Adsorptive Pattern Using Drinking Water Treatment Residual for Organic Effluent Abatement from Aqueous Solutions
Abstract
:1. Introduction
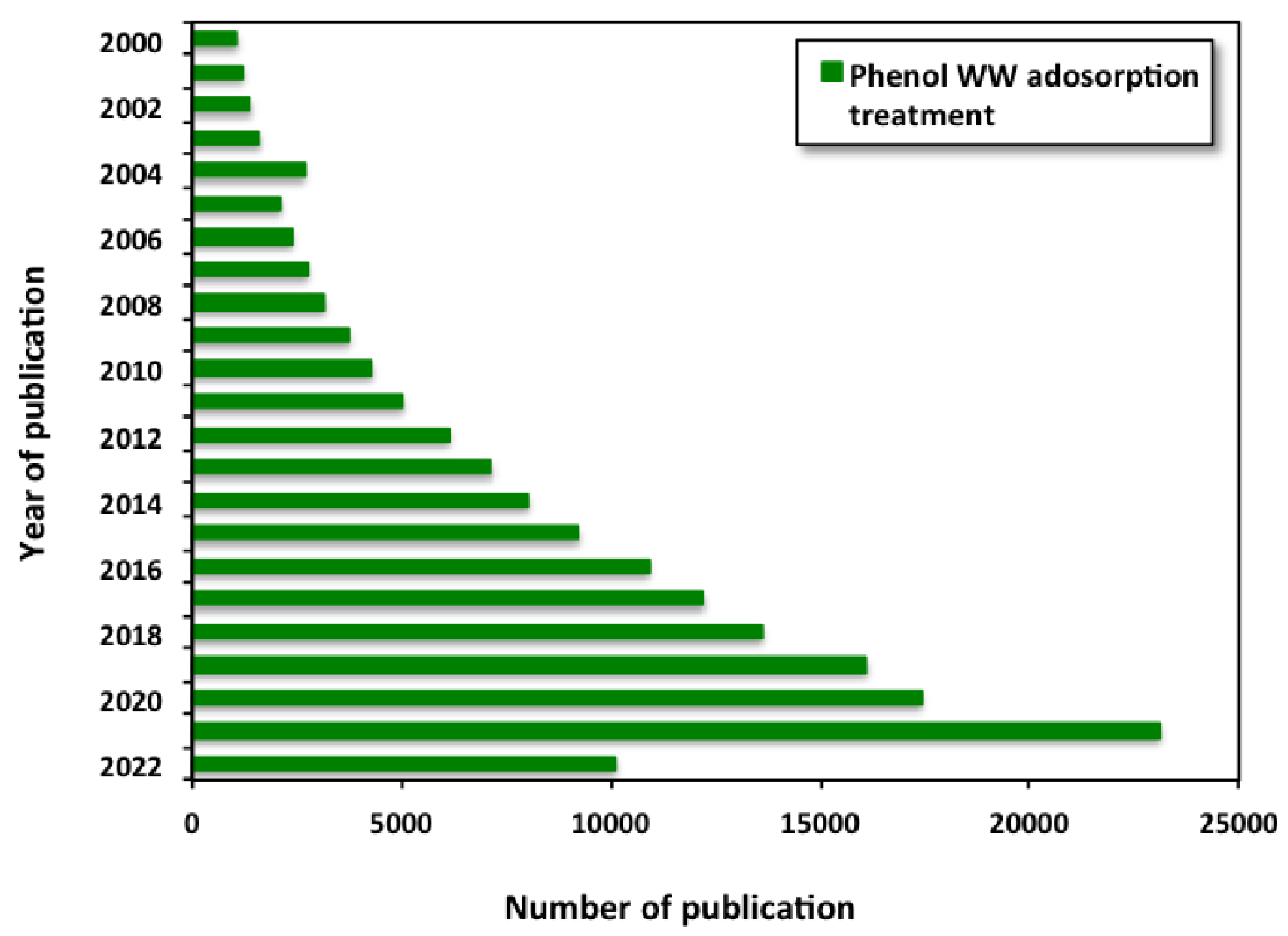
1.1. Bibliometric Technique
1.2. Box–Behnken Design (BBD) Model
1.3. Isotherm Mathematical Models
2. Materials and Methods
2.1. Field Sampling of Aluminium-Based Waste
2.2. Additional Adsorbents
2.3. Phenolic Wastewater
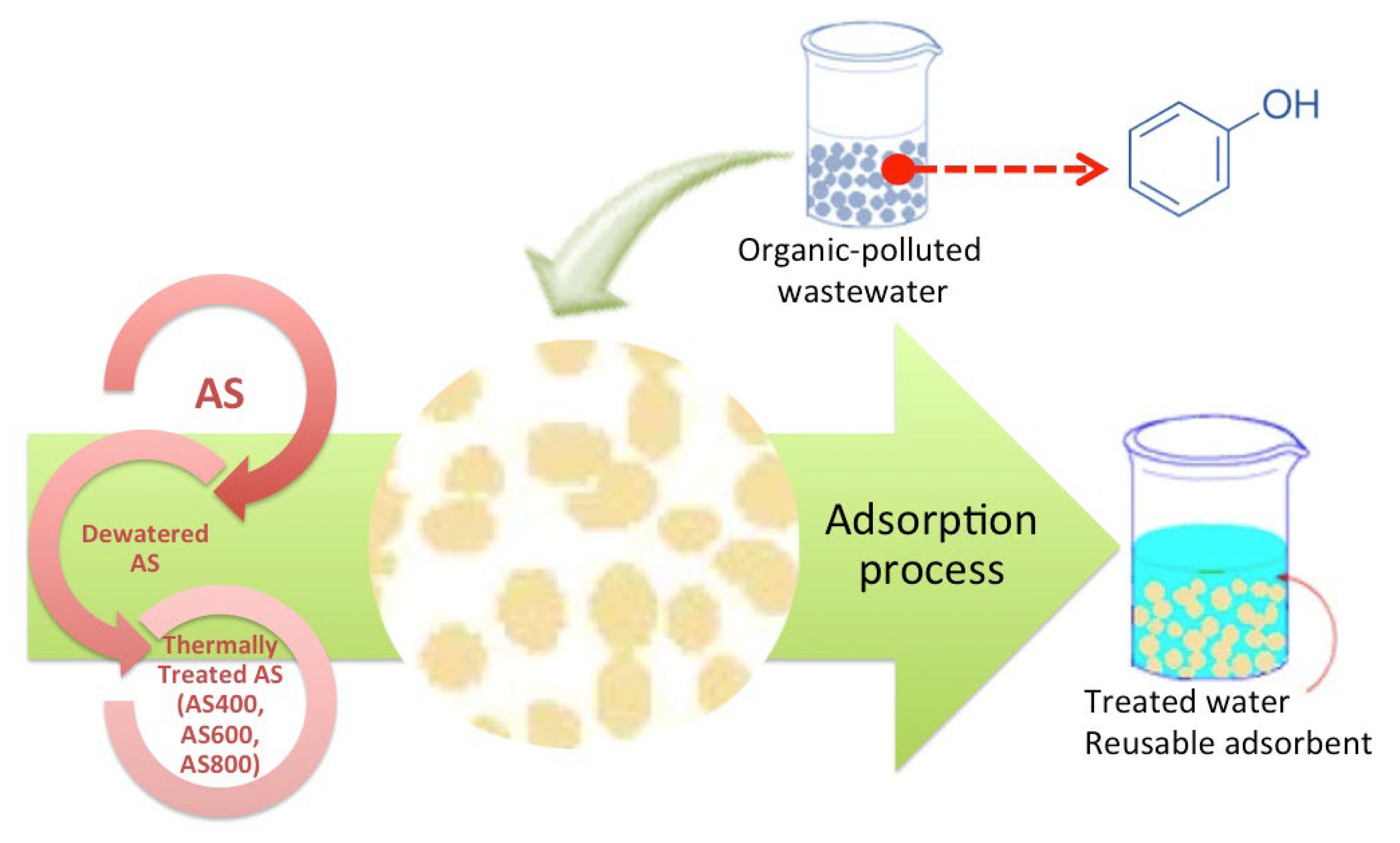
2.4. Experimental Methodology
2.5. Characterization Study
3. Results and Discussion
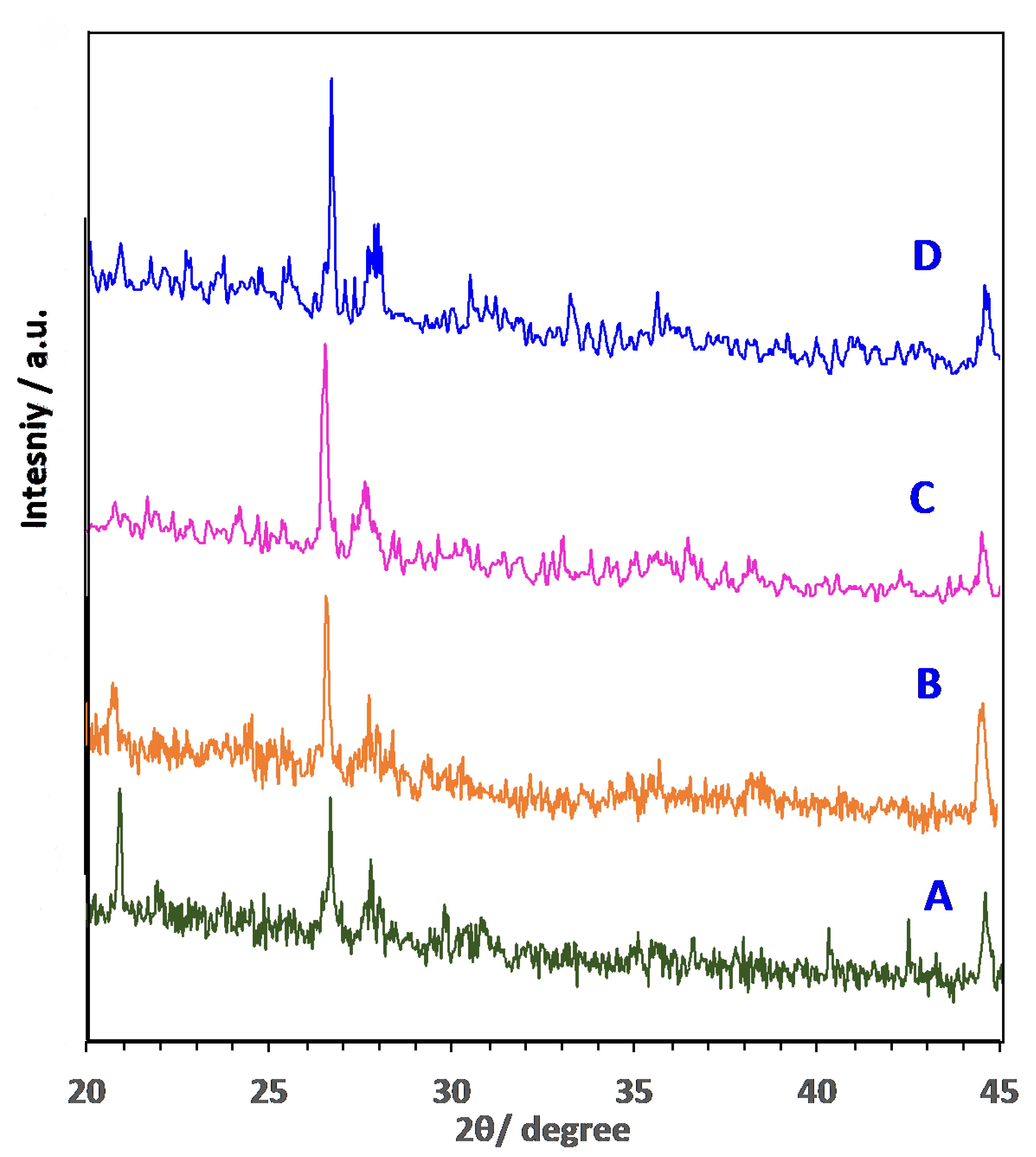
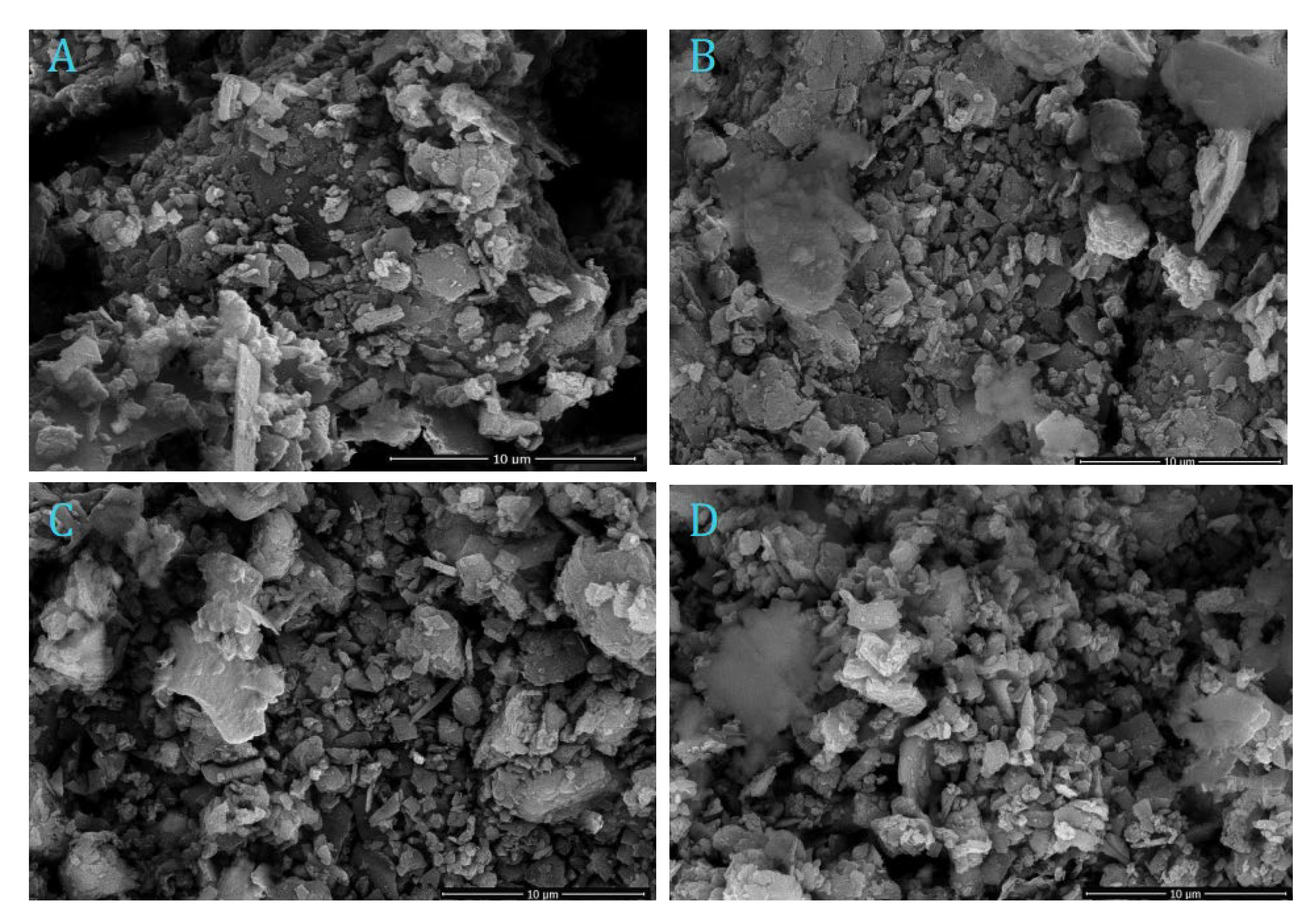
3.1. Characterization of AS adsorbent Materials
3.2. Assessment of Organic Effluent Removal by AS Adsorbent Materials
3.2.1. Contact Time for Phenol Adsorption
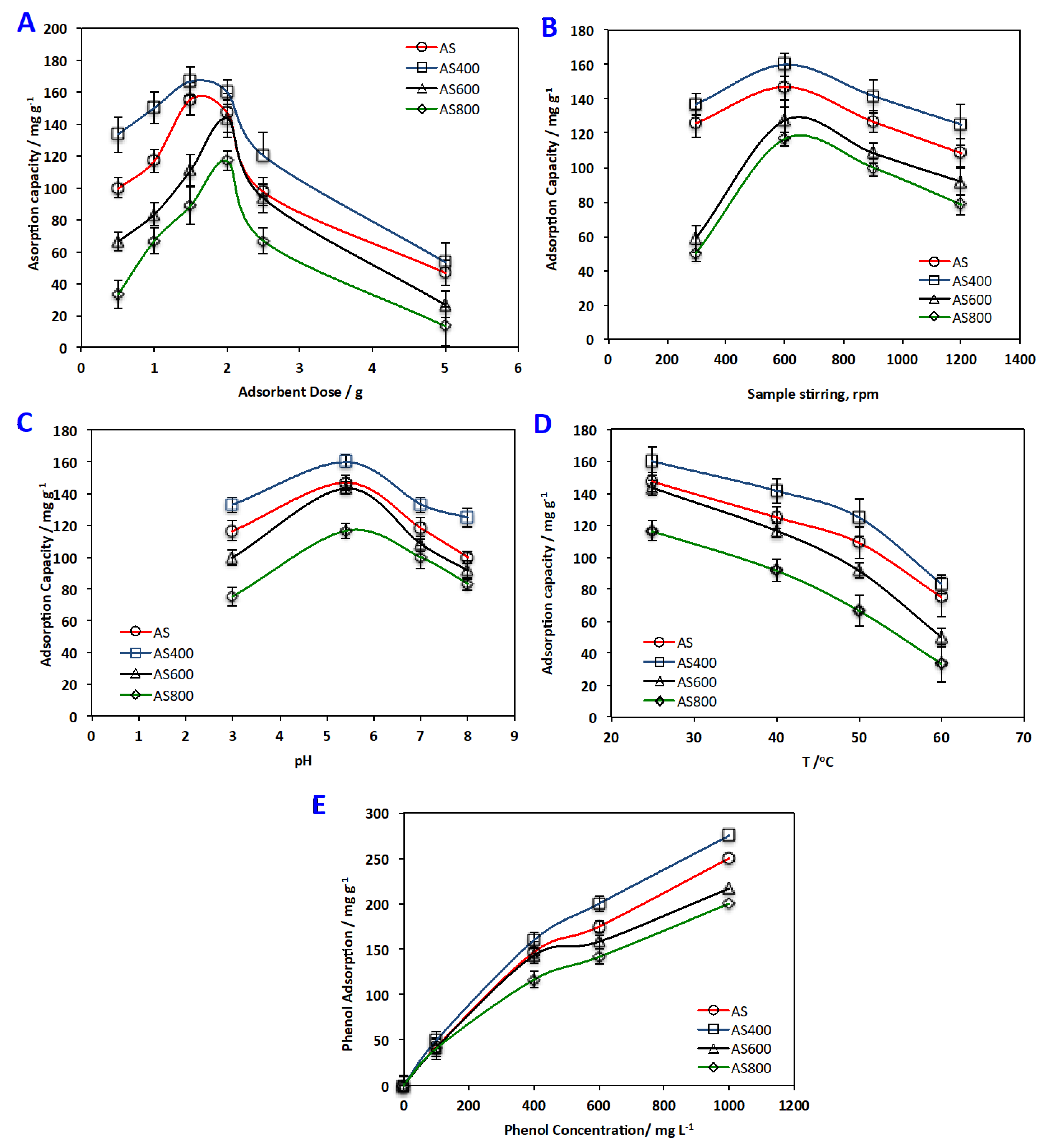
3.2.2. Multivariate-Parameters Effect on Adsorption Capacity
3.2.3. BBD Factorial Design and ANOVA Testing:
3.2.4. Adsorption Isotherm Models
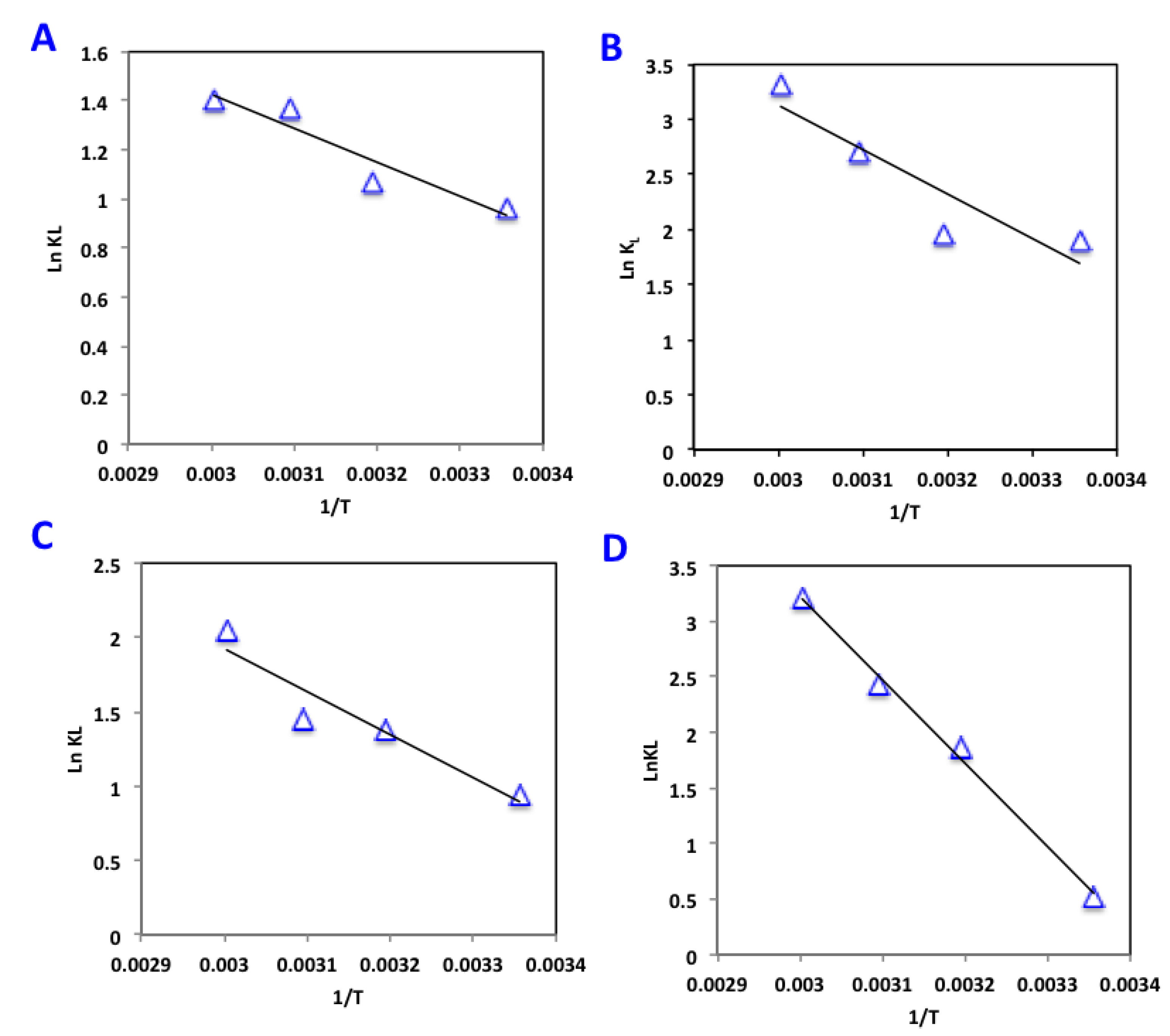
3.2.5. Thermodynamic Investigation
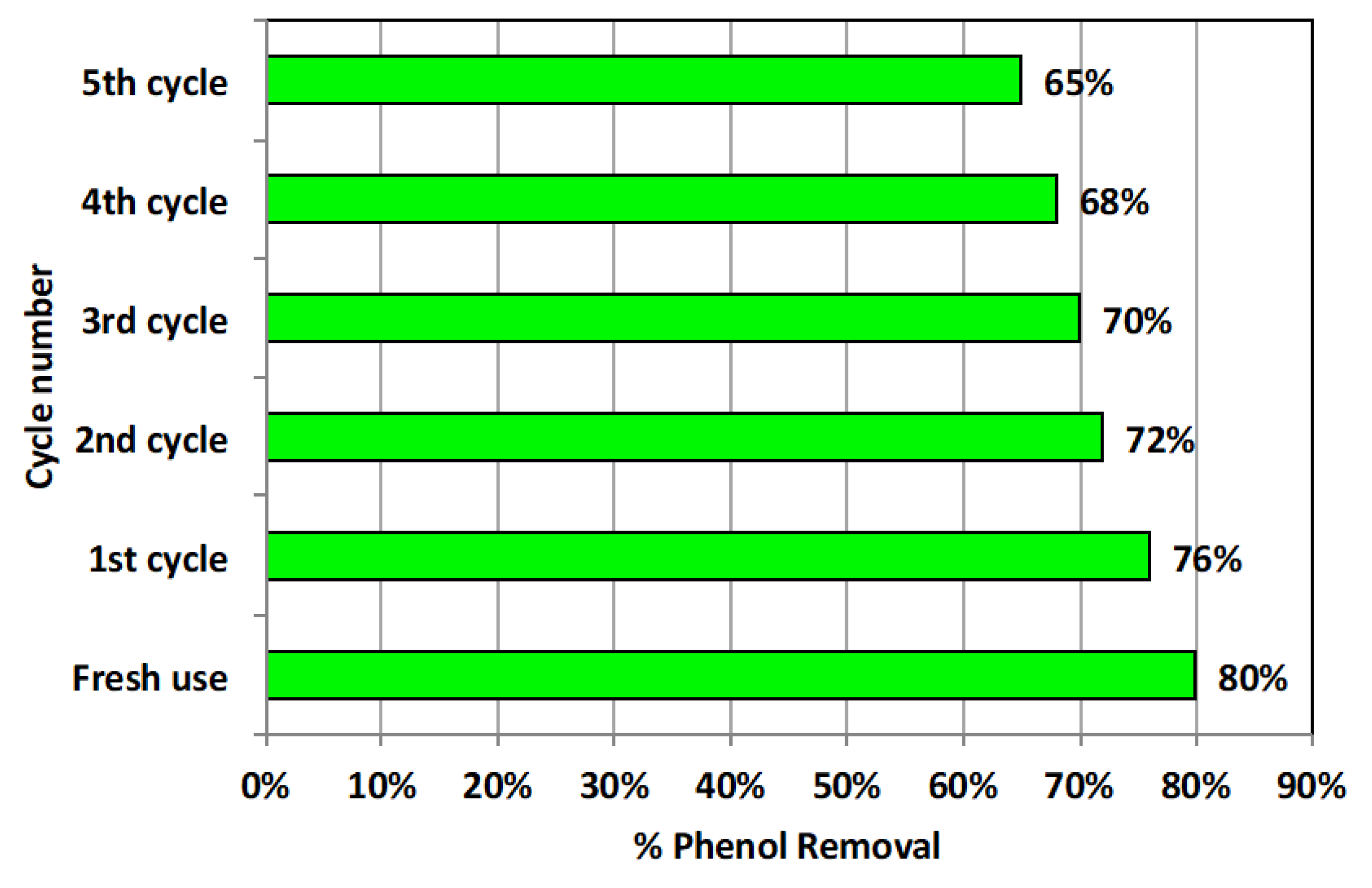
3.2.6. Adsorbent Stability and Reusability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.Q. Involvement of gypsum (CaSO4·2H2O) in water treatment sludge dewatering: A potential benefit in disposal and reuse. Sep. Sci. Technol. 2006, 41, 2785–2794. [Google Scholar] [CrossRef] [Green Version]
- Tony, M.A. Solar concentration for green environmental remediation opportunity—International review: Advances, constraints and their practice in wastewater treatment. Int. J. Environ. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Tony, M.A.; Lin, L.-S. Iron recovery from acid mine drainage sludge as Fenton source for municipal wastewater treatment. Int. J. Environ. Anal. Chem. 2022, 102, 1245–1260. [Google Scholar] [CrossRef]
- Tony, M.A.; Zhao, Y.Q.; El-Sherbiny, M.F. Fenton and Fenton-like AOPs for alum sludge conditioning: Effectiveness comparison with different Fe2+ and Fe3+ salts. Chem. Eng. Commun. 2010, 198, 442–452. [Google Scholar] [CrossRef] [Green Version]
