Abstract
Metal oxide semiconductor gas sensors are widely used to detect toxic and inflammable gases in industrial production and daily life. The main research hotspot in this field is the synthesis of gas sensing materials. Previous studies have shown that incorporating two or more metal oxides to form a heterojunction interface can exhibit superior gas sensing performance in response and selectivity compared with single phase. This review focuses on mainly the synthesis methods and gas sensing mechanisms of metal oxide heterostructures. A significant number of heterostructures with different morphologies and shapes have been fabricated, which exhibit specific sensing performance toward a specific target gas. Among these synthesis methods, the hydrothermal method is noteworthy due to the fabrication of diverse structures, such as nanorod-like, nanoflower-like, and hollow sphere structures with enhanced sensing properties. In addition, it should be noted that the combination of different synthesis methods is also an efficient way to obtain metal oxide heterostructures with novel morphologies. Despite advanced methods in the metal oxide semiconductors and nanotechnology field, there are still some new issues which deserve further investigation, such as long-term chemical stability of sensing materials, reproducibility of the fabrication process, and selectivity toward homogeneous gases. Moreover, the gas sensing mechanism of metal oxide heterostructures is controversial. It should be clarified so as to further integrate laboratory theory research with practical exploitation.
1. Introduction
As people pay increasingly more attention to the environmental protection, the detection of toxic, inflammable, and explosive gases is of vital significance [1,2,3]. Gas sensors are devices which are capable of achieving this requirement. Among these, resistive-type gas sensors based on metal-oxide-semiconductor (MOS) configurations are more attractive and widely used. The electrical resistance of the MOS gas sensing material is correspondingly changed with various types and concentrations of gases, which makes it convenient for gas testing. Sensitivity, selectivity, response and recovery times, and stability are the critical parameters of gas sensors. Considerable studies have been adopted to improve these gas sensing parameters by the modification of the nanostructures of the sensing materials, adding catalyst as well as synthesizing nanocomposites [4]. Because of easy fabrication, high sensitivity, and stability of MOS sensors under ambient atmosphere compared with other electronic devices, the correlative research on MOS-based gas sensors has become a hotspot in this area.
According to the different conductive behaviors, MOSs are classified mainly into two types, which are referred to as n-type and p-type. SnO2 [5] and ZnO [6] are the most representative MOS, and they both exhibit n-type oxide conductivity feature. Other n-type MOSs such as TiO2 [7], Fe2O3 [8], and In2O3 [9] are also widely studied by researchers for investigating their gas sensing behaviors. In contrast, p-type MOSs such as NiO, CuO, Co3O4, and Cr2O3 have received relatively less attention because of their lower response to target gases compared with n-type MOS. Some scholars such as M. Hübner et al. [10] suggested that with the identical morphological structures, the response of an n-type MOS-based gas sensor to target gases is equal to the square of that of a p-type MOS-based gas sensor. This indicates that the responses of p-type MOS-based sensors should be enhanced so as to ameliorate their gas sensing properties.
Nevertheless, regardless of whether MOS perform n-type or p-type conductive behavior, there exists the intractable problem of poor selectivity among some reduction gases due to their cross-sensitivity property, which makes it difficult to simultaneously quantify the concentration of target gases in the presence of interfering gases [11,12,13]. More attention should be paid that most p-type MOS exhibit excellent catalytic performance to promote selective oxidation of some volatile organic compounds (VOCs) [14,15]. Additionally, by compounding p-type and n-type MOS materials, p–n heterojunctions are created at the interface between these materials, whereby the transport behavior of their respective charge carriers will be changed. The combination of these two or more dissimilar components allows the integration of their different properties; therefore, it will enhance their comprehensive sensing performances and weaken their respective intrinsic sensing defects, so that the sensing parameters such as selectivity and sensitivity can be significantly improved.
The material structure incorporating two dissimilar components is often referred to as a heterostructure. The nanomaterials which constitute a heterostructure always have different Fermi levels. By creating electrical contact at the interface when contacting dissimilar semiconducting materials, the Fermi levels can equilibrate to the same energy, which results in a carrier transfer and the formation of a charge depletion layer [11,16]. This is the basic gas sensing enhancement mechanism of these heterostructures and will be discussed later.
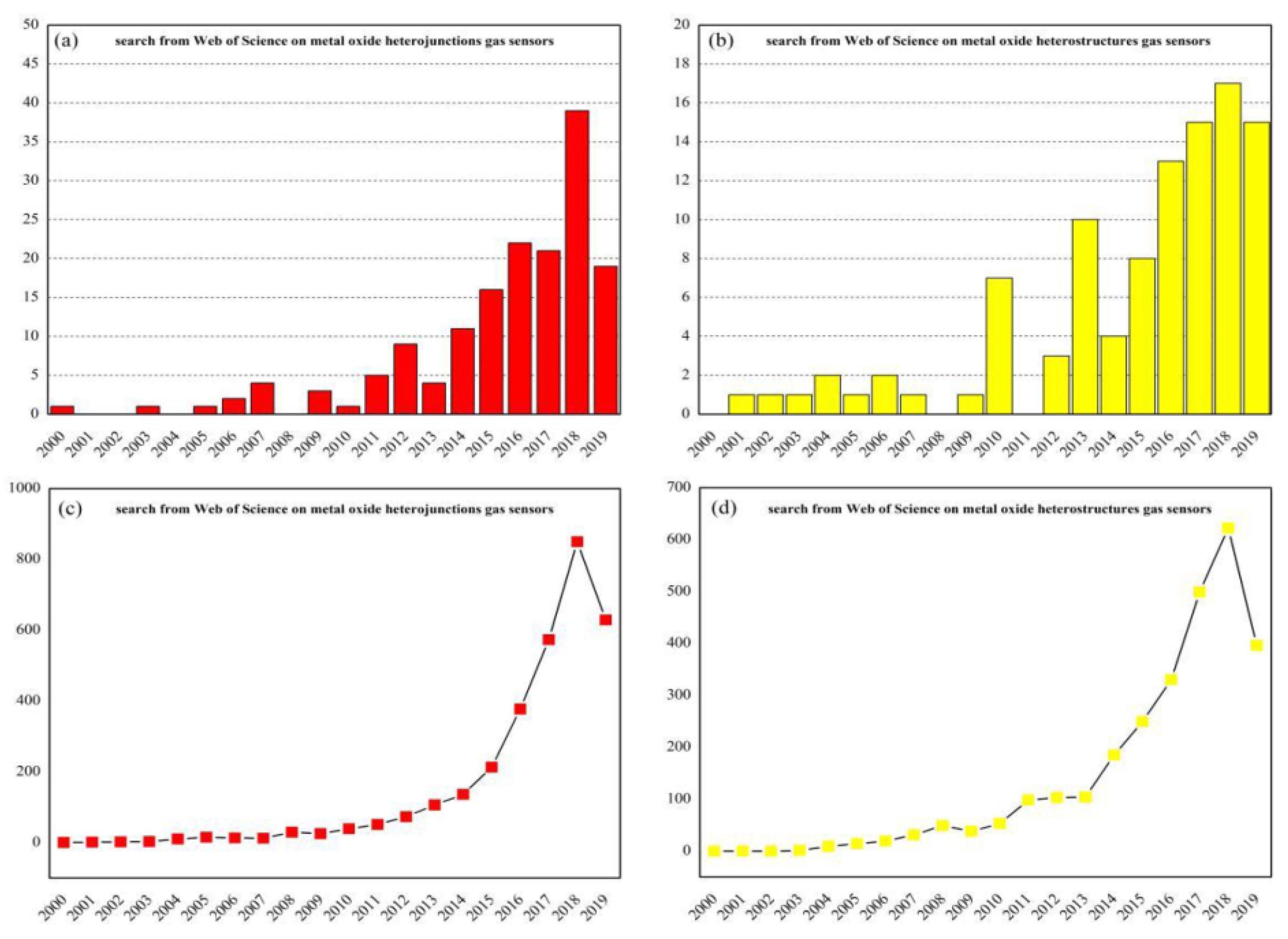
In recent years, there are several review articles which have been published about MOS nanomaterials applied in the gas sensing field. However, this review article specifically focuses on the MOS-heterostructured gas sensing materials [17,18]. Many researchers have found that the selectivity and other sensing parameters of resistive-type MOS gas sensors can be improved by synthesizing nanocomposites [19,20,21,22,23,24]. Attracted by the superior performances of gas sensors with heterostructures, researchers still maintain a high interest in nanostructured MOS which are used as based blocks to fabricate the heterostructures. Analyzing the data of the search from the Web of Science since the year 2000 (Figure 1), it can clearly be seen that the number of published papers on this topic is continually increasing. In addition, the number of times papers corresponding to heterojunctions or heterostructures gas sensors are cited is also gradually increasing.
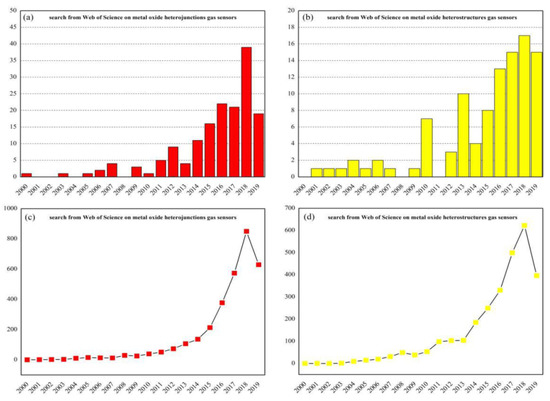
Figure 1.
Records of the number of metal-oxide-heterojunctions-related published papers (a) or metal-oxide-heterostructures-related published papers (b) and the number of their respective times cited (c,d) since the year 2000. The search string: TITLE-ABS-KEY (metal and oxide and heterojunctions/heterostructures and gas and sensors) (internet search of Web of Science on 28 June 2019).
2. Gas Sensing Mechanisms of Metal Oxide Semiconductors
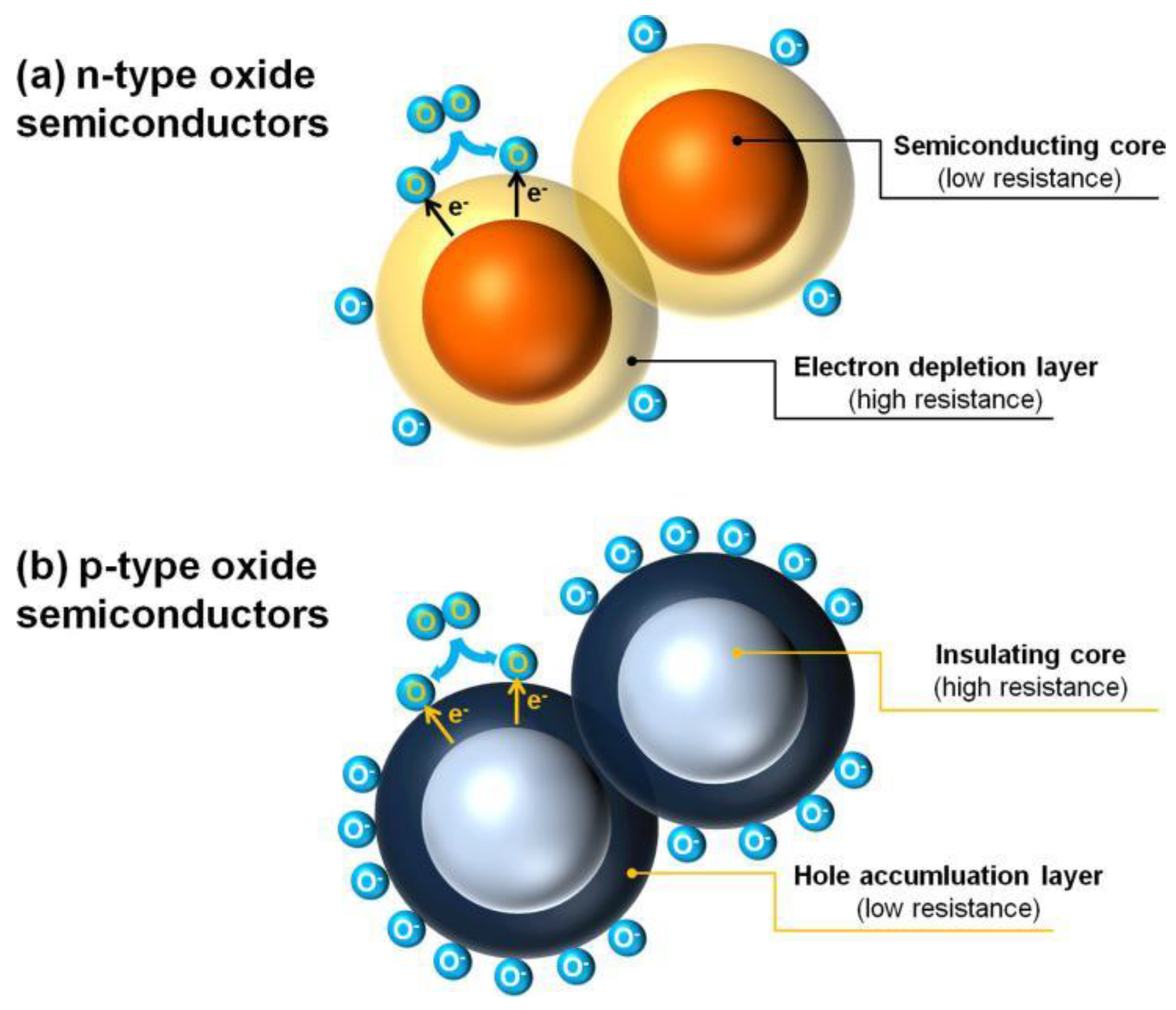
Electrons and holes are the main charge carriers of MOS for conductivity. According to the differences of their relative contents, n-type MOS are materials which carry more electrons than holes, while the relative content of internal carriers of p-type MOS is the opposite. When the electronic affinity of gas molecules is greater than the work function of MOS surface, some oxidizing gases (e.g., O2) are able to capture electrons from the MOS surface; thus, ionized oxygen anions (i.e., O2−, O−, and O2−) will form with the difference of temperature range. Furthermore, if the electronic affinity of gas molecules is less than the work function of MOS surface, electrons can release to the surface from the gas molecular, forming cations adsorption, such as reducing gases (CO and H2) substituting for oxidizing gases absorbed. Therefore, for an n-type MOS, when exposed to an oxidizing gas, electrons are captured and combined with the gas molecular, thus constructing an electron depletion layer on the surface of the material to establish electrical core–shell structure (Figure 2a), which exhibits a high resistance characteristic. When an n-type MOS is exposed to a reducing gas, adsorbed oxygen ions are able to be oxidized, the trapped electrons will re-inject into the MOS surface, thereby increasing the charge carrier concentration and obviously decreasing the sensor resistance. It is widely noted that there is a certain functional relationship between the response of MOS gas sensor and the gas concentration [12,25,26,27,28,29,30]. Thus, it will be helpful for gas quantitative analysis.
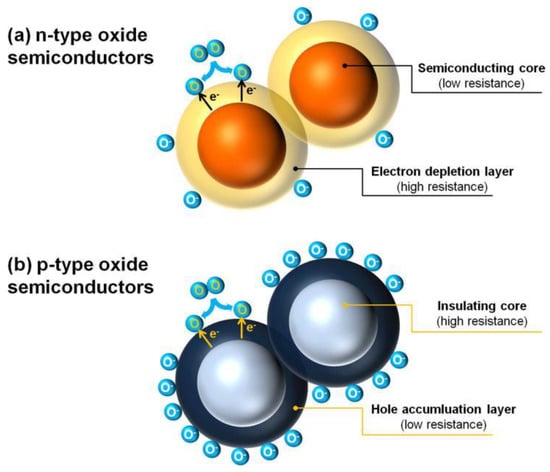
Figure 2.
