Influence of Intrinsic Physicochemical Properties of Agroforestry Waste on Its Pyrolysis Characteristics and Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation and Characterization
2.2. Pyrolysis Experiments and Thermogravimetric Analysis
2.3. Kinetic Analysis
2.4. Mechanism Model f(α) Prediction
2.5. Data Analysis
3. Results and Discussion
3.1. Intrinsic Physicochemical Properties of Samples
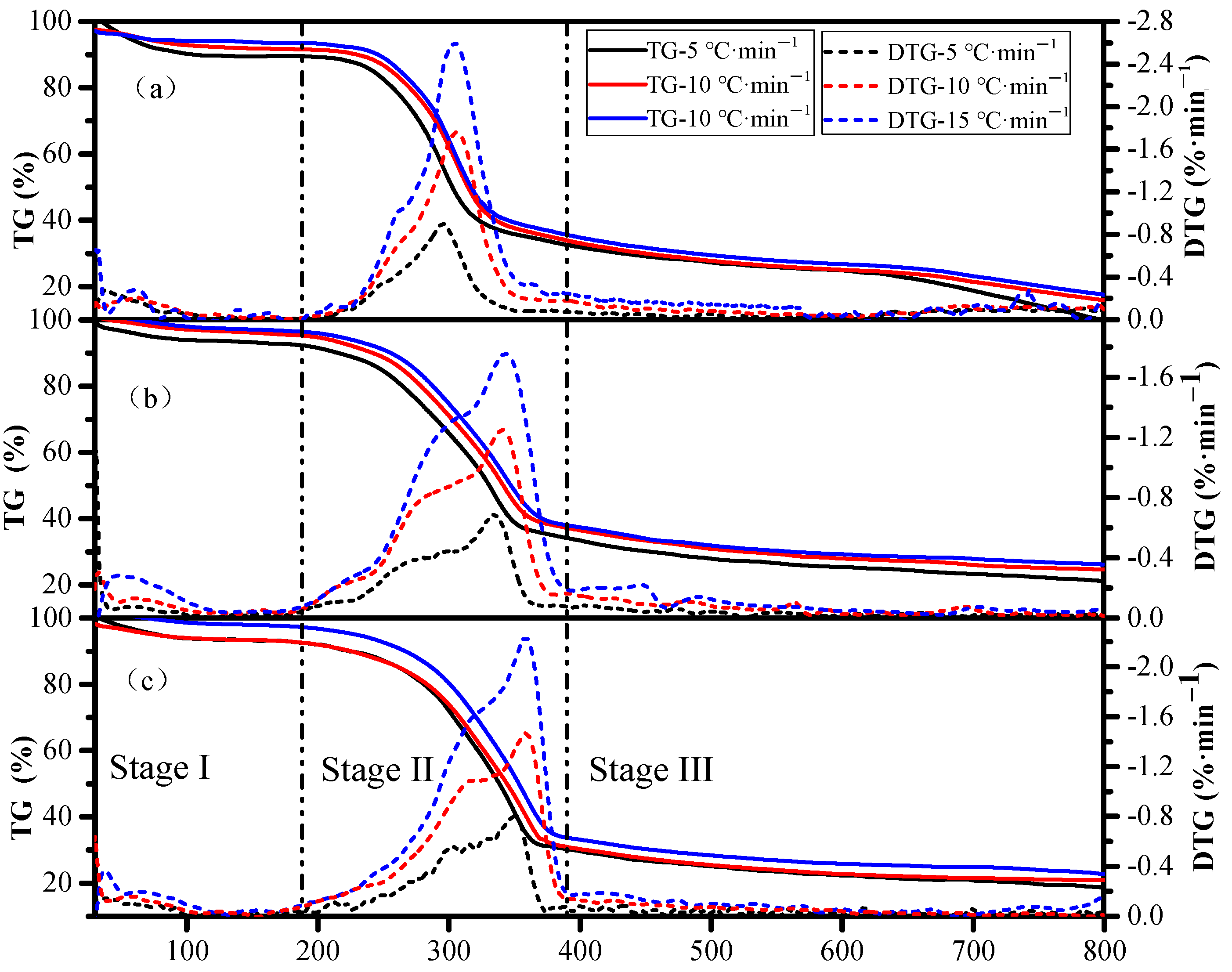
3.2. TG and DTG Curves
3.3. Pyrolysis Characteristics
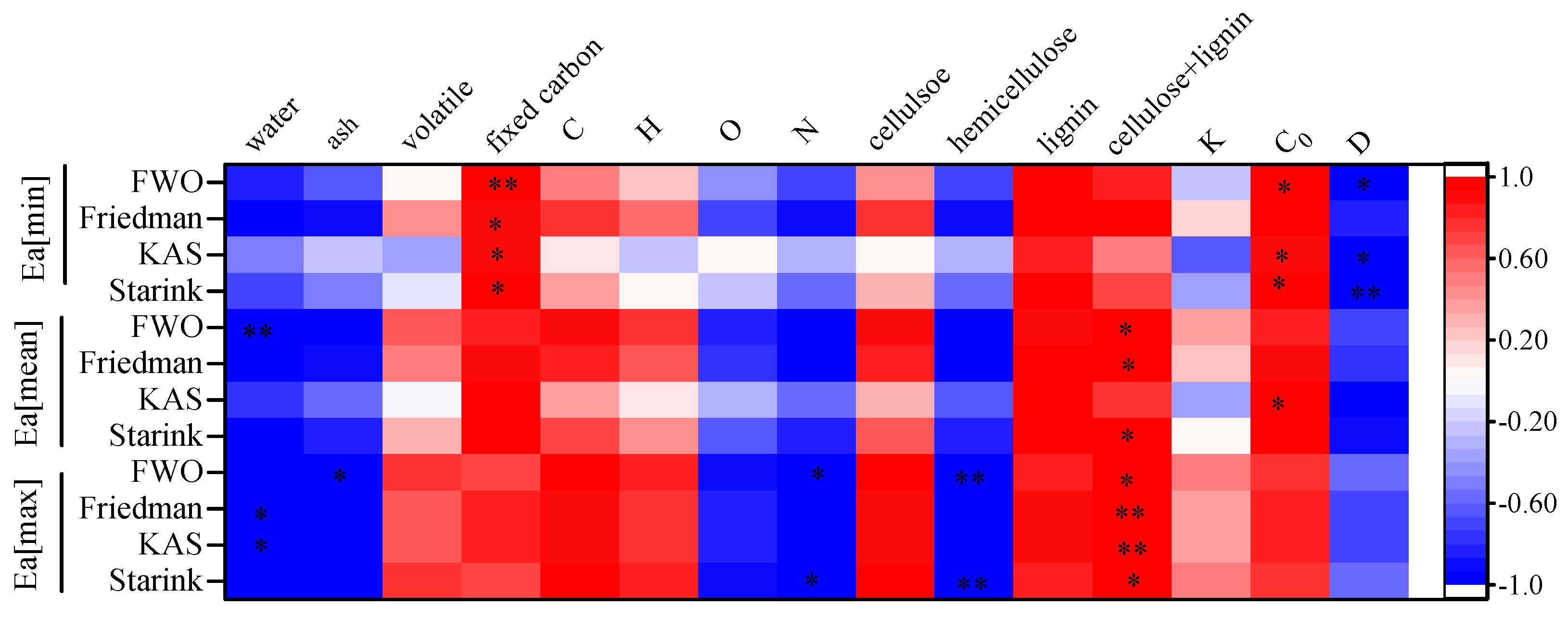
3.4. Correlation between the Intrinsic Physicochemical Properties and Thermal Characteristics
3.5. Kinetic Parameter Analysis
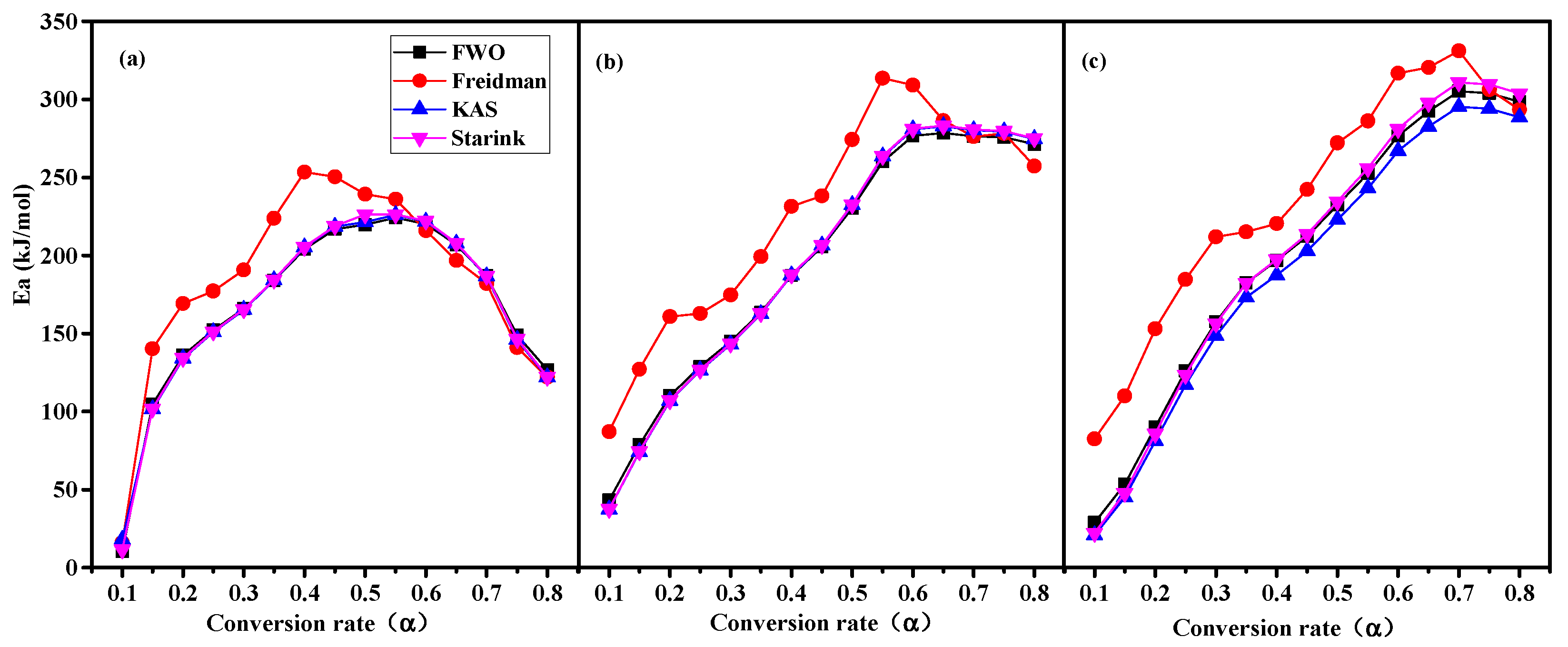
3.5.1. Ea Analysis
3.5.2. Pre-Exponential Factors (A) Analysis
3.5.3. Estimation of the Reaction Mechanism [f(α)]
