Luminescence Properties and Energy Transfer of Eu3+, Bi3+ Co-Doped LuVO4 Films Modified with Pluronic F-127 Obtained by Sol–Gel
Abstract
:1. Introduction
2. Materials and Methods
Synthesis
3. Results and Discussion
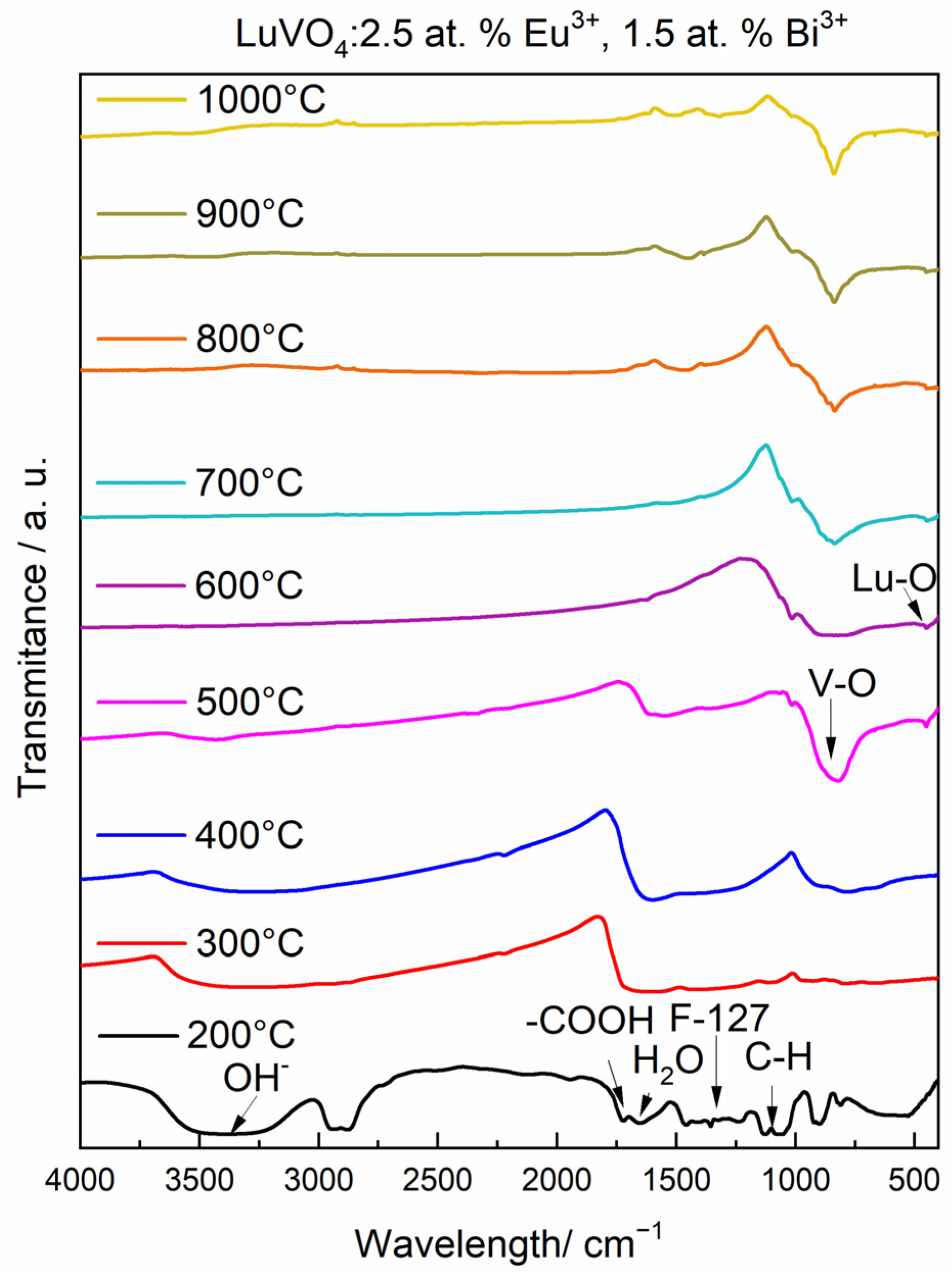
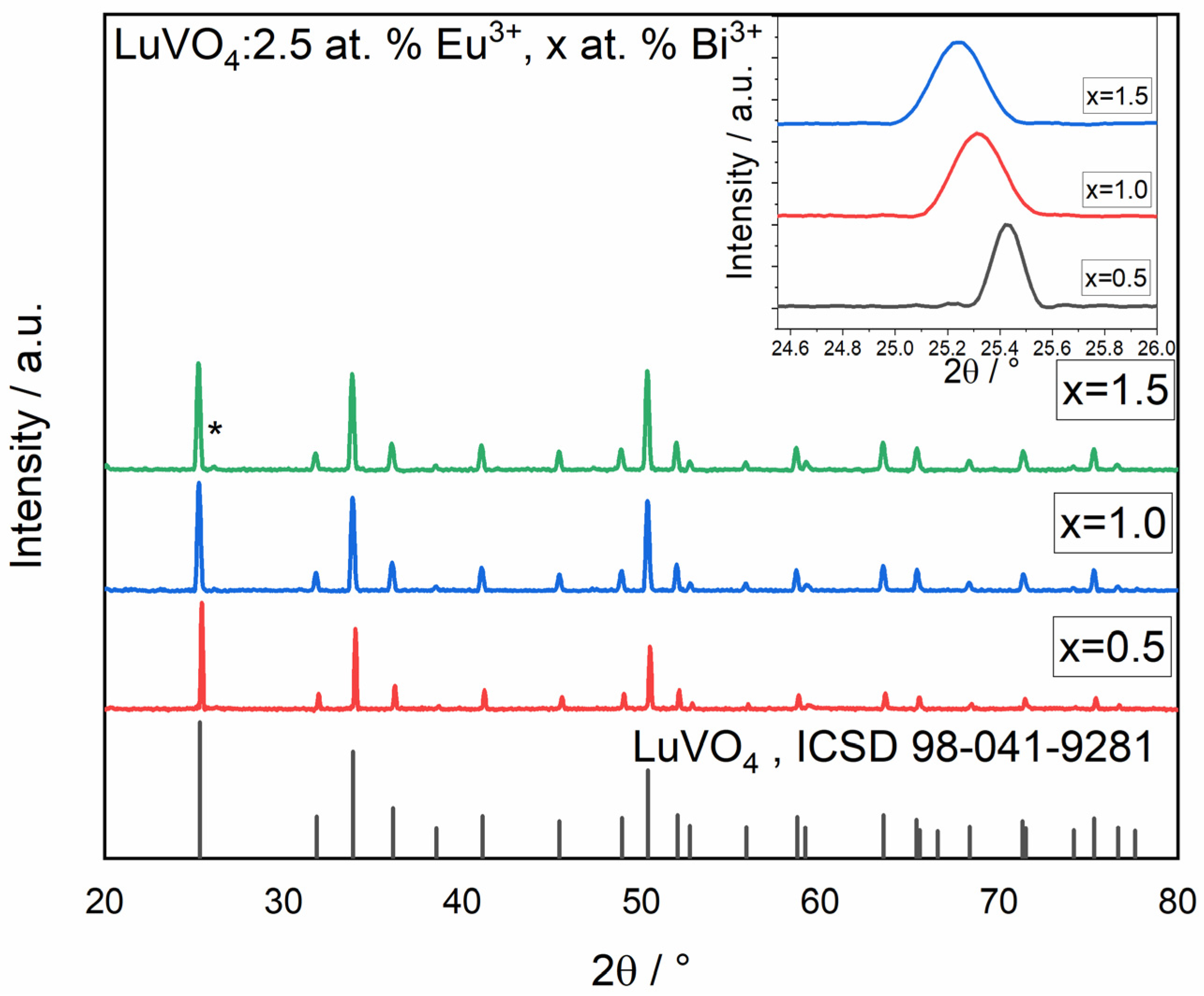
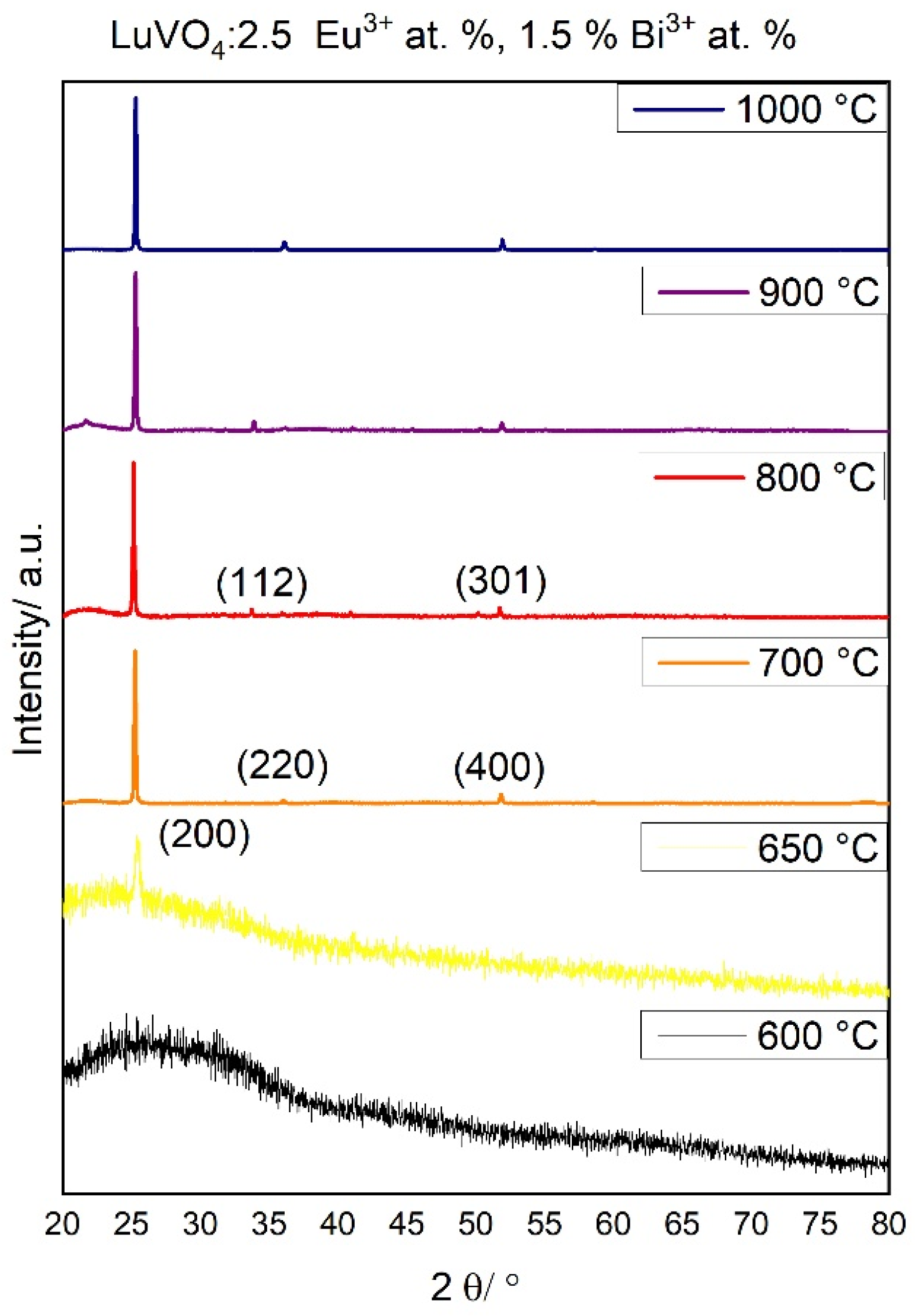
Chemical Evolution and Structural Properties
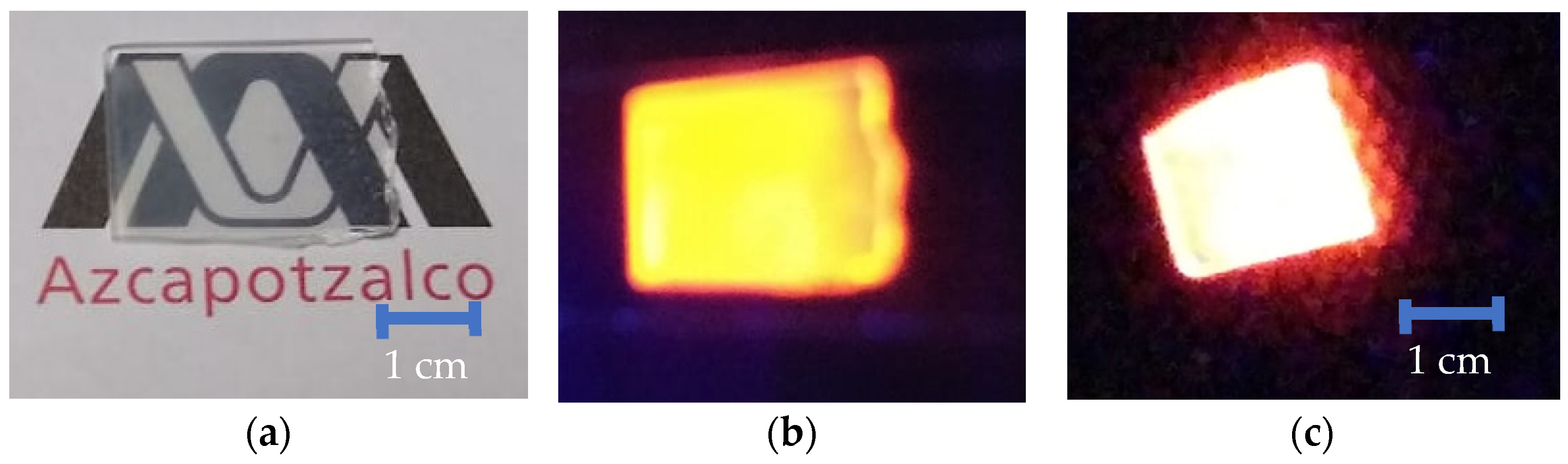
4. Morphological Studies
5. Photoluminescence Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, Y.; Hu, J.; Li, B.; Lin, L.; Li, Y.; Liu, F.; Li, X.Y. Tailoring the Coordination Environment of Cobalt in a Single-Atom Catalyst through Phosphorus Doping for Enhanced Activation of Peroxymonosulfate and thus Efficient Degradation of Sulfadiazine. Appl. Catal. B 2022, 312, 121408. [Google Scholar] [CrossRef]
- Chen, D.; Yu, Y.; Huang, P.; Lin, H.; Shan, Z.; Zeng, L.; Yang, A.; Wang, Y. Color-tunable luminescence for Bi3+/Ln3+: YVO4 (Ln = Eu, Sm, Dy, Ho) nanophosphors excitable by near-ultraviolet light. J. Eur. Ceram. Soc. 2010, 35, 859–869. [Google Scholar] [CrossRef]
- Chen, D.; Xiang, W.; Liang, X.; Zhong, J.; Yu, H.; Ding, M.; Lu, H.; Ji, Z. Advances in transparent glass-ceramic phosphors for white light-emitting diodes—A review. J. Eur. Ceram. Soc. 2014, 35, 859–869. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, S.; Wang, F.; Yan, H. Layer-Dependent Electronic and Optical Properties of 2D Black Phosphorus: Fundamentals and Engineering. Laser Photonics Rev. 2021, 15, 2000399. [Google Scholar] [CrossRef]
- Ueda, J.; Tanabe, S. Review of luminescent properties of Ce3+-doped garnet phosphors: New insight into the effect of crystal and electronic structure. Opt. Mater. 2019, 1, 100018. [Google Scholar] [CrossRef]
- Gupta, A.D.; Rene, E.R.; Giri, B.S.; Pandey, A.; Singh, H. Adsorptive and photocatalytic properties of metal oxides towards arsenic remediation from water: A review. J. Environ. Chem. Eng. 2021, 9, 106376. [Google Scholar] [CrossRef]
- Smet, P.F.; Moreels, I.; Hens, Z.; Poelman, D. Luminescence in sulfides: A rich history and a bright future. Materials 2010, 3, 2834–2883. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Li, G.; Zhao, M.; Yang, E.; Li, L. Lattice defect quenching effects on luminescence properties of Eu3+ doped YVO4 nanoparticles. J. Nanopart. Res. 2013, 1, 1996. [Google Scholar] [CrossRef]
- Stopikowska, N.; Runowski, M.; Woźny, P.; Lis, S.; Du, P. Generation of Pure Green Up-Conversion Luminescence in Er3+ Doped and Yb3+-Er3+ Co-Doped YVO4 Nanomaterials under 785 and 975 nm Excitation. J. Nanomater. 2022, 12, 799. [Google Scholar] [CrossRef]