- Thabet, R.H.; Fouad, M.K.; Ali, I.A.; El Sherbiny, S.A.; Tony, M.A. Synthesis, characterization and potential application of magnetized nanoparticles for photocatalysis of Levafix CA reactive azo-dye in aqueous effluent. Water Environ. J. 2022, 36, 245–260. [Google Scholar] [CrossRef]
- Djebbar, M.; Djafri, F.; Bouchekara, M.; Djafri, A. Adsorption of phenol on natural clay. Appl. Water Sci. 2012, 2, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Markandeya; Shukla, S.P.; Dhiman, N.; Mohan, D.; Kisku, G.C.; Roy, S. An efficient removal of disperse dye from wastewater using zeolite synthesized from cenospheres. J. Hazard. Toxic Radioact. Waste 2017, 21, 04017017. [Google Scholar] [CrossRef]
- Thabet, R.H.; Fouad, M.K.; El Sherbiney, S.A.; Tony, M.A. Solar assisted green photocatalysis for deducing carbamate insecticide from agriculture stream into water reclaiming opportunity. Int. J. Environ. Anal. Chem. 2022. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Yang, Y. Extending the use of dewatered alum sludge as a P-trapping material in effluent purification: Study on two separate water treatment sludges. J. Environ. Sci. Health Part A 2010, 45, 1234–1239. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Sreekumari, S.S.; Bringle, C.D. Removal of phenols from water and petroleum industry refinery effluents by activated carbon obtained from coconut coir pith. Adsorption 2009, 15, 439–451. [Google Scholar] [CrossRef]
- Elsayed, S.A.; El-Sayed, I.E.T.; Tony, M.A. Impregnated chitin biopolymer with magnetic nanoparticles to immobilize dye from aqueous media as a simple, rapid and efficient composite photocatalyst. Appl. Water Sci. 2022, 12, 252. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, M.; Zhang, G.; Zhao, W.; Han, G. Enhanced adsorption of sulfide and xanthate on smithsonite surfaces by lead activation and implications for flotation intensification. Sep. Purif. Technol. 2023, 307, 122772. [Google Scholar] [CrossRef]