4. Conclusions
- (1)
- There were significant differences in the intrinsic physicochemical properties of the three agroforestry wastes. The rich lignocellulose content; low moisture, ash, and N element content; and good thermophysical properties indicate that the agroforestry waste has good prospects for pyrolysis.
- (2)
- The intrinsic physicochemical properties of agroforestry waste have a significant influence on their pyrolysis characteristics. Moisture is positively correlated with the weight loss in the first stage. The second stage is the main stage of pyrolysis, and the pyrolysis characteristics are influenced by the combination of several intrinsic physicochemical properties. The content of fixed carbon was positively correlated with Prate. WL2 was positively correlated with C and cellulose content and negatively correlated with O content. R2 was positively correlated with the sum of cellulose and lignin content. RSS was positively correlated with lignin content. In addition, C0 and D also had an effect on Prate, but not significantly at the lower heating rate conditions. This indicates that the intrinsic physicochemical properties of the biomass on the pyrolysis characteristics are also influenced by pyrolysis conditions.
- (3)
- The Ea[min] values were significantly positively correlated with C0 and negatively correlated with D. The Ea[mean] and Ea[max] were significantly positively correlated with the sum content of cellulose and lignin, indicating that the contents of cellulose and lignin determine the energy required for the pyrolysis process of agroforestry waste.
