Synthesis and Characterization of Novel Ternary-Hybrid Nanoparticles as Thermal Additives
Abstract
1. Introduction
The Rationale behind the Ternary-Hybrid Synthesis
2. Materials and Methods
2.1. Synthesis of Ternary-Hybrid Nanoparticles
2.1.1. Preparation of GO-TiO2-Ag and rGO-TiO2-Ag Nanocomposites
2.1.2. Materials’ Characterization
2.2. Synthesis of Hybrid Nanofluids
2.3. Thermal Conductivity Measurement
2.4. Dynamic Viscosity Measurement
3. Results
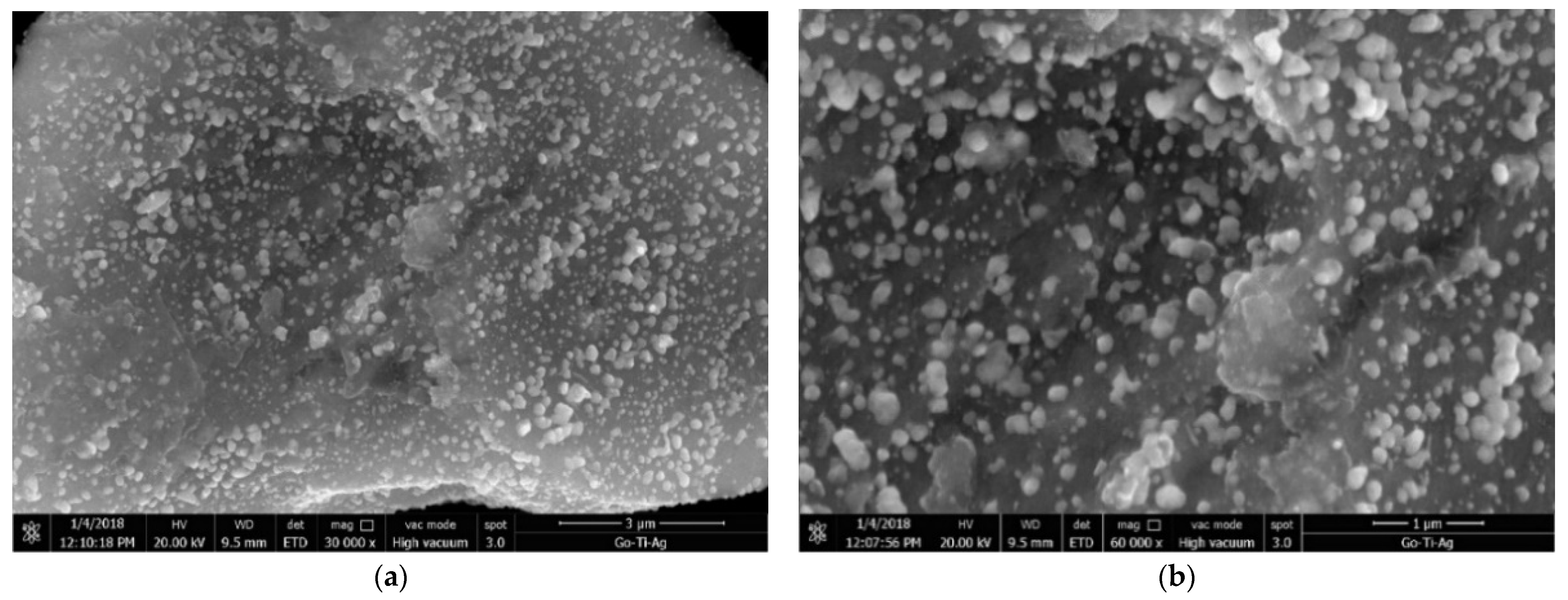
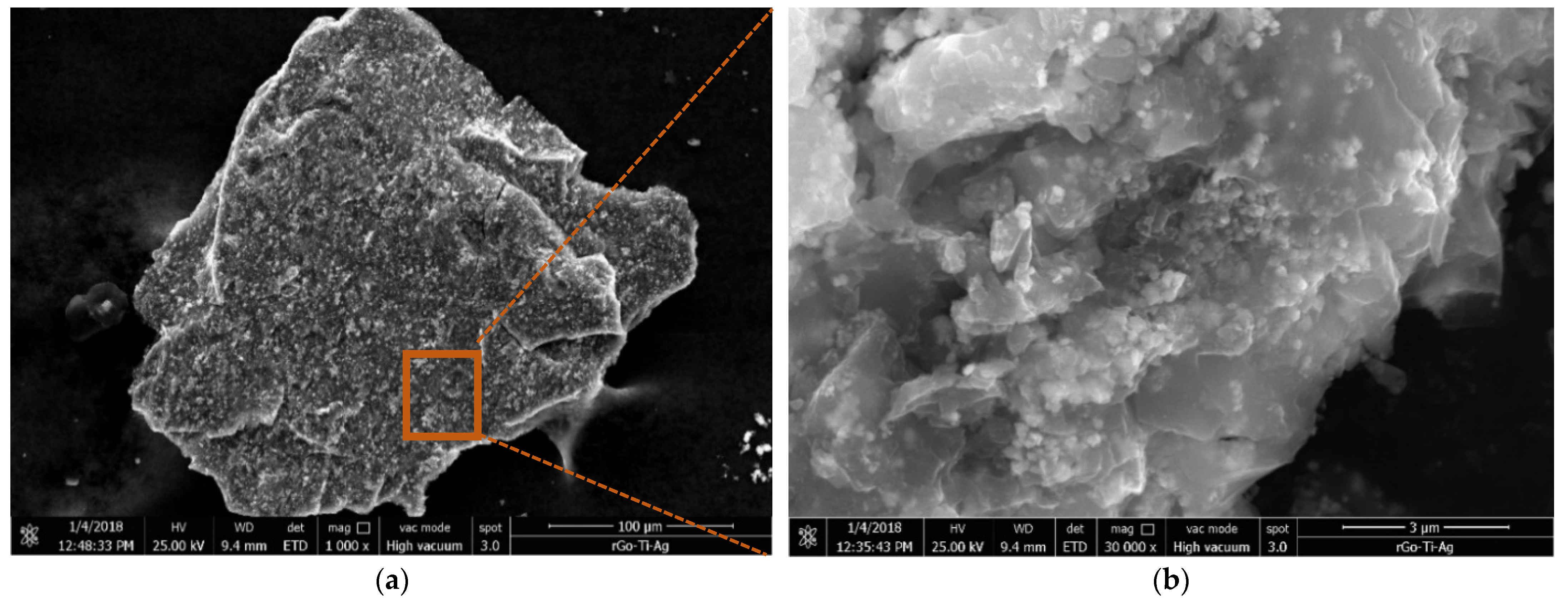
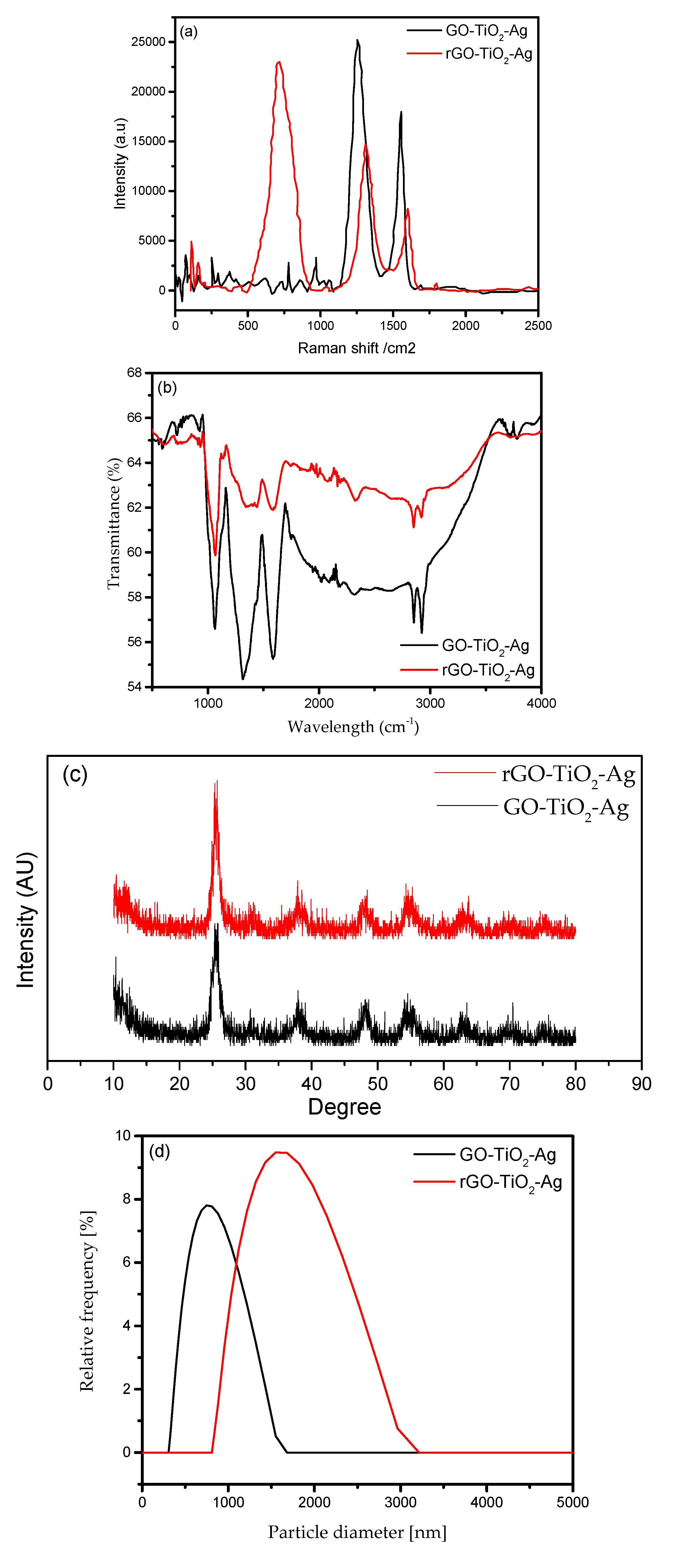
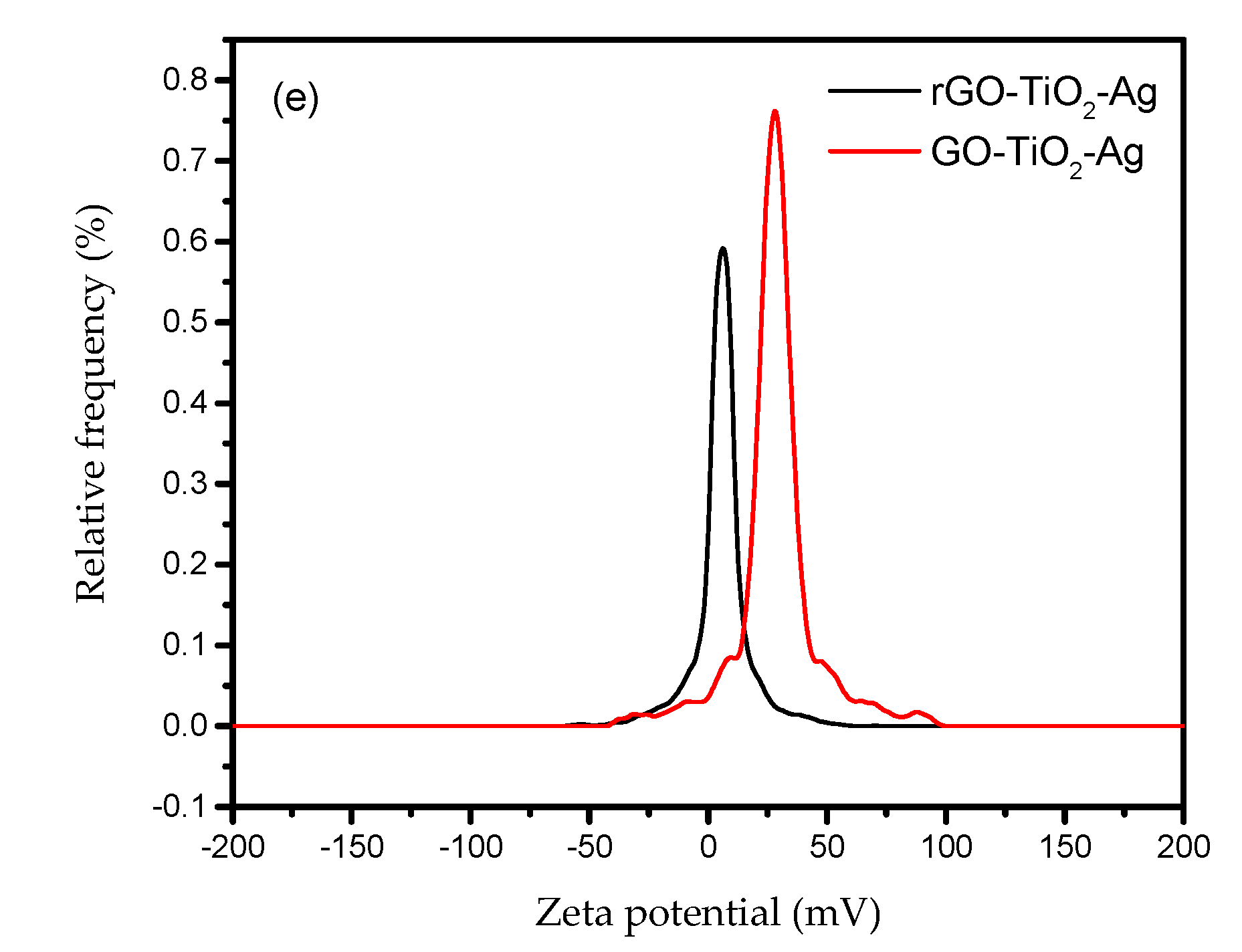
3.1. Characterization Results
3.2. Thermal Conductivity Measurements of Synthesized GO-TiO2-Ag and rGO-TiO2-Ag
3.2.1. Effect of Concentration
3.2.2. Effect of Temperature
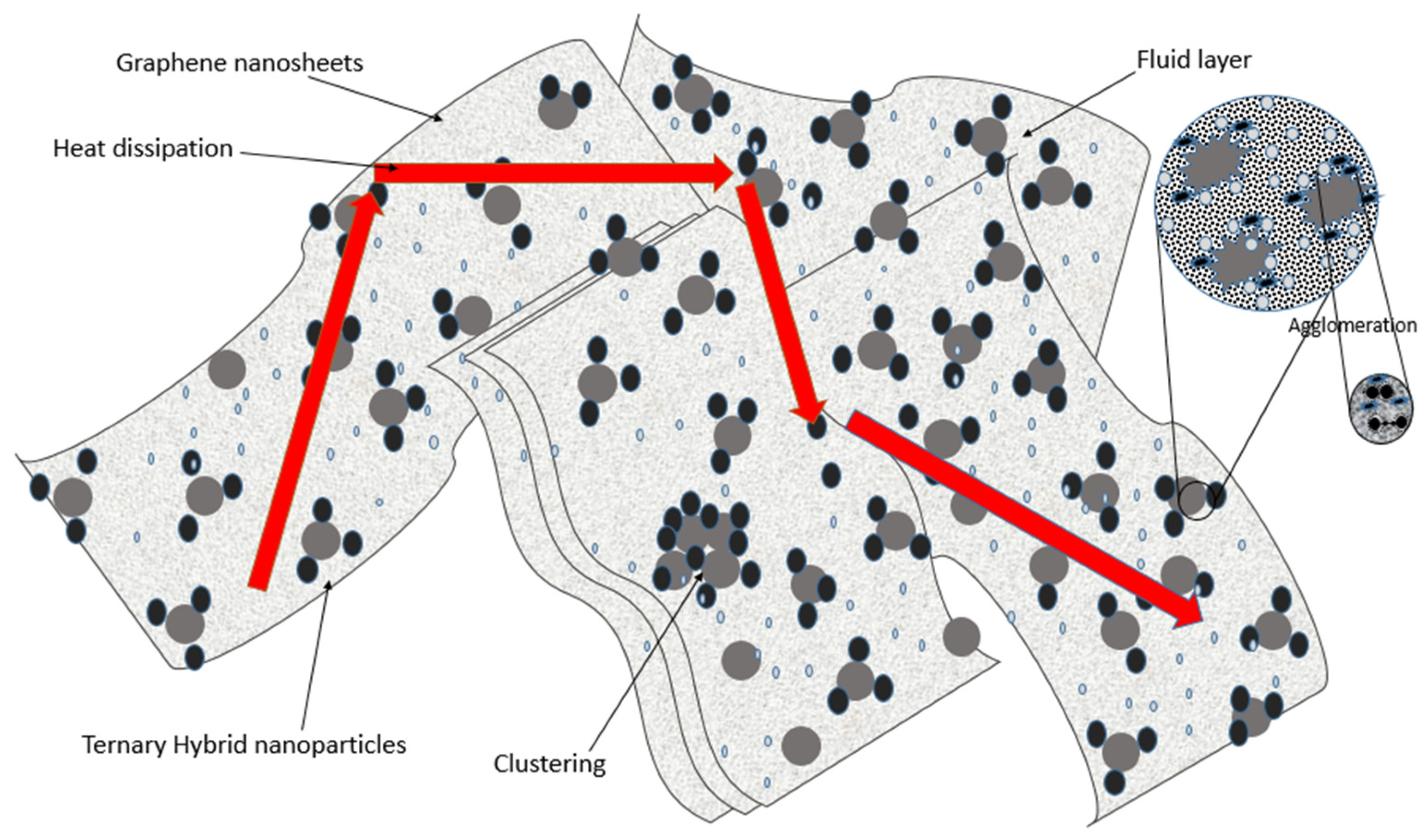
3.3. Mechanisms Influencing the Thermal Conductivity Enhancements
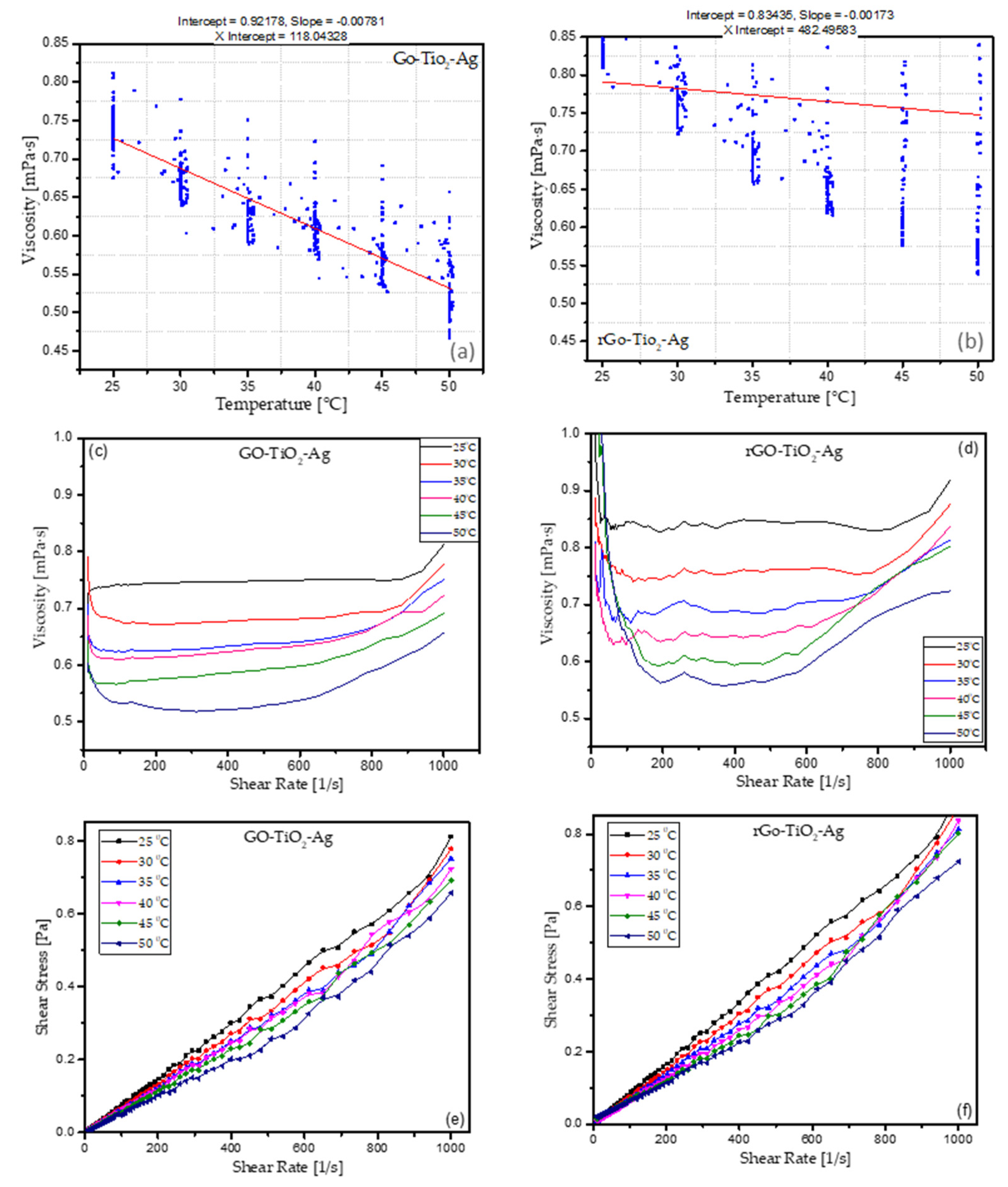
3.4. Viscosity Measurements of GO-TiO2-Ag and rGO-TiO2-Ag
3.4.1. Viscosity vs. Temperature
3.4.2. Viscosity vs. Shear Rate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chrystal, G. A Treatise on Electricity and Magnetism an Elementary Treatise on Electricity; Clarendon Press: Oxford, UK, 1882; Volume 25. [Google Scholar]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Wen, D.; Ding, Y. Experimental investigation into convective heat transfer of nanofluids at the entrance region under laminar flow conditions. Int. J. Heat Mass Transf. 2004, 47, 5181–5188. [Google Scholar] [CrossRef]
- Sajid, M.U.; Ali, H.M. Thermal conductivity of hybrid nanofluids: A critical review. Int. J. Heat Mass Transf. 2018, 126, 211–234. [Google Scholar] [CrossRef]
- Motevasel, M.; Nazar, A.R.S.; Jamialahmadi, M. The effect of nanoparticles aggregation on the thermal conductivity of nanofluids at very low concentrations: Experimental and theoretical evaluations. Heat Mass Transf. Und Stoffuebertragung 2018, 54, 125–133. [Google Scholar] [CrossRef]