- Silva, E.M.; Rogez, H.; Larondelle, Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep. Purif. Technol. 2007, 55, 381–387. [Google Scholar] [CrossRef]
- Babatunde, A.O.; Zhao, Y.Q.; Zhao, X.H. Alum sludge-based constructed wetland system for enhanced removal of P and OM from wastewater: Concept, design and performance analysis. Bioresour. Technol. 2010, 101, 6576–6579. [Google Scholar] [CrossRef] [Green Version]
- Thabet, R.H.; Tony, M.A.; El Sherbiny, S.A.; Ali, I.A.; Fouad, M.K. Catalytic oxidation over nanostructured heterogeneous process as an effective tool for environmental remediation. IOP Conf. Ser. Mater. Sci. Eng. 2020, 975, 012004. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.; Wang, H.; Feng, Q. Sulfidization regulation of cuprite by pre-oxidation using sodium hypochlorite as an oxidant. Int. J. Min. Sci. Technol. 2021, 31, 1117–1128. [Google Scholar] [CrossRef]
- Cheng, W.-P.; Chen, P.-H.; Yu, R.-F.; Ho, W.-N. Treating ammonium-rich wastewater with sludge from water treatment plant to produce ammonium alum. Sustain. Environ. Res. 2016, 26, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Carvallho, M.N.; da Silva, K.S.; Sales, D.C.S.; Freire, E.M.P.L.; Sobrinho, M.A.M.; Ghislandi, M.G. Dye removal from textile industrial effluents by adsorption on exfoliated graphite nanoplatelets: Kinetic and equilibrium studies. Water Sci. Technol. 2016, 73, 2189–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashour, E.A.; Tony, M.A. Eco-friendly removal of hexavalent chromium from aqueous solution using natural clay mineral: Activation and modification effects. SN Appl. Sci. 2020, 2, 2042. [Google Scholar] [CrossRef]