- Piotrowski, W.; Dalipi, L.; Elzbieciak-Piecka, K.; Bednarkiewicz, A.; Fond, B.; Marciniak, L. Self-Referenced Temperature Imaging with Dual Light Emitting Diode Excitation and Single-Band Emission of AVO4: Eu3+ (A = Y, La, Lu, Gd) Nanophosphors. Adv. Photonics 2022, 3, 2100139. [Google Scholar] [CrossRef]
- Getz, M.N.; Nilsen, O.; Hansen, P.-A. Sensors for optical thermometry based on luminescence from layered YVO4: Ln3+ (Ln = Nd, Sm, Eu, Dy, Ho, Er, Tm, Yb) thin films made by atomic layer deposition. Sci. Rep. 2019, 9, 10247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; He, H.; Zhu, W.; Zheng, A. LuVO4:Ln3+ (Ln = Sm, Eu, Dy, Er, and Tm) with high uniform size and morphology: Controlled synthesis, growth mechanism and optical properties. CrystEngComm 2011, 13, 6471–6480. [Google Scholar] [CrossRef]
- Fang, C.; Wu, D.; Xie, J.; Li, Y.; Lou, Y.; Pu, Y. Preparation and luminescence properties of Ca2+-doped LaVO4: Dy3+ phosphors. J. Mol. Struct. 2022, 1266, 133497. [Google Scholar] [CrossRef]
- Cao, L.; Wang, Z.; Cai, P.; Hu, X.; Zhang, B.; Chu, X.; Li, S. Up-conversion luminescence properties of LuVO4: Yb3+/Tm3+/Er3+ submicron materials for high sensitivity temperature probing. J. Mater. Sci. Mater. Electron. 2021, 32, 28088–28097. [Google Scholar] [CrossRef]
- Tymiński, A.; Grzyb, T. Enhancement of the up-conversion luminescence in LaVO4 nanomaterials by doping with M2+, M4+ (M2+ = Sr2+, Ba2+, Mg2+; M4+= Sn4+) ions. J. Alloys Compd. 2019, 782, 69–80. [Google Scholar] [CrossRef]
- Bai, Y.; Bai, C.; Mo, G.; Guo, Y.; Jia, M.; Huo, X. LuVO4: Eu3+ nanorods: Synthesis and luminescence. J. Mater. Sci. Mater. Electron. 2018, 29, 3189–3193. [Google Scholar] [CrossRef]
- de Morales-Ramírez, A.J.; Domínguez, F.S.; Medina, D.; David, J.-V.; Margarita, G.-H.; Dorantes-Rosales, H. Synthesis and photoluminescent properties of Y2O3:Eu3+ thin films prepared from F127-containing solution. J. Ceram. Soc. Jpn. 2014, 122, 701–707. [Google Scholar] [CrossRef] [Green Version]
- de Morales-Ramírez, A.J.; García Hernández, M.; Yepez Ávila, J.; Carrillo Romo, F.; García Murillo, A.; de la Rosa, E.; Garibay Febles, V.; Reyes Miranda, J. Eu3+, Bi3+ codoped Lu2O3 nanopowders: Synthesis and luminescent properties. Mater. Res. 2013, 18, 1365–1371. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Yang, C.; Ji, B.; Lin, J.; Tomie, T. Theory of multiphoton photoemission disclosing excited states in conduction band of individual TiO2 nanoparticles. Chin. Phys. B 2021, 30, 114214. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.; Jiang, L. Luminescent LuVO4:Ln3+ (Ln = Eu, Sm, Dy, Er) hollow porous spheres for encapsulation of biomolecules. Opt. Mater. 2015, 48, 18–24. [Google Scholar] [CrossRef]
