Mordenite-Supported Ag+-Cu2+-Zn2+ Trimetallic System: A Variety of Nanospecies Obtained via Thermal Reduction in Hydrogen Followed by Cooling in an Air or Hydrogen Atmosphere
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Composition and Characterization of the Exchanged Samples
3.2. Thermal Reduction of the Exchanged Samples Followed by Cooling in either Air or Hydrogen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nezamzadeh-Ejhieh, A.; Karimi-Shamsabadi, M. Decolorization of a binary azo dyes mixture using CuO incorporated nanozeolite-X as a heterogeneous catalyst and solar irradiation. Chem. Eng. J. 2013, 228, 631–641. [Google Scholar] [CrossRef]
- Sapawe, N.; Jalil, A.A.; Triwahyono, S.; Sah, R.; Jusoh, N.W.C.; Hairom, N.H.H.; Efendi, J. Electrochemical strategy for grown ZnO nanoparticles depositedontoHYzeolite with enhanced photodecolorization of methylene blue: Effect of the formation of Si-O-Zn bonds. Appl. Catal. A Gen. 2013, 456, 144–158. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Khorsandi, S. Photocatalytic degradation of 4-nitrophenol with ZnO supported nano-clinoptilolite zeolite. J. Ind. Eng. Chem. 2014, 20, 937–946. [Google Scholar] [CrossRef]
- Chotigkrai, N.; Tannititam, P.; Piticharoenphun, S.; Triamnak, N.; Praserthdam, S.; Praserthdam, P. The effect of Zn doping on active Cu species and its location of Cu-exchanged mordenite for the stepwise oxidation of methane to methanol. Korean J. Chem. Eng. 2022, 39, 920–927. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Hanxue, Z.; Fuzhen, Y.; Ahui, T.; Dongxu, H.; Junxiu, J.; Jin, W.; Li, Z.; Zhang, Z. Recent progress in performance optimization of Cu-SSZ-13 catalyst for selective catalytic reduction of NOx. Front. Chem. 2022, 10, 1033255. [Google Scholar] [CrossRef]
- Ramírez-Garza, R.E.; Rodríguez-Iznaga, I.; Simakov, A.; Farías, M.H.; Castillón-Barraza, F.F. Cu-Ag/mordenite catalysts for NO reduction: Effect of silver on catalytic activity and hydrothermal stability. Mater. Res. Bull. 2018, 97, 369–378. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Moghaddam, M.J.; Salavatiyan, T. Synthesis and characterization of CuO–montmorillonite nanocomposite by thermal decomposition method and antibacterial activity of nanocomposite. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 125, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kaali, P.; Pérez-Madrigal, M.M.; Strömberg, E.; Aune, R.E.; Czél, G.; Karlsson, S. The influence of Ag+, Zn2+ and Cu2+ exchanged zeolite on antimicrobial and long term in vitro stability of medical grade polyether polyurethane. Express Polym. Lett. 2011, 5, 1028–1040. [Google Scholar] [CrossRef]
- Contreras-Arzate, D.; Islas-Espinoza, M.; Fall, C.; Alcántara-Díaz, D.; Olguin, M.T.; López-Callejas, R.; Peña-Eguiluz, R. Microbial mortality behavior promoted by silver (Ag+/Ago)-modified zeolite-rich tuffs for water disinfection. J. Environ. Health Sci. Eng. 2020, 18, 755–768. [Google Scholar] [CrossRef]
- Ferreira, A.N.; D’Souza, K.; Aras, M.; Chitre, V.; Parsekar, S.; Pinto, M.J.W. Long term antifungal efficacy of silver-zinc zeolite nanoparticles incorporated in two soft denture liners—An in vitro assessment. Dent. Res. J. 2022, 19, 12. [Google Scholar] [CrossRef]
- Oheix, E.; Reicher, C.; Nouali, H.; Michelin, L.; Josien, L.; Daou, T.J.; Pieuchot, L. Rational Design and Characterisation of Novel Mono- and Bimetallic Antibacterial Linde Type A Zeolite Materials. J. Funct. Biomater. 2022, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Pérez, N.C.; Miró, E.E.; Zamaro, J.M. Microreactors based on CuO–CeO2/zeolite films synthesized onto brass microgrids for the oxidation of CO. Appl. Catal. B Environ. 2013, 129, 416–425. [Google Scholar] [CrossRef]
- Chang-Yong, L.; Tae-Hwan, J.; Baik-Hyon, H. Characteristics of CuO—Cr2O3/mordenite and its catalytic activity for combustion and NO decomposition. Appl. Catal. B Environ. 1996, 9, 77–91. [Google Scholar] [CrossRef]
- Quiñones, L.; Martínez-Iñesta, M. Effect of the precursor and reduction methods on the synthesis of supported Pt nanostructures in zeolite mordenita. J. Mater. Sci. 2011, 46, 7289–7297. [Google Scholar] [CrossRef]
- Mathisen, K.; Nilsen, M.H.; Nordhei, C.; Nicholson, D.G. Irreversible Silver(I) Interconversion in Ag:ZSM-5 and Ag:SAPO-5 by Propene and Hydrogen. J. Phys. Chem. C 2012, 116, 171–184. [Google Scholar] [CrossRef]
- Quiñones, L.; Grazul, J.; Martínez-Iñesta, M.M. Synthesis of platinum nanostructures in zeolite mordenite using a solid-state reduction method. Mater. Lett. 2009, 63, 2684–2686. [Google Scholar] [CrossRef]