- Nour, M.M.; Tony, M.A.; Nabwey, H.A. Immobilization of Magnetic Nanoparticles on Cellulosic Wooden Sawdust for Competitive Nudrin Elimination from Environmental Waters as a Green Strategy: Box–Behnken Design Optimization. Int. J. Environ. Res. Public Health 2022, 19, 15397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, M.; Yang, B.; Feng, Q.; Liu, D. Enhanced sulfidization flotation mechanism of smithsonite in the synergistic activation system of copper–ammonium species. Miner. Eng. 2022, 187, 107796. [Google Scholar] [CrossRef]
- Hilder, M.; Winther-Jensen, O.; Winther-Jensen, B.; MacFarlane, D.R. Graphene/zinc nano-composites by electrochemical co-deposition. Phys. Chem. Chem. Phys. 2012, 14, 14034–14040. [Google Scholar] [CrossRef]
- Tony, M.A. Valorization of undervalued aluminum-based waterworks sludge waste for the science of “The 5 Rs’ criteria”. Appl. Water Sci. 2022, 12, 20. [Google Scholar] [CrossRef]
- Parker, H.L.; Budarin, V.L.; Clark, J.H.; Hunt, A.J. Use of starbon for the adsorption and desorption of phenols. ACS Sustain. Chem. Eng. 2013, 1, 1311–1318. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, D.-H.; Yang, J.-S.; Baek, K. Adsorption characteristics of As(III) and As(V) on alum sludge from water purification facilities. Sep. Sci. Technol. 2012, 47, 2211–2217. [Google Scholar]
- Tony, M.A. Sun in a Box” Day-to-Night Solar Energy Storage for Heating and Cooling Applications Utilizing Zeolite Synthesized from Waste Residues towards Energy Density Enhancement. Int. J. Appl. Energy Syst. 2020, 2, 1–5. [Google Scholar] [CrossRef]
- Tony, M.A.; Eltabey, M.M. End-of-life waste criteria: Synthesis and utilization of Mn–Zn ferrite nanoparticles as a superparamagnetic photocatalyst for synergistic wastewater remediation. Appl. Water Sci. 2022, 12, 21. [Google Scholar] [CrossRef]
- Oliva-Teles, M.T.; Paıga, P.; Delerue-Matos, C.M.; Alvim-Ferraz, M.d.C.M. Determination of free formaldehyde in foundry resins as its 2,4-dinitrophenylhydrazone by liquid chromatography. Anal. Chim. Acta 2002, 467, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Weng, C.-H.; Pan, Y.-F. Adsorption of a cationic dye (methylene blue) onto spent activated clay. J. Hazard. Mater. 2007, 144, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, M.A. Characterization and pozzolanic properties of calcined alum sludge. Mater. Res. Bull. 2015, 61, 415–421. [Google Scholar] [CrossRef]