Formation of core–shell structures of charge carriers in (a) n-type and (b) p-type oxide semiconductors. Reproduced with permission from Ref. [25].
In contrast, by adsorbing oxygen, the charge carriers of p-type MOS are mainly holes which assemble on the surface of MOS to form a hole accumulation layer with low resistance; in contrast, the MOS performs a high resistance characteristic, which also establishes the core–shell configuration (Figure 2b). Under this configuration, when adsorbing reducing gases, the electrons are released to the MOS through the reaction between the reducing gases and the ionized oxygen anions on the surface of the materials, which, in turn, decreases the concentration of holes in the shell layer and increases the material resistance. Table 1 summarizes the converse gas sensing behaviors of n-type and p-type MOS materials.

Table 1.
Gas sensing behaviors of n-type and p-type materials to reducing and oxidizing gases.
On the basis of the aforementioned MOS gas sensing mechanisms, Yamazoe firstly proposed the grain-size effect which is illustrated as follows [31]. First, we introduce two concepts. One is ‘D’ which represents the grain size of the particles; the other is the Debye length (LD), which is defined as the furthest distance to which a fixed charge can provide a force to the surrounding charges. Additionally, LD is approximately equal to the thickness of electron depletion layer or hole accumulation layer. The grain size mainly determines the response value of sensing materials if the grain size is greater than twice of the Debye length (D > 2LD), whereas it will bring some influence to the gas response of p-type MOS in which the conduction mostly occurs along the shell layer. Under this condition, the slight change of the concentration of holes in the shell layer because of the gain or loss of the electrons will not bring notable variation of the sensor resistance. This is the main reason why the response value of p-type semiconductor is lower than that of n-type semiconductor.
3. Heterostructure Classification
Before introducing the heterostructure synthesis methods and elucidating the sensing mechanisms of different heterostructural materials, it is worth describing the major classifications of heterostructural compound materials. The combination of these materials fabricates diverse heterojunctions, such as p–n, n–n, and p–p nanojunctions according to the semiconducting properties of sensing materials. The sensing mechanisms of these different heterojunctions will be separately described in the following parts.
The relationship between the structure and distribution state of the constituents can effectively influence the sensing performance of materials. Thus, it is worth describing three structure–architecture types of heterostructure by the following nomenclature.
- -
- A dash between the names of two or more constituents such as SnO2-Co3O4 represents a simple mixture of SnO2 and Co3O4, which are not controlled and randomly distributed.
- -
- An “@” sign between two or more constituents such as SnO2@CuO represents the base material SnO2 with a second material CuO adding on it in some ways. For example, CuO is coated on SnO2 in the ways such as sputtering, dipping, etc.
- -
- A forward slash between constituents’ names such as ZnO/NiO represents a clear partition or a well-defined interface between these two materials. For example, ZnO/NiO could represent a bi-layer structure or core–shell ZnO/NiO nanorods.
3.1. Simple Mixed Compound Structures
This synthetic method is an easy way to obtain the mixed compound structure by mixing the existing oxide powders. B. Lyson-Sypien et al. [32] detected that by mechanical mixing with different contents (0%, 2%, 10%, 50%, and 80%) of TiO2 and SnO2, the sensing response to H2 gradually increases with the increase of TiO2 content and reaches the maximum with 50% TiO2, which is attributed to the presence of most heterojunctions in this composition. It should be noted that even though the nominal component of two heterostructural materials is identical, the gas sensing behavior can be quite different. The dispersion state of heterostructures, which depends on the processing routes, has a significant influence on the behavior of the sensor material. D. Shaposhnik et al. [33] studied the gas sensing behavior of TiO2-doped SnO2 by comparing co-precipitation with mechanical mixing method. The results showed that the optimum composition which performed the best sensitivity to H2 was 10 mol% TiO2 for co-precipitation and 20 mol% TiO2 for mechanical mixing.
3.2. Layered Structures
Heterostructures based on bi-layers or multi-layers exhibit well-defined interfaces, which are suitable for characterizing and modeling because of their simple stacked 2D structure. Thanks to this structure, it becomes easier to characterize their electronic properties at the interface. In addition, it is easy to study the thermal stability which includes the possible growth of mixed phases and the diffusion across the interface [34]. However, these structures are less popular for some applications due to the lower specific surface area ratio, which brings less gas-accessibility to the heterojunction interface, thus influencing the gas sensor response. Dandeneau et al. [35] optimized the porosity and crystallinity of the top CuO film by changing the pyrolysis temperature through the sol-gel process of n-ZnO/p-CuO heterojunctions so as to rapidly analyze the gas diffusion rate to the interface of heterojunctions.
3.3. Structures Decorated with Second-Phase Particles
Another common class of heterostructures is the decoration of the host oxide material with nanoparticles of a second phase, which includes oxide materials [36,37,38], metals [39,40] or carbon-based materials [41,42]. These structures are usually used in photocatalysis [43] and photovoltaics, of which the secondary particle phases can perform as catalysts or sensitizers so as to enhance the sensing performance. Moreover, noble metal nanoparticles such as Au, Ag, Pt, and Pd are also used as the second-phase particles which are added into host oxides [44,45,46,47] and which act as catalysts and activators to increase the dissociation and reaction rate of gas molecular by reducing the activation energy of the reactions [48]. However, these noble metal nanoparticles can increase the cost and instability issues, such as catalytic poisoning effect because of the activity decrease and phenomena of coarsening or clustering at high temperatures [49].
3.4. One-Dimensional Structures
These nanostructures include mainly nanowires, nanorods, and nanofibers, which usually possess large specific surface areas. The selectivity and gas sensing properties can be significantly improved by modifying the secondary-phase material on one-dimensional nanostructures, such as nanowires modified by nanocrystals [50,51,52], core–shell nanowires [53,54], core–shell nanotubes [55,56], and composite nanofibers [57,58,59,60,61]. Generally speaking, the gas sensing enhancement mechanisms attribute mainly to three aspects including one-dimensional structure model, heterojunction effect, and catalytic effect. One-dimensional heterostructure usually has a high length–diameter ratio, which means that more surface atoms can participate in the gas–solid reaction compared with other heterostructures [62]. The actual effective heterojunction area of these one-dimensional composites varies with the process types and synthetic methods, which will be introduced later.
3.5. Core–Shell Structures
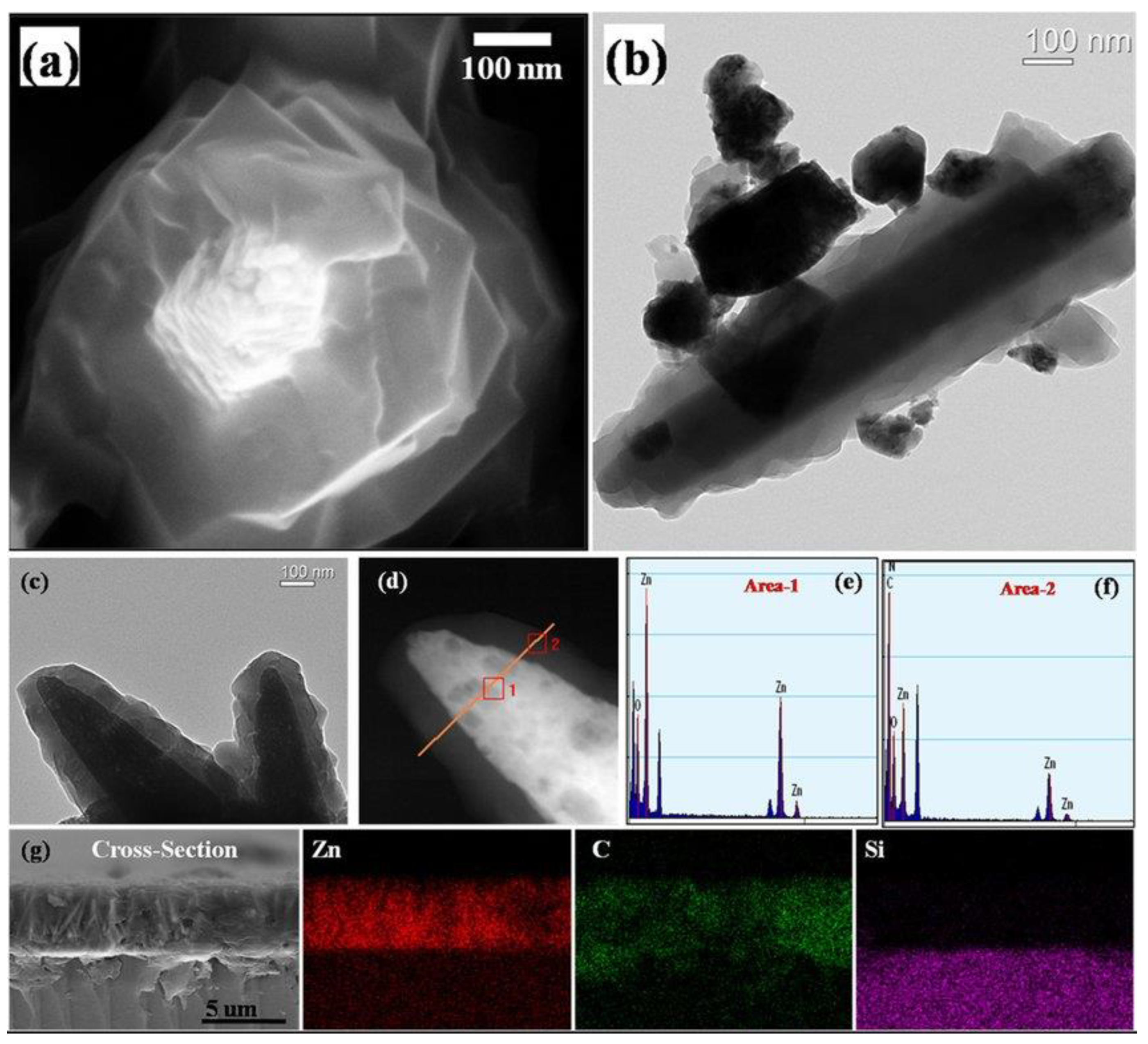
The last morphology to which we often pay attention is core–shell structures. Among all types of heterostructures for gas sensing applications, core–shell structures are the most promising types and will attract future researchers. This morphology can provide a maximized interfacial area with the help of completely covering the host material with a secondary phase while minimizing the amount of the material as bulk. Because of their unique structural features, core–shell structures integrate the properties of both internal and external materials and also compensate for their respective shortcomings. Scholars have conducted much research on core–shell structures recently. Wu et al. [63] found that the zeolitic imidazolate framework-8 (ZIF-8) shell, which is a stable metal–organic framework (MOF) porous material, had fine grains and was completely coated on the intact ZnO nanorod core, as shown in Figure 3a. The coating of the ZIF-8 shell was uniform and continuous, and the interfacial area between ZIF-8 shell and ZnO core was totally maximized, as shown in Figure 3b,c. It can be seen in Figure 3d–f that the core–shell structure of ZnO@ZIF-8 was particularly clear and the successful transition of ZnO core to ZIF-8 shell was found by EDS element scanning. The cross-section image and EDXS mappings of Figure 3g showed that ZIF-8 shell fully covered the ZnO nanorods, and the porous ZIF-8 shell could control access of the gas species to the ZnO core so as to improve the gas selectivity of the sensing materials.
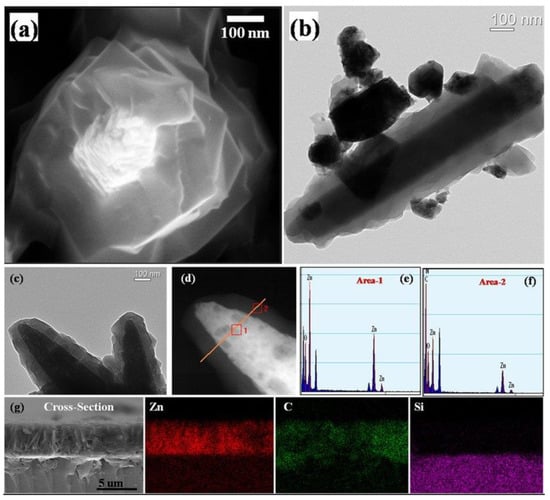
Figure 3.
Microstructure of ZnO@ZIF-8 core–shell nanorod film: (a) SEM image; (b,c) TEM images; (d) HAADF-STEM image; (e,f) EDS element scanning of area-1 and area-2 in image (d); (g) cross-section image and EDXS mappings (reprinted with permission from Ref. [63]).
4. Overview of Synthesis Methods
With the development of the research on metal-oxide-based heterostructures, different technologies have been employed to fabricate these materials. The preparation of these heterostructures demands various factors such as structural affection and chemical homogeneity, which lead to the rapid development of synthesis methods. This investigation will introduce some common fabrication techniques, making it possible to develop various heterostructural nanomaterials by combining different types of MOS materials.
4.1. Sol-Gel Method
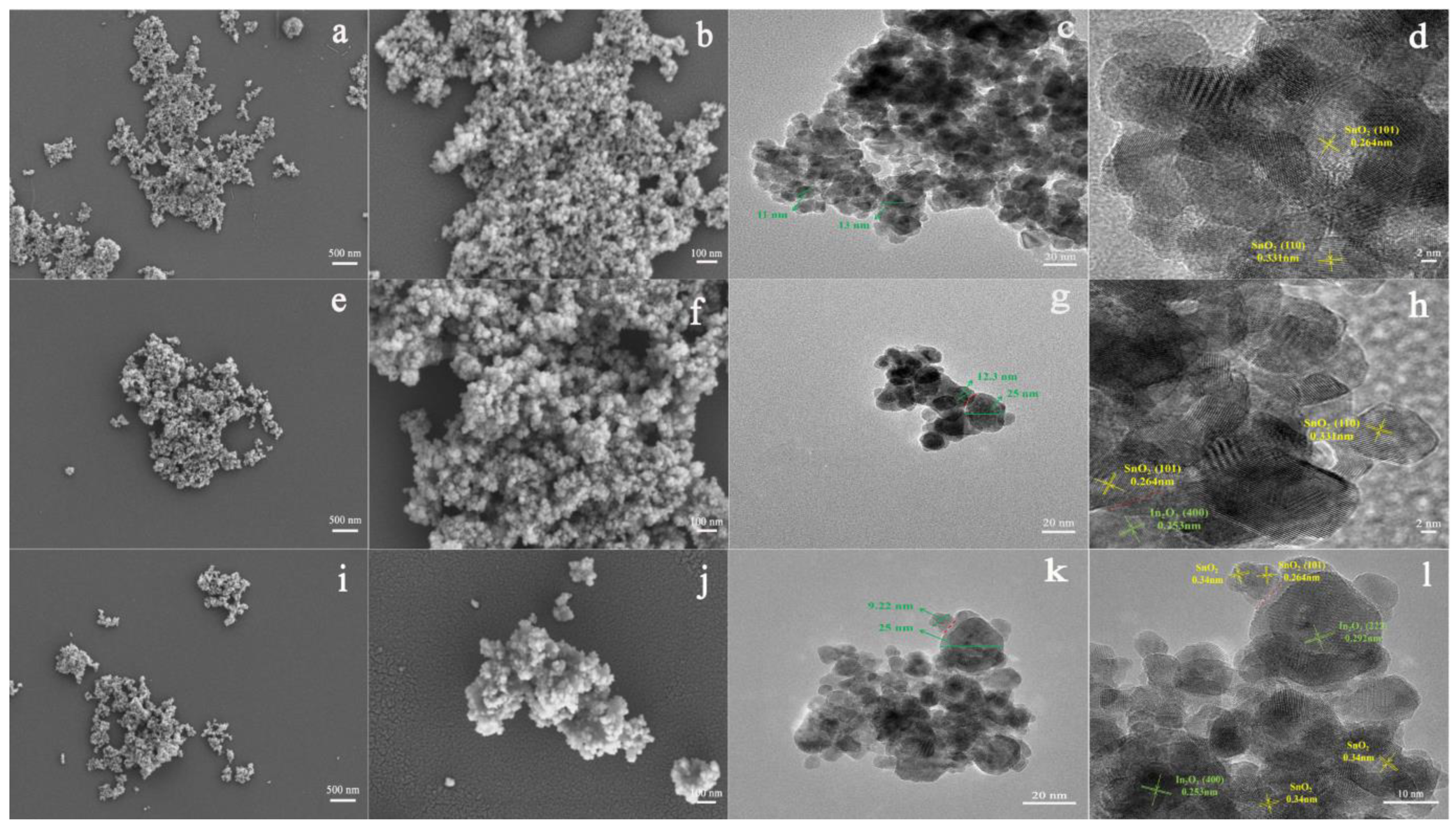
The sol-gel method has become one of the most preferable methods for fabricating MOS-based heterostructures. The compounds with high chemical activity act as precursors, which are evenly mixed in a liquid environment by adding surface active agent, forming a stable and transparent sol system by internal chemical reactions. The stagnant gel subsequently forms by slow polymerization. Finally, the nanostructured materials can be synthesized by drying and sintering methods. Jiang et al. studied the effect of polyethylene glycol on the microstructures of TiO2 thin films using the sol-gel method [64]. They reported that porous and fine-grained TiO2 films can form when adding more or with a high molecular weight of polyethylene glycol. They drew the conclusion that the sol-gel method could control the shape and size of fabrication materials by changing the solution composition and the synthesized conditions. In this aspect, many research groups synthesized different shapes of MOS heterostructures. Hernández et al. fabricated heterostructural materials based on TiO2 nanoparticles and ZnO nanowires by the sol-gel method [65]. Referring to our previous work, we successfully prepared pristine SnO2 (Figure 4a–d), SnO2-In2O3 heterostructure (Figure 4e–h), and In3+-doped SnO2-In2O3 heterostructure (Figure 4i–l) via the sol-gel method [66]. The structure morphology of the SnO2-In2O3 nanocomposite was not affected after In3+ doping modification. The particle size of SnO2 was changed by doping with In3+, which could improve sensing performance towards CO gas.