- Rodríguez, I.; Petranovskii, V. Thermal reduction of Cu2+-Ag+-Zn2+ trimetallic system on mordenite. In Proceedings of the 16th International Zeolite Conference Joint with the 7th International Mesostructured Materials Symposium, Sorrento, Italy, 4–9 July 2010; Colella, C., Apresa, P., de Gennaro, B., Liguori, B., Eds.; De Frede Editore: Naples, Italy, 2010; p. 1662. [Google Scholar]
- Rodríguez-Iznaga, I.; Petranovskii, V.; Castillón-Barraza, F.; Concepción-Rosabal, B. Copper-Silver Bimetallic System on Natural Clinoptilolite: Thermal Reduction of Cu2+ and Ag+ Exchanged. J. Nanosci. Nanotechnol. 2011, 11, 5580–5586. [Google Scholar] [CrossRef]
- Rodríguez-Iznaga, I.; Petranovskii, V.; Castillón, F.; Farias, M.H. Effect of the Zn(II) on the reduction of Cu(II) in natural clinoptilolite. Opt. Mater. 2006, 29, 105–109. [Google Scholar] [CrossRef]
- Petranovskii, V.; Bogdanchikova, N. Reduction of binary silver-copper ion mixture in mordenite: An example of synergetic behavior. Stud. Surf. Sci. Catal. 2002, 141, 569–574. [Google Scholar]
- Rodríguez-Iznaga, I.; Petranovskii, V.; Chávez-Rivas, F.; Shelyapina, M.G. Bimetallic Copper-Silver Systems Supported on Natural Clinoptilolite: Long-Term Changes in Nanospecies Composition and Stability. Inorganics 2022, 10, 34. [Google Scholar] [CrossRef]
- Chajar, Z.; Denton, P.; de Bernard, F.B.; Primet, M.; Praliaud, H. Influence of silver on the catalytic activity of Cu-ZSM-5 for NO SCR by propane. Effect of the presence of water and hydrothermal agings. Catal. Lett. 1998, 55, 217–222. [Google Scholar] [CrossRef]
- Sánchez-López, P.; Kotolevich, Y.; Miridonov, S.; Chávez-Rivas, F.; Fuentes, S.; Petranovskii, V. Bimetallic AgFe Systems on Mordenite: Effect of Cation Deposition Order in the NO Reduction with C3H6/CO. Catalysts 2019, 9, 58. [Google Scholar] [CrossRef]
- Song, H.; Wan, X.; Dai, M.; Zhang, J.; Li, F.; Song, H. Deep desulfurization of model gasoline by selective adsorption over Cu–Ce bimetal ion-exchanged Y zeolite. Fuel Process. Technol. 2013, 116, 52–62. [Google Scholar] [CrossRef]