- (4)
- Vast variations in A value and several reaction models (diffusion model, growth model, and geometrical contraction model) involved in the pyrolysis process indicate that the pyrolysis of agroforestry waste is a complex process due to the heterogeneity of its intrinsic physicochemical properties.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Lin, J.; Song, T.; Su, H. Anaerobic Digestion of Waste for Biogas Production. Waste-to-Energy 2022, 177–206. [Google Scholar]
- Pardo, G.; Moral, R.; Del Prado, A. SIMSWASTE-AD-A modelling framework for the environmental assessment of agricultural waste management strategies: Anaerobic digestion. Sci. Total Environ. 2017, 574, 806–817. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, J.; Zeng, Y. Knowledge domain and emerging trends of agricultural waste management in the field of social science: A scientometric review. Sci. Total Environ. 2019, 670, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Bach, Q.V.; Chen, W.H. Pyrolysis characteristics and kinetics of microalgae via thermogravimetric analysis (TGA): A state-of-the-art review. Bioresour. Technol. 2017, 246, 88–100. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Memon, M.Z.; Ji, G.; Raheem, A.; Jia, T.Z.; Dupont, V.; Zhao, M. Thermal characteristics and kinetic analysis of woody biomass pyrolysis in the presence of bifunctional alkali metal ceramics. ACS Sustain. Chem. Eng. 2018, 7, 238–248. [Google Scholar] [CrossRef]
- Segurado, R.; Pereira, S.; Correia, D.; Costa, M. Techno-economic analysis of a trigeneration system based on biomass gasification. Renew. Sustain. Energy Rev. 2019, 103, 501–514. [Google Scholar] [CrossRef]
- Correa, C.R.; Hehr, T.; Voglhuber-Slavinsky, A.; Rauscher, Y.; Kruse, A. Pyrolysis vs. hydrothermal carbonization: Understanding the effect of biomass structural components and inorganic compounds on the char properties. J. Anal. Appl. Pyrolysis 2019, 140, 137–147. [Google Scholar] [CrossRef]
- He, Q.; Guo, Q.; Ding, L.; Wei, J.; Yu, G. Rapid co-pyrolysis of lignite and biomass blends: Analysis of synergy and gasification reactivity of residue char. J. Anal. Appl. Pyrolysis 2019, 143, 104688. [Google Scholar] [CrossRef]
- Sun, D.; Cai, W.; Li, C.; Lu, J. Experimental study on atomization characteristics of high-energy-density fuels using a fuel slinger. Energy 2021, 234, 121222. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Pandey, S.P.; Kumar, S. Valorisation of argemone mexicana seeds to renewable fuels by thermochemical conversion process. J. Environ. Chem. Eng. 2020, 8, 104271. [Google Scholar] [CrossRef]
- Cai, W.; Dai, L.; Liu, R. Catalytic fast pyrolysis of rice husk for bio-oil production. Energy 2018, 154, 477–487. [Google Scholar] [CrossRef]
- Han, B.; Chen, Y.; Wu, Y.; Hua, D.; Chen, Z.; Feng, W.; Yang, M.; Xie, Q. Co-pyrolysis behaviors and kinetics of plastics–biomass blends through thermogravimetric analysis. J. Therm. Anal. Calorim. 2014, 115, 227–235. [Google Scholar] [CrossRef]