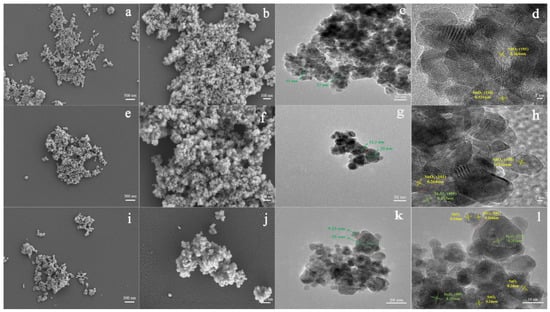
Figure 4.
FESEM and TEM images of (a–d) pristine SnO2, (e–h) 20 mol%In2O3-SnO2 nanocomposite and (i–l) 20 mol%In2O3-Sn0.92In0.08O2 nanocomposite (reprinted with permission from Ref. [66]).
4.2. Hydrothermal–Solvothermal Synthesis Method
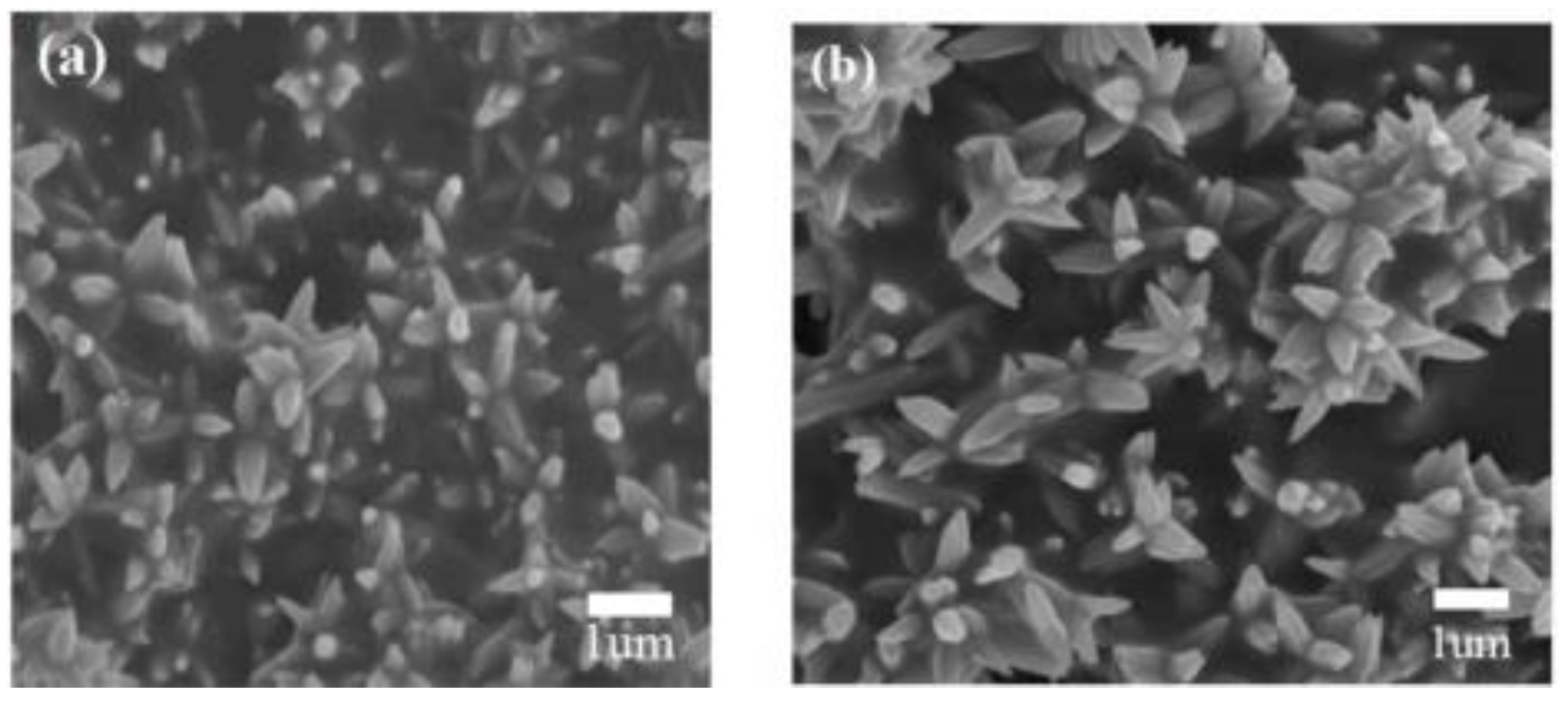
Because of the morphology and structure of MOS-based heterostructures that play an important role in improving their gas sensing properties, many researchers have been making effort to fabricate heterostructures using novel methods. Among these, the hydrothermal–solvothermal synthesis method is considered to be a powerful and efficient route for the fabrication of diverse kinds of heterostructural semiconductor nanomaterials, which can precisely control the structure and morphology of MOS nanomaterials, achieving the goals of fabricating a wide spectrum of metal oxide heterojunctions. This method is performed in an autoclave, where the solution concentration, reaction time ,and temperature can be automatically controlled [67]. Therefore, phases with diverse morphology and properties are fabricated by controlling the reaction process and crystal growth. Due to allowing the fabrication of a wide spectrum of metal oxide heterostructures, this method is widely studied by most researchers. Recently, Liu et al. fabricated a flower-like structure composed of NiO-decorated ZnO nanostructures via a one-step hydrothermal procedure [68] (Figure 5). In addition, by varying the synthesis temperature and the solution concentration, a two-step hydrothermal method can construct heterostructures of the same materials with diverse shapes. Wang et al. studied the effect of hydrothermal temperature on the morphology of metal oxide heterostructures and successfully synthesized a nanobelt-like structure of SnO2-TiO2 at relatively high temperatures [69]. Liu et al. controlled the reaction time and temperature by means of solvothermal method and then obtained NiO/ZnO hollow spheres materials [70]. The aforementioned introductions illustrate that a precise control over the hydrothermal synthetic conditions is the key factor for the construction of high-quality metal oxide heterostructures with diverse shapes.
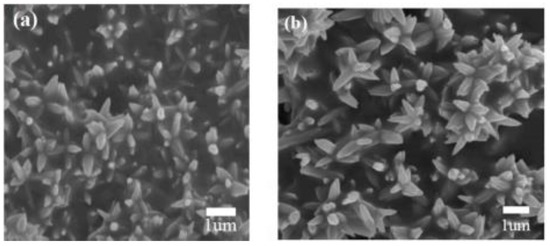
Figure 5.
(a) FESEM image of pure ZnO nanoflowers; (b) FESEM image of NiO-decorated ZnO nanostructures (reprinted with permission from Ref. [68]).
4.3. Vapor Deposition Method
This method mainly consists of chemical vapor deposition (CVD) or physical vapor deposition (PVD). CVD technology uses mainly one or several gaseous compounds or elementary substances containing film elements to produce films by chemical reaction on the substrate surface. CVD method can be used to purify materials, fabricate new crystals, as well as deposit monocrystal, polycrystal, glassy inorganic films, etc. The physical properties of materials can be precisely controlled by the process of vapor deposition. Additionally, high purity samples with different structure and morphology can be obtained by CVD method through precise control of vapor deposition process, such as the operating temperature, the pressure in the reactor, the template material, as well as the composition of the gas-phase [71]. Recently, by preparing SnO2/ZnO superlattice nanowires, Jiang et al. found that Au loading is helpful for the adsorption of Zn/Sn vapor and the formation of SnO2 superlattice on the ZnO lattice surface [72].
Another well-developed technique to synthesize novel heterostructures is PVD. Different catalyst layers could be deposited on the substrates to improve the nucleation of oxide materials. The catalyst type, the patterned template, the temperature and pressure inside the furnace, and the carrier gas composition and its flow affect the morphology of materials prepared by PVD method [73]. The PVD method is often carried out at high temperatures in a high-vacuum or inert-gas environment, which obtains low dimensional metal oxide heterostructures. Choi et al. deposited a thin Au layer on the surface of ZnO nanofiber stems to promote the growth of SnO2 nanowires by PVD method and successfully fabricated the ZnO-SnO2 nanofiber–nanowire stem–branch heterostructure [74].
4.4. Electrospinning Method
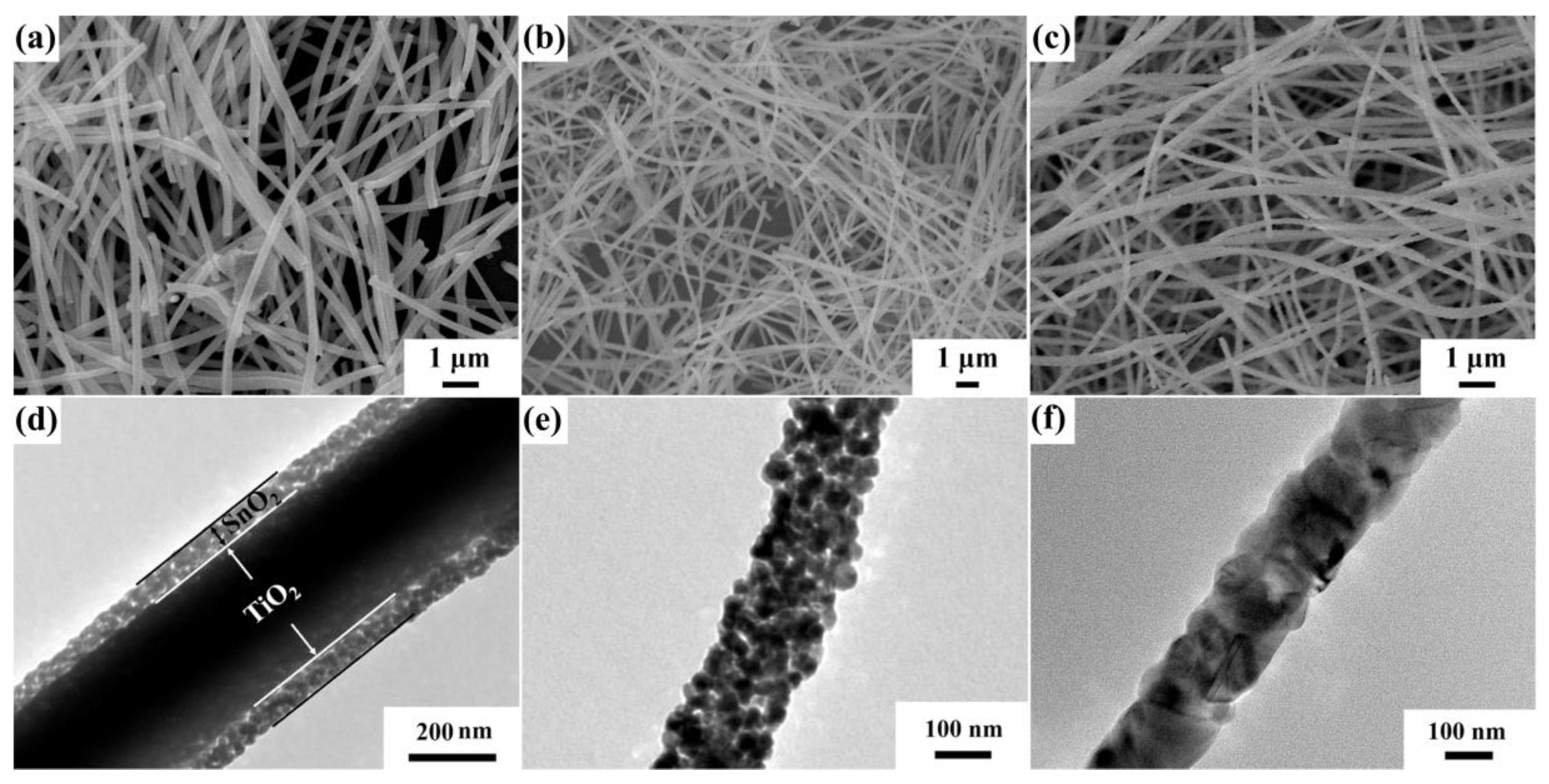
Electrospinning is a technique which utilizes high voltage electrostatic field force to fabricate nanofibers. In this way, the polymer and related materials can be prepared into one-dimensional nanofibers with high specific surface area, controllable composition and shape, and porous structure after calcination. The working mechanism of this technology is that the polymer or solution is electrified with the help of a high-voltage electrostatic field, and the liquid drop at the tip of the nozzle will form a suspended liquid drop (i.e., “Taylor cone”) and extend from the tip of the cone to obtain the fiber filament. Meanwhile, the liquid drops are subject to surface tension and surface charge repulsion force caused by the electrostatic field. When the surface charge repulsion force is greater than the surface tension, the micro flow of polymer will be ejected from the solution surface. The liquid flow is stretched and dragged by electric field force, then the solvent volatilizes and solidifies, and finally the sample is deposited on the prepared substrate to form polymer fiber [75]. Nanopolymer filaments can be fabricated by electrospinning. Feng et al. [76] prepared TiO2-SnO2 composite heterojunction materials with core–shell nanofiber structure by the electrospinning method (Figure 6).