- Esfe, M.H.; Alirezaie, A.; Rejvani, M. An applicable study on the thermal conductivity of SWCNT-MgO hybrid nanofluid and price-performance analysis for energy management. Appl. Therm. Eng. 2017, 111, 1202–1210. [Google Scholar] [CrossRef]
- Esfahani, N.N.; Toghraie, D.; Afrand, M. A new correlation for predicting the thermal conductivity of ZnO–Ag (50%–50%)/water hybrid nanofluid: An experimental study. Powder Technol. 2018, 323, 367–373. [Google Scholar] [CrossRef]
- Akhgar, A.; Toghraie, D. An experimental study on the stability and thermal conductivity of water-ethylene glycol/TiO2-MWCNTs hybrid nanofluid: Developing a new correlation. Powder Technol. 2018, 338, 806–818. [Google Scholar] [CrossRef]
- Nabil, M.F.; Azmi, W.H.; Hamid, K.A.; Mamat, R.; Hagos, F.Y. An experimental study on the thermal conductivity and dynamic viscosity of TiO2-SiO2 nanofluids in water: Ethylene glycol mixture. Int. Commun. Heat Mass Transf. 2017, 86, 181–189. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Yan, Y.; Reay, D. A review on graphene based nanofluids: Preparation, characterization and applications. J. Mol. Liq. 2019, 279, 444–484. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Rheological behaviour of nanofluids: A review. Renew. Sustain. Energy Rev. 2016, 53, 779–791. [Google Scholar] [CrossRef]
- Deepa, C.; Rajeshkumar, L.; Ramesh, M. Preparation, synthesis, properties and characterization of graphene-based 2D nano-materials for biosensors and bioelectronics. J. Mater. Res. Technol. 2022, 19, 2657–2694. [Google Scholar] [CrossRef]
- Thamilselvi, V.; Radha, K.V. A Review on the Diverse Application of Silver Nanoparticle. IOSR J. Pharm. 2017, 7, 21–27. [Google Scholar] [CrossRef]
- Naphon, P.; Thongjing, C. Pool boiling heat transfer characteristics of refrigerant-nanoparticle mixtures. Int. Commun. Heat Mass Transf. 2014, 52, 84–89. [Google Scholar] [CrossRef]
