Yield-Point Phenomenon and Plastic Bands in Ferrite–Pearlite Steels
Abstract
1. Introduction
- Studies on the yield-point phenomenon:
- (1)
- The micromechanism of the yield point.Shen et al. [7], Akama [8], and Tsuchiyama [9] investigated the yield-point phenomenon in low-carbon steels, and they found that carbon segregation [7] and grain boundary strengthening [8] significantly affect the occurrence of the yield point, and that the yield point is a dislocation emission phenomenon caused by dislocation sources that exist at the grain boundaries [9].
- (2)
- The development of yield-point models.
- (3)
- Effects of the second or third phases on the yield point in multiple phases.Commercial steels are generally composed of multiple phases. The effects of the second phase or third phase on the yield point have been investigated, such as cementite particles in ferrite–cementite steel [13,14], martensite islands in ferrite–martensite steel [13], and cementite in pearlite steel [15].
- (4)
- Influence of the yield point on the material’s behavior.Žerovnik et al. [16] investigated the effect of the yield-point phenomenon on the evolution of the cyclic plasticity of a low-alloy steel.
- (5)
- Elimination of the yield point.
- Studies on the plastic band:
- (1)
- The development of a micromechanical model of plastic bands.Schwab and Ruff [19] proposed a micromechanical model that revealed the relationship between the formation of plastic band and the upper and lower yield point.
- (2)
- In situ observations of moving plastic bands.
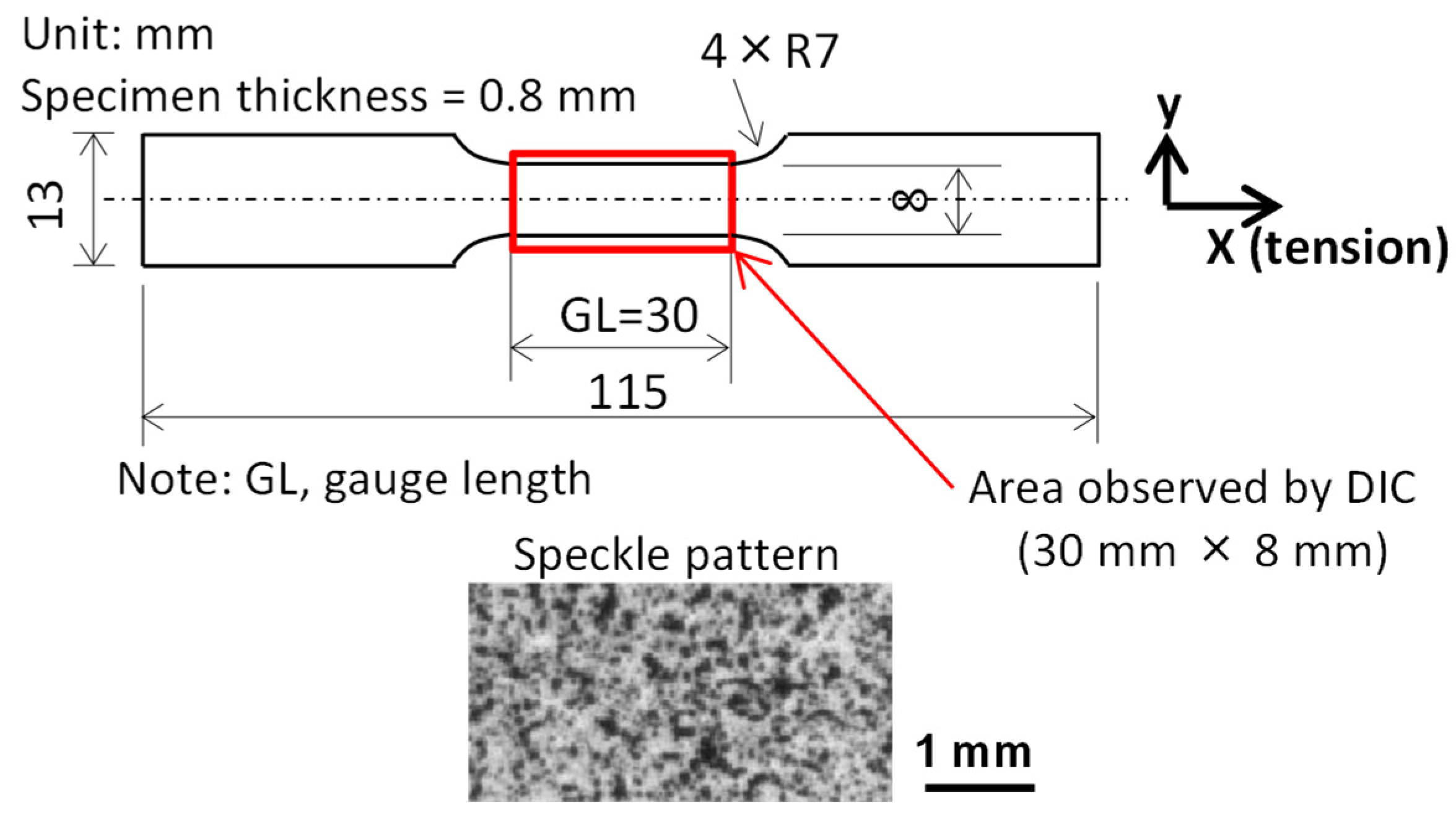
2. Materials, Tension Test Conditions, and Deformation Evaluation Methods
2.1. Materials
2.2. Tension Test Conditions
2.3. Deformation Evaluation Methods
3. Results and Discussion
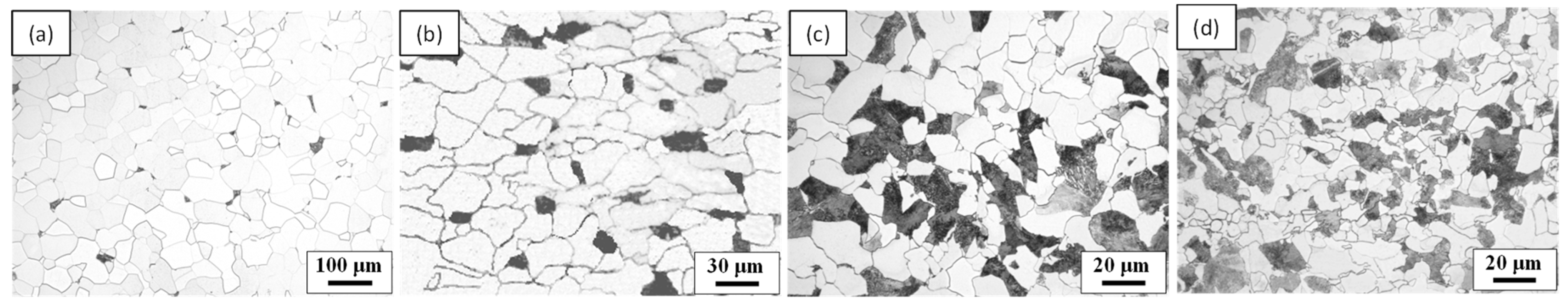
3.1. Microstructure
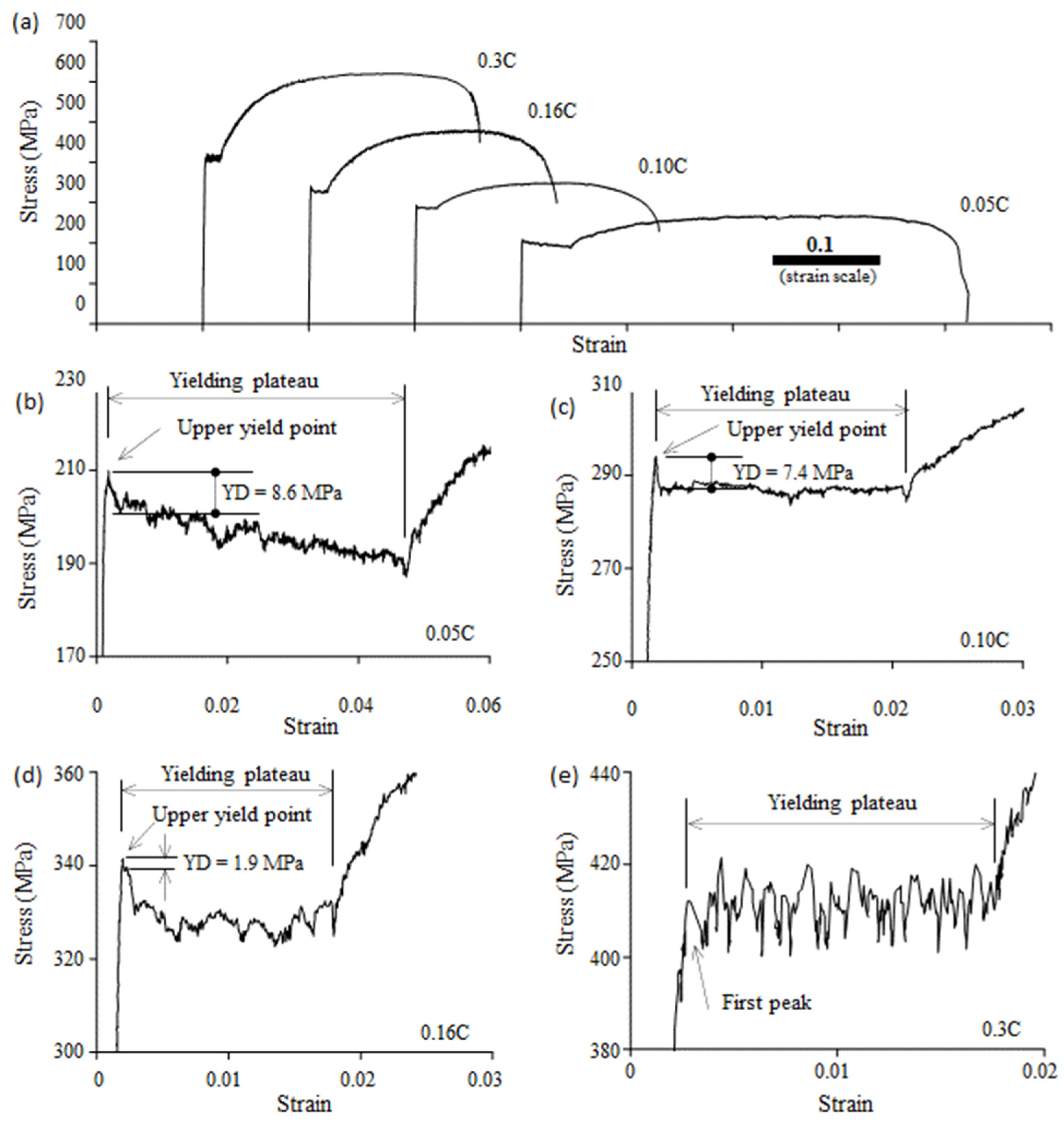
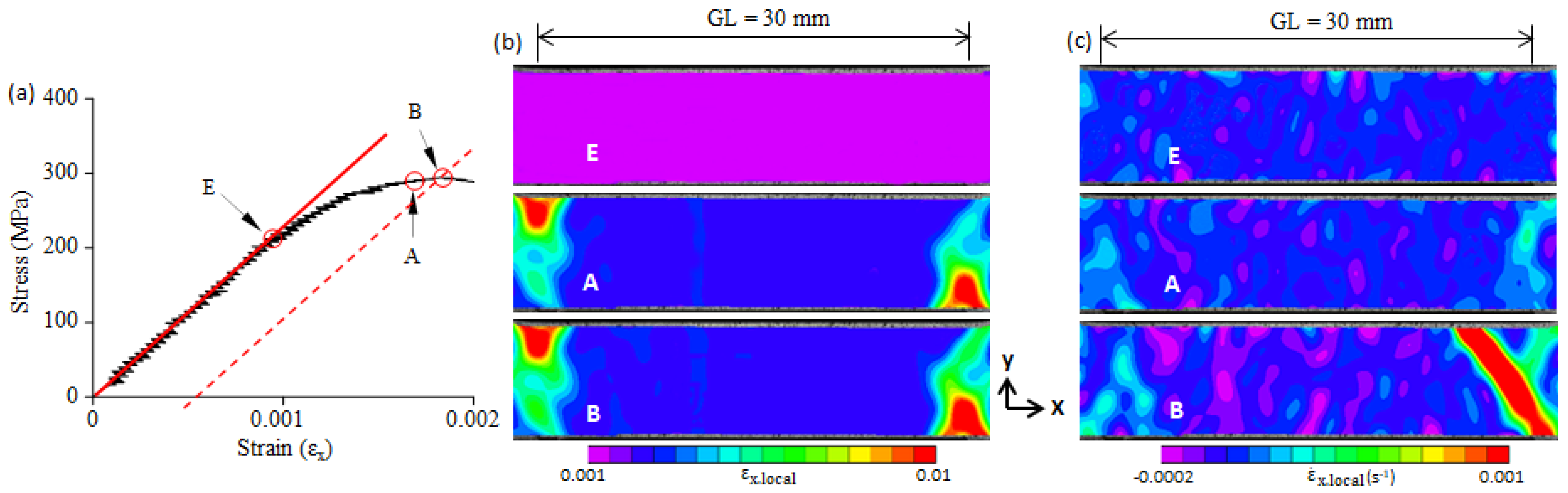
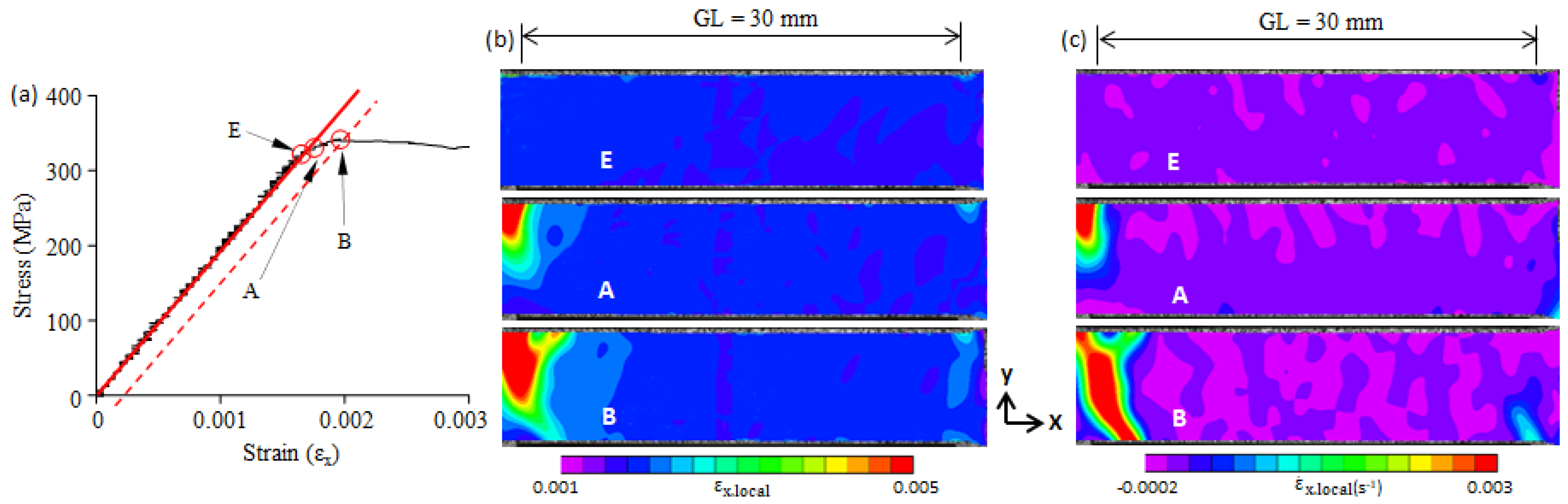
3.2. Yield-Point Phenomenon
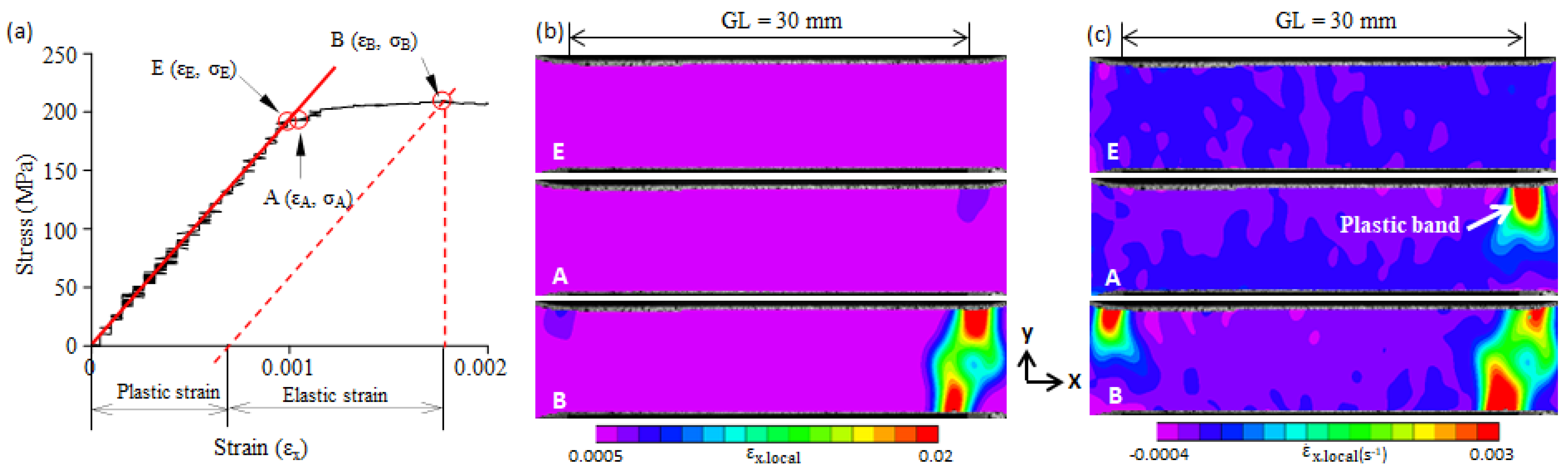
3.3. Plastic Band
4. Conclusions
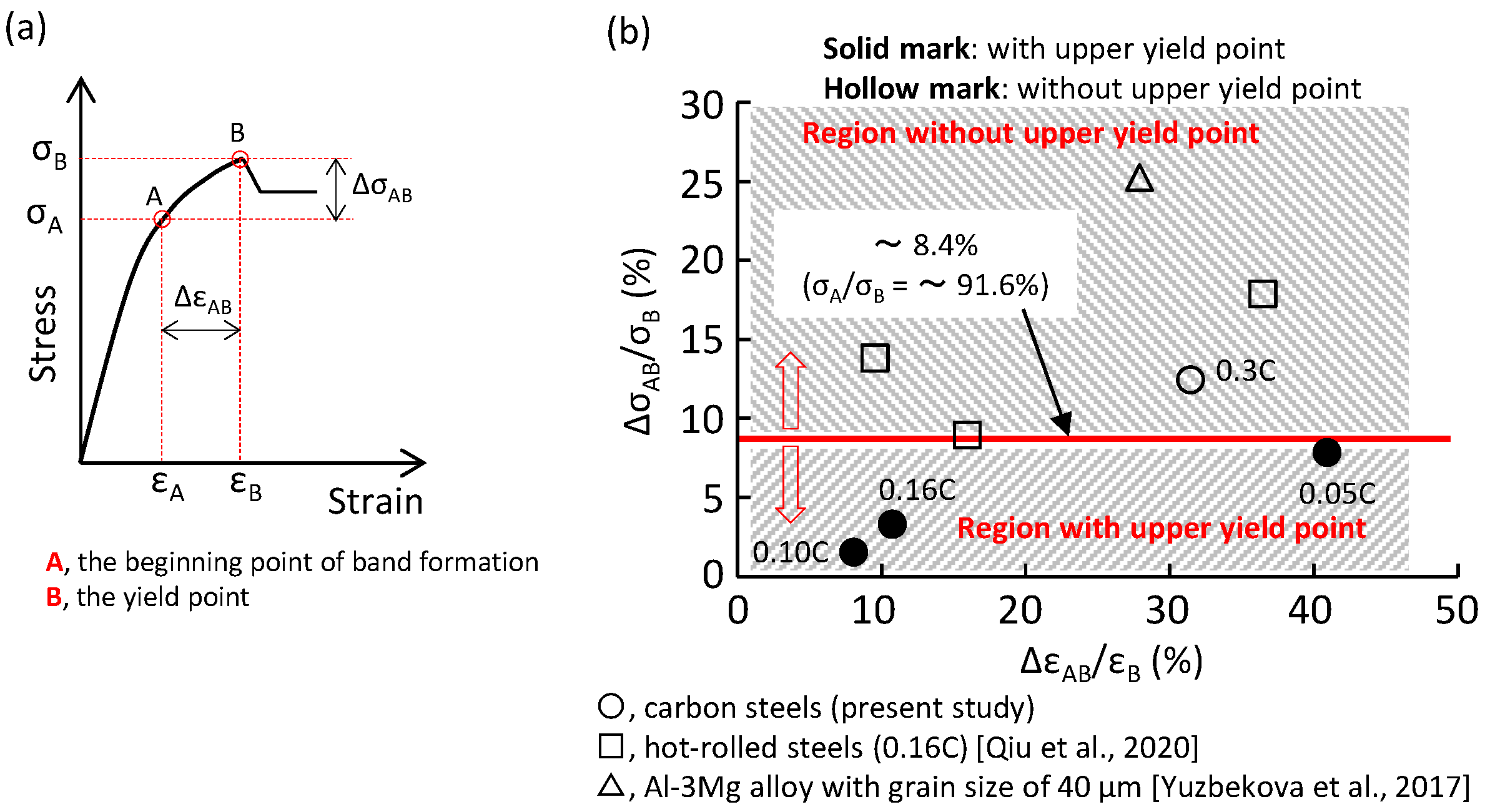
- The degree of the yield drop decreased with an increase in the pearlite fraction. When the pearlite fraction was sufficiently large, the upper yield point disappeared.
- Large local plastic strain was present at the yield point. It was much larger than the average plastic strain over the gauge length, and it was smaller than the Lüders strain.
- The yield-point phenomenon was related to the formation of a plastic band. A plastic band is formed at a certain stress level (i.e., the critical initiation stress) that is smaller than the upper yield stress (σB). When the critical initiation stress was larger than 0.92σB, the upper yield point disappeared.