | - | - | 0.57 (2.00) |
| 12 | 3.53 (55.48) A | 9.78 (11.70) A | 0.36 (4.35) A | |
| 24 | 2.10 (16.21) AB | 7.63 (13.44) B | 0.37 (4.29) A | |
| 48 | 1.48 (28.36) B | 5.52 (23.30) C | 0.38 (3.98) A | |
| Vochysia ferruginea | Untreated | - | - | 0.38 (3.40) |
| 12 | 6.49 (13.32) A | 12.70 (9.48) A | 0.20 (3.82) A | |
| 24 | 6.09 (8.96) A | 12.00 (9.22) A | 0.20 (2.40) A | |
| 48 | 4.21 (35.20) B | 8.30 (34.05) B | 0.20 (2.05) A | |
| Vochysia guatemalensis | Untreated | - | - | 0.37 (3.01) |
| 12 | 5.38 (15.50) A | 14.15 (10.00) A | 0.27 (7.59) A | |
| 24 | 6.36 (48.96) A | 17.78 (45.12) A | 0.29 (4.10) A | |
| 48 | 4.11 (15.06) A | 11.07 (10.13) A | 0.28 (5.61) A |
| Treatment (h) | Nanoparticle Size (nm) | ||
|---|---|---|---|
| Pinus oocarpa | Vochysia ferruginea | Vochysia guatemalensis | |
| 12 | 7.27 (3.13) B | 19.23 (1.98) A | 12.58 (1.78) A |
| 24 | 7.84 (2.15) AB | 18.19 (2.45) AB | 12.74 (2.51) A |
| 48 | 8.49 (2.78) A | 17.88 (1.96) B | 12.58 (2.65) A |
| Species | Treatment (h) | Ms (emu/g) | Hc (Oe) | Mr (emu/g) | Experimental Percentage (%) |
|---|---|---|---|---|---|
| Pinus oocarta | 12 | 1.05 B | 4.39 A | 0.01 B | 6.83 B |
| 24 | 1.83 B | 3.87 A | 0.01 B | 11.88 B | |
| 48 | 1.10 B | 3.76 A | 0.02 B | 7.16 B | |
| Vochysia ferruginea | 12 | 2.98 B | 2.45 A | 0.12 B | 19.40 B |
| 24 | 2.34 B | 0.73 A | 0.00 B | 15.22 B | |
| 48 | 2.21 B | 2.34 A | 0.02 B | 9.56 B | |
| Vochysia guatamalensis | 12 | 3.74 B | 11.87 A | 0.17 A | 24.35 B |
| 24 | 3.05 B | 7.08 A | 0.12 A | 19.87 B | |
| 48 | 3.29 B | 17.73 A | 0.25 A | 21.38 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya, R.; Gaitán-Álvarez, J.; Berrocal, A.; Merazzo, K.J. In Situ Synthesis of Fe3O4 Nanoparticles and Wood Composite Properties of Three Tropical Species. Materials 2022, 15, 3394. https://doi.org/10.3390/ma15093394
Moya R, Gaitán-Álvarez J, Berrocal A, Merazzo KJ. In Situ Synthesis of Fe3O4 Nanoparticles and Wood Composite Properties of Three Tropical Species. Materials. 2022; 15(9):3394. https://doi.org/10.3390/ma15093394
Chicago/Turabian StyleMoya, Roger, Johanna Gaitán-Álvarez, Alexander Berrocal, and Karla J. Merazzo. 2022. "In Situ Synthesis of Fe3O4 Nanoparticles and Wood Composite Properties of Three Tropical Species" Materials 15, no. 9: 3394. https://doi.org/10.3390/ma15093394
APA StyleMoya, R., Gaitán-Álvarez, J., Berrocal, A., & Merazzo, K. J. (2022). In Situ Synthesis of Fe3O4 Nanoparticles and Wood Composite Properties of Three Tropical Species. Materials, 15(9), 3394. https://doi.org/10.3390/ma15093394