- Khatamian, M.; Hashemian, S.; Yavari, A.; Saket, M. Preparation of metal ion (Fe3+ and Ni2+) doped TiO2 nanoparticles supported on ZSM-5 zeolite and investigation of its photocatalytic activity. Mater. Sci. Eng. B 2012, 177, 1623–1627. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, P.S.; Cho, B.K.; Nam, I.S.; Oh, S.H. Enhanced NOx reduction and byproduct removal by (HC + OHC)/SCR over multifunctional dual-bed monolith catalyst. Catal. Today 2012, 184, 95–106. [Google Scholar] [CrossRef]
- Sriningsih, W.; Saerodji, M.G.; Trisunaryanti, W.; Armunanto, T.R.; Falah, I.I. Fuel Production from LDPE Plastic Waste over Natural Zeolite Supported Ni, Ni-Mo, Co and Co-Mo Metals. Procedia Environ. Sci. 2014, 20, 215–224. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Krylova, E.A.; Zhukov, Y.M.; Zvereva, I.A.; Rodriguez-Iznaga, I.; Petranovskii, V.; Fuentes-Moyado, S. Comprehensive Analysis of the Copper Exchange Implemented in Ammonia and Protonated Forms of Mordenite Using Microwave and Conventional Methods. Molecules 2019, 24, 4216. [Google Scholar] [CrossRef]
- Sherman, J.D. Ion Exchange Separations with Molecular Sieve Zeolites. In Zeolites: Science and Technology; Ribeiro, F.R., Rodrigues, A., Rollmann, D., Naccache, C., Eds.; NATO ASI Series: Series E, The Hague; Springer: Dordrecht, The Netherlands, 1984; Volume 80, pp. 583–623. [Google Scholar] [CrossRef]
- Shi, C.; Cheng, M.; Qu, Z.; Bao, X. On the correlation between microstructural changes of Ag-H-ZSM-5 catalysts and their catalytic performances in the selective catalytic reduction of NOx by methane. J. Mol. Catal. A Chem. 2005, 235, 35–43. [Google Scholar] [CrossRef]
- Li, Z.; Flytzani-Stephanopoulos, M. On the Promotion of Ag–ZSM-5 by Cerium for the SCR of NO by Methane. J. Catal. 1999, 182, 313–327. [Google Scholar] [CrossRef]
- Keshavaraja, A.; She, X.; Flytzani-Stephanopoulos, M. Selective catalytic reduction of NO with methane over Ag-alumina catalysts. Appl. Catal. B Environ. 2000, 27, L1–L9. [Google Scholar] [CrossRef]
- Ju, W.S.; Matsuoka, M.; Anpo, M. Incorporation of silver (I) ions within zeolite cavities andtheir photocatalytic reactivity for the decompositionof N2O into N2 and O2. Int. J. Photoenergy 2003, 5, 17–19. [Google Scholar] [CrossRef]
- Petrov, O.E. Cation exchange in clinoptilolite: An X-ray powder diffraction analysis. In Natural Zeolites 93: Occurrence, Properties, Use; Ming, D.W., Mumpton, F.A., Eds.; Brockport: New York, NY, USA, 1995; p. 271. [Google Scholar]