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naka, T.; Torikai, G.; Hino, R.; Yoshida, F. The effects of temperature and forming speed on the forming limit diagram for type 5083 aluminum-magnesium alloy sheet. J. Mater. Proc. Technol. 2001, 113, 648–653. [Google Scholar] [CrossRef]
- Pepelnjak, T.; Baristic, B. Analysis and elimination of the stretcher strains on TH415 tinplate rings in the stamping process. J. Mater. Proc. Technol. 2007, 186, 111–119. [Google Scholar] [CrossRef]
- Cottrell, A.H.; Bilby, B.A. Dislocation theory of yielding and strain ageing of iron. Phys. Soc. 1949, 62, 49–62. [Google Scholar] [CrossRef]
- Hahn, G.T. A model for yielding with special reference to the yield-point phenomena of iron and related bcc metals. Acta Met. 1962, 10, 727–738. [Google Scholar] [CrossRef]
- Van Rooyen, G.T. Basic factors which influence the Lüders strain during discontinuous yielding. Mater. Sci. Eng. 1971, 7, 37–48. [Google Scholar] [CrossRef]
- Van Rooyen, G.T. The stress and strain distribution in a propagating Lüders front accompanying the yield-point phenomenon in iron. Mater. Sci. Eng. 1968, 3, 105–117. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Wang, B.L.; Liang, G.F.; Zhang, Y.H.; Han, K.; Song, C.J. Grain boundary pop-in, yield point phenomenon and carbon segregation in aged low carbon steel. ISIJ Int. 2018, 58, 373–375. [Google Scholar] [CrossRef]