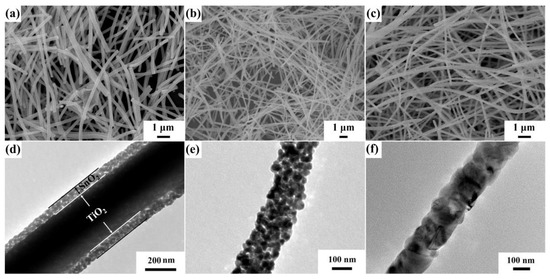
Figure 6.
Field emission scanning electron microscopy (FESEM) images of (a) TiO2-SnO2 core–shell nanofibers (NFs), (b) SnO2 NFs, and (c) TiO2 core–shell nanofibers, transmission electron microscopy (TEM) images of (d) TiO2-SnO2 core–shell NFs, (e) SnO2 NFs, and (f) TiO2 core–shell NFs (reprinted with permission from Ref. [76]).
5. Mechanisms of Gas Sensing Enhancement with Heterostructures
5.1. Working Mechanisms of Gas Sensing Materials
The sensing mechanism of MOS materials consists mainly in the interactions of the target gas molecules with the pre-absorbed oxygen species on the surface of gas sensing materials [77,78]. Referring to a resistive-type metal oxide gas sensor, the signal transmission mechanism is dependent on the change of the electrical resistance or conductance of sensitive materials when interacting with the analytic gas. The resistance of MOS gas sensors may increase or decrease on exposure to the gas depending on the type of metal oxide and gas analyte [79,80,81]. Different semiconducting materials can form various heterojunctions, which, in turn, integrate their respective conduction properties. Compared with single gas sensing materials, the construction of heterostructured compound materials can improve the sensing performance of gas sensors. Therefore, to understand the gas sensing mechanisms of materials composed of heterojunctions, exploring the junction sensing behavior is of vital importance.
5.2. Role of Heterojunction at the Interface
Two different solid-state materials can construct an electronic junction at the interface between them, which is always called a heterojunction. The study on heterojunction interface is significant when analyzing the mechanism of composite semiconducting materials. The p–n junctions are the most common heterojunctions used to modulate gas sensing properties.
Referring to the heterostructures gas sensing literature, n-type MOS gas sensors are more attractive to researchers in comparison with p-type MOS gas sensors because of several considerations such as better stability and higher sensitivity [82,83]. The combination of these two types of sensing materials opens a novel way to better improve the comprehensive sensing performance of gas sensors. In addition, these materials can be constructed into different structure–architecture types of heterostructure [84,85,86,87]. For reference, some sensing materials with their respective conduction types are listed in Table 2.

Table 2.
Conduction types of MOS and semiconductors (adapted from Ref. [88]).
Because of the difference in the Fermi energy (EF) between two different sensing materials—for example, the EF of n-type semiconductor material is always higher than that of the p-type semiconductor material when forming heterostructure—if they connect to form a heterostructure, the electrons at the higher energies will flow across the interface to unoccupied lower energies states until EF reach equilibrium, which results in the recombination between electron and hole in the vicinity of a p–n junction. This decreases the concentration of charge carriers and leads to the creation of a charge carrier depleted zone at the interface called the depletion region. This phenomenon is often called “Fermi level-mediated charge transfer”. Because of the band bending, a potential energy barrier will develop at the interface, which is caused by the difference in the original EF of the materials. Therefore, in order to pass through the interface, charge carriers must overcome this potential energy barrier. According to the aforementioned conduction types of sensing materials, the mechanisms of p–n, n–n, and p–p heterojunctions on gas sensing will be discussed in the following sections.
5.2.1. p–n Nanojunctions
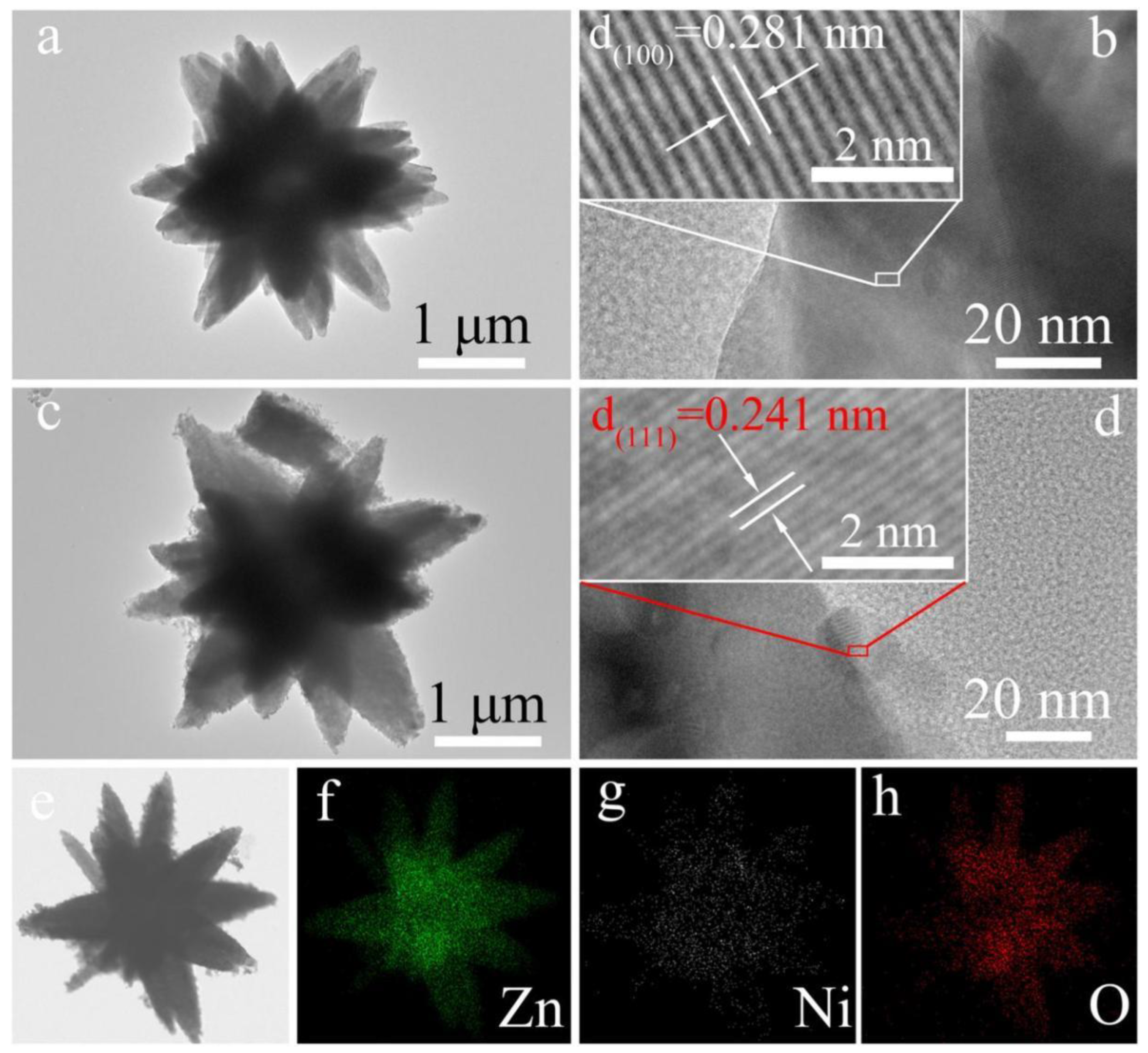
Considering a flower-like n-type ZnO decorated with p-type NiO nanoparticles (as shown in Figure 7) [89], the normal ambient resistance of NiO-decorated ZnO flower-like heterojunctions in air is higher than that of single pure ZnO microflowers. However, when acetone is introduced, a large decrease of the resistance (Rg) may occur, resulting in a large change of gas response for sensing materials; this is because the initial value of Ra is exceedingly high, as the gas response is defined as Ra/Rg for reducing gas [90]. Therefore, the response to target gas is obviously increased. The likely reasons can be interpreted as follows. First, increasing the concentration of initial absorbed oxygen ions helps to improve the gas sensitivity. NiO generally exhibits better oxygen-adsorption ability. With the help of the catalytic function of NiO, oxygen ions can be easily absorbed on the surface of NiO, and Ni2+ can be oxidized to a higher oxidation state (Ni3+), which significantly enhances the concentration of surface adsorbed oxygen. Secondly, the conduction of charge carriers across the heterojunction interface can contribute to the gas sensing performance. Due to the formation of an energy barrier potential at the interface between n-type ZnO and p-type NiO, the conduction capability of electrons and holes across the p–n interface will be weakened. When absorbing acetone, the height of energy barrier potential efficiently decreases, contributing to the conduction of charge carriers and increasing the sensing response.
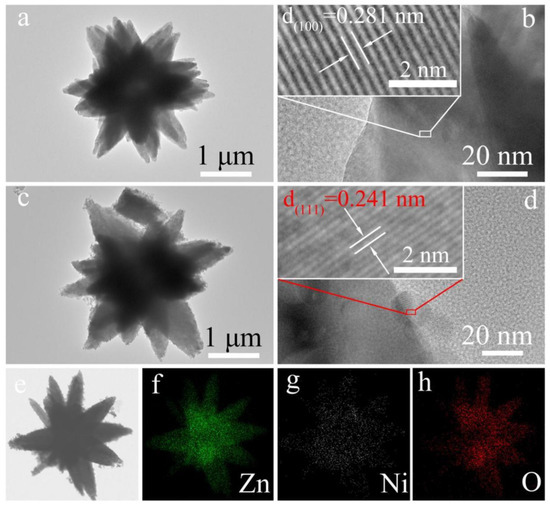
Figure 7.
(a,b) TEM image and corresponding HRTEM image of a single pure ZnO microflower; (c,d) TEM image and corresponding HRTEM image of NiO-decorated ZnO to form NiO-ZnO composite microflower; and (e–h) The corresponding EDS elemental mapping images. (Reprinted with permission from Ref. [89]).
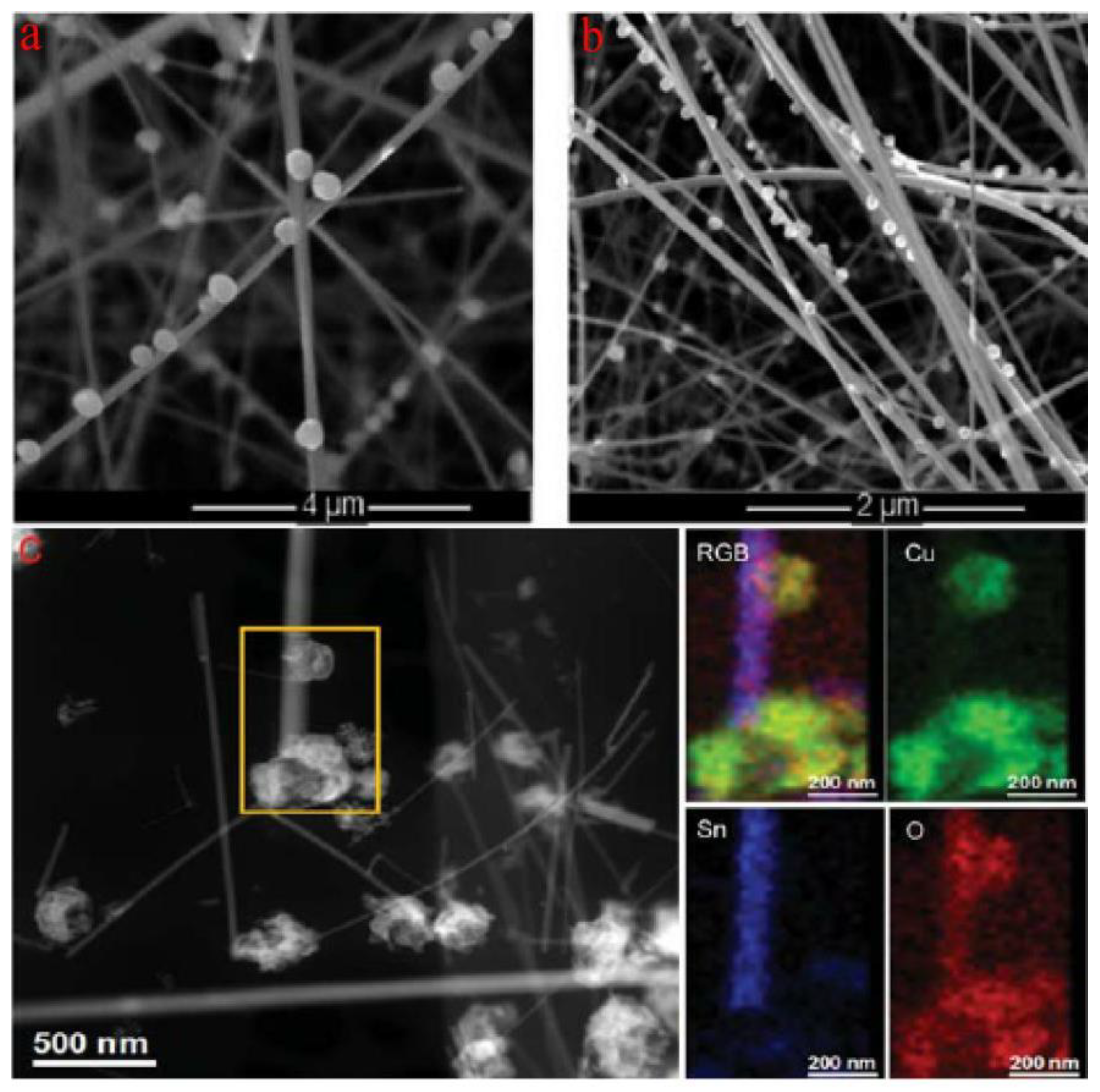
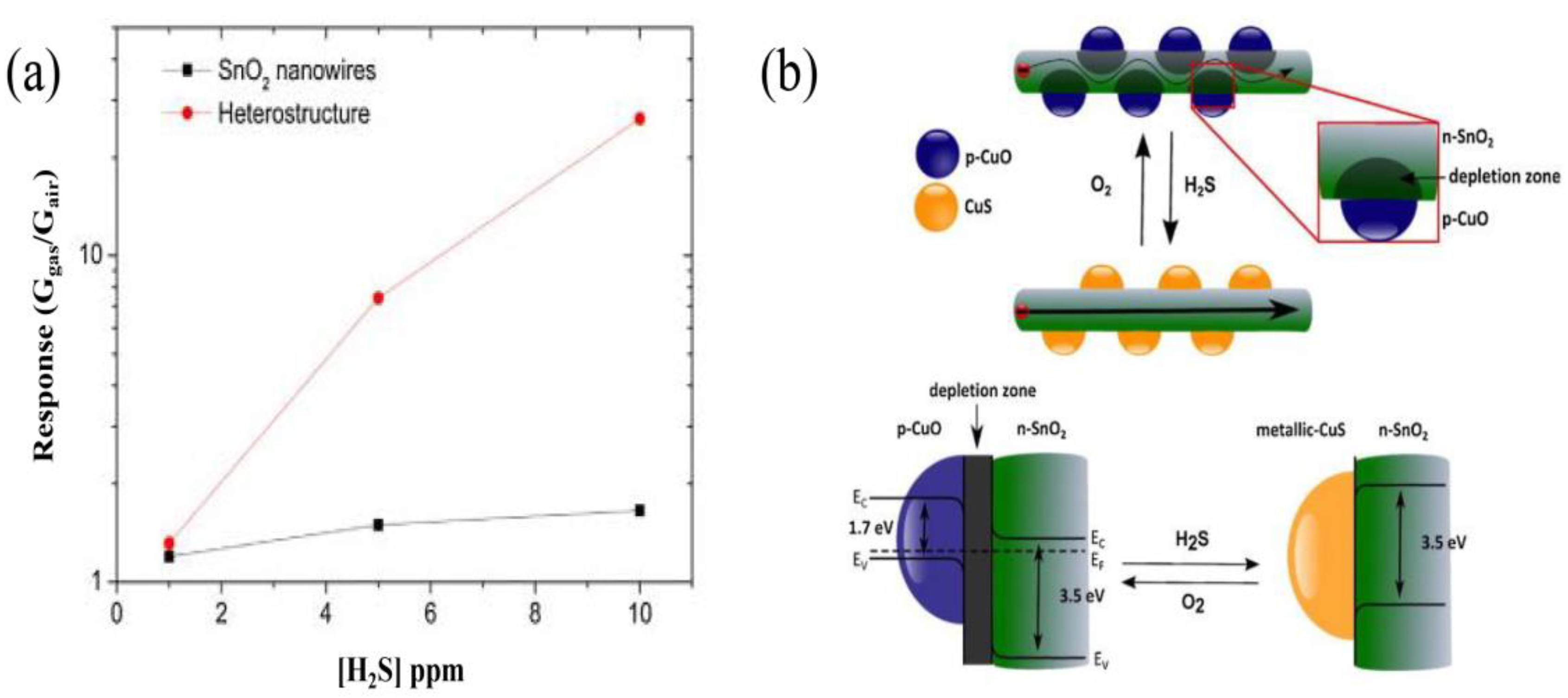
Irina et al. reported the synthesis of p-type CuO nanoparticles decorating SnO2 nanowires, constructing SnO2@CuO nanowires by chemical vapor deposition (CVD) for enhancing the detection of H2S [91]. Figure 8a,b show the successful synthesis of SnO2 nanowires decorated with CuO nanoparticles. It is also shown in Figure 8c that the successful formation of CuO-SnO2 heterostructure by STEM and EDS element analysis. This research has certified that the CuO decorating narrows the conduction channel of SnO2 when forming CuO-SnO2 heterostructures, increasing the initial resistance of sensors in air. Quite interestingly, when absorbing H2S, p-type CuO will react with H2S and be sulfuretted into CuS, destroying the potential energy barrier at the interface. Additionally, the hydrogen ions will transfer on the surface of the host material, reacting with the adsorbed oxygen ions and, thus, also decreasing the electrical resistance [92]. These are all attributed to the strong chemical interaction between H2S and CuO, as shown in Figure 9b. Irina et al. also performed a gas sensitivity test. It can be seen in Figure 9a that the response of CuO-SnO2 heterostructure is obviously higher than that of pristine SnO2 nanowires, certifying the sensing enhancement in heterostructures. The same phenomenon was also reported in the form of CuO-ZnO heterostructures for H2S testing. The potential energy barrier height of heterojunctions was efficiently reduced because of the chemical interaction between H2S and CuO [93].