- Krumpel, H.A.; Van der Kolk, E.; Cavalli, E.; Boutinaud, P.; Bettinelli, M.; Dorenbos, P. Lanthanide 4f-level location in AVO4:Ln3+ (A = La, Gd, Lu) crystals. J. Condens. Matter Phys. 2009, 21, 11550–11558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leow, T.; Liu, H.; Hussin, R.; Ibrahim, Z.; Deraman, K.; Lintang, H.O.; Shamsuri, W.N.W. Effects of Eu3+ and Dy3+ doping or co-doping on optical and structural properties of BaB2Si2O8 phosphor for white LED applications. J. Rare Earths 2016, 34, 21–29. [Google Scholar] [CrossRef]
- Babu, J.K.; Rao, B.S.; Suresh, K.; Sridhar, M.; Murthy, K.V.R. 3Photoluminescence study of activator ions (Eu, Tb) co-doped in different host environments (CaO, CaSiO3, CaAl2O4 and CaSiAl2O6). Mater. Today Proc. 2019, 18, 2530–2539. [Google Scholar] [CrossRef]
- Alvarez-Ramos, M.E.; Carrillo-Torres, R.C.; Sánchez-Zeferino, R.; Caldiño, U.; Alvarado-Rivera, J. Co-emission and energy transfer of Sm3+ and/or Eu3+ activated zinc-germanate-tellurite glass as a potential tunable orange to reddish-orange phosphor. J. Non-Cryst. Solids 2019, 521, 119462. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, Y.; Wu, X.; Du, L.; Pei, M.; Hai, O. Luminescence characteristics of a novel Tm/Eu co-doped polychromatic tunable phosphor. J. Solid State Chem. 2021, 294, 121869. [Google Scholar] [CrossRef]
- Wen, F.; Sun, L.; Kim, J. Photoluminescent properties of Sb3+-doped and (Sb3+, Eu3+) co-doped YBO3 phosphors prepared via hydrothermal method and solid-state process. Front. Chem. Sci. Eng. 2011, 5, 429–434. [Google Scholar] [CrossRef]
- Yashodha, S.R.; Dhananjaya, N.; Yogananda, H.S.; Vinutha, K.; Ravikumar, C.R. Preparation and characterization of multifunctional Li+ co-doped LaOCl: Eu3+ for sensor, phosphor and catalytic applications. Inorg. Chem. Commun. 2022, 139, 109402. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Guo, Z.; Mo, F.; Wu, Z.C. A zero-thermal-quenching and color-tunable phosphor LuVO4: Bi3+, Eu3+ for NUV LEDs. Dyes Pigm. 2018, 156, 67–73. [Google Scholar] [CrossRef]
- de Morales-Ramírez, A.J.; Margarita, G.-H.; Medina-Velázquez, D.; Ruiz-Guerrero, R.; Juárez-López, F.; Reyes-Miranda, J. Luminescence properties of co-doped Eu3+, Bi3+ Lu2O3/polyvinylpyrrolidone films. Coatings 2018, 8, 434. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Zhang, H.; Luo, Z.; Liang, J.; Wu, X. Facile Preparation of YVO4: RE Films and the Investigation of Photoluminescence. Coatings 2022, 12, 461. [Google Scholar] [CrossRef]