- Perera, S.D.; Mariano, P.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J., Jr. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Bobrowska, A.; Jagoda, E.; Domonik, A.; Ryżyński, G. Thermomechanical properties of detrital limestone from the Nowe Brusno town (Poland). Resour. Policy 2022, 77, 102698. [Google Scholar] [CrossRef]
- Godson, L.; Raja, B.; Lal, D.M.; Wongwises, S. Experimental investigation on the thermal conductivity and viscosity of silver-deionized water nanofluid. Exp. Heat Transf. 2010, 23, 317–332. [Google Scholar] [CrossRef]
- Leong, K.H.; Sim, L.C.; Bahnemann, D.; Jang, M.; Ibrahim, S.; Saravanan, P. Reduced graphene oxide and Ag wrapped TiO2 photocatalyst for enhanced visible light photocatalysis. APL Mater. 2015, 3, 104503. [Google Scholar] [CrossRef]
- Choi, B.-K.; Choi, W.-K.; Park, S.-J.; Seo, M.-K. One-Pot Synthesis of Ag-TiO2/Nitrogen-Doped Graphene Oxide Nanocomposites and Its Photocatalytic Degradation of Methylene Blue. J. Nanosci. Nanotechnol. 2018, 18, 6075–6080. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Park, J.H.; Kim, E.; Choi, Y.J.; Kwon, D.N.; Kim, J.H. Reduced graphene oxide-silver nanoparticle nanocomposite: A potential anticancer nanotherapy. Int. J. Nanomed. 2015, 10, 6257–6276. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Li, N.; Xia, J.; Meng, Q.; Ding, J.; Lu, J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241–34251. [Google Scholar] [CrossRef]
- Thomas, R.T.; Rasheed, P.A.; Sandhyarani, N. Synthesis of nanotitania decorated few-layer graphene for enhanced visible light driven photocatalysis. J. Colloid Interface Sci. 2014, 428, 214–221. [Google Scholar] [CrossRef]
- Fan, B.; Guo, H.; Shi, J.; Shi, C.; Jia, Y.; Wang, H.; Chen, D.; Yang, Y.; Lu, H.; Xu, H.; et al. Facile one-pot preparation of silver/reduced graphene oxide nanocomposite for cancer photodynamic and photothermal therapy. J. Nanosci. Nanotechnol. 2016, 16, 7049–7054. [Google Scholar] [CrossRef]