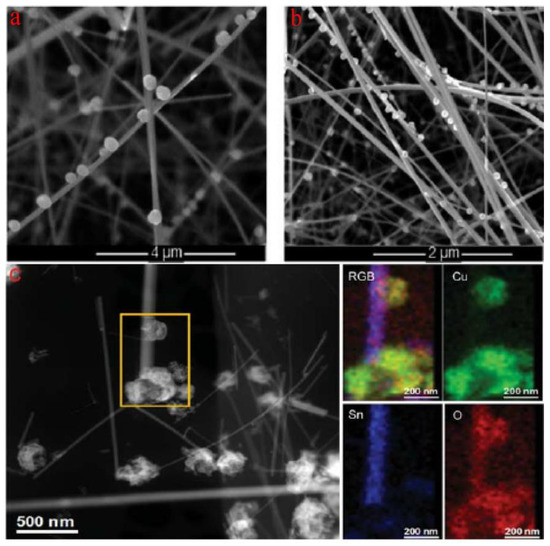
Figure 8.
(a,b) SEM micrographs of CuO particle-decorated SnO2 nanowires deposited on Al2O3 substrate; (c) STEM image of SnO2@CuO heterostructures (left) and EDS elemental maps (right) (reprinted with permission from Ref. [91]).
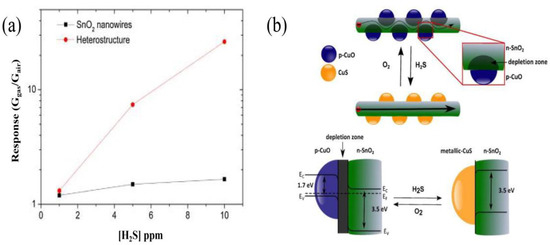
Figure 9.
(a) A significant improvement in sensor response to H2S is observed when forming p–n junctions. (b) Sensing mechanism of CuO sensitivity to H2S is explained. At the interface between SnO2 and CuO, a depletion zone forms in air. After H2S joins in, p-CuO particles react with it and transforms to CuS, resulting in decreasing the depletion region. (Reprinted with permission from Ref. [92]).
It is worth noting that Mashock et al. reported an opposite heterostructure configuration of SnO2 nanoparticles coating CuO nanowires [94]. Similarly, an energy barrier potential forms at the interface between n-type SnO2 and p-type CuO. Since p-type CuO is dominated by surface conduction because of the accumulation layer formed in air, the effect of SnO2 deposition is worth considering. The study results showed that with the deposition of SnO2 nanoparticles coating, a significant increase in the electrical resistance occurred when forming CuO-SnO2 heterojunctions. Furthermore, by doubling the deposition time of SnO2, they could create a continuous coating and a core–shell structure was successfully constructed. Prolonging the deposition times contributed to bringing an even higher resistance, which also produced a smaller increase in response to NH3 compared with the nanowires of shorter deposition time. The authors came up with two possible mechanisms to explain this phenomenon: (1) NH3 lowers the hole concentration of CuO nanowire by electron transfer, which increases the resistance; and/or (2) the increase of electron concentration in SnO2 nanoparticle due to removing absorbed oxygen enhances the p–n junction. This strong nanojunction is able to block the hole transport and increase the resistance.
5.2.2. n–n and p–p Nanojunctions
Just like p–n heterojunctions, band bending of energy levels can also appear in n–n and p–p heterojunctions [95,96,97,98,99,100]. Among these reports, n–n heterostructures are widely studied by researchers for gas sensing. Different from a p–n junction with fewer electrons at the interface due to electron-hole recombination that increases the resistance, electrons are not exhausted in an n–n junction, and electrons from the material with a high Fermi level can easily transfer to the one with a low Fermi level, which then forms an accumulation layer rather than a depletion layer. While this accumulation layer will be depleted by the subsequent oxygen adsorption on the surface, further increasing the potential energy barrier at the interface to enhance the sensing performance.
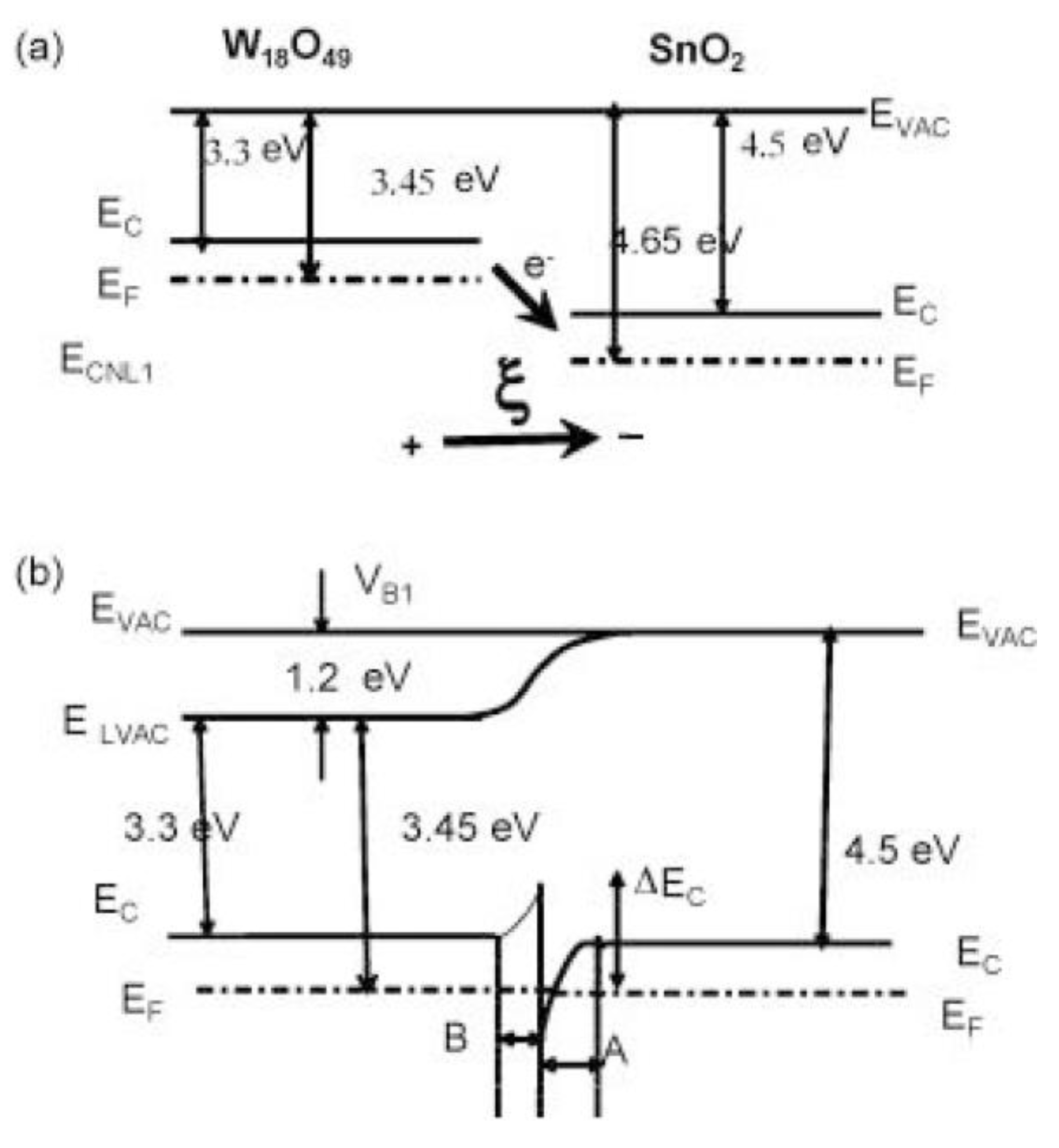
Considering small n-type W18O49 nanowires grown on larger n-type SnO2 nanowires (Figure 10) [95], the energy conduction band of SnO2 is higher than that of W18O49. When they reach an equilibrium state with each other, electrons will transfer from the high energy conduction band of SnO2 to W18O49 until their Fermi energy levels achieve equilibrium, leading to the band bending at the interface between them. When exposed to the air, oxygen species can adsorb on the surface of SnO2/W18O49 nano-heterostructures. By capturing free electrons on the surface of heterostructures, these oxygen species can be ionized into oxygen ions, leading to the formation of an electron depletion layer, increasing the energy barrier height at the interface, which improves the H2S sensing performance.
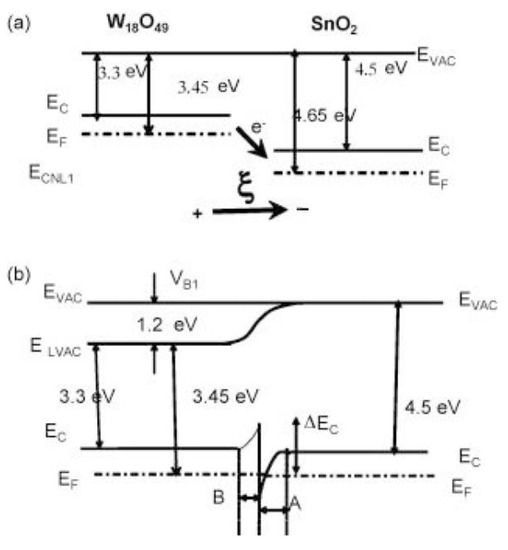
Figure 10.
The energy band diagram of the gas sensing mechanism for (a) W18O49 and SnO2 materials and (b) the SnO2/W18O49 nanostructures. Reprinted with permission from Ref. [95].
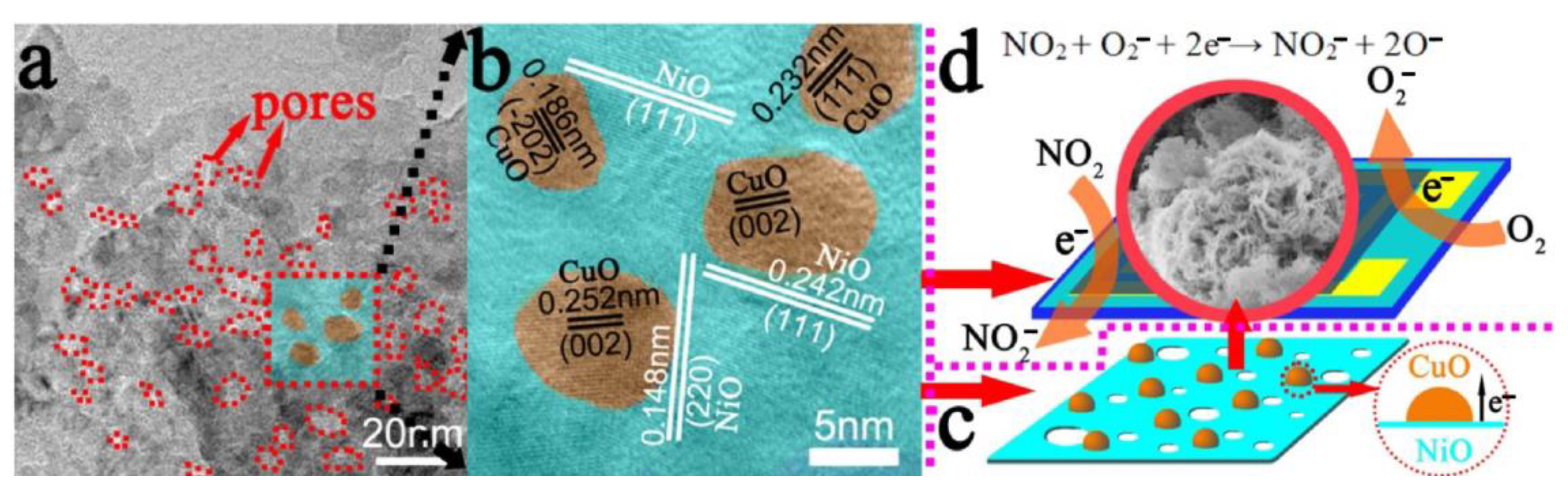
On the other hand, when referring to p-p heterostructure gas sensors, there are rare reports in the literature since the year 2000. Quite interestingly, recently in 2017, a paper was published on highly sensitive NO2 sensors based on NiO@CuO nanocomposite worked at room temperature [98]. The increased gas sensing property towards the detection of NO2 may be caused by two factors. Firstly, mesoporous hierarchical flower-like NiO nanosheet owns a large surface area, which performs as the catalyst and allows NO2 to adsorb and desorb effectively on the surface of the pores. Secondly, p-type CuO and p-type NiO are able to form the p–p heterojunction which plays a vital role in improving the sensing performance (Figure 11a–c). It helps to efficiently transfer the electrons from NiO to CuO. When the Fermi energy arrives at equilibrium state, the hole accumulation layer will form at the interface between NiO and CuO. O2 molecules will act as electron acceptors and capture electrons from the conduction band of the sensing materials by being absorbed on the surface of the sensing materials in air, which increases the holes concentration and decreases the resistance of the sensors. If these sensors are exposed to NO2, the NO2 molecules could interact with the chemisorbed oxygen ions on the surface of the materials and directly be adsorbed on the surface, which can decrease the resistance under NO2 atmosphere (Figure 11d). It may provide a novel method to detect NO2 gas at room temperature by constructing NiO@CuO heterostructure gas sensors.