- Tsotetsi, D.; Dhlamini, M.; Mbule, P. Sol-gel derived mesoporous TiO2: Effects of non-ionic co-polymers on the pore size, morphology, specific surface area and optical properties analysis. Results Mater. 2022, 14, 100266. [Google Scholar] [CrossRef]
- Arconada, N.; Castro, Y.; Durán, A. Photocatalytic properties in aqueous solution of porous TiO2-anatase films prepared by sol-gel process. Appl. Catal. A Gen. 2010, 385, 101–107. [Google Scholar] [CrossRef]
- Obregón, S.; Rodríguez-González, V. Photocatalytic TiO2 thin films and coatings prepared by sol-gel processing: A brief review. J. Sol-Gel Sci. Technol. 2021, 102, 125–141. [Google Scholar] [CrossRef]
- Yang, J.; Peterlik, H.; Lomoschitz, M.; Schubert, U. Preparation of mesoporous titania by surfactant-assisted sol-gel processing of acetaldoxime-modified titanium alkoxides. J. Non-Cryst. Solids 2010, 356, 1217–1227. [Google Scholar] [CrossRef]
- Youn, J.; Choi, J.H.; Lee, S.; Lee, S.W.; Moon, B.K.; Song, J.E.; Khang, G. Pluronic F-127/silk fibroin for enhanced mechanical property and sustained release drug for tissue engineering biomaterial. Materials 2021, 14, 1287. [Google Scholar] [CrossRef]
- de Morales-Ramírez, A.J.; García-Hernández, M.; Murillo, A.G.; Carrillo-Romo, F.; Palmerín, J.M.; Medina-Velázquez, D.; Carrera-Jota, M.L. Structural and Luminescence Properties of Lu2O3:Eu3+ F127 Tri-Block Copolymer Modified Thin Films Prepared by Sol-Gel Method. Materials 2013, 6, 713–725. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Song, H.; Yan, D.; Zhu, H.; Wang, Y.; Xu, S.; Biao, D.; Liu, Y. YVO4: Eu 3+, Bi 3+ UV to visible conversion nano-films used for organic photovoltaic solar cells. J. Mater. Chem. 2011, 21, 12331–12336. [Google Scholar] [CrossRef]
- Luo, M.; Sun, T.Y.; Wang, J.H.; Yang, P.; Gan, L.; Liang, L.L.; Yu, X.F.; Gong, X.H. Synthesis of carboxyl-capped and bright YVO4: Eu, Bi nanoparticles and their applications in immunochromatographic test strip assay. Mater. Res. Bull. 2013, 48, 4454–4459. [Google Scholar] [CrossRef]
- Zeng, L.; Tang, Q.; Lin, B.; Zhou, H.; Liu, G.; Li, Y.; Chen, D. Fabrication of Bi3+/Ln3+: LuVO4 (Ln = Eu, Sm, Dy, Ho) nanophosphors and its color-tunable optical performance. J. Lumin. 2018, 194, 667–674. [Google Scholar] [CrossRef]