- Liu, H.; Duan, C.; Su, X.; Dong, X.; Huang, Z.; Shen, W.; Zhu, Z. A hemoglobin encapsulated titania nanosheet modified reduced graphene oxide nanocomposite as a mediator-free biosensor. Sens. Actuators B Chem. 2014, 203, 303–310. [Google Scholar] [CrossRef]
- Ghadimi, A.; Metselaar, I.H. The influence of surfactant and ultrasonic processing on improvement of stability, thermal conductivity and viscosity of titania nanofluid. Exp. Therm. Fluid Sci. 2013, 51, 1–9. [Google Scholar] [CrossRef]
- Jabbari, F.; Rajabpour, A.; Saedodin, S. Thermal conductivity and viscosity of nanofluids: A review of recent molecular dynamics studies. Chem. Eng. Sci. 2017, 174, 67–81. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; Miglietta, P.; Milanese, M.; de Risi, A. Thermal conductivity, viscosity and stability of Al2O3-diathermic oil nanofluids for solar energy systems. Energy 2016, 95, 124–136. [Google Scholar] [CrossRef]
- Eapen, J.; Rusconi, R.; Piazza, R.; Yip, S. The Classical Nature of Thermal Conduction in Nanofluids. J. Heat Transf. 2010, 132, 102402. [Google Scholar] [CrossRef]
- Afrand, M. Experimental study on thermal conductivity of ethylene glycol containing hybrid nano-additives and development of a new correlation. Appl. Therm. Eng. 2017, 110, 1111–1119. [Google Scholar] [CrossRef]
- Esfe, M.H.; Arani, A.A.A.; Rezaie, M.; Yan, W.M.; Karimipour, A. Experimental determination of thermal conductivity and dynamic viscosity of Ag-MgO/water hybrid nanofluid. Int. Commun. Heat Mass Transf. 2015, 66, 189–195. [Google Scholar] [CrossRef]
- Esfe, M.H.; Yan, W.M.; Akbari, M.; Karimipour, A.; Hassani, M. Experimental study on thermal conductivity of DWCNT-ZnO/water-EG nanofluids. Int. Commun. Heat Mass Transf. 2015, 68, 248–251. [Google Scholar] [CrossRef]
- Esfe, M.H.; Esfandeh, S.; Saedodin, S.; Rostamian, H. Experimental evaluation, sensitivity analyzation and ANN modeling of thermal conductivity of ZnO-MWCNT/EG-water hybrid nanofluid for engineering applications. Appl. Therm. Eng. 2017, 125, 673–685. [Google Scholar] [CrossRef]