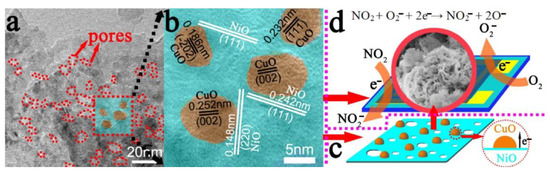
Figure 11.
(a) TEM image of mesoporous NiO@CuO nanosheets; (b,c) heterojunction between NiO nanosheets and CuO nanoparticles at the interface; (d) sensing mechanism of NiO@CuO gas sensors exposed to air and NO2. Reprinted with permission from Ref. [98].
5.3. Synergistic Effect
An additional mechanism that should be considered to enhance gas sensing performance is synergistic behavior which occurs in the heterostructured composite materials. These materials synthesize the advantages of each component, which results in a special synergistic effect between each components to improve the gas sensing performance of the materials. Generally speaking, it is when two different components of a material respectively contact with the gas phase and each provides a different purpose which is complementary to the other [11]. Ivanovskaya et al. found that composite oxide materials which simultaneously have acidic and alkaline active sites could decompose the organic gas molecules more completely due to the diverse redox properties of these composite oxide materials [101]. Costello et al. found that SnO2 could completely oxidize butanol to butyral and that ZnO had no effect on butanol decomposition while it could easily decompose butyral; moreover, ZnO-SnO2 composite oxides could synergistically decompose butanol and performed the highest sensing response [102]. Recently, Kamble et al. proposed that the synergism of Cr and noble metal Pt-activated SnO2 gas sensors could exhibit enhanced sensitivity and improved selectivity toward CO gas, which, respectively, synthesize the advantages of Cr for improving selectivity and Pt for enhancing sensitivity [103].
5.4. Catalyzed Spill-Over Effect
The catalyzed spill-over effect is another important sensing behavior often mentioned in the literature [29,104,105,106,107]. In general, the target gas molecules firstly react with one of the heterostructure composite constituents, forming a secondary product which remains adsorbed on the surface of the other constituent and directly affects the sensing properties. This phenomenon often occurs in CuO composite materials for H2S detection [91,92,108,109]. As aforementioned, with regard to CuO/SnO2 nanojunction composites, H2S can react with CuO nanoparticles and transform them into CuS. Then the left-over hydrogen spills over on the surface of the composite material, acting as a reducing agent and reacting with the other host material, thus decreasing the resistance [91]. Meanwhile, CuO plays a part in improving the sensitivity of host materials to H2S. Moreover, the spill-over effect can improve the sensing response by removing the depletion layer. Shao et al. [91] discovered that CuO was firstly combined with SnO2 to form a p–n heterojunction, which increased the resistance in air. However, when H2S was introduced in this p–n junction, CuO was converted to CuS and this new product acted as a moderate conductor between CuO and SnO2, When the conversion was completed, an ohmic junction could form between CuS and SnO2, eliminating the depletion region barrier of CuO/SnO2, thus favoring the conduction across the interface.
In addition, the spill-over effect applies to noble metals which are used to catalyze MOS materials. For example, noble metal Pt performs a strong catalytic effect at the surface of SnO2 [110]. It can catalyze the dissociation of O2 and spill over the oxygen ions which adsorbed on the surface of SnO2. The main advantage of Pt is to absorb exceptionally large number of gas molecules and convert them into adsorbed ions, decreasing the activation energy of MOS needed for reaction, hence reducing the response and recovery time and lowering the operating temperature [88].
5.5. Response Inversion Effect
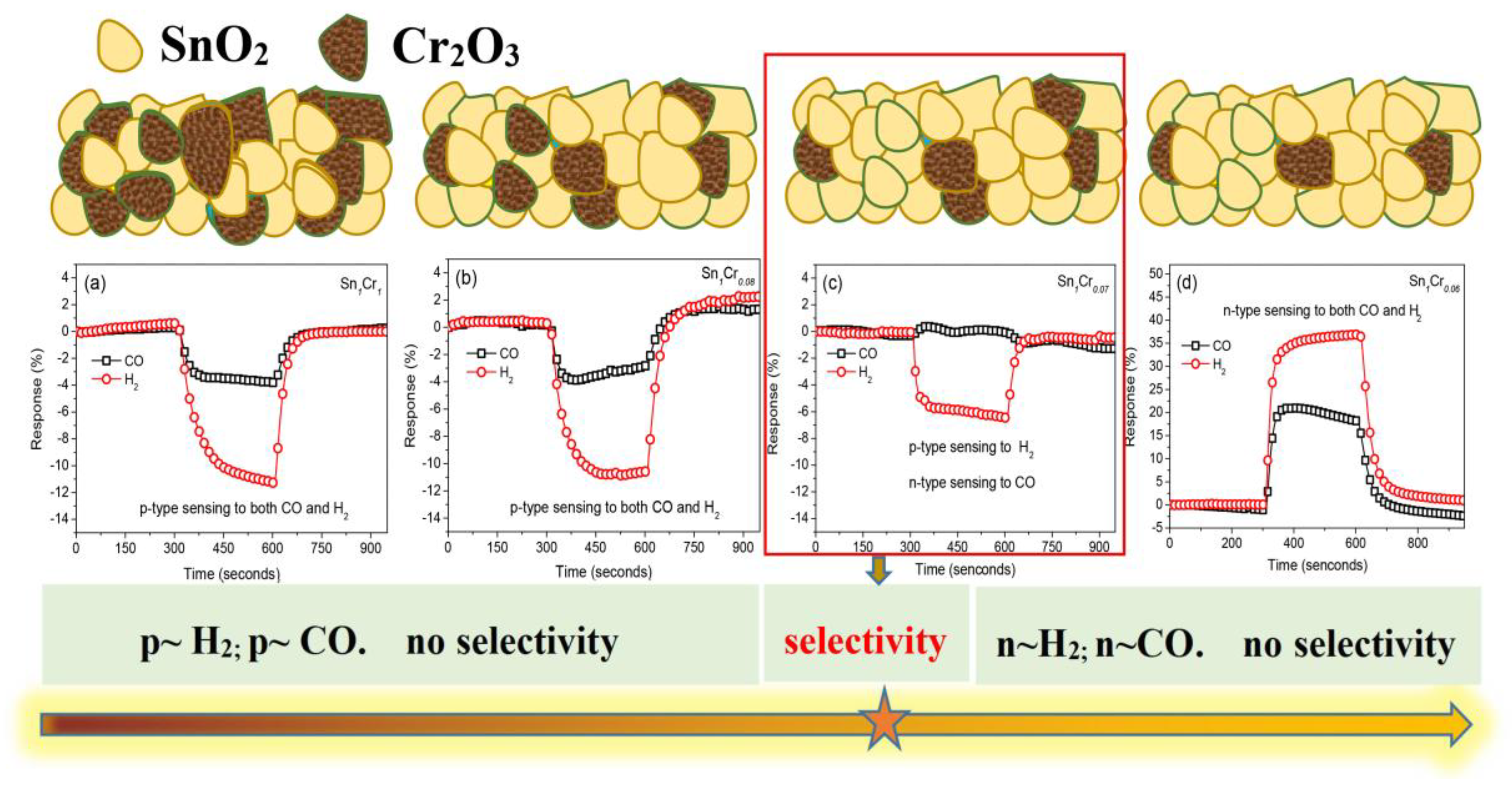
The addition of a p-type material to an n-type material can form an n–p composite oxide material. It should be realized that under certain conditions, the p-type constituent can counteract the resistance change of the n-type constituent to target gases. Sometimes the p-type constituent can dominate the sensing properties over a certain range of constitution, making the composite oxide material present a p-type response. Several studies reported the n–p or n–p–n response inversion phenomena [111,112]. Kosc et al. [111] studied a bi-phase TiO2/NiO sputtered film which showed p-type conductivity. When absorbing a certain concentration of H2, the holes in the NiO layer would be fully compensated by the added electrons from broken oxygen bonds, resulting in the response type inversion, then the sensor presented n-type behavior. Additionally, Huang et al. [112] synthesized the ZnO-modified SnO2 nanorods, which showed a typical n–p–n response inversion to H2. Furthermore, it is worth noting that the response inversion phenomenon also occurs in p–n heterostructure nanocomposites when exposed to homogeneous gases by adjusting the metal oxide ratio. Yin’s research group has overcome the drawback of poor selectivity of MOS-based gas sensors to homogeneous gases such as CO and H2 [113,114]. As exhibited in Figure 12, due to the different adsorption tendency of CO and H2 on the surface of SnO2 and Cr2O3, under the optimal heterojunction composition (Figure 12c), H2 molecules prefer to adsorb on Cr2O3, causing the reduction of hole accumulation layer (HAL) on Cr2O3, which is greater than the decrease of electron depletion layer (EDL) on SnO2, and the increase of electron content is lower than the decrease of hole content. The hole content variation dominates the conductivity of n-SnO2-p-Cr2O3 heterostructure, while CO gas prefers to adsorb on SnO2, which brings about the opposite change tendency of conductivity; thus, sensing behavior under this proportion presents a p-type response and n-type response toward H2 and CO, respectively, successfully distinguishing these two homogeneous gases.
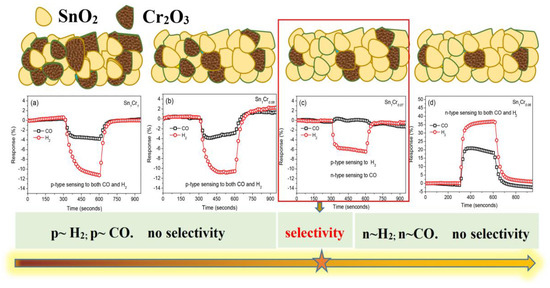
Figure 12.
(a–d) Gas sensing response of SnO2-Cr2O3 heterostructure nanocomposite towards CO and H2 via changing Cr2O3 content. Reprinted with permission from Ref. [114].
6. Conclusions and Future Outlook
The present work reported here has started to reveal novel and innovative ways to synthesize MOS heterostructural materials for improving chemical gas sensor performance. In this paper, a series of works by researchers in this field have been summarized. It is demonstrated throughout this review that it is possible to utilize different synthesis methods for preparing metal oxide heterostructures with different morphologies and shapes, each of them exhibiting a specific sensing performance towards particular target gas.
This literature review summarizes four different techniques which have been widely adopted for fabricating metal oxide heterostructures, namely core–shell, bi-layer, hollow spheres, and branched ones. The aforementioned methods have some advantages, such as the obvious heterostructure interface between different phases, the selective growth on the substrates, and the fabrication of multi-layered structures or composites. In recent years, more attention has been paid to the hydrothermal method due to the development of MOS materials with diverse morphologies and structures, such as nanorod-like, nanoflower-like, nanobelt-like, or hollow spheres structures by precisely controlling the reaction process. In addition, it seems that the combination of different fabrication methods is a more efficient way to acquire metal oxide heterostructures with novel morphologies or dimensions (core–shell nanostructures, one-dimensional heterostructures, two-dimensional layered heterostructures, or three-dimensional hierarchical heterostructures).
Furthermore, gas sensing enhancement mechanisms of MOS-based heterostructure materials have been demonstrated, which will make it useful to fabricate the corresponding heterostructure toward a specific target gas. However, despite novel and advanced technologies in the MOS field, nanotechnology and these complex configurations are now facing new challenges and issues, such as long-term stability, reproducibility, gas selectivity, characterization of the key parameters, establishing the gas sensing mechanism, and functional integration, which should be overcome in time. Recent works have provided us with new ideas for fabricating metal oxide heterostructures as gas sensing materials. Going forward, the synthesis of novel materials and the design of heterostructures will require further study of the influencing factors and the gas sensing enhancement mechanisms. Further study on MOS heterostructural semiconductor gas sensors should focus mainly on (1) novel materials and new heterojunction interfaces, and (2) the mechanisms which contribute to the gas sensing performance.
Although the present research focuses mostly on the combination of binary compounds, it should be realized that the interface of heterojunction is not limited to binary compounds, as there are many other configurations which could be used to prepare heterojunctions. In particular, composite materials obtained by the combination of metal oxides have proved to be extremely interesting for gas sensing applications. In addition, further research on heterojunction should not be limited to metal oxide, as organic and hybrid organic/inorganic composite materials also show great potential as gas sensors. Core–shell structure has much potential due to the maximization of the interfacial heterojunction area, where the electronic interaction is the most dominant. Self-assembly of 0D, 1D, and 2D structures can form unique morphologies and shapes. At present, it is still necessary to develop new synthesis methods to self-assemble these types of structures to fabricate special heterostructures.
Moreover, there are different opinions on the gas sensing mechanism, among which the most prominent ones are the grain boundary–barrier model, heterojunction structure, surface synergistic effect, catalyzed spill-over effect, response inversion effect, and separation of charge carriers effect. It is possible to design complex structures with the rapid development of new fabrication techniques. Therefore, it becomes essential to clarify the gas sensing mechanisms in order to properly select the morphology and heterostructure for a given application. On the basis of the first principles, the construction of gas adsorption model of metal oxide heterojunction is expected to clarify the gas sensing mechanism qualitatively.