- Pawlik, N.; Szpikowska-Sroka, B.; Pisarska, J.; Goryczka, T.; Pisarski, W.A. Reddish-Orange Luminescence from BaF2: Eu3+ Fluoride Nanocrystals Dispersed in Sol-Gel Materials. Materials 2019, 12, 3735. [Google Scholar] [CrossRef]
- Amakali, T.; Daniel, L.S.; Uahengo, V.; Dzade, N.Y.; De Leeuw, N.H. Structural and optical properties of ZnO thin films prepared by molecular precursor and sol-gel methods. Crystals 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Hwang, K.S.; Kim, T.S. The microscopic origin of residual stress for flat self-actuating piezoelectric cantilevers. Nanoscale Res. Lett. 2011, 6, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soli, J.; Kachbouri, S.; Elaloui, E.; Charnay, C. Role of surfactant type on morphological, textural, optical, and photocatalytic properties of ZnO nanoparticles obtained by modified sol-gel. J. Sol-Gel Sci. Technol. 2021, 100, 271–285. [Google Scholar] [CrossRef]
- Feng, Q.; Qian, Y.; Wang, H.; Hou, W.; Peng, X.; Xie, S.; Wang, S.; Xie, L. Donor arylmethylation toward horizontally oriented TADF emitters for efficient electroluminescence with 37% external quantum efficiency. Adv. Opt. Mater. 2022, 10, 2102441. [Google Scholar] [CrossRef]
- Song, J.; Kim, K.H.; Kim, E.; Moon, C.K.; Kim, Y.H.; Kim, J.J.; Yoo, S. Lensfree OLEDs with over 50% external quantum efficiency via external scattering and horizontally oriented emitters. Nat. Commun. 2018, 9, 3207. [Google Scholar] [CrossRef]
- Murugan, C.; Subramani, K.; Subash, R.; Sathish, M.; Pandikumar, A. High-Performance High-Voltage Symmetric Supercapattery Based on a Graphitic Carbon Nitride/Bismuth Vanadate Nanocomposite. Energy Fuels 2020, 34, 16858–16869. [Google Scholar] [CrossRef]
- Liang, Z.; Cao, Y.; Qin, H.; Jia, D. Low-heating solid-state chemical synthesis of monoclinic scheelite BiVO4 with different morphologies and their enhanced photocatalytic property under visible light. Mater. Res. Bull. 2016, 84, 397–402. [Google Scholar] [CrossRef]
- Kumar, V.; Khan, A.F.; Chawla, S. Intense red-emitting multi-rare-earth doped nanoparticles of YVO4 for spectrum conversion towards improved energy harvesting by solar cells. J. Phys. D 2013, 46, 365101. [Google Scholar] [CrossRef]
- Brack, P.; Sagu, J.S.; Peiris, T.N.; McInnes, A.; Senili, M.; Wijayantha, K.U.; Marken, F.; Selli, E. Aerosol—assisted CVD of bismuth vanadate thin films and their photoelectrochemical properties. Chem. Vap. Depos. 2015, 21, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Ghosh, S.P.; Pradhan, D.; Sahu, P.K.; Kar, J.P. Morphological and electrical study of porous TiO2 films with various concentrations of Pluronic F-127 additive. J. Porous Mater. 2021, 28, 231–238. [Google Scholar] [CrossRef]