- Parker, H.L.; Hunt, A.J.; Budarin, V.L.; Shuttleworth, P.S.; Miller, K.L.; Clark, J.H. The importance of being porous: Polysaccharide-derived mesoporous materials for use in dye adsorption. RSC Adv. 2012, 2, 8992–8997. [Google Scholar] [CrossRef]
- Gómez-Avilés, A.; Peñas-Garzón, M.; Bedia, J.; Rodriguez, J.J.; Belver, C. C-modified TiO2 using lignin as carbon precursor for the solar photocatalytic degradation of acetaminophen. Chem. Eng. J. 2019, 358, 1574–1582. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tony, M.A.; Ali, I.A. Mechanistic implications of redox cycles solar reactions of recyclable layered double hydroxides nanoparticles for remazol brilliant abatement. Int. J. Environ. Sci. Technol. 2022, 19, 9843–9860. [Google Scholar] [CrossRef]
- Ashour, A.; Tony, M.A.; Purcell, P.J. Use of agriculture-based waste for basic dye sorption from aqueous solution: Kinetics and isotherm studies. Am. J. Chem. Eng. 2014, 2, 92–98. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, J.; Zhou, J.; Lei, J. Study on adsorption of methylene blue by a novel composite material of TiO2 and alum sludge. RSC Adv. 2018, 8, 32799–32807. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.K.; Cho, K.; Kim, S.K.; Lim, J.S.; Kim, J.-N. Control of Water-Adsorption Properties of Mesoporous Silica and MOF by Ion Exchange and Salt Impregnation. Clean Technol. 2018, 24, 55–62. [Google Scholar]
- Saha, P.; Chowdhury, S.; Gupta, S.; Kumar, I. Insight into adsorption equilibrium, kinetics and thermodynamics of Malachite Green onto clayey soil of Indian origin. Chem. Eng. J. 2010, 165, 874–882. [Google Scholar] [CrossRef]
- Schrank, S.G.; Dos Santos, J.N.R.; Souza, D.S.; Souza, E.E.S. Decolourisation effects of Vat Green 01 textile dye and textile wastewater using H2O2/UV process. J. Photochem. Photobiol. A Chem. 2007, 186. [Google Scholar] [CrossRef]
