Plasma Nitriding of Inner Surface of Slender Tubes using Small Diameter Helicon Plasma
Abstract
1. Introduction
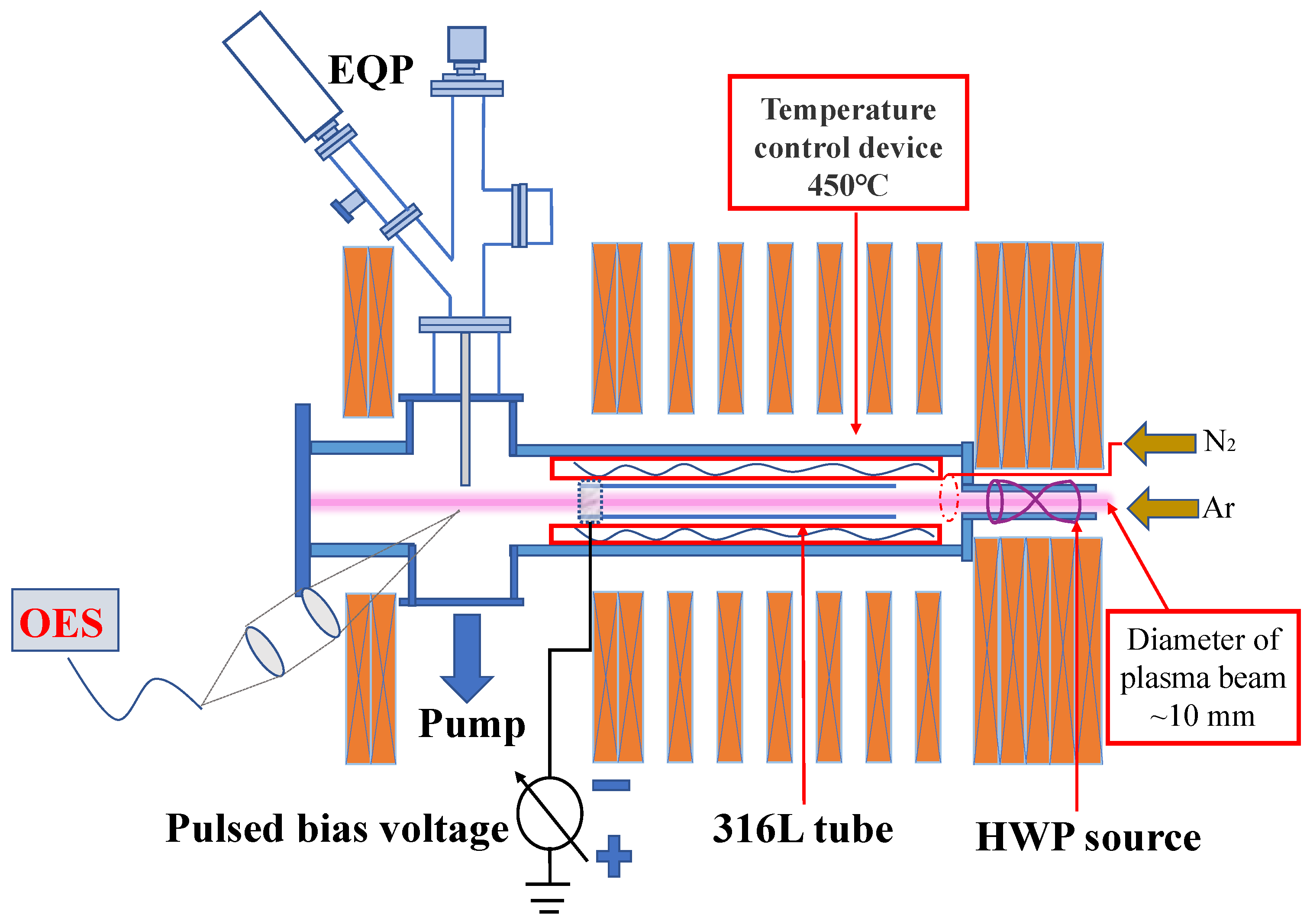
2. Experimental Methods
3. Results and Discussion
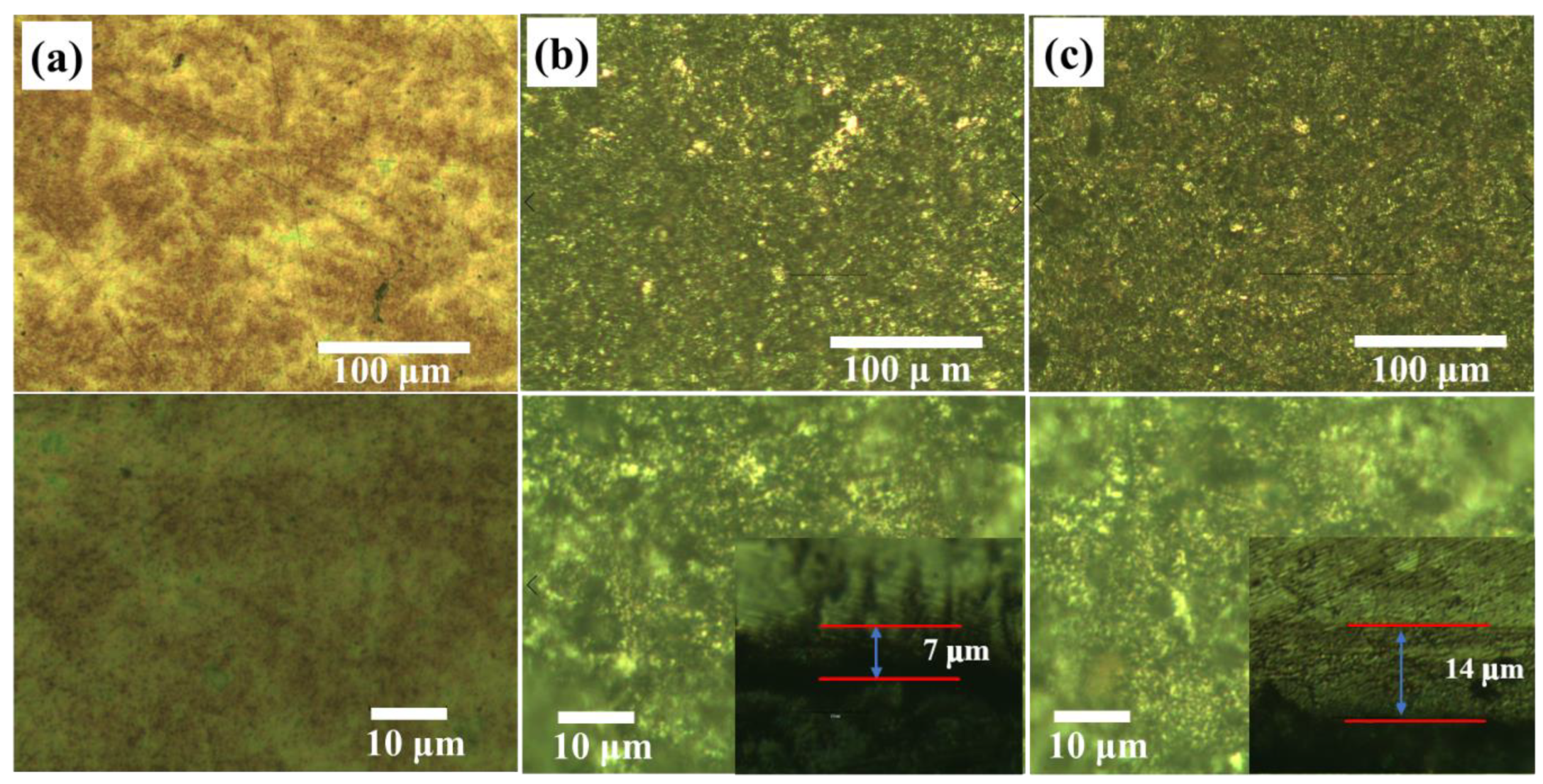