- González-Penguelly, B.; de Morales-Ramírez, A.J.; Rodríguez-Rosales, M.G.; Rodríguez-Nava, O.; Carrera-Jota, M.L. New infrared-assisted method for sol-gel derived ZnO: Ag thin films: Structural and bacterial inhibition properties. Mater. Sci. Eng. C 2017, 78, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.; Peng, M.; Zhang, Q.; Qiu, J. Abnormal Anti-Quenching and Controllable Multi-Transitions of Bi3+ Luminescence by Temperature in a Yellow-Emitting LuVO4:Bi3+ Phosphor for UV-Converted White LEDs. Chem. Eur. J. 2014, 20, 11522–11530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xia, Z.; Liu, H.; Mi, R.; Hui, Z. Luminescence properties and energy transfer of Bi3+/Eu3+ codoped Ca10(PO4)6F2 phosphors. Mater. Res. Bull. 2013, 48, 3513–3517. [Google Scholar] [CrossRef]
- Narro-García, R.; Desirena, H.; López-Luke, T.; Guerrero-Contreras, J.; Jayasankar, C.K.; Quintero-Torres, R.; Rosa, E.D.L. Spectroscopic properties of Eu3+/Nd3+ co-doped phosphate glasses and opaque glass-ceramics. Opt. Mater. 2015, 46, 34–39. [Google Scholar] [CrossRef]
- Deopa, N.; Kaur, S.; Prasad, A.; Joshi, B.; Rao, A.S. Spectral studies of Eu3+ doped lithium lead alumino borate glasses for visible photonic applications. Opt. Laser Technol. 2018, 108, 434–440. [Google Scholar] [CrossRef]
- Wu, X.; Du, L.; Ren, Q.; Hai, O. Luminescence properties and energy transfer of Tm3+–Eu3+ double-doped LiLaSiO4 phosphors. J. Mater. Sci. Mater. 2021, 32, 17662–17673. [Google Scholar] [CrossRef]
- Bispo-Jr, A.G.; Lima, S.A.M.; Pires, A.M. Energy transfer between terbium and europium ions in barium orthosilicate phosphors obtained from sol-gel route. J. Lumin. 2018, 199, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, O.G.; Fei, M.; Wei, R.; Teng, L.; Zheng, Z.; Guo, H. Energy transfer and white luminescence in Bi3+/Eu3+ co-doped oxide glasses. J. Lumin. 2020, 219, 116918. [Google Scholar] [CrossRef]
- Ruelle, N.; Fouassier, M.P.T. Cathodoluminescent properties and energy transfer in red calcium sulfide phosphors (CaS: Eu, Mn). Jpn. J. Appl. Phys. 1992, 31, 2786. [Google Scholar] [CrossRef]
- Delgado, T.; Gartmann, N.; Waltfort, B.; LaMattina, F.; Pollnau, M.; Rosspeintne, A.; Afshani, J.; Olchowka, J.; Hageman, H. Fundamental Loading-Curve Characteristics of the Persistent Phosphor SrAl2O4:Eu2+,Dy3+,B3+: The Effect of Temperature and Excitation Density. Adv. Photonics Res. 2022, 4, 2100179. [Google Scholar] [CrossRef]