- Lü, G.; Hao, J.; Liu, L.; Ma, H.; Fang, Q.; Wu, L.; Wei, M.; Zhang, Y. The adsorption of phenol by lignite activated carbon. Chin. J. Chem. Eng. 2011, 19, 380–385. [Google Scholar] [CrossRef]
- Tony, M.A.; Lin, L.-S. Performance of acid mine drainage sludge as an innovative catalytic oxidation source for treating vehicle-washing wastewater. J. Dispers. Sci. Technol. 2021, 43, 50–60. [Google Scholar] [CrossRef]
- Arimi, M.M. Modified natural zeolite as heterogeneous Fenton catalyst in treatment of recalcitrants in industrial effluent. Prog. Nat. Sci. Mater. Int. 2017, 27, 275–282. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Ho, Y.-S. Equilibrium sorption of anionic dye from aqueous solution by palm kernel fibre as sorbent. Dye. Pigment. 2007, 74, 60–66. [Google Scholar] [CrossRef]
- Gamaralalage, D.; Sawai, O.; Nunoura, T. Reusing the generated sludge as Fe source in Fenton process for treating crepe rubber wastewater. J. Mater. Cycles Waste Manag. 2019, 21, 248–257. [Google Scholar] [CrossRef]
- Pan, J.R.; Huang, C.; Lin, S. Reuse of fresh water sludge in cement making. Water Sci. Technol. 2004, 50, 183–188. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Uber die adsorption in losungen. Z. Phys. Chem. 1906, 57U, 385–470. [Google Scholar] [CrossRef]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Rio, S.; Le Coq, L.; Faur, C.; Lecomte, D.; Le Cloirec, P. Preparation of adsorbents from sewage sludge by steam activation for industrial emission treatment. Process Saf. Environ. Prot. 2006, 84, 258–264. [Google Scholar] [CrossRef]
- Han, W.; Luo, L.; Zhang, S. Adsorption of bisphenol A on lignin: Effects of solution chemistry. Int. J. Environ. Sci. Technol. 2012, 9, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Streat, M.; Patrick, J.W.; Camporro Perez, M.J. Sorption of phenol and para-chlorophenol from water using conventional and novel activated carbons. Water Res. 1995, 29, 467–472. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Vijaya, J.J.; Sekaran, G.; Kayalvizhi, K. Equilibrium, kinetic and thermodynamic studies on the adsorption of m-cresol onto micro-and mesoporous carbon. J. Hazard. Mater. 2007, 149, 134–143. [Google Scholar] [CrossRef]