- Nezamzadeh-Ejhieh, A.; Amiri, M. CuO supported Clinoptilolite towards solar photocatalytic degradation of p-aminophenol. Powder Technol. 2013, 235, 279–288. [Google Scholar] [CrossRef]
- Rodríguez, I.; Petranovskii, V.; Rodríguez Fuentes, G.; Mendoza, C.; Benítez, A. Exchange and reduction of Cu2+ ions in clinoptilolite. J. Colloid Interface Sci. 2007, 316, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Concepción-Rosabal, B.; Rodríguez-Fuentes, G.; Bogdanchikova, N.; Bosch, P.; Avalos, M.; Lara, V.H. Comparative study of natural and synthetic clinoptilolites containing silver in different states. Microporous Mesoporous Mater. 2005, 86, 249–255. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Petranovskii, V.; Fuentes, S.; Paukshtis, E.; Sugi, Y.; Licea-Claverie, A. Role of mordenite acid properties in silver cluster stabilization. Mater. Sci. Eng. A 2000, 276, 236–242. [Google Scholar] [CrossRef]
- Gurin, V.S.; Bogdanchikova, N.E.; Petranovskii, V.P. Few-Atomic Silver Clusters in Zeolites: Ab Initio MO LCAO Calculation and Optical Spectroscopy. J. Phys. Chem. B 2000, 104, 12105–12110. [Google Scholar] [CrossRef]
- Gurin, V.S.; Petranovskii, V.P.; Bogdanchikova, N.E. Silver and copper nanostructures within the erionite regular lattice: Interplay between intra- and extra-crystalline locations. Mater. Sci. Eng. C 2003, 23, 81–85. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Petranovskii, V.; Machorro, R.; Sugi, Y.; Soto, V.M.; Fuentes, S. Stability of silver clusters in mordenites with different SiO2/Al2O3 molar ratio. Appl. Surf. Sci. 1999, 150, 58–64. [Google Scholar] [CrossRef]
- Gurin, V.S.; Petranovskii, V.; Hernandez, M.A.; Bogdanchikova, N.E.; Alexeenko, A.A. Silver and copper clusters and small particles stabilized within nanoporous silicate-based materials. Mater. Sci. Eng. A 2005, 391, 71–76. [Google Scholar] [CrossRef]
- Aspromonte, S.G.; Mizrahi, M.D.; Schneeberger, F.A.; Ramallo-Lopez, J.M.; Boix, A.V. Study of the Nature and Location of Silver in Ag-Exchanged Mordenite Catalysts. Characterization by Spectroscopic Techniques. J. Phys. Chem. C 2013, 117, 25433–25442. [Google Scholar] [CrossRef]
- Heo, N.H.; Kim, Y.; Kim, J.J.; Seff, K. Surprising Intrazeolitic Chemistry of Silver. J. Phys. Chem. C 2016, 120, 5277–5287. [Google Scholar] [CrossRef]