- Dutta, S.; Soma, S.; Sharma, K. Excitation spectra and luminescence decay analysis of K+ compensated Dy3+ doped CaMoO4 phosphors. RSC Adv. 2015, 10, 7380–7387. [Google Scholar] [CrossRef]
- Ninjbadgar, T.; Garnweitner, G.; Börger, A.; Goldenberg, L.; Sakhno, O.; Stumpe, J. Synthesis of Luminescent ZrO2:Eu3+ Nanoparticles and Their Holographic Sub-Micrometer Patterning in Polymer Composites. Adv. Funct. Mater. 2009, 19, 1819–1825. [Google Scholar] [CrossRef]
- Byrd, R.H.; Hribar, M.E.; Nocedal, J. An interior point algorithm for large-scale nonlinear programming. SIAM J. Optim. 1999, 9, 877–900. [Google Scholar] [CrossRef]
- Coleman, T.F.; Li, Y. On the convergence of interior-reflective Newton methods for nonlinear minimization subject to bounds. Math. Program. 1994, 67, 189–224. [Google Scholar] [CrossRef]
- Zhou, W.; Deng, S.; Rong, C.; Xie, Q.; Lian, S.; Zhang, J.; Lia, C.; Yu, L. Synthesis, crystal structure and luminescence of a near ultraviolet-green to red spectral converter BaY2S4:Eu2+, Er3+. RSC Adv. 2013, 3, 16781–16787. [Google Scholar] [CrossRef]
- Broadbent, A.D. A critical review of the development of the CIE1931 RGB colormatching functions. Color Res. Appl. 2004, 29, 267–272. [Google Scholar] [CrossRef]
- Schanda, J. Colorimetry: Understanding the CIE System; Wiley-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Jin, M.; Li, X.; Yan, F.; Chen, W.; Jiang, L.; Zhang, X. The Effects of Low-Color-Temperature Dual-Primary-Color Light-Emitting Diodes on Three Kinds of Retinal Cells. J. Photochem. Photobiol. 2021, 214, 112099. Available online: https://n9.cl/nta6t (accessed on 8 December 2022). [CrossRef]
- Wang, S.; Wang, G.; Liang, X.; Li, D.; Zhang, Z.; Guo, K.; Li, J.; Miao, Y.; Wang, H. Dyes and pigments: Introduction of chlorine into spiro[fluorene-9,9′-xanthene] based luminophore for high color purity single-molecule white emitter. Dye. Pigment. 2022, 204, 110450. Available online: https://n9.cl/jb25m (accessed on 8 December 2022). [CrossRef]
- Krishnan, M.L.; Neethish, M.M.; Kumar, V.R.K. Structural and optical studies of rare earth-free bismuth silicate glasses for white light generation. J. Lumin. 2018, 201, 442–450. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Z.; Zhu, M.; Zhang, Y.; Li, J.; Dou, C.; Sun, S.; Teng, B.; Che, Y.; Zhong, D. Full-visible-spectrum emission with high color rendering index and low correlated color temperature enabled by a sin-gle-phased phosphor of α-Sr2V1.98P0.02O7: 0.5% Eu3+. Mater. Res. Bull. 2021, 141, 111344. Available online: https://n9.cl/lwvsx (accessed on 8 December 2022). [CrossRef]