- Mankar, S.V.; Garcia Gonzalez, M.N.; Warlin, N.; Valsange, N.G.; Rehnberg, N.; Lundmark, S.; Jannasch, P.; Zhang, B. Synthesis, life cycle assessment, and polymerization of a vanillin-based spirocyclic diol toward polyesters with increased glass-transition temperature. ACS Sustain. Chem. Eng. 2019, 7, 19090–19103. [Google Scholar] [CrossRef]



| Variable | Symbols | Range and Levels | |||
|---|---|---|---|---|---|
| Natural | Coded | −1 | 0 | 1 | |
| Sample stirring (rpm) | e1 | ξ1 | 400 | 600 | 800 |
| pH | e2 | ξ2 | 4.0 | 5.0 | 6.0 |
| AS mass (mg/L) | e3 | ξ3 | 1.0 | 1.5 | 2.0 |
| Experimental Runs | Factors | ||
|---|---|---|---|
| ξ3 | ξ2 | ξ3 | |
| 1 | −1 | −1 | 0 |
| 2 | −1 | 1 | 0 |
| 3 | 1 | −1 | 0 |
| 4 | 1 | 1 | 0 |
| 5 | 0 | −1 | −1 |
| 6 | 0 | −1 | 1 |
| 7 | 0 | 1 | −1 |
| 8 | 0 | 1 | 1 |
| 9 | −1 | 0 | −1 |
| 10 | 1 | 0 | −1 |
| 11 | −1 | 0 | 1 |
| 12 | 1 | 0 | 1 |
| 13 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 |
| Source | Degree of Freedom (df) | Sum of Squares (SS) | Mean Squares (MS) | Fisher F-Values | Probability p-Values |
|---|---|---|---|---|---|
| Model | 9 | 1700.22 | 188.910 | 42.179 | 0.0003 |
| Linear | 3 | 1099.94 | 1099.93 | 245.58 | 0.2387 |
| Square | 3 | 660.870 | 660.868 | 147.55 | 0.0710 |
| Interaction | 3 | 0.65550 | 1.65555 | 0.3693 | 2.2761 |
| Error | 5 | 22.3943 | 4.47887 | ||
| Total | 14 | 1722.60 |
| Adsorbent Material | Adsorption Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Langmuir | Freundlich | Dubinin—Radushkevich | |||||||||
| aL (L/mg) ×10−2 | KL | Qo (mg/g) | r2 | KF | n | r2 | qm (mol/g) | K′ (mol2/J2) ×10−4 | E (kJ/mol) | r2 | |
| AS | 0.89 | 2.62 | 294.12 | 0.98 | 1.99 | 1.92 | 0.97 | 188.67 | 0.60 | 0.091 | 0.92 |