- Li, M.; Li, G.; Fan, Y.; Jiang, J.; Ding, Q.; Dai, X.; Mai, K. Effect of nano-ZnO-supported 13X zeolite on photo-oxidation degradation and antimicrobial properties of polypropylene random copolymer. Polym. Bull. 2014, 71, 2981–2997. [Google Scholar] [CrossRef]
- Gayatri, R.; Agustina, T.E.; Bahrin, D.; Moeksin, R.; Gustini, G. Preparation and Characterization of ZnO-Zeolite Nanocomposite for Photocatalytic Degradation by Ultraviolet Light. J. Ecol. Eng. 2020, 22, 178–186. [Google Scholar] [CrossRef]
- Qumar, U.; Hassan, J.Z.; Bhatti, R.A.; Raza, A. Photocatalysis vs. adsorption by metal oxide nanoparticles. J. Mater. Sci. Technol. 2022, 131, 122–166. [Google Scholar] [CrossRef]
- Martínez-Vieyra, C.; Gutiérrez-Segura, E.; López-Tellez, G.; Alcántara-Díaz, D.; Olguín, M.T. Antimicrobial composites of nanoparticles generated by gamma irradiation supported in clinoptilolite-rich tuff. Appl. Nanosci. 2021, 11, 1183–1195. [Google Scholar] [CrossRef]
- Partoazar, A.; Fatemeh, R.B.; Takzaree, N.; Dallal, M.M.S. Antibiofilm activity of ZnO/zeolite nanocomposite (ZnO/zeonc) against klebsiella pneumoniae and its biocompatibility in an animal model. Anti-Infect. Agents 2021, 19, 174–181. [Google Scholar] [CrossRef]
- Susarrey-Arce, A.; Hernández-Espinosa, M.-A.; Rojas-González, F.; Reed, C.; Petranovskii, V.; Licea, A. Inception and trapping of ZnO nanoparticles within desilicated mordenite and ZSM-5 zeolites. Part. Part. Syst. Charact. 2010, 27, 100–111. [Google Scholar] [CrossRef]
- Susarrey-Arce, A.; Petranovskii, V.; Hernández-Espinosa, M.A.; Portillo, R.; De La Cruz, W. Optical properties of ZnO nanoparticles on the porous structure of mordenites and ZSM-5. J. Nanosci. Nanotechnol. 2011, 11, 5574–5579. [Google Scholar] [CrossRef]
- Zhao, D.; Tian, X.; Doronkin, D.E.; Han, S.; Kondratenko, V.A.; Grunwaldt, J.-D.; Perechodjuk, A.; Vuong, T.H.; Rabeah, J.; Eckelt, R.; et al. In situ formation of ZnOx species for efficient propane dehydrogenation. Nature 2021, 599, 234–238. [Google Scholar] [CrossRef]
- Brazlauskas, M.; Kitrys, S. Synthesis and Properties of CuO/Zeolite Sandwich Type Adsorbent-Catalysts. Chin. J. Catal. 2008, 29, 25–30. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, J.; Huang, C.; Lei, Z.; Chen, B. Adsorptive separation of dimethyl disulfide from liquefied petroleum gas by different zeolites and selectivity study via FT-IR. Sep. Purif. Technol. 2014, 125, 247–255. [Google Scholar] [CrossRef]