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.W.; Bridgwater, A.V. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715. [Google Scholar] [CrossRef] [Green Version]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, J.; Zhang, Q. Effects of heating rate on slow pyrolysis behavior, kinetic parameters and products properties of moso bamboo. Bioresour. Technol. 2014, 169, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Dacres, O.D.; Tong, S.; Li, X.; Sun, Y.; Wang, F.; Luo, G.; Liu, H.; Hu, H.; Yao, H. Gas-pressurized torrefaction of biomass wastes: The effect of varied pressure on pyrolysis kinetics and mechanism of torrefied biomass. Fuel 2020, 276, 118132. [Google Scholar] [CrossRef]
- Gözke, G.; Açıkalın, K. Pyrolysis characteristics and kinetics of sour cherry stalk and flesh via thermogravimetric analysis using isoconversional methods. J. Therm. Anal. Calorim. 2021, 146, 893–910. [Google Scholar] [CrossRef]
- de Paula Protásio, T.; Scatolino, M.V.; de Araújo, A.C.C.; de Oliveira, A.F.C.F.; de Figueiredo, I.C.R.; de Assis, M.R.; Trugilho, P.F. Assessing proximate composition, extractive concentration, and lignin quality to determine appropriate parameters for selection of superior Eucalyptus firewood. BioEnergy Res. 2019, 12, 626–641. [Google Scholar] [CrossRef]
- Pang, C.H.; Gaddipatti, S.; Tucker, G.; Lester, E.; Wu, T. Relationship between thermal behaviour of lignocellulosic components and properties of biomass. Bioresour. Technol. 2014, 172, 312–320. [Google Scholar] [CrossRef]
- Peduzzi, E.; Boissonnet, G.; Maréchal, F. Biomass modelling: Estimating thermodynamic properties from the elemental composition. Fuel 2016, 181, 207–217. [Google Scholar] [CrossRef]
- Babu, B.V.; Chaurasia, A.S. Parametric study of thermal and thermodynamic properties on pyrolysis of biomass in thermally thick regime. Energy Convers. Manag. 2004, 45, 53–72. [Google Scholar] [CrossRef]
- Dupont, C.; Chiriac, R.; Gauthier, G.; Toche, F. Heat capacity measurements of various biomass types and pyrolysis residues. Fuel 2014, 115, 644–651. [Google Scholar] [CrossRef]
- Yildiz, G.; Ronsse, F.; Venderbosch, R.; van Duren, R.; Kersten, S.R.; Prins, W. Effect of biomass ash in catalytic fast pyrolysis of pine wood. Appl. Catal. B. 2015, 168, 203–211. [Google Scholar] [CrossRef]
- Demirbas, A. Effect of initial moisture content on the yields of oily products from pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2004, 71, 803–815. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A critical review on hemicellulose pyrolysis. Energy Technol. 2017, 5, 52–79. [Google Scholar] [CrossRef]
- Song, Y.; Hu, J.; Evrendilek, F.; Buyukada, M.; Liang, G.; Huang, W.; Liu, J. Reaction mechanisms and product patterns of Pteris vittata pyrolysis for cleaner energy. Renew. Energ. 2021, 167, 600–612. [Google Scholar] [CrossRef]