- Esfe, M.H.; Arani, A.A.A.; Firouzi, M. Empirical study and model development of thermal conductivity improvement and assessment of cost and sensitivity of EG-water based SWCNT-ZnO (30%:70%) hybrid nanofluid. J. Mol. Liq. 2017, 244, 252–261. [Google Scholar] [CrossRef]
- Rostamian, S.H.; Biglari, M.; Saedodin, S.; Esfe, M.H. An inspection of thermal conductivity of CuO-SWCNTs hybrid nanofluid versus temperature and concentration using experimental data, ANN modeling and new correlation. J. Mol. Liq. 2017, 231, 364–369. [Google Scholar] [CrossRef]
- Das, S.K.; Choi, S.U.S.; Yu, W.; Pradeep, T. Nanofluids: Science and Technology; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Karthikeyan, N.R.; Philip, J.; Raj, B. Effect of clustering on the thermal conductivity of nanofluids. Mater. Chem. Phys. 2008, 109, 50–55. [Google Scholar] [CrossRef]
- Philip, J.; Shima, P.D. Thermal properties of nanofluids. Adv. Colloid Interface Sci. 2012, 183–184, 30–45. [Google Scholar] [CrossRef]
- Keblinski, P.; Phillpot, S.R.; Choi, S.U.S.; Eastman, J.A. Mechanisms of heat flow in suspensions of nano-sized particles (nanofluids). Int. J. Heat Mass Transf. 2001, 45, 855–863. [Google Scholar] [CrossRef]
- Loulijat, H.; Zerradi, H. The effect of the liquid layer around the spherical and cylindrical nanoparticles in enhancing thermal conductivity of nanofluids. J. Heat Transf. 2019, 141, 032401. [Google Scholar] [CrossRef]
- Xue, L.; Keblinski, P.; Phillpot, S.R.; Choi, S.U.S.; Eastman, J.A. Effect of liquid layering at the liquid-solid interface on thermal transport. Int. J. Heat Mass Transf. 2004, 47, 4277–4284. [Google Scholar] [CrossRef]
- Das, P.K. A review based on the effect and mechanism of thermal conductivity of normal nanofluids and hybrid nanofluids. J. Mol. Liq. 2017, 240, 420–446. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, A.K.; Tiwari, S.; Prakash, R. Viscosity of hybrid nanofluids: Measurement and comparison. J. Mech. Eng. Sci. 2018, 12, 3614–3623. [Google Scholar] [CrossRef]