- Tao, L.; Lee, I.; Sanchez-Sanchez, M. Cu oxo nanoclusters for direct oxidation of methane to methanol: Formation, structure and catalytic performance. Catal. Sci. Technol. 2020, 10, 7124–7141. [Google Scholar] [CrossRef]
- Martini, A.; Signorile, M.; Negri, C.; Kvande, K.; Lomachenko, K.A.; Svelle, S.; Beato, P.; Berlier, G.; Borfecchia, E.; Bordiga, S. EXAFS wavelet transform analysis of Cu-MOR zeolites for the direct methane to methanol conversion. Phys. Chem. Chem. Phys. 2020, 22, 18950–18963. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Liu, L.; Liu, Z. Recent Insights into Cu-Based Catalytic Sites for the Direct Conversion of Methane to Methanol. Molecules 2022, 27, 7146. [Google Scholar] [CrossRef]
- Palagin, D.; Knorpp, A.J.; Pinar, A.B.; Ranocchiari, M.; van Bokhoven, J.A. Assessing the relative stability of copper oxide clusters as active sites of a CuMOR zeolite for methane to methanol conversion: Size matters? Nanoscale 2017, 9, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Bogdanchikova, N.E.; Paukshtis, E.A.; Dulin, M.N.; Petranovskii, V.P.; Shugi, I.; Hanaoka, T.; Matsuzaki, T.; Tue, H.; Shin, S. The crucial role of the SiO2-AL2O3 ratio in stabilization of silver clusters in mordenita. Inorg. Mater. 1995, 31, 451–456. [Google Scholar]
- Petranovskii, V.; Gurin, V.; Bogdanchikova, N.; Sugi, Y. Controlling copper reducibility in mordenites by varying the SiO2/Al2O3 molar ratio. Mater. Lett. 2003, 57, 1781–1785. [Google Scholar] [CrossRef]
- Petranovskii, V.; Stoyanov, E.; Gurin, V.; Katada, N.; Hernandez, M.-A.; Avalos, M.; Pestryakov, A.; Rivas, F.C.; Zamorano Ulloa, R.; Portillo, R. Formation of copper nanoparticles in mordenites with variable SiO2/Al2O3 molar ratios under redox treatments. Rev. Mex. Física 2013, 59, 170–185. [Google Scholar]
- Antúnez-García, J.; Galván, D.H.; Petranovskii, V.; Posada-Amarillas, A. A DFT study of copper-oxide clusters embedded in dry and water-immersed siliceous mordenita. Comput. Mater. Sci. 2015, 106, 140–148. [Google Scholar] [CrossRef]