- Akama, D.; Nakada, N.; Tsuchiyama, T.; Takaki, S.; Hironaka, A. Discontinuous yielding induced by the addition of nickel to interstitial-free steel. Scripta Mater. 2014, 82, 13–16. [Google Scholar] [CrossRef]
- Tsuchiyama, T.; Araki, S.; Takaki, S. Interpretation of upper yield point in polycrystalline ferritic steel based on the critical grain boundary shear stress. Tetsu-to-Hagané 2020, 106, 382–390. (In Japanese) [Google Scholar] [CrossRef]
- Yoshida, F. A constitutive model of cyclic plasticity. Int. J. Plasticity 2000, 16, 359–380. [Google Scholar] [CrossRef]
- Blagoveshchenskii, V.V.; Panin, I.G. On the problem of a yield tooth. Phys. Metals Metallography. 2010, 109, 286–288. [Google Scholar] [CrossRef]
- Petukhov, B.V. A Theory of sharp yield point in low-dislocation crystals. Tech. Phys. 2001, 46, 1389–1395. [Google Scholar] [CrossRef]
- Mohsenzadeh, M.S.; Mazinani, M. On the yield point phenomenon in low-carbon steels with ferrite-cementite microstructure. Mater. Sci. Eng. A 2016, 673, 193–203. [Google Scholar] [CrossRef]
- Wang, Y.X.; Tomota, Y.; Ohmura, T.; Gong, W.; Harjo, S.; Tanaka, M. Continuous and discontinuous yielding behaviors in ferrite-cementite steels. Acta Mater. 2020, 196, 565–575. [Google Scholar] [CrossRef]