- Ohta, N.; Robertson, A. Colorimetry Fundamentals and Applications; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2005; pp. 63–114. [Google Scholar]
- Fu, K.; He, J.; Yang, X. Few-shot learning-based RGB-D salient object detection: A case study. Neurocomputing 2022, 512, 142–152. Available online: https://n9.cl/j0s2zy (accessed on 8 December 2022). [CrossRef]
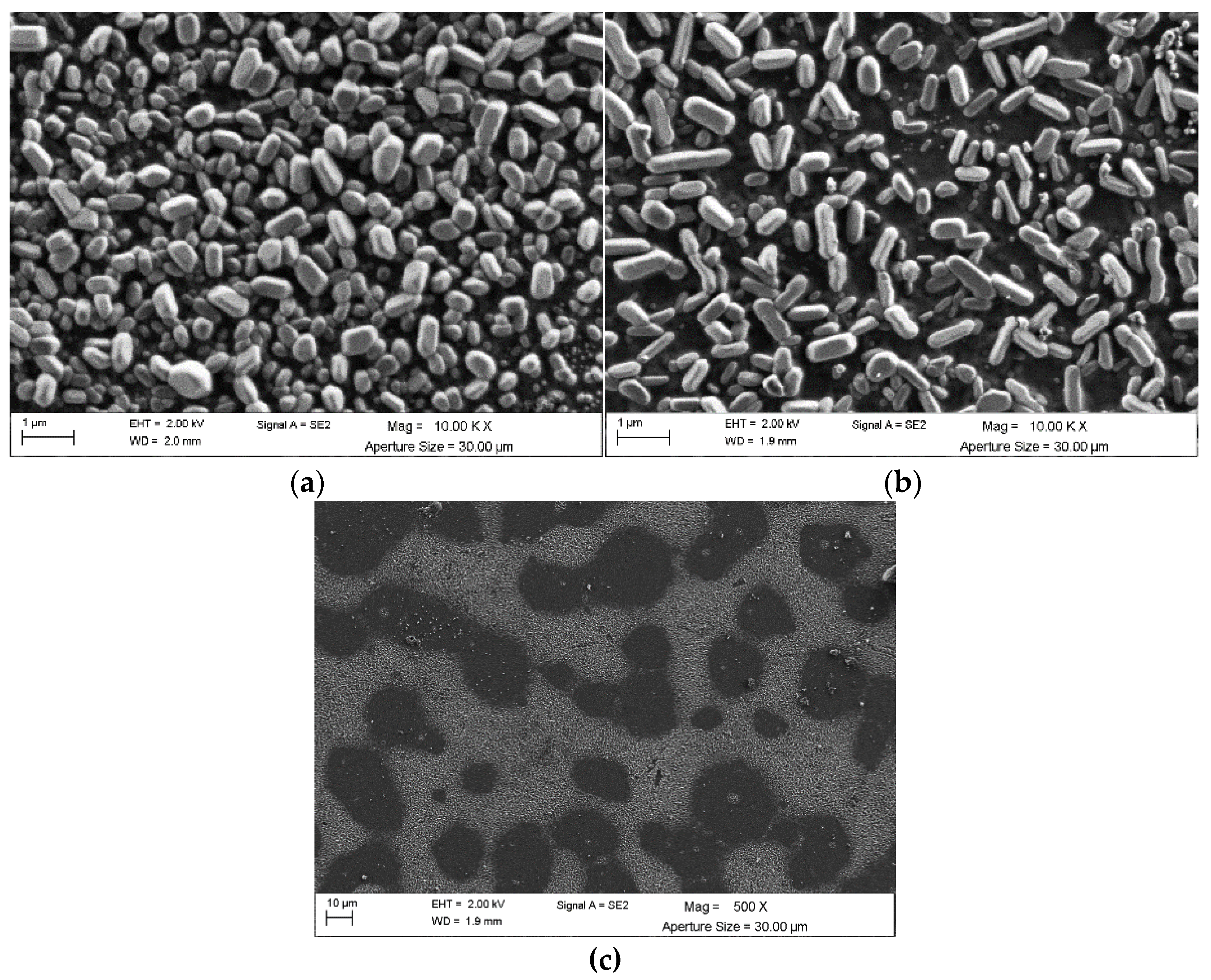
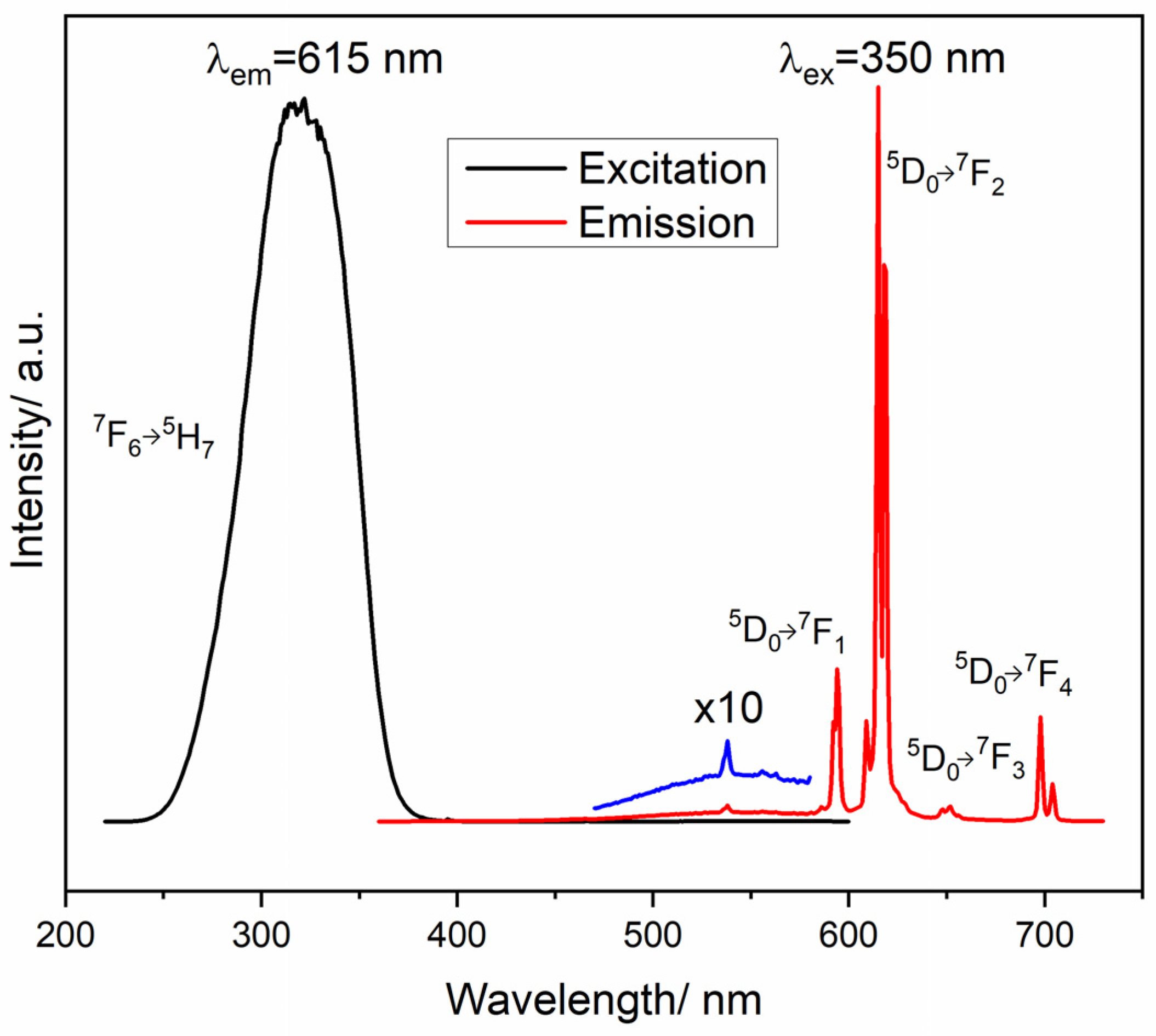

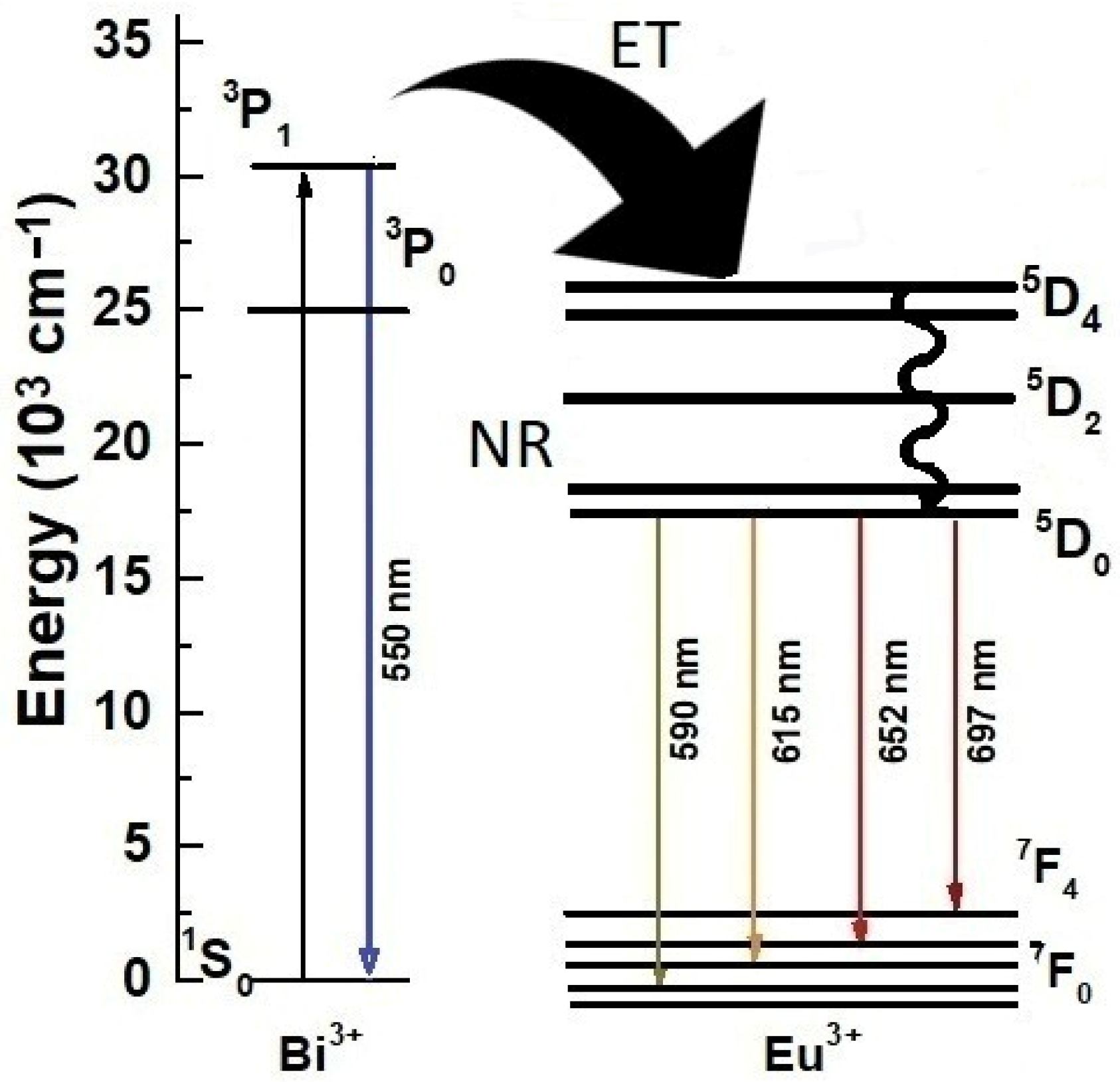
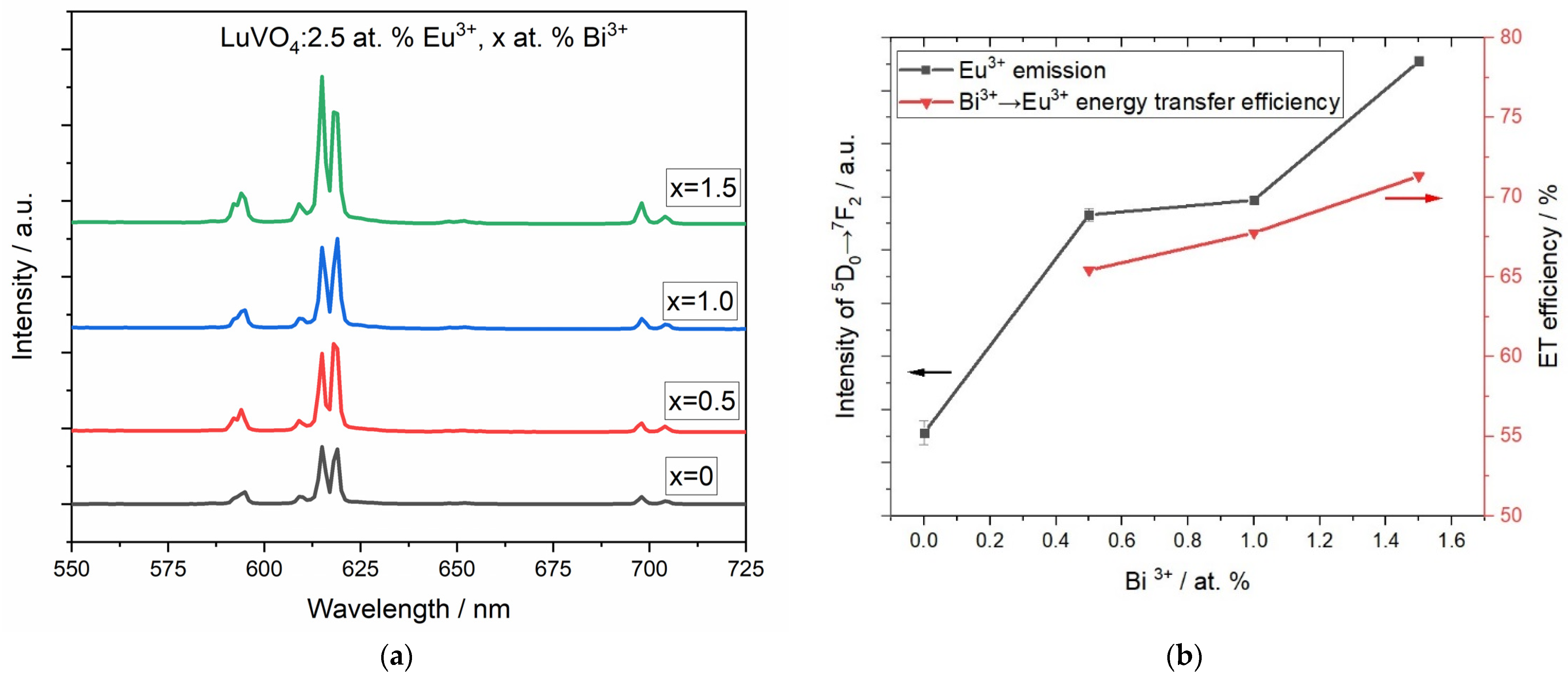
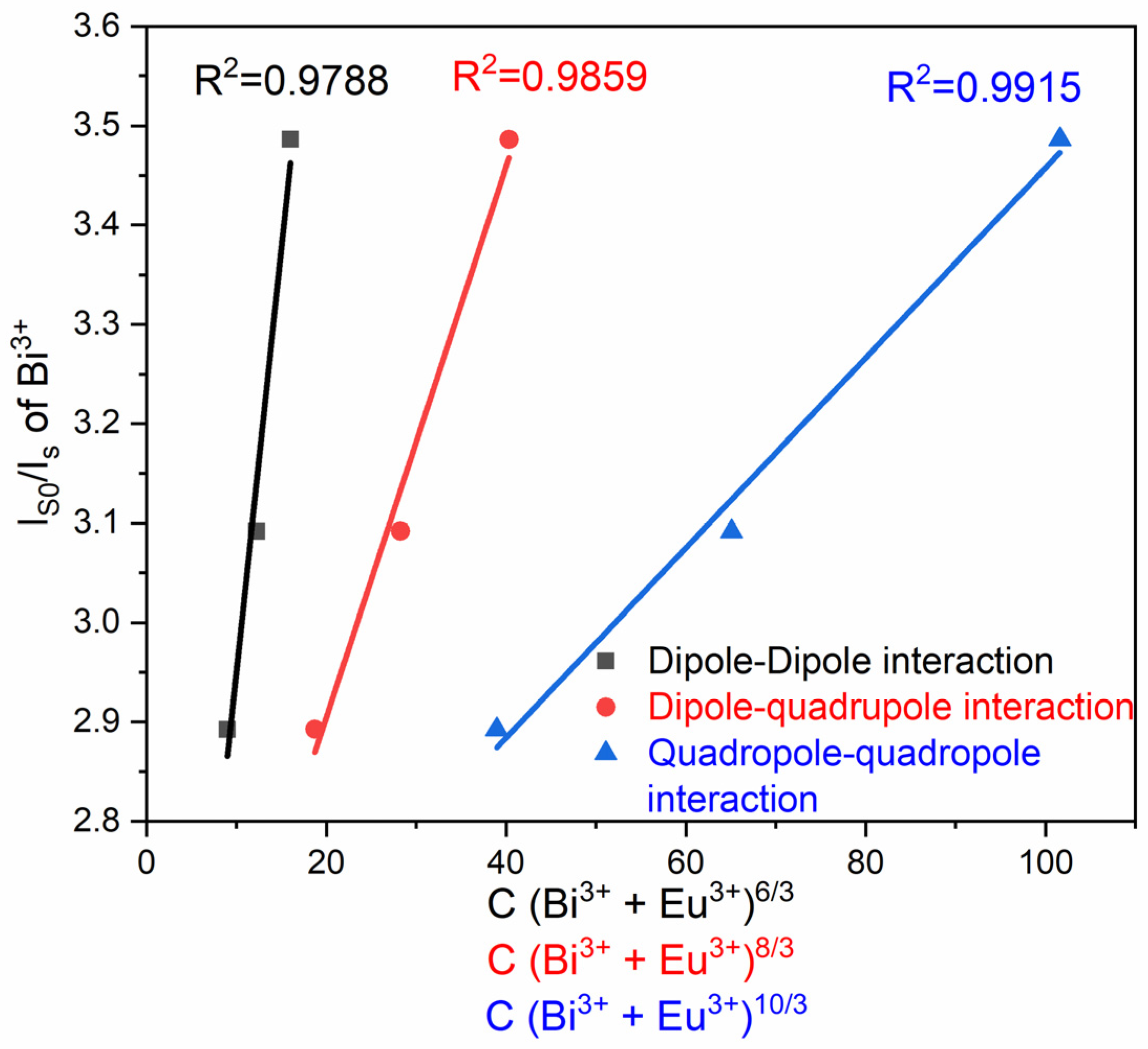

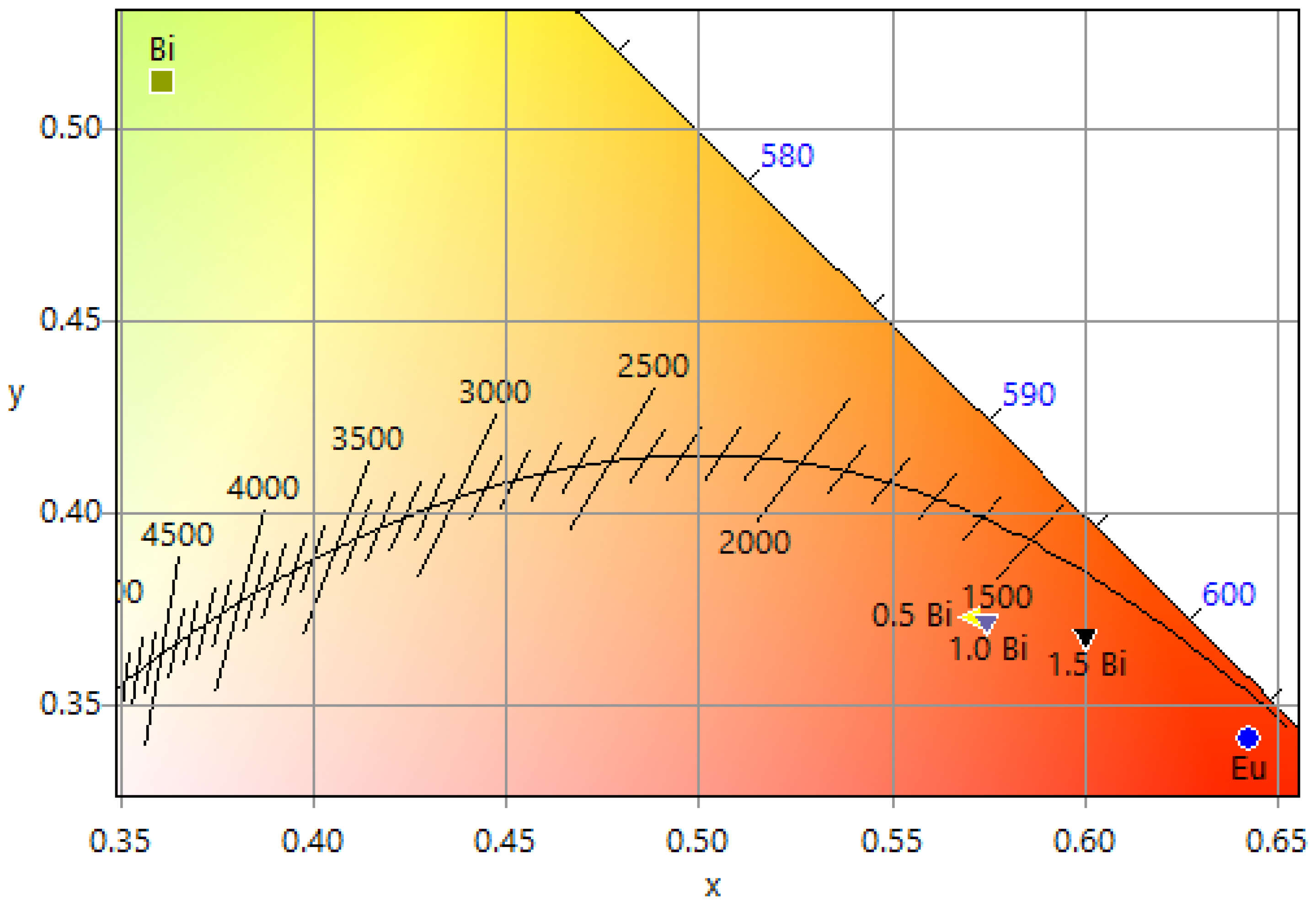
| System | Annealing Temperature (°C) | Crystallite Size (nm) | Preferential Orientation Index α200 |
|---|---|---|---|
| LuVO4: Eu3+ 2.5 at. %, Bi3+ 1.5 at. % | 700 | 68.2 ± 6.2 | 0.92 |
| 800 | 71.6 ± 7.8 | 0.91 | |
| 900 | 66.9 ± 4.2 | 0.91 | |
| 1000 | 80.8 ± 6.1 | 0.90 |
| Sample | CIEE Color Coordinates (x, y) | CTTK | CP | RGB | Hex | Hex Color | ||
|---|---|---|---|---|---|---|---|---|
| R | G | B | ||||||
| Eu3+ mono-doped | (0.6423, 0.3416) | 1026 | 0.92 | 255 | 34 | 0 | #FF2200 |  |
| Bi3+ mono-doped | (0.3611, 0.5124) | 4939 | 0.59 | 189 | 255 | 80 | #BDFF50 |  |
| Eu3+ 2.5 at. %, Bi3+ 0.5 at. % | (0.5713, 0.3734) | 1620 | 0.78 | 255 | 97 | 33 | #FF6121 |  |
| Eu3+ 2.5 at. %, Bi3+ 1.0 at. % | (0.5721, 0.3724) | 1620 | 0.79 | 255 | 96 | 33 | #FF0021 |  |
| Eu3+ 2.5 at. %, Bi3+ 1.5 at. % | (0.5999, 0.3684) | 1680 | 0.86 | 255 | 81 | 0 | #FF5100 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Penguelly, B.; Pérez-Sánchez, G.G.; Medina-Velázquez, D.Y.; Martínez-Falcón, P.; Morales-Ramírez, A.d.J. Luminescence Properties and Energy Transfer of Eu3+, Bi3+ Co-Doped LuVO4 Films Modified with Pluronic F-127 Obtained by Sol–Gel. Materials 2023, 16, 146. https://doi.org/10.3390/ma16010146
González-Penguelly B, Pérez-Sánchez GG, Medina-Velázquez DY, Martínez-Falcón P, Morales-Ramírez AdJ. Luminescence Properties and Energy Transfer of Eu3+, Bi3+ Co-Doped LuVO4 Films Modified with Pluronic F-127 Obtained by Sol–Gel. Materials. 2023; 16(1):146. https://doi.org/10.3390/ma16010146
Chicago/Turabian StyleGonzález-Penguelly, Brenely, Grethell Georgina Pérez-Sánchez, Dulce Yolotzin Medina-Velázquez, Paulina Martínez-Falcón, and Angel de Jesús Morales-Ramírez. 2023. "Luminescence Properties and Energy Transfer of Eu3+, Bi3+ Co-Doped LuVO4 Films Modified with Pluronic F-127 Obtained by Sol–Gel" Materials 16, no. 1: 146. https://doi.org/10.3390/ma16010146