Author Contributions
Conceptualization, methodology, software, investigation, writing—original draft, and writing—review and editing were completed by F.-J.M.; conceptualization, methodology, validation, resources, supervision, and writing—review and editing were completed by R.-F.X.; validation, resources, supervision were completed by S.-X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Somov, A.; Baranov, A.; Savkin, A.; Spirjakin, D.; Spirjakin, A.; Passerone, R. Development of Wireless Sensor Network for Combustible Gas Monitoring. Sens. Actuators A Phys. 2011, 171, 398–405. [Google Scholar] [CrossRef]
- Aliyu, F.; Sheltami, T. Development of an Energy-Harvesting Toxic and Combustible Gas Sensor for Oil and Gas Industries. Sens. Actuators B Chem. 2016, 231, 265–275. [Google Scholar] [CrossRef]
- Kim, I.-D.; Rothschild, A.; Tuller, H.L. Advances and New Directions in Gas-Sensing Devices. Acta Mater. 2013, 61, 974–1000. [Google Scholar] [CrossRef]
- Lin, Z.; Li, N.; Chen, Z.; Fu, P. The Effect of Ni Doping Concentration on the Gas Sensing Properties of Ni Doped SnO2. Sens. Actuators B Chem. 2017, 239, 501–510. [Google Scholar] [CrossRef]
- Kolmakov, A.; Zhang, Y.; Cheng, G.; Moskovits, M. Detection of CO and O2 Using Tin Oxide Nanowire Sensors. Adv. Mater. 2003, 15, 997–1000. [Google Scholar] [CrossRef]
- Jing, Z.; Zhan, J. Fabrication and Gas-Sensing Properties of Porous ZnO Nanoplates. Adv. Mater. 2008, 20, 4547–4551. [Google Scholar] [CrossRef]
- Wilson, R.L.; Simion, C.E.; Blackman, C.S.; Carmalt, C.J.; Stanoiu, A.; Di Maggio, F.; Covington, J.A. The Effect of Film Thickness on the Gas Sensing Properties of Ultra-Thin TiO2 Films Deposited by Atomic Layer Deposition. Sensors 2018, 18, 735. [Google Scholar] [CrossRef]
- Wang, H.; Yan, L.; Li, S.; Li, Y.; Liu, L.; Du, L.; Duan, H.; Cheng, Y. Acetone Sensors Based on Microsheet-Assembled Hierarchical Fe2O3 with Different Fe3+ Concentrations. Appl. Phys. A 2018, 124, 212. [Google Scholar] [CrossRef]
- Han, D.; Zhai, L.; Gu, F.; Wang, Z. Highly Sensitive NO2 Gas Sensor of Ppb-Level Detection Based on In2O3 Nanobricks at Low Temperature. Sens. Actuators B Chem. 2018, 262, 655–663. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Tomescu-Stănoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of Humidity on CO Sensing with P-Type CuO Thick Film Gas Sensors. Sens. Actuators B Chem. 2011, 153, 347–353. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale Metal Oxide-Based Heterojunctions for Gas Sensing: A Review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Guo, X.M.; Zhao, J.T.; Yin, X.T.; Huang, S.L. Sensitivity and Selectivity of SnO2-Based Sensor for CO and H2 Detections: A Novel Method to Detect Simultaneously the CO and H2 Concentrations. Adv. Sci. Technol. 2017, 99, 40–47. [Google Scholar] [CrossRef]
- Huo, L.; Yang, X.; Liu, Z.; Tian, X.; Qi, T.; Wang, X.; Yu, K.; Sun, J.; Fan, M. Modulation of Potential Barrier Heights in Co3O4/SnO2 Heterojunctions for Highly H2-Selective Sensors. Sens. Actuators B Chem. 2017, 244, 694–700. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Cretu, V.; Wolff, N.; Duppel, V.; Kienle, L.; Adelung, R. Single and Networked CuO Nanowires for Highly Sensitive P-Type Semiconductor Gas Sensor Applications. Phys. Status Solidi-Rapid Res. Lett. 2016, 10, 260–266. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.; Jian, P.; Wang, L.; Yan, X. Hollow Urchin-like NiO/NiCo2O4 Heterostructures as Highly Efficient Catalysts for Selective Oxidation of Styrene. J. Colloid Interface Sci. 2018, 526, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Zappa, D.; Galstyan, V.; Kaur, N.; Munasinghe Arachchige, H.M.M.; Sisman, O.; Comini, E. “Metal Oxide-Based Heterostructures for Gas Sensors”—A Review. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef]
- Xue, S.; Cao, S.; Huang, Z.; Yang, D.; Zhang, G. Improving Gas-Sensing Performance Based on MOS Nanomaterials: A Review. Materials 2021, 14, 4263. [Google Scholar] [CrossRef]
- Joshi, N.; Braunger, M.L.; Shimizu, F.M.; Riul, A.R., Jr.; Oliveira, O.N. Insights into Nano-Heterostructured Materials for Gas Sensing: A Review. Multifunct. Mater. 2021, 4, 032002. [Google Scholar] [CrossRef]
- Moon, W.J.; Yu, J.H.; Choi, G.M. The CO and H2 Gas Selectivity of CuO-Doped SnO2–ZnO Composite Gas Sensor. Sens. Actuators B Chem. 2002, 87, 464–470. [Google Scholar] [CrossRef]
- Patil, D.R.; Patil, L.A. Cr2O3-Modified ZnO Thick Film Resistors as LPG Sensors. Talanta 2009, 77, 1409–1414. [Google Scholar] [CrossRef]
- Yin, M.; Yao, Y.; Fan, H.; Liu, S. WO3-SnO2 Nanosheet Composites: Hydrothermal Synthesis and Gas Sensing Mechanism. J. Alloys Compd. 2018, 736, 322–331. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Fan, H.; Li, M.; Ma, L. Zeolitic Imidazolate Framework Coated ZnO Nanorods as Molecular Sieving to Improve Selectivity of Formaldehyde Gas Sensor. ACS Sens. 2016, 1, 243–250. [Google Scholar] [CrossRef]
- Absalan, S.; Nasresfahani, S.; Sheikhi, M.H. High-Performance Carbon Monoxide Gas Sensor Based on Palladium/Tin Oxide/Porous Graphitic Carbon Nitride Nanocomposite. J. Alloys Compd. 2019, 795, 79–90. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly Sensitive and Selective Gas Sensors Using P-Type Oxide Semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, L.; Bian, Y.; Ma, X.; Han, N.; Chen, Y. Coupling P+n Field-Effect Transistor Circuits for Low Concentration Methane Gas Detection. Sensors 2018, 18, 787. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, K.; Wang, X.; Sui, Y.; Zou, B.; Zheng, W.; Zou, G. Rapid and Selective H2S Detection of Hierarchical ZnSnO3 Nanocages. Sens. Actuators B Chem. 2011, 159, 245–250. [Google Scholar] [CrossRef]
- Xu, L.; Xing, R.; Song, J.; Xu, W.; Song, H. ZnO–SnO2 Nanotubes Surface Engineered by Ag Nanoparticles: Synthesis, Characterization, and Highly Enhanced HCHO Gas Sensing Properties. J. Mater. Chem. C 2013, 1, 2174–2182. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Sun, Y.; Li, P.; Ji, J.; Chen, Y.; Zhang, W.; Hu, J. Fabrication and Gas Sensing Properties of Au-Loaded SnO2 Composite Nanoparticles for Highly Sensitive Hydrogen Detection. Sens. Actuators B Chem. 2017, 240, 664–673. [Google Scholar] [CrossRef]
- Yin, X.-T.; Zhou, W.-D.; Li, J.; Wang, Q.; Wu, F.-Y.; Dastan, D.; Wang, D.; Garmestani, H.; Wang, X.-M.; Ţălu, Ş. A Highly Sensitivity and Selectivity Pt-SnO2 Nanoparticles for Sensing Applications at Extremely Low Level Hydrogen Gas Detection. J. Alloys Compd. 2019, 805, 229–236. [Google Scholar] [CrossRef]
- Yamazoe, N.; Kurokawa, Y.; Seiyama, T. Effects of Additives on Semiconductor Gas Sensors. Sens. Actuators 1983, 4, 283–289. [Google Scholar] [CrossRef]
- Lyson-Sypien, B.; Czapla, A.; Lubecka, M.; Kusior, E.; Zakrzewska, K.; Radecka, M.; Kusior, A.; Balogh, A.G.; Lauterbach, S.; Kleebe, H.-J. Gas Sensing Properties of TiO2–SnO2 Nanomaterials. Sens. Actuators B Chem. 2013, 187, 445–454. [Google Scholar] [CrossRef]
- Shaposhnik, D.; Pavelko, R.; Llobet, E.; Gispert-Guirado, F.; Vilanova, X. Hydrogen Sensors on the Basis of SnO2-TiO2 Systems. Procedia Eng. 2011, 25, 1133–1136. [Google Scholar] [CrossRef]
- Vasiliev, R.B.; Rumyantseva, M.N.; Podguzova, S.E.; Ryzhikov, A.S.; Ryabova, L.I.; Gaskov, A.M. Effect of Interdiffusion on Electrical and Gas Sensor Properties of CuO/SnO2 Heterostructure. Mater. Sci. Eng. B 1999, 57, 241–246. [Google Scholar] [CrossRef]
- Dandeneau, C.S.; Jeon, Y.-H.; Shelton, C.T.; Plant, T.K.; Cann, D.P.; Gibbons, B.J. Thin Film Chemical Sensors Based on P-CuO/n-ZnO Heterocontacts. Thin Solid Films 2009, 517, 4448–4454. [Google Scholar] [CrossRef]
- Zhu, Y.; Su, H.; Chen, Y.; Jin, Z.; Xu, J.; Zhang, D. A Facile Synthesis of PdO-Decorated SnO2 Nanocomposites with Open Porous Hierarchical Architectures for Gas Sensors. J. Am. Ceram. Soc. 2016, 99, 3770–3774. [Google Scholar] [CrossRef]
- Vuong, N.M.; Chinh, N.D.; Huy, B.T.; Lee, Y.-I. CuO-Decorated ZnO Hierarchical Nanostructures as Efficient and Established Sensing Materials for H2S Gas Sensors. Sci. Rep. 2016, 6, 26736. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhang, H.; Gao, X.; Yang, J.; Wang, L. Brookite TiO2 Decorated α-Fe2O 3 Nanoheterostructures with Rod Morphologies for Gas Sensor Application. J. Mater. Chem. A 2014, 2, 7935–7943. [Google Scholar] [CrossRef]
- Asad, M.; Sheikhi, M.H. Surface Acoustic Wave Based H2S Gas Sensors Incorporating Sensitive Layers of Single Wall Carbon Nanotubes Decorated with Cu Nanoparticles. Sens. Actuators B Chem. 2014, 198, 134–141. [Google Scholar] [CrossRef]
- Lü, R.; Zhou, W.; Shi, K.; Yang, Y.; Wang, L.; Pan, K.; Tian, C.; Ren, Z.; Fu, H. Alumina Decorated TiO2 Nanotubes with Ordered Mesoporous Walls as High Sensitivity NOx Gas Sensors at Room Temperature. Nanoscale 2013, 5, 8569–8576. [Google Scholar] [CrossRef]
- Cao, J.; Qin, C.; Wang, Y.; Zhang, H.; Sun, G.; Zhang, Z. Solid-State Method Synthesis of SnO2-Decorated g-C3N4 Nanocomposites with Enhanced Gas-Sensing Property to Ethanol. Materials 2017, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Y.; Zhang, Z.; Cao, J. Highly Sensitive Acetone Gas Sensor Based on G-C3N4 Decorated MgFe2O4 Porous Microspheres Composites. Sensors 2018, 18, 2211. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Fan, Q.-F.; Li, G.-L.; Huang, Y.-H.; Gao, Z.; Fan, X.-M.; Zhang, C.-L.; Zhou, Z.-W. Syntheses of ZnO Nano-Arrays and Spike-Shaped CuO/ZnO Heterostructure. Acta Phys. Chim. Sin. 2015, 31, 783–792. [Google Scholar] [CrossRef]
- Katoch, A.; Byun, J.-H.; Choi, S.-W.; Kim, S.S. One-Pot Synthesis of Au-Loaded SnO2 Nanofibers and Their Gas Sensing Properties. Sens. Actuators B Chem. 2014, 202, 38–45. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Jun Hong, Y.; Kang, Y.C.; Lee, J.-H. High Performance Chemiresistive H2S Sensors Using Ag-Loaded SnO2 Yolk–Shell Nanostructures. RSC Adv. 2014, 4, 16067–16074. [Google Scholar] [CrossRef]
- Yin, X.-T.; Lv, P.; Li, J. Study on Simultaneous Detection of CO and H2 with (Pd, Fe)-Modified SnO2 and Pt-Loaded SnO2 Sensors. J. Mater. Sci. Mater. Electron. 2018, 29, 18935–18940. [Google Scholar] [CrossRef]
- Liewhiran, C.; Tamaekong, N.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Ultra-Sensitive H2 Sensors Based on Flame-Spray-Made Pd-Loaded SnO2 Sensing Films. Sens. Actuators B Chem. 2013, 176, 893–905. [Google Scholar] [CrossRef]
- Arafat, M.M.; Dinan, B.; Akbar, S.A.; Haseeb, A.S.M.A. Gas Sensors Based on One Dimensional Nanostructured Metal-Oxides: A Review. Sensors 2012, 12, 7207–7258. [Google Scholar] [CrossRef]
- Korotcenkov, G. Gas Response Control through Structural and Chemical Modification of Metal Oxide Films: State of the Art and Approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Q.H.; Chen, Y.J.; Wang, T.H.; He, X.L.; Li, J.P.; Lin, C.L. Fabrication and Ethanol Sensing Characteristics of ZnO Nanowire Gas Sensors. Appl. Phys. Lett. 2004, 84, 3654–3656. [Google Scholar] [CrossRef]
- Hamidi, S.M.; Mosivand, A.; Mahboubi, M.; Arabi, H.; Azad, N.; Jamal, M.R. New Generation of α-MnO2 Nanowires @PDMS Composite as a Hydrogen Gas Sensor. Appl. Phys. A 2018, 124, 253. [Google Scholar] [CrossRef]
- Caicedo, N.; Leturcq, R.; Raskin, J.-P.; Flandre, D.; Lenoble, D. Detection Mechanism in Highly Sensitive ZnO Nanowires Network Gas Sensors. Sens. Actuators B Chem. 2019, 297, 126602. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low Power-Consumption CO Gas Sensors Based on Au-Functionalized SnO2-ZnO Core-Shell Nanowires. Sens. Actuators B Chem. 2018, 267, 597–607. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Kim, S.-J.; Choi, J.-K.; Choi, J.; Ji, H.; Kim, G.-T.; Lee, J.-H. ZnO-SnO2 Core-Shell Nanowire Networks and Their Gas Sensing Characteristics. In Proceedings of the 2011 IEEE Nanotechnology Materials and Devices Conference, Jeju, Korea, 18–21 October 2011; pp. 396–397. [Google Scholar]
- Tian, X.; Wang, Q.; Chen, X.; Yang, W.; Wu, Z.; Xu, X.; Jiang, M.; Zhou, Z. Enhanced Performance of Core-Shell Structured Polyaniline at Helical Carbon Nanotube Hybrids for Ammonia Gas Sensor. Appl. Phys. Lett. 2014, 105, 203109. [Google Scholar] [CrossRef]
- Jin, W.; Chen, W.; Lu, Y.; Zhao, C.; Dai, Y. V2O5/Polypyrrole Core–Shell Nanotubes for Gas Sensor. J. Nanosci. Nanotechnol. 2011, 11, 10834–10838. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Yang, Z.; Chen, Y.; Zhang, J.; Wang, Q.; Huang, F.; Wei, Q. A Room Temperature Ammonia Gas Sensor Based on Cellulose/TiO2/PANI Composite Nanofibers. Colloids Surf. A 2016, 494, 248–255. [Google Scholar] [CrossRef]
- Li, C.; Chartuprayoon, N.; Bosze, W.; Low, K.; Lee, K.H.; Nam, J.; Myung, N.V. Electrospun Polyaniline/Poly(Ethylene Oxide) Composite Nanofibers Based Gas Sensor. Electroanalysis 2014, 26, 711–722. [Google Scholar] [CrossRef]