| AS400 | 2.40 | 6.74 | 285.71 | 0.98 | 3.34 | 42.67 | 0.71 | 5.51 | 0.01 | 0.707 | 0.91 |
| AS600 | 1.04 | 2.56 | 243.9 | 0.94 | 2.18 | 12.85 | 0.79 | 172.36 | 0.70 | 0085 | 0.94 |
| AS800 | 0.72 | 1.68 | 232.56 | 0.98 | 2.22 | 11.11 | 0.91 | 150.22 | 0.80 | 0.079 | 0.90 |
| Adsorbent | ΔG (KJ mol−1) | ΔH (KJ mol−1) | ΔS (J mol−1 K−1) | |||
|---|---|---|---|---|---|---|
| 25 °C | 40 °C | 50 °C | 60 °C | |||
| AS | −2.38 | −2.78 | −3.67 | −3.89 | −11.51 | 46.37 |
| AS400 | −4.73 | −5.12 | −7.28 | −9.17 | −33.54 | 126.64 |
| AS600 | −2.33 | −3.58 | −3.90 | −5.68 | −24.02 | 88.06 |
| AS800 | −1.29 | −4.84 | −6.52 | −8.89 | −62.38 | 213.93 |
| Adsorbent | Chromium Metal | Adsorption Capacity | Temperature, K | Ref. |
|---|---|---|---|---|
| Zeolite (ZSM-12) from aluminium waste | Phenol | 275 | 298 | Current study |
| coir pith carbon | 2-chlorophenol | 18 | 298 | [53] |
| Lignin from black liquor | Bisphenol-A | 85 | 298 | [51] |
| Starbon (from natural polysaccharides) | Phenolic compound | 87 | 298 | [54] |
| Carbonized biological sludge | phenol | 50 | 298 | [50] |
| Coconut coir pith | phenol | 37 | 303 | [11] |
| Straw | p-chlorophenol | 129 | 294 | [52] |
| Coconut shell | p-chlorophenol | 334 | 294 | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nour, M.M.; Tony, M.A.; Nabwey, H.A. Adsorptive Pattern Using Drinking Water Treatment Residual for Organic Effluent Abatement from Aqueous Solutions. Materials 2023, 16, 247. https://doi.org/10.3390/ma16010247
Nour MM, Tony MA, Nabwey HA. Adsorptive Pattern Using Drinking Water Treatment Residual for Organic Effluent Abatement from Aqueous Solutions. Materials. 2023; 16(1):247. https://doi.org/10.3390/ma16010247
Chicago/Turabian StyleNour, Manasik M., Maha A. Tony, and Hossam A. Nabwey. 2023. "Adsorptive Pattern Using Drinking Water Treatment Residual for Organic Effluent Abatement from Aqueous Solutions" Materials 16, no. 1: 247. https://doi.org/10.3390/ma16010247