- Shimokawa, T.; Oto, T.; Niiyama, T. Molecular dynamics simulation of the effect of cementite decomposition on yield phenomena in pearlite microstructure. ISIJ Int. 2022, 62, 343–352. [Google Scholar] [CrossRef]
- Žerovnik, A.; Pepel, V.; Prebil, I.; Kunc, R. The yield-point phenomenon and cyclic plasticity of the uniaxially loaded specimens. Mater. Design. 2016, 92, 971–977. [Google Scholar] [CrossRef]
- Yoshida, F.; Kaneda, Y.; Yamamoto, S. A plasticity model describing yield-point phenomena of steels and its application to FE simulation of temper rolling. Int. J. Plasticity 2008, 24, 1792–1818. [Google Scholar] [CrossRef]
- Shi, H.G.; Li, J.X.; Mao, J.W.; Lu, W.J. The elimination of the yield point phenomenon in a new zirconium alloy: Influence of degree of recrystallization on the tensile properties. Scripta Mater. 2019, 169, 28–32. [Google Scholar] [CrossRef]
- Schwab, R.; Ruff, V. On the nature of the yield point phenomenon. Acta Materialia 2013, 61, 1798–1808. [Google Scholar] [CrossRef]
- Qiu, H.; Inoue, T.; Ueji, R. In-situ observation of Lüders band formation in hot-rolled steel via digital image correlation. Metals 2020, 10, 530. [Google Scholar] [CrossRef]