- Signorile, M.; Borfecchia, E.; Bordiga, S.; Berlier, G. Influence of ion mobility on the redox and catalytic properties of Cu ions in zeolites. Chem. Sci. 2022, 13, 10238–10250. [Google Scholar] [CrossRef] [PubMed]
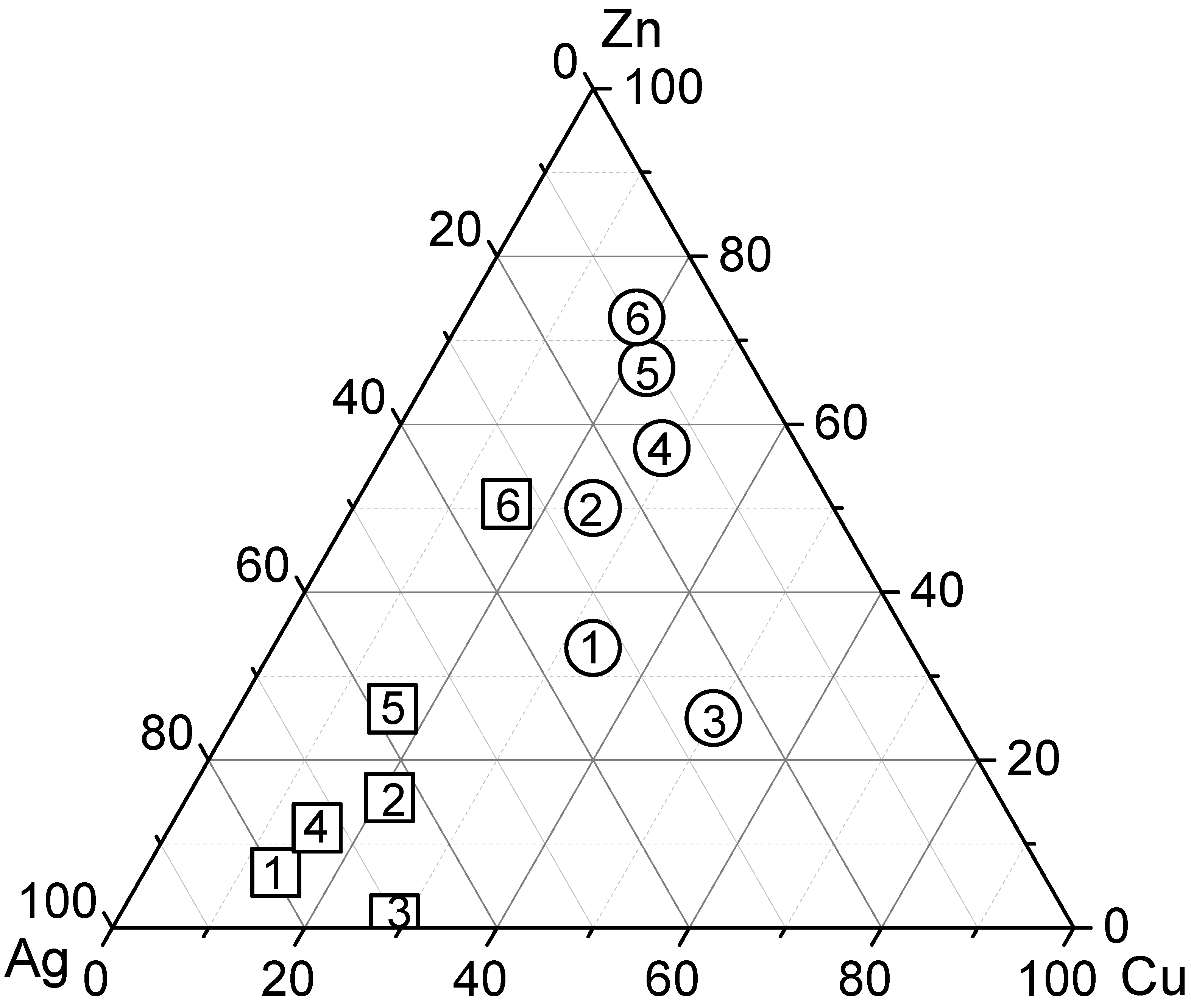

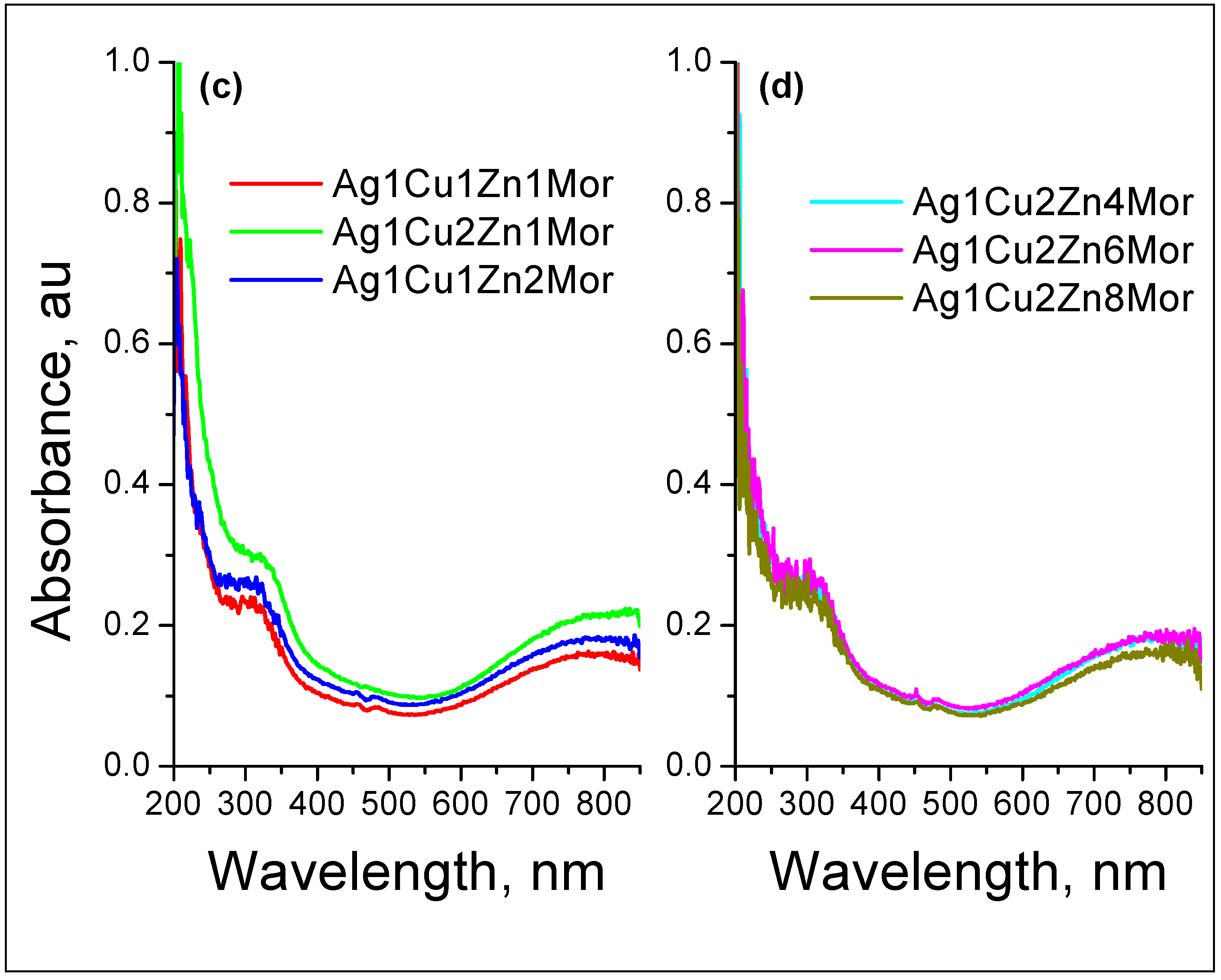

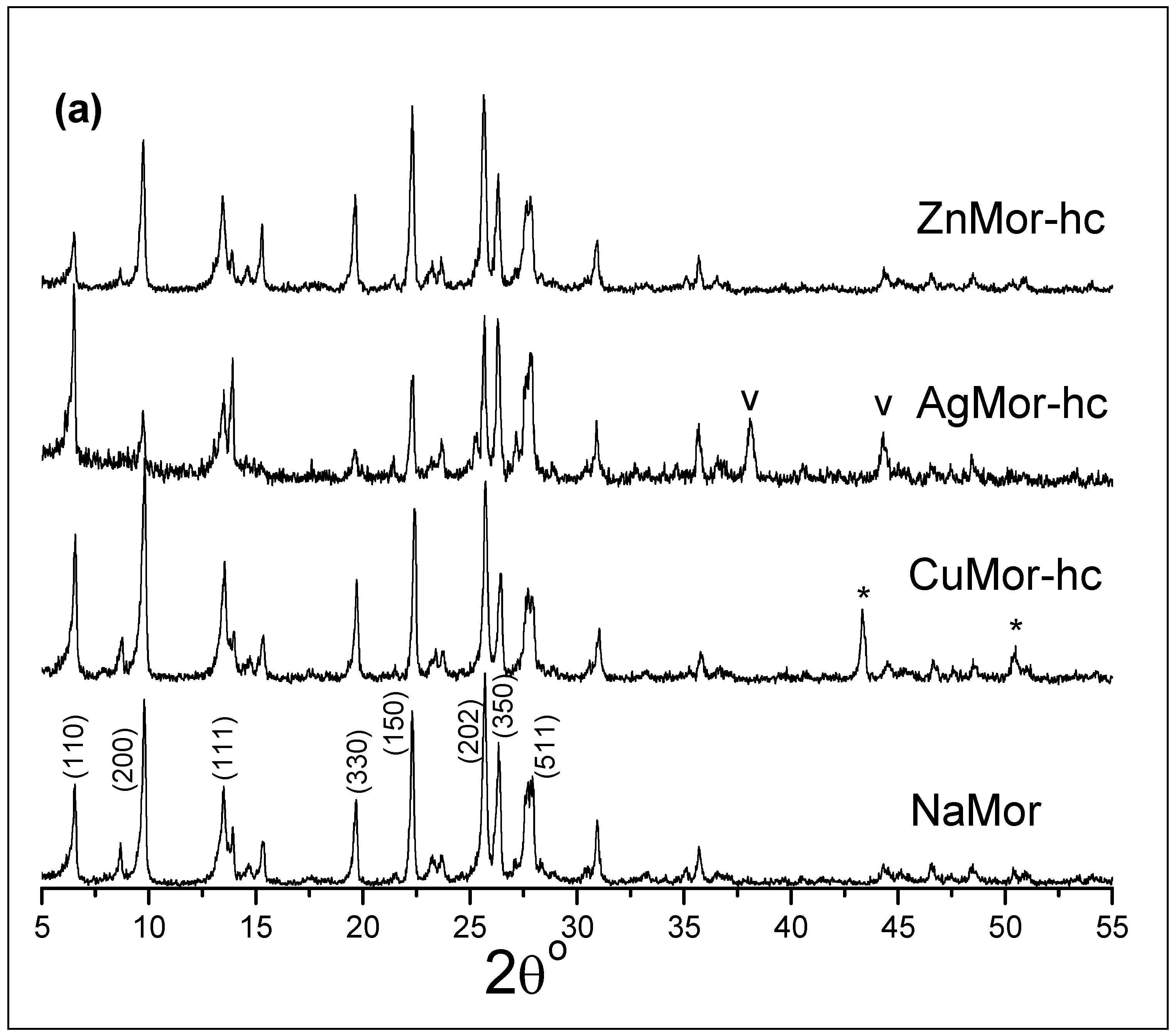
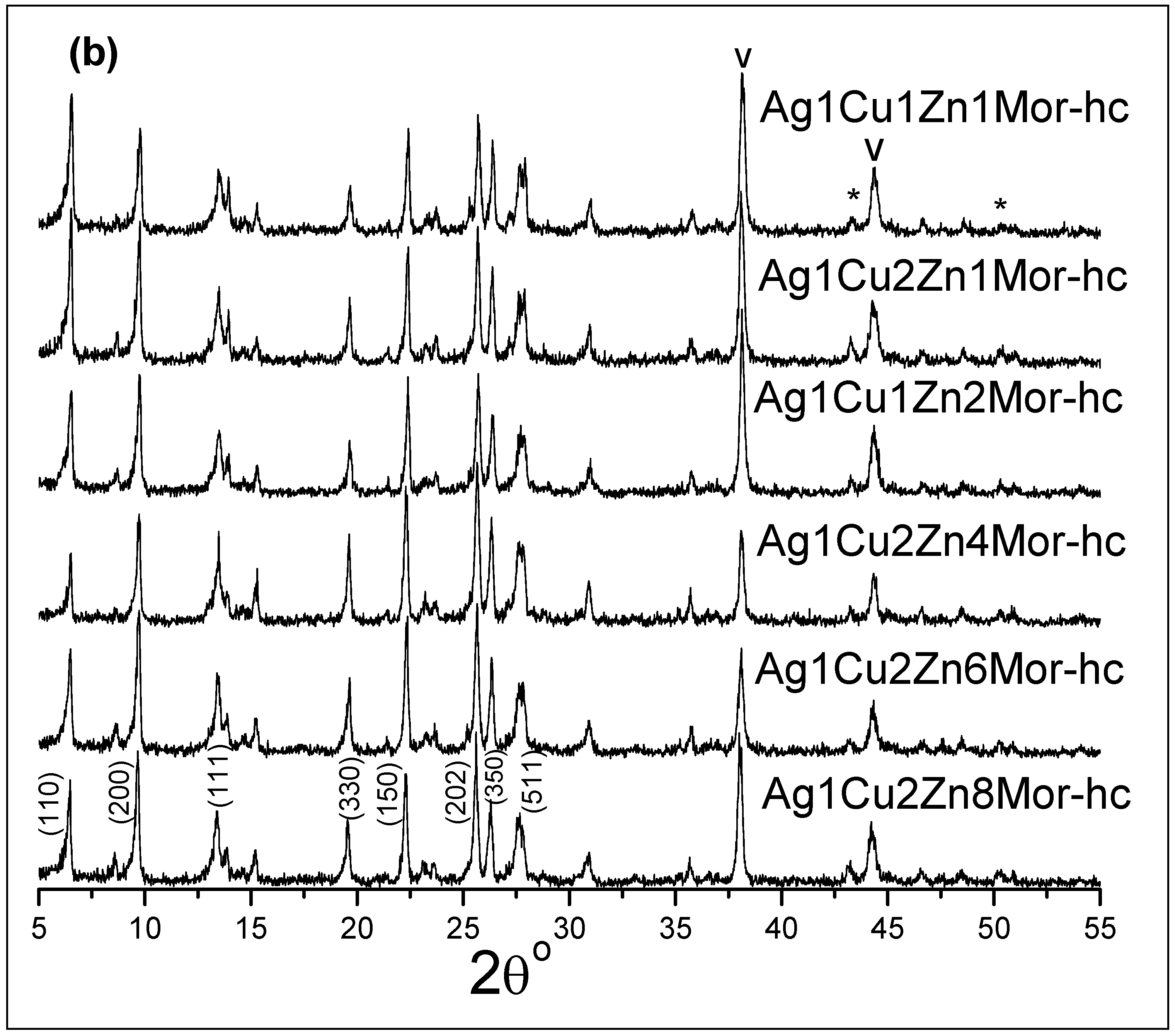

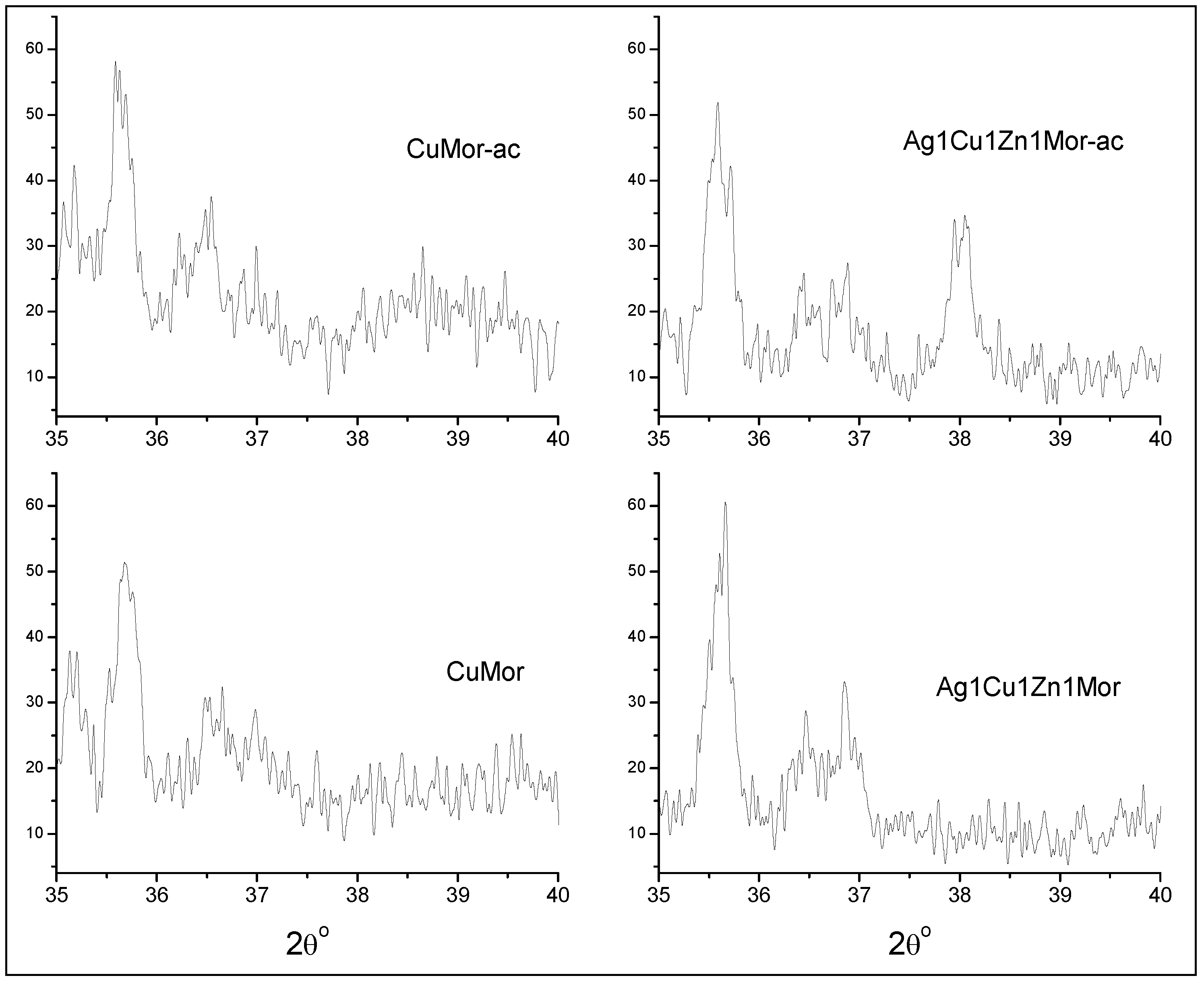
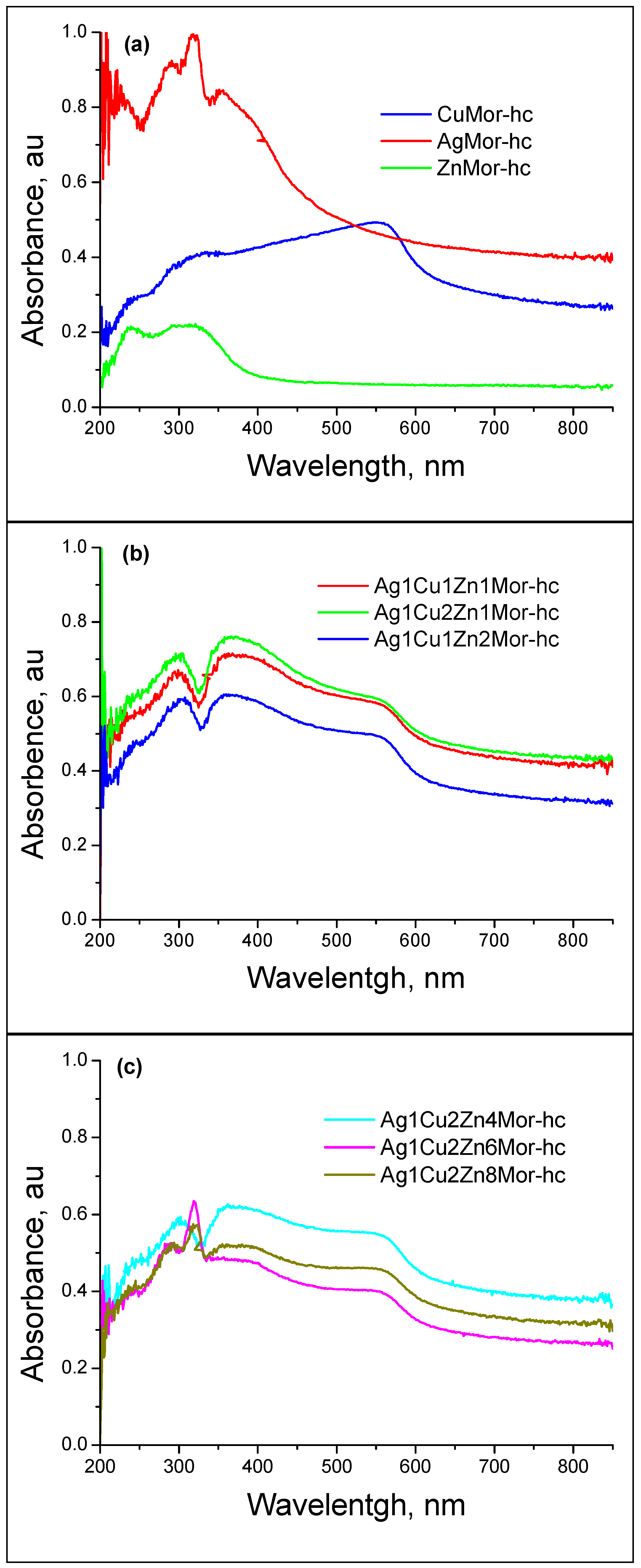


| Samples | Ag | Cu | Zn |
|---|---|---|---|
| AgMor | 12.75 | ||
| CuMor | 4.07 | ||
| ZnMor | 3.58 | ||
| Ag1Cu1Zn1Mor | 8.08 | 1.39 | 0.67 |
| Ag1Cu1Zn2Mor | 7.15 | 1.51 | 1.17 |
| Ag1Cu2Zn1Mor | 6.79 | 2.79 | 0.10 |
| Ag1Cu2Zn4Mor | 4.70 | 1.56 | 1.15 |
| Ag1Cu2Zn6Mor | 3.88 | 1.08 | 1.75 |
| Ag1Cu2Zn8Mor | 2.37 | 1.11 | 3.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Iznaga, I.; Petranovskii, V.; Castillón-Barraza, F.F.; Fuentes-Moyado, S.; Chávez-Rivas, F.; Pestryakov, A. Mordenite-Supported Ag+-Cu2+-Zn2+ Trimetallic System: A Variety of Nanospecies Obtained via Thermal Reduction in Hydrogen Followed by Cooling in an Air or Hydrogen Atmosphere. Materials 2023, 16, 221. https://doi.org/10.3390/ma16010221
Rodríguez-Iznaga I, Petranovskii V, Castillón-Barraza FF, Fuentes-Moyado S, Chávez-Rivas F, Pestryakov A. Mordenite-Supported Ag+-Cu2+-Zn2+ Trimetallic System: A Variety of Nanospecies Obtained via Thermal Reduction in Hydrogen Followed by Cooling in an Air or Hydrogen Atmosphere. Materials. 2023; 16(1):221. https://doi.org/10.3390/ma16010221
Chicago/Turabian StyleRodríguez-Iznaga, Inocente, Vitalii Petranovskii, Felipe F. Castillón-Barraza, Sergio Fuentes-Moyado, Fernando Chávez-Rivas, and Alexey Pestryakov. 2023. "Mordenite-Supported Ag+-Cu2+-Zn2+ Trimetallic System: A Variety of Nanospecies Obtained via Thermal Reduction in Hydrogen Followed by Cooling in an Air or Hydrogen Atmosphere" Materials 16, no. 1: 221. https://doi.org/10.3390/ma16010221
APA StyleRodríguez-Iznaga, I., Petranovskii, V., Castillón-Barraza, F. F., Fuentes-Moyado, S., Chávez-Rivas, F., & Pestryakov, A. (2023). Mordenite-Supported Ag+-Cu2+-Zn2+ Trimetallic System: A Variety of Nanospecies Obtained via Thermal Reduction in Hydrogen Followed by Cooling in an Air or Hydrogen Atmosphere. Materials, 16(1), 221. https://doi.org/10.3390/ma16010221