- Fan, Y.; Cai, Y.; Li, X.; Yin, H.; Yu, N.; Zhang, R.; Zhao, W. Rape straw as a source of bio-oil via vacuum pyrolysis: Optimization of bio-oil yield using orthogonal design method and characterization of bio-oil. J. Anal. Appl. Pyrolysis 2014, 106, 63–70. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, B.; Zhang, X.; Li, L.; Zhao, Y.; Li, Y.; Duan, K. Investigating Biochar-Derived Dissolved Organic Carbon (DOC) Components Extracted Using a Sequential Extraction Protocol. Materials 2022, 15, 3865. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P.; Jewaratnam, J. Thermophysical characterization of oil palm shell (OPS) and OPS char synthesized by the microwave pyrolysis of OPS. Appl. Therm. Eng. 2016, 105, 605–612. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Pandey, D.; Patil, T.; Sawarkar, A.N. Pyrolysis of banana leaves biomass: Physico-chemical characterization, thermal decomposition behavior, kinetic and thermodynamic analyses. Bioresour. Technol. 2020, 310, 123464. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef]
- Pimenidou, P.; Dupont, V. Characterisation of palm empty fruit bunch (PEFB) and pinewood bio-oils and kinetics of their thermal degradation. Bioresour. Technol. 2012, 109, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Mason, P.E.; Darvell, L.I.; Jones, J.M.; Williams, A. Comparative study of the thermal conductivity of solid biomass fuels. Energy Fuels 2016, 30, 2158–2163. [Google Scholar] [CrossRef] [Green Version]
- Gotor, F.J.; Criado, J.M.; Malek, J.; Koga, N. Kinetic analysis of solid-state reactions: The universality of master plots for analyzing isothermal and nonisothermal experiments. J. Phys. Chem. A 2000, 104, 10777–10782. [Google Scholar] [CrossRef]
- Sahoo, A.; Kumar, S.; Kumar, J.; Bhaskar, T. A detailed assessment of pyrolysis kinetics of invasive lignocellulosic biomasses (Prosopis juliflora and Lantana camara) by thermogravimetric analysis. Bioresour. Technol. 2021, 319, 124060. [Google Scholar] [CrossRef]
- Xiao, R.; Yang, W.; Cong, X.; Dong, K.; Xu, J.; Wang, D.; Yang, X. Thermogravimetric analysis and reaction kinetics of lignocellulosic biomass pyrolysis. Energy 2020, 201, 117537. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Sahoo, A.; Kumar, S.; Mohanty, K. A comprehensive characterization of non-edible lignocellulosic biomass to elucidate their biofuel production potential. Biomass Convers. Biorefin. 2022, 12, 5087–5103. [Google Scholar] [CrossRef]
- Chong, C.T.; Mong, G.R.; Ng, J.H.; Chong, W.W.F.; Ani, F.N.; Lam, S.S.; Ong, H.C. Pyrolysis characteristics and kinetic studies of horse manure using thermogravimetric analysis. Energy Convers. Manag. 2019, 180, 1260–1267. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Effect of heating rate on the pyrolysis yields of rapeseed. Renew. Energ. 2006, 31, 803–810. [Google Scholar] [CrossRef]
- Fu, J.; Mao, X.; Siyal, A.A.; Liu, Y.; Ao, W.; Liu, G.; Dai, J. Pyrolysis of furfural residue pellets: Physicochemical characteristics of pyrolytic pellets and pyrolysis kinetics. Renew. Energ. 2021, 179, 2136–2146. [Google Scholar] [CrossRef]