- Ahammed, N.; Asirvatham, L.G.; Wongwises, S. Effect of volume concentration and temperature on viscosity and surface tension of graphene-water nanofluid for heat transfer applications. J. Therm. Anal. Calorim. 2016, 123, 1399–1409. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Estellé, P. A state of the art review on viscosity of nanofluids. Renew. Sustain. Energy Rev. 2017, 76, 1134–1152. [Google Scholar] [CrossRef]
- Hwang, K.S.; Lee, J.H.; Jang, S.P. Buoyancy-driven heat transfer of water-based Al2O3 nanofluids in a rectangular cavity. Int. J. Heat Mass Transf. 2007, 50, 4003–4010. [Google Scholar] [CrossRef]
- Bahrami, M.; Akbari, M.; Karimipour, A.; Afrand, M. An experimental study on rheological behavior of hybrid nanofluids made of iron and copper oxide in a binary mixture of water and ethylene glycol: Non-Newtonian behavior. Exp. Therm. Fluid Sci. 2016, 79, 231–237. [Google Scholar] [CrossRef]
- Nadooshan, A.A.; Eshgarf, H.; Afrand, M. Measuring the viscosity of Fe3O4-MWCNTs/EG hybrid nanofluid for evaluation of thermal efficiency: Newtonian and non-Newtonian behavior. J. Mol. Liq. 2018, 253, 169–177. [Google Scholar] [CrossRef]
- Motahari, K.; Moghaddam, M.A.; Moradian, M. Experimental investigation and development of new correlation for influences of temperature and concentration on dynamic viscosity of MWCNT-SiO2 (20–80)/20W50 hybrid nano-lubricant. Chin. J. Chem. Eng. 2018, 26, 152–158. [Google Scholar] [CrossRef]
- Zawawi, N.N.M.; Azmi, W.H.; Redhwan, A.A.M.; Sharif, M.Z.; Sharma, K.V. Propriétés thermo-physiques du nanolubrifiant composite Al2O3-SiO2/PAG pour les systèmes frigorifiques. Int. J. Refrig. 2017, 80, 1–10. [Google Scholar] [CrossRef]
 ,
,  ,
,  ,
,  , and
, and  are the data from Afrand et al. [29] hybrid nanoparticle, while
are the data from Afrand et al. [29] hybrid nanoparticle, while  ,