- Yuzbekova, D.; Mogucheva, A.; Zhemchuzhnikova, D.; Lebedkina, T.; Lebyodkin, M.; Kaibyshev, R. Effect of microstructure on continuous propagation of the Portevine–Le Chatelier deformation bands. Inter. J. Plasticity 2017, 96, 210–226. [Google Scholar] [CrossRef]
- Qiu, H.; Inoue, T.; Ueji, R. Experimental measurement of the variables of Lüders deformation in hot-rolled steel via digital image correlation. Mater. Sci. Eng. A 2020, 790, 139756. [Google Scholar] [CrossRef]
- Tsuchida, N.; Torizuka, S.; Nagai, K.; Ueji, R. Effect of cementite volume fraction on static tensile properties in ultrafine-grained ferrite-cementite steels. Tetsu-to-Hagané 2010, 96, 42–50. [Google Scholar] [CrossRef][Green Version]
- Mizuno, R.; Matsuda, H.; Funakawa, Y.; Tanaki, Y. Influence of microstructure on yield strength of ferrite-pearlite steels. Tetsu-to-Hagagé 2010, 96, 414–423. [Google Scholar] [CrossRef][Green Version]
- Massardier, V.; Merlin, J. Analysis of the parameters influencing the quench-aging behavior of ultra-low-carbon steels. Metall. Mater. Trans. A 2009, 40, 1100–1109. [Google Scholar] [CrossRef]
- García de Andrés, C.; Caballero, F.G.; Capdevila, C.; Bhadeshia, H.K.D.H. Modelling of kinetics and dilatometric behavior of non-isothermal pearlite-to-austenite transformation in an eutectoid steel. Scripta Materialia 1998, 39, 791–796. [Google Scholar] [CrossRef]