- Antal, M.J., Jr.; Allen, S.G.; Dai, X.; Shimizu, B.; Tam, M.S.; Grønli, M. Attainment of the theoretical yield of carbon from biomass. Ind. Eng. Chem. Res. 2000, 39, 4024–4031. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chin, T.; Liang, D.T.; Chen, H.; Zheng, C. Thermogravimetric analysis− Fourier transform infrared analysis of palm oil waste pyrolysis. Energy Fuels 2004, 18, 1814–1821. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Wu, Y.; Hou, Z.; Jiang, E. Influence of biomass components, temperature and pressure on the pyrolysis behavior and biochar properties of pine nut shells. Bioresour. Technol. 2020, 313, 123682. [Google Scholar] [CrossRef]
- Hashimoto, K.; Hasegawa, I.; Hayashi, J.; Mae, K. Correlations of kinetic parameters in biomass pyrolysis with solid residue yield and lignin content. Fuel 2011, 90, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA–FTIR and model-free integral methods. Energy Convers. Manag. 2015, 89, 251–259. [Google Scholar] [CrossRef]
- Aup-Ngoen, K.; Noipitak, M. Effect of carbon-rich biochar on mechanical properties of PLA-biochar composites. Sustain. Chem. Pharm. 2020, 15, 100204. [Google Scholar] [CrossRef]
- Chen, D.Y.; Zhang, D.; Zhu, X.F. Heat/mass transfer characteristics and nonisothermal drying kinetics at the first stage of biomass pyrolysis. J. Therm. Anal. Calorim. 2012, 109, 847–854. [Google Scholar] [CrossRef]
- Vasudev, V.; Ku, X.; Lin, J. Pyrolysis of algal biomass: Determination of the kinetic triplet and thermodynamic analysis. Bioresour. Technol. 2020, 317, 124007. [Google Scholar] [CrossRef] [PubMed]
- Poletto, M.; Zattera, A.J.; Santana, R.M. Thermal decomposition of wood: Kinetics and degradation mechanisms. Bioresour. Technol. 2012, 126, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, M.; Hu, W.; Chen, Z.; Liu, S.; Hu, Z.; Xiao, B. Thermogravimetric kinetic study of agricultural residue biomass pyrolysis based on combined kinetics. Bioresour. Technol. 2016, 219, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, M.; Konwar, K.; Bhuyan, N.; Borah, R.C.; Kalita, A.C.; Nath, H.P.; Saikia, N. Assessments of pyrolysis kinetics and mechanisms of biomass residues using thermogravimetry. Bioresour. Technol. Rep. 2018, 4, 40–49. [Google Scholar] [CrossRef]

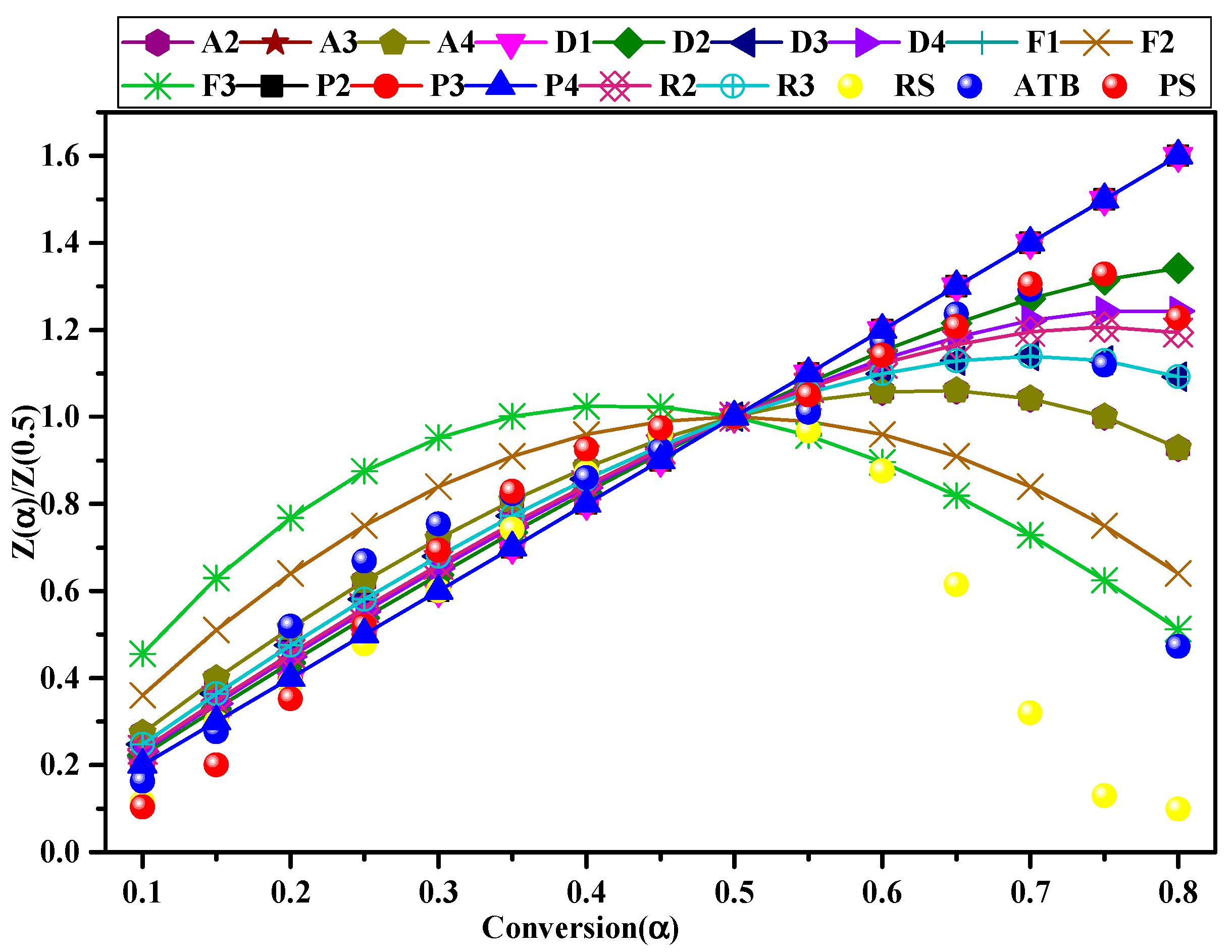
| Kinetic Models | f(α) | g(α) | |
|---|---|---|---|
| Nucleation models or growth model | |||
| Power law | P2 | ||
| P3 | |||
| P4 | |||
| Avarami–Erofeev | A2 | ||
| A3 | |||
| A4 | |||
| Diffusion model | |||
| One-dimensional diffusion | D1 | ||
| Two-dimensional (Valensi model) | D2 | ||
| Diffusion control (Jander model) | D3 | ||
| Diffusion control (Ginstling model) | D4 | ||
| Order-based model | |||
| First order | F1 | ||
| Second order | F2 | ||
| Third order | F3 | ||
| Geometrical contraction model | |||
| Contracting area | R2 | ||
| Contracting sphere | R3 | ||