,  ,
,  ,
,  , and
, and  are Nabil et al. [9] data.
are Nabil et al. [9] data.  ,
,  ,
,  ,
,  , and
, and  are Esfahani et al. [7] data, while
are Esfahani et al. [7] data, while  ,
,  ,
,  ,
,  , and
, and  are Hemmat et al. [30] data.
are Hemmat et al. [30] data.  ,
,  ,
,  ,
,  , and
, and  are Hemmat et al. [31] data, also
are Hemmat et al. [31] data, also  ,
,  ,
,  ,
,  , and
, and  Hemmat et al. [32] data as well as
Hemmat et al. [32] data as well as  ,
,  ,
,  ,
,  , and
, and  Hemmat et al. [33].
Hemmat et al. [33].  ,
,  ,
,  ,
,  , and
, and  are Rostamian et al. [34] data.
are Rostamian et al. [34] data.  ,
,  ,
,  ,
,  , and
, and  are the data of rGO-TiO2-Ag at concentration A (5 × 10−1 wt.%), B (5 × 10−2 wt.%), C (5 × 10−3 wt.%), D (5 × 10−4 wt.%), and E (5 × 10−5 wt.%), respectively.
are the data of rGO-TiO2-Ag at concentration A (5 × 10−1 wt.%), B (5 × 10−2 wt.%), C (5 × 10−3 wt.%), D (5 × 10−4 wt.%), and E (5 × 10−5 wt.%), respectively.
 ,
,  ,
,  ,
,  , and
, and  are the data from Afrand et al. [29] hybrid nanoparticle, while
are the data from Afrand et al. [29] hybrid nanoparticle, while  ,
,  ,
,  ,
,  , and
, and  are Nabil et al. [9] data.
are Nabil et al. [9] data.  ,
,  ,
,  ,
,  , and
, and  are Esfahani et al. [7] data, while
are Esfahani et al. [7] data, while  ,
,  ,
,  ,
,  , and
, and  are Hemmat et al. [30] data.
are Hemmat et al. [30] data.  ,
,  ,
,  ,
,  , and
, and  are Hemmat et al. [31] data, also
are Hemmat et al. [31] data, also  ,
,  ,
,  ,
,  , and
, and  Hemmat et al. [32] data as well as
Hemmat et al. [32] data as well as  ,
,  ,
,  ,
,  , and
, and  Hemmat et al. [33].
Hemmat et al. [33].  ,
,  ,
,  ,
,  , and
, and  are Rostamian et al. [34] data.
are Rostamian et al. [34] data.  ,
,  ,
,  ,
,  , and
, and  are the data of rGO-TiO2-Ag at concentration A (5 × 10−1 wt.%), B (5 × 10−2 wt.%), C (5 × 10−3 wt.%), D (5 × 10−4 wt.%), and E (5 × 10−5 wt.%), respectively.