- TANG, W.; WANG, J.; YAO, P.-J.; DU, H.-Y.; SUN, Y.-H. Preparation, Characterization and Gas Sensing Mechanism of ZnO-Doped SnO2 Nanofibers. Acta Phys. Chim. Sin. 2014, 30, 781–788. [Google Scholar] [CrossRef]
- Zhuang, H.J.; Wang, D.; Wang, X.Y.; Wang, P.P.; Zheng, X.J. Preparation and Gas Sensing Properties of Porous CuO/ZnO Composite Nanofibers. J. Synth. Cryst. 2015, 206, 161–166. [Google Scholar]
- Guo, L.; Kou, X.; Ding, M.; Wang, C.; Dong, L.; Zhang, H.; Feng, C.; Sun, Y.; Gao, Y.; Sun, P.; et al. Reduced Graphene Oxide/α-Fe2O3 Composite Nanofibers for Application in Gas Sensors. Sens. Actuators B Chem. 2017, 244, 233–242. [Google Scholar] [CrossRef]
- Li, T.; Zeng, W.; Wang, Z. Quasi-One-Dimensional Metal-Oxide-Based Heterostructural Gas-Sensing Materials: A Review. Sens. Actuators B Chem. 2015, 221, 1570–1585. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, S.; Mao, Z.; Hu, S.; Long, X. A Designed ZnO@ZIF-8 Core–Shell Nanorod Film as a Gas Sensor with Excellent Selectivity for H2 over CO. Chemistry 2017, 23, 7969–7975. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Bao, Z. Effect of Polyethylene Glycol on Microstructure and Photocatalysis of TiO2 Thin Films by Non-Hydrolytic Sol-Gel Processing. Chin. Ceram. Soc. 2008, 36, 187–191. [Google Scholar]
- Hernández, S.; Cauda, V.; Chiodoni, A.; Dallorto, S.; Sacco, A.; Hidalgo, D.; Celasco, E.; Pirri, C.F. Optimization of 1D ZnO@TiO2 Core–Shell Nanostructures for Enhanced Photoelectrochemical Water Splitting under Solar Light Illumination. ACS Appl. Mater. Interfaces 2014, 6, 12153–12167. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.-J.; Guo, X.-M. Tuning the Oxygen Defects and Fermi Levels via In3+ Doping in SnO2-In2O3 Nanocomposite for Efficient CO Detection. Sens. Actuators B Chem. 2022, 357, 131412. [Google Scholar] [CrossRef]
- Galstyan, V. Porous TiO2-Based Gas Sensors for Cyber Chemical Systems to Provide Security and Medical Diagnosis. Sensors 2017, 17, 2947. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Q.; Zhang, Q.; Hong, C.; Xu, L.; Jin, L.; Chen, W. Synthesis, Characterization and Enhanced Sensing Properties of a NiO/ZnO p–n Junctions Sensor for the SF6 Decomposition Byproducts SO2, SO2F2, and SOF2. Sensors 2017, 17, 913. [Google Scholar] [CrossRef]
- Chen, G.; Ji, S.; Li, H.; Kang, X.; Chang, S.; Wang, Y.; Yu, G.; Lu, J.; Claverie, J.; Sang, Y.; et al. High-Energy Faceted SnO2-Coated TiO2 Nanobelt Heterostructure for Near-Ambient Temperature-Responsive Ethanol Sensor. ACS Appl. Mater. Interfaces 2015, 7, 24950–24956. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.; Wang, B.; Sun, P.; Wang, Q.; Gao, Y.; Liang, X.; Zhang, T.; Lu, G. Acetone Gas Sensor Based on NiO/ZnO Hollow Spheres: Fast Response and Recovery, and Low (Ppb) Detection Limit. J. Colloid Interface Sci. 2017, 495, 207–215. [Google Scholar] [CrossRef]
- Gao, P.; Wang, Z.L. Self-Assembled Nanowire−Nanoribbon Junction Arrays of ZnO. J. Phys. Chem. B 2002, 106, 12653–12658. [Google Scholar] [CrossRef]
- Jiang, S.; Ge, B.; Xu, B.; Wang, Q.; Cao, B. In Situ Growth of ZnO/SnO2 (ZnO:Sn) m Binary/Superlattice Heterojunction Nanowire Arrays. CrystEngComm 2018, 20, 556–562. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Concina, I.; Faglia, G.; Falasconi, M.; Ferroni, M.; Galstyan, V.; Gobbi, E.; Ponzoni, A.; Vomiero, A.; et al. Metal Oxide Nanoscience and Nanotechnology for Chemical Sensors. Sens. Actuators B Chem. 2013, 179, 3–20. [Google Scholar] [CrossRef]
- Choi, S.-W.; Katoch, A.; Sun, G.-J.; Kim, S.S. Synthesis and Gas Sensing Performance of ZnO–SnO2 Nanofiber–Nanowire Stem-Branch Heterostructure. Sens. Actuators B Chem. 2013, 181, 787–794. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G. Study on TiO2-SnO2 Core-Shell Heterostructure Nanofibers with Different Work Function and Its Application in Gas Sensor. Sens. Actuators B Chem. 2017, 248, 812–819. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, Y.; Wang, W.; Zhao, J.; Wang, X. Au/CuO/Cu2O Heterostructures for Conductometric Triethylamine Gas Sensing. Sens. Actuators B Chem. 2022, 371, 132515. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Kaur, N.; Comini, E.; Poli, N.; Zappa, D.; Sberveglieri, G. NiO/ZnO Nanowire-Heterostructures by Vapor Phase Growth for Gas Sensing. Procedia Eng. 2016, 168, 1140–1143. [Google Scholar] [CrossRef]
- Kaur, N.; Comini, E.; Zappa, D.; Poli, N.; Sberveglieri, G. Nickel Oxide Nanowires: Vapor Liquid Solid Synthesis and Integration into a Gas Sensing Device. Nanotechnology 2016, 27, 205701. [Google Scholar] [CrossRef]
- Zappa, D.; Comini, E.; Sberveglieri, G. Thermally Oxidized Zinc Oxide Nanowires for Use as Chemical Sensors. Nanotechnology 2013, 24, 444008. [Google Scholar] [CrossRef]
- Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Ultra-Sensitive H2S Sensors Based on Hydrothermal/Impregnation-Made Ru-Functionalized WO3 Nanorods. Sens. Actuators B Chem. 2015, 215, 630–636. [Google Scholar] [CrossRef]
- Raksa, P.; Gardchareon, A.; Chairuangsri, T.; Mangkorntong, P.; Mangkorntong, N.; Choopun, S. Ethanol Sensing Properties of CuO Nanowires Prepared by an Oxidation Reaction. Ceram. Int. 2009, 35, 649–652. [Google Scholar] [CrossRef]
- Xu, Q.-H.; Xu, D.-M.; Guan, M.-Y.; Guo, Y.; Qi, Q.; Li, G.-D. ZnO/Al2O3/CeO2 Composite with Enhanced Gas Sensing Performance. Sens. Actuators B Chem. 2013, 177, 1134–1141. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C. Pt-Activated TiO2-MoS2 Nanocomposites for H2 Detection at Low Temperature. J. Alloys Compd. 2018, 747, 550–557. [Google Scholar] [CrossRef]
- Song, L.; Yang, L.; Wang, Z.; Liu, D.; Luo, L.; Zhu, X.; Xi, Y.; Yang, Z.; Han, N.; Wang, F.; et al. One-Step Electrospun SnO2/MOx Heterostructured Nanomaterials for Highly Selective Gas Sensor Array Integration. Sens. Actuators B Chem. 2019, 283, 793–801. [Google Scholar] [CrossRef]
- Liang, J.; Yang, R.; Zhu, K.; Hu, M. Room Temperature Acetone-Sensing Properties of Branch-like VO2 (B)@ZnO Hierarchical Hetero-Nanostructures. J. Mater. Sci. Mater. Electron. 2018, 29, 3780–3789. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal Oxides for Solid-State Gas Sensors: What Determines Our Choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Liu, C.; Wang, B.; Liu, T.; Sun, P.; Gao, Y.; Liu, F.; Lu, G. Facile Synthesis and Gas Sensing Properties of the Flower-like NiO-Decorated ZnO Microstructures. Sens. Actuators B Chem. 2016, 235, 294–301. [Google Scholar] [CrossRef]
- San, X.; Wang, G.; Liang, B.; Song, Y.; Gao, S.; Zhang, J.; Meng, F. Catalyst-Free Growth of One-Dimensional ZnO Nanostructures on SiO2 Substrate and in Situ Investigation of Their H2 Sensing Properties. J. Alloys Compd. 2015, 622, 73–78. [Google Scholar] [CrossRef]
- Giebelhaus, I.; Varechkina, E.; Fischer, T.; Rumyantseva, M.; Ivanov, V.; Gaskov, A.; Ramon Morante, J.; Arbiol, J.; Tyrra, W.; Mathur, S. One-Dimensional CuO–Sn2 p–n Heterojunctions for Enhanced Detection of H2S. J. Mater. Chem. A 2013, 1, 11261–11268. [Google Scholar] [CrossRef]
- Shao, F.; Hoffmann, M.W.G.; Prades, J.D.; Zamani, R.; Arbiol, J.; Morante, J.R.; Varechkina, E.; Rumyantseva, M.; Gaskov, A.; Giebelhaus, I.; et al. Heterostructured P-CuO (Nanoparticle)/n-SnO2 (Nanowire) Devices for Selective H2S Detection. Sens. Actuators B Chem. 2013, 181, 130–135. [Google Scholar] [CrossRef]
- Liu, X.; Du, B.; Sun, Y.; Yu, M.; Yin, Y.; Tang, W.; Chen, C.; Sun, L.; Yang, B.; Cao, W.; et al. Sensitive Room Temperature Photoluminescence-Based Sensing of H2S with Novel CuO–ZnO Nanorods. ACS Appl. Mater. Interfaces 2016, 8, 16379–16385. [Google Scholar] [CrossRef] [PubMed]
- Mashock, M.; Yu, K.; Cui, S.; Mao, S.; Lu, G.; Chen, J. Modulating Gas Sensing Properties of CuO Nanowires through Creation of Discrete Nanosized p–n Junctions on Their Surfaces. ACS Appl. Mater. Interfaces 2012, 4, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Kanitkar, P.; Sharma, A.; Muthe, K.P.; Rath, A.; Deshpande, S.K.; Kaur, M.; Aiyer, R.C.; Gupta, S.K.; Yakhmi, J.V. Growth of SnO2/W18O49 Nanowire Hierarchical Heterostructure and Their Application as Chemical Sensor. Sens. Actuators B Chem. 2010, 147, 453–460. [Google Scholar] [CrossRef]
- Sonker, R.K.; Sharma, A.; Tomar, M.; Gupta, V.; Yadav, B.C. Low Temperature Operated NO2 Gas Sensor Based on SnO2–ZnO Nanocomposite Thin Film. Adv. Sci. Lett. 2014, 20, 911–916. [Google Scholar] [CrossRef]
- Manjula, N.; Selvan, G.; Rajan, S.T.; Ayeshamariam, A.; Muthuraja, S.; Jayachandran, M. Properties of SnO2-TiO2 Composite Films Deposited Using Jet Nebulizer Spray Pyrolysis for Gas Sensors. Mater. Sci. Forum 2015, 832, 94–101. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Rehman, A.U.; Gong, L.; Kan, K.; Li, L.; Shi, K. Synthesis of NiO@CuO Nanocomposite as High-Performance Gas Sensing Material for NO2 at Room Temperature. Appl. Surf. Sci. 2017, 412, 230–237. [Google Scholar] [CrossRef]
- Lou, Z.; Li, F.; Deng, J.; Wang, L.; Zhang, T. Branch-like Hierarchical Heterostructure (α-Fe2O3/TiO2): A Novel Sensing Material for Trimethylamine Gas Sensor. ACS Appl. Mater. Interfaces 2013, 5, 12310–12316. [Google Scholar] [CrossRef]
- Deng, J.; Yu, B.; Lou, Z.; Wang, L.; Wang, R.; Zhang, T. Facile Synthesis and Enhanced Ethanol Sensing Properties of the Brush-like ZnO–TiO2 Heterojunctions Nanofibers. Sens. Actuators B Chem. 2013, 184, 21–26. [Google Scholar] [CrossRef]
- Ivanovskaya, M.; Kotsikau, D.; Faglia, G.; Nelli, P.; Irkaev, S. Gas-Sensitive Properties of Thin Film Heterojunction Structures Based on Fe2O3–In2O3 Nanocomposites. Sens. Actuators B Chem. 2003, 93, 422–430. [Google Scholar] [CrossRef]
- de Lacy Costello, B.P.J.; Ewen, R.J.; Jones, P.R.H.; Ratcliffe, N.M.; Wat, R.K.M. A Study of the Catalytic and Vapour-Sensing Properties of Zinc Oxide and Tin Dioxide in Relation to 1-Butanol and Dimethyldisulphide. Sens. Actuators B Chem. 1999, 61, 199–207. [Google Scholar] [CrossRef]
- Kamble, V.B.; Umarji, A.M. Achieving Selectivity from the Synergistic Effect of Cr and Pt Activated SnO2 Thin Film Gas Sensors. Sens. Actuators B Chem. 2016, 236, 208–217. [Google Scholar] [CrossRef]
- Van Tong, P.; Hoa, N.D.; Van Duy, N.; Le, D.T.T.; Van Hieu, N. Enhancement of Gas-Sensing Characteristics of Hydrothermally Synthesized WO3 Nanorods by Surface Decoration with Pd Nanoparticles. Sens. Actuators B Chem. 2016, 223, 453–460. [Google Scholar] [CrossRef]
- Yin, X.-T.; Guo, X.-M. Sensitivity and Selectivity of (Au, Pt, Pd)-Loaded and (In, Fe)-Doped SnO2 Sensors for H2 and CO Detection. J. Mater. Sci. Mater. Electron. 2014, 25, 4960–4966. [Google Scholar] [CrossRef]
- Yin, X.-T.; Guo, X.-M. Selectivity and Sensitivity of Pd-Loaded and Fe-Doped SnO2 Sensor for CO Detection. Sens. Actuators B Chem. 2014, 200, 213–218. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, Q.; Xu, L.; Gui, Y.; Zhao, Z.; Tang, C.; Chen, W. Synthesis and Characterization of Highly Sensitive Hydrogen (H2) Sensing Device Based on Ag Doped SnO2 Nanospheres. Materials 2018, 11, 492. [Google Scholar] [CrossRef]
- Kim, S.-J.; Na, C.W.; Hwang, I.-S.; Lee, J.-H. One-Pot Hydrothermal Synthesis of CuO–ZnO Composite Hollow Spheres for Selective H2S Detection. Sens. Actuators B Chem. 2012, 168, 83–89. [Google Scholar] [CrossRef]
- Kim, S.S.; Na, H.G.; Choi, S.-W.; Kwak, D.S.; Kim, H.W. Novel Growth of CuO-Functionalized, Branched SnO2 Nanowires and Their Application to H2S Sensors. J. Phys. D Appl. Phys. 2012, 45, 205301. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L.; Umar, A.; Chen, W.; Kumar, R. Pt Nanoparticles Decorated SnO2 Nanoneedles for Efficient CO Gas Sensing Applications. Sens. Actuators B Chem. 2018, 256, 656–664. [Google Scholar] [CrossRef]
- Kosc, I.; Hotovy, I.; Rehacek, V.; Griesseler, R.; Predanocy, M.; Wilke, M.; Spiess, L. Sputtered TiO2 Thin Films with NiO Additives for Hydrogen Detection. Appl. Surf. Sci. 2013, 269, 110–115. [Google Scholar] [CrossRef]
- Huang, H.; Gong, H.; Chow, C.L.; Guo, J.; White, T.J.; Tse, M.S.; Tan, O.K. Low-Temperature Growth of SnO2 Nanorod Arrays and Tunable n–p–n Sensing Response of a ZnO/SnO2 Heterojunction for Exclusive Hydrogen Sensors. Adv. Funct. Mater. 2011, 21, 2680–2686. [Google Scholar] [CrossRef]
- Yin, X.-T.; Wu, S.-S.; Dastan, D.; Nie, S.; Liu, Y.; Li, Z.-G.; Zhou, Y.-W.; Li, J.; Faik, A.; Shan, K.; et al. Sensing Selectivity of SnO2-Mn3O4 Nanocomposite Sensors for the Detection of H2 and CO Gases. Surf. Interfaces 2021, 25, 101190. [Google Scholar] [CrossRef]
- Yin, X.-T.; Li, J.; Wang, Q.; Dastan, D.; Shi, Z.-C.; Alharbi, N.; Garmestani, H.; Tan, X.-M.; Liu, Y.; Ma, X.-G. Opposite Sensing Response of Heterojunction Gas Sensors Based on SnO2–Cr2O3 Nanocomposites to H2 against CO and Its Selectivity Mechanism. Langmuir 2021, 37, 13548–13558. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).