- Allain, S.; Bouaziz, O. Microstructure based modelling for the mechanical behavior of ferrite-pearlite steels suitable to capture isotropic and kinematic hardening. Mater. Sci. Eng. A 2008, 496, 329–336. [Google Scholar] [CrossRef]
- Guziewski, M.; Coleman, S.P.; Weinberger, C.R. Atomistic investigation into the atomic structure and energetics of the ferrite-cementite interface: The Bagaryatskii orientation. Acta Materialia 2016, 119, 184–192. [Google Scholar] [CrossRef]
- Shimokawa, T.; Niiyama, T.; Okabe, M.; Sawakoshi, J. Interfacial-dislocation-controlled deformation and fracture in nanolayered composites: Toward higher ductility of drawn pearlite. Acta Materialia 2019, 164, 602–617. [Google Scholar] [CrossRef]
- Liang, L.W.; Wang, Y.J.; Chen, Y.; Wang, H.Y.; Dai, L.H. Dislocation nucleation and evolution at the ferrite-cementite interface under cyclic loadings. Acta Materialia 2020, 186, 267–277. [Google Scholar] [CrossRef]
- Euser, V.K.; Martinez, D.T.; Valdez, J.A.; Trujillo, C.P.; Cady, C.M.; Jones, D.R.; Fensin, S.J. The influence of pearlite fraction on the shock properties of ferrite–pearlite steel microstructures: Insight into the effect of second-phase particles. J. Appl. Phys. 2022, 131, 115902. [Google Scholar] [CrossRef]


| Chemical Composition (in wt%) | Heat-Treatment Procedure | |||