are the data of rGO-TiO2-Ag at concentration A (5 × 10−1 wt.%), B (5 × 10−2 wt.%), C (5 × 10−3 wt.%), D (5 × 10−4 wt.%), and E (5 × 10−5 wt.%), respectively.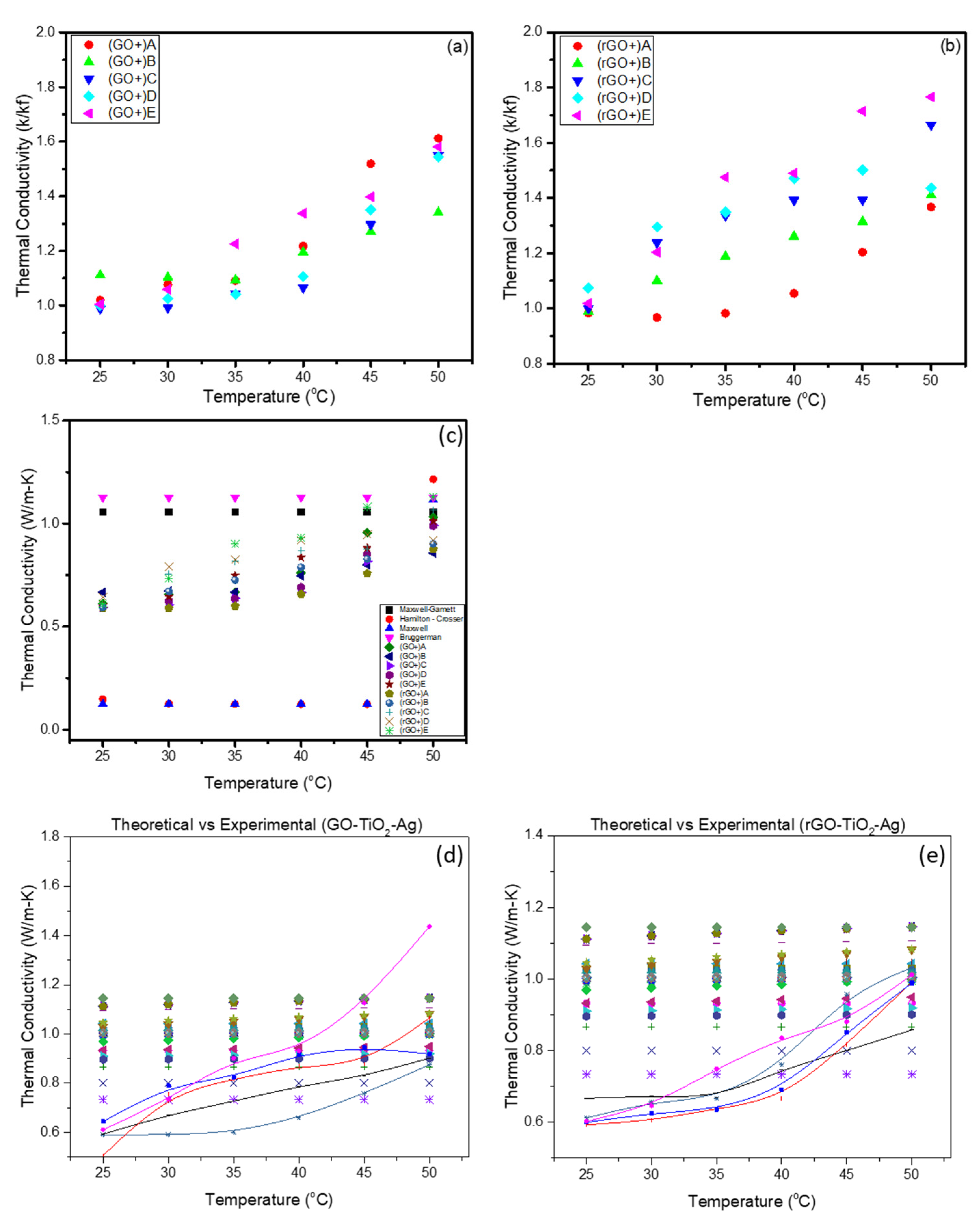
| Nanoparticle | Base Fluid | Empirical Correlation | Range | Authors |
|---|---|---|---|---|
| SiO2-TiO2 | water and EG (60:40) | 1:4 to 4:1 30 to 80 °C | Afrand et al. [29] | |
| SiO2-TiO2 | water and EG (60:40) | 0.5% to 3% 30 to 80 °C | Nabil et al. [9] | |
| ZnO-Ag | water | 0.125% to 2% 25 to 50 °C | Esfahani et al. [7] | |
| Ag-MgO | water | 0 to 3% | Hemmat et al. [30] | |
| DWCNTs-ZnO | water and EG (60:40) | 0.025% to 1% 30 to 50 °C | Hemmat et al. [31] | |
| ZnO-MWCNTs | water and EG (50:50) | 0.02% to 1% 30 to 50 °C | Hemmat et al. [32] | |
| SWCNTs-ZnO (30:70) | water and EG | 0.05% to 1.6% 26 to 50 °C | Hemmat et al. [33] | |
| SWCNTs-CuO | water and EG (60:40) | 0.02% to 0.75% 20 to 50 °C | Rostamian et al. [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed Zayan, J.; Rasheed, A.K.; John, A.; Faris, W.F.; Aabid, A.; Baig, M.; Alallam, B. Synthesis and Characterization of Novel Ternary-Hybrid Nanoparticles as Thermal Additives. Materials 2023, 16, 173. https://doi.org/10.3390/ma16010173
Mohammed Zayan J, Rasheed AK, John A, Faris WF, Aabid A, Baig M, Alallam B. Synthesis and Characterization of Novel Ternary-Hybrid Nanoparticles as Thermal Additives. Materials. 2023; 16(1):173. https://doi.org/10.3390/ma16010173
Chicago/Turabian StyleMohammed Zayan, Jalal, Abdul Khaliq Rasheed, Akbar John, Waleed Fekry Faris, Abdul Aabid, Muneer Baig, and Batoul Alallam. 2023. "Synthesis and Characterization of Novel Ternary-Hybrid Nanoparticles as Thermal Additives" Materials 16, no. 1: 173. https://doi.org/10.3390/ma16010173
APA StyleMohammed Zayan, J., Rasheed, A. K., John, A., Faris, W. F., Aabid, A., Baig, M., & Alallam, B. (2023). Synthesis and Characterization of Novel Ternary-Hybrid Nanoparticles as Thermal Additives. Materials, 16(1), 173. https://doi.org/10.3390/ma16010173