| Samples | RS | ATB | PS | |
|---|---|---|---|---|
| Proximate analysis (%) | water | 5.01 ± 0.08 a | 2.63 ± 0.12 b | 2.24 ± 0.33 b |
| ash | 3.49 ± 0.03 a | 2.32 ± 0.02 b | 1.55 ± 0.02 c | |
| volatile matter | 80.02 ± 0.54 b | 80.0 ± 0.42 b | 82.72 ± 0.39 a | |
| fixed carbon | 11.47 ± 0.23 c | 15.04 ± 0.17 a | 13.49 ± 0.16 b | |
| Ultimate analysis (%) | C | 42.24 ± 0.02 c | 46.19 ± 0.08 b | 51.27 ± 0.07 a |
| H | 5.52 ± 0.03 c | 5.69 ± 0.06 b | 6.55 ± 0.01 a | |
| O | 47.79 ± 0.05 a | 45.25 ± 0.17 b | 40.33 ± 0.06 c | |
| N | 0.97 ± 0.01 a | 0.55 ± 0.01 b | 0.30 ± 0.02 c | |
| Samples | RS | ATB | PS | |
|---|---|---|---|---|
| Lignocellulosic contents (%) | cellulose | 41.08 ± 2.26 c | 46.22 ± 1.30 b | 54.69 ± 2.01 a |
| hemicellulose | 27.95 ± 5.13 a | 16.68 ± 0.55 b | 11.08 ± 2.65 c | |
| lignin | 16.05 ± 3.08 c | 30.73 ± 0.97 a | 26.43 ± 1.22 b | |
| Thermophysical properties | K (W·m−1·°C−1) | 0.112 ± 0.005 a | 0.110 ± 0.005 a | 0.116 ± 0.004 a |
| C0 (MJ·m−3·°C−1) | 0.539 ± 0.013 b | 0.652 ± 0.027 a | 0.606 ± 0.017 a | |
| D (m2·s−1) | 0.208 ± 0.006 a | 0.168 ± 0.005 c | 0.191 ± 0.002 b | |
| Pyrolysis Stages | RS | ATB | PS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 5 | 10 | 15 | 5 | 10 | 15 | ||
| The initial stage | FT1 (°C) | 176 | 178 | 190 | 165 | 170 | 180 | 160 | 162 | 172 |
| R1 (°C) | 146 | 148 | 160 | 135 | 140 | 150 | 130 | 132 | 142 | |
| WL1 (%) | 10.39 | 8.34 | 6.46 | 7.16 | 4.35 | 3.4 | 6.79 | 6.69 | 2.34 | |
| The second stage | FT2 (°C) | 353 | 362 | 380 | 373 | 380 | 395 | 378 | 380 | 402 |
| R2 (°C) | 177 | 184 | 190 | 208 | 210 | 215 | 218 | 218 | 230 | |
| WL2 (%) | 54.06 | 55.28 | 58.3 | 57.3 | 57.62 | 58.88 | 62.2 | 63.37 | 64.69 | |
| PT (°C) | 295.99 | 306.45 | 304.91 | 335.84 | 342.43 | 343.79 | 351.28 | 358.62 | 358.12 | |
| Prate (%/°C) | 0.9 | 1.77 | 2.6 | 0.68 | 1.25 | 1.76 | 0.79 | 1.46 | 2.23 | |
| The final stage | R3 (°C) | 447 | 438 | 420 | 427 | 420 | 405 | 422 | 420 | 398 |
| WL3 (%) | 25.27 | 20.62 | 23.94 | 14.28 | 13.34 | 11.57 | 12.35 | 11.01 | 10.42 | |
| WLtotal (%) | 90.15 | 84.25 | 88.71 | 78.75 | 75.31 | 73.85 | 81.34 | 81.07 | 77.45 | |
| RSS (%) | 9.85 | 15.75 | 11.29 | 21.24 | 24.69 | 26.15 | 18.66 | 18.93 | 22.55 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhao, B.; Zhang, X.; Zhang, Y. Influence of Intrinsic Physicochemical Properties of Agroforestry Waste on Its Pyrolysis Characteristics and Behavior. Materials 2023, 16, 222. https://doi.org/10.3390/ma16010222
Liu H, Zhao B, Zhang X, Zhang Y. Influence of Intrinsic Physicochemical Properties of Agroforestry Waste on Its Pyrolysis Characteristics and Behavior. Materials. 2023; 16(1):222. https://doi.org/10.3390/ma16010222
Chicago/Turabian StyleLiu, Hui, Baowei Zhao, Xin Zhang, and Yin Zhang. 2023. "Influence of Intrinsic Physicochemical Properties of Agroforestry Waste on Its Pyrolysis Characteristics and Behavior" Materials 16, no. 1: 222. https://doi.org/10.3390/ma16010222