|---|---|---|---|---|
| C | Mn | Si | ||
| 0.05C steel | 0.05 | 0.008 | 0.002 | 900 °C, 5 min → furnace cooling to room temperature |
| 0.10C steel | 0.10 | 1.53 | 0.44 | 900 °C, 5 min → furnace cooling to room temperature |
| 0.16C steel | 0.16 | 1.46 | 0.44 | 900 °C, 5 min → furnace cooling to room temperature |
| 0.3C steel | 0.3 | 1.5 | 0.3 | 850 °C, 5 min → furnace cooling to room temperature |
| DPF (μm) | fce (%) * | fPF/fP | |
|---|---|---|---|
| 0.05C steel | 30 | 0.8 | 98.8/1.2 |
| 0.10C steel | 18 | 1.5 | 92/8 |
| 0.16C steel | 9 | 2.4 | 83/17 |
| 0.3C steel | 4 | 4.6 | 68/32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, H.; Ueji, R.; Inoue, T. Yield-Point Phenomenon and Plastic Bands in Ferrite–Pearlite Steels. Materials 2023, 16, 195. https://doi.org/10.3390/ma16010195
Qiu H, Ueji R, Inoue T. Yield-Point Phenomenon and Plastic Bands in Ferrite–Pearlite Steels. Materials. 2023; 16(1):195. https://doi.org/10.3390/ma16010195
Chicago/Turabian StyleQiu, Hai, Rintaro Ueji, and Tadanobu Inoue. 2023. "Yield-Point Phenomenon and Plastic Bands in Ferrite–Pearlite Steels" Materials 16, no. 1: 195. https://doi.org/10.3390/ma16010195
APA StyleQiu, H., Ueji, R., & Inoue, T. (2023). Yield-Point Phenomenon and Plastic Bands in Ferrite–Pearlite Steels. Materials, 16(1), 195. https://doi.org/10.3390/ma16010195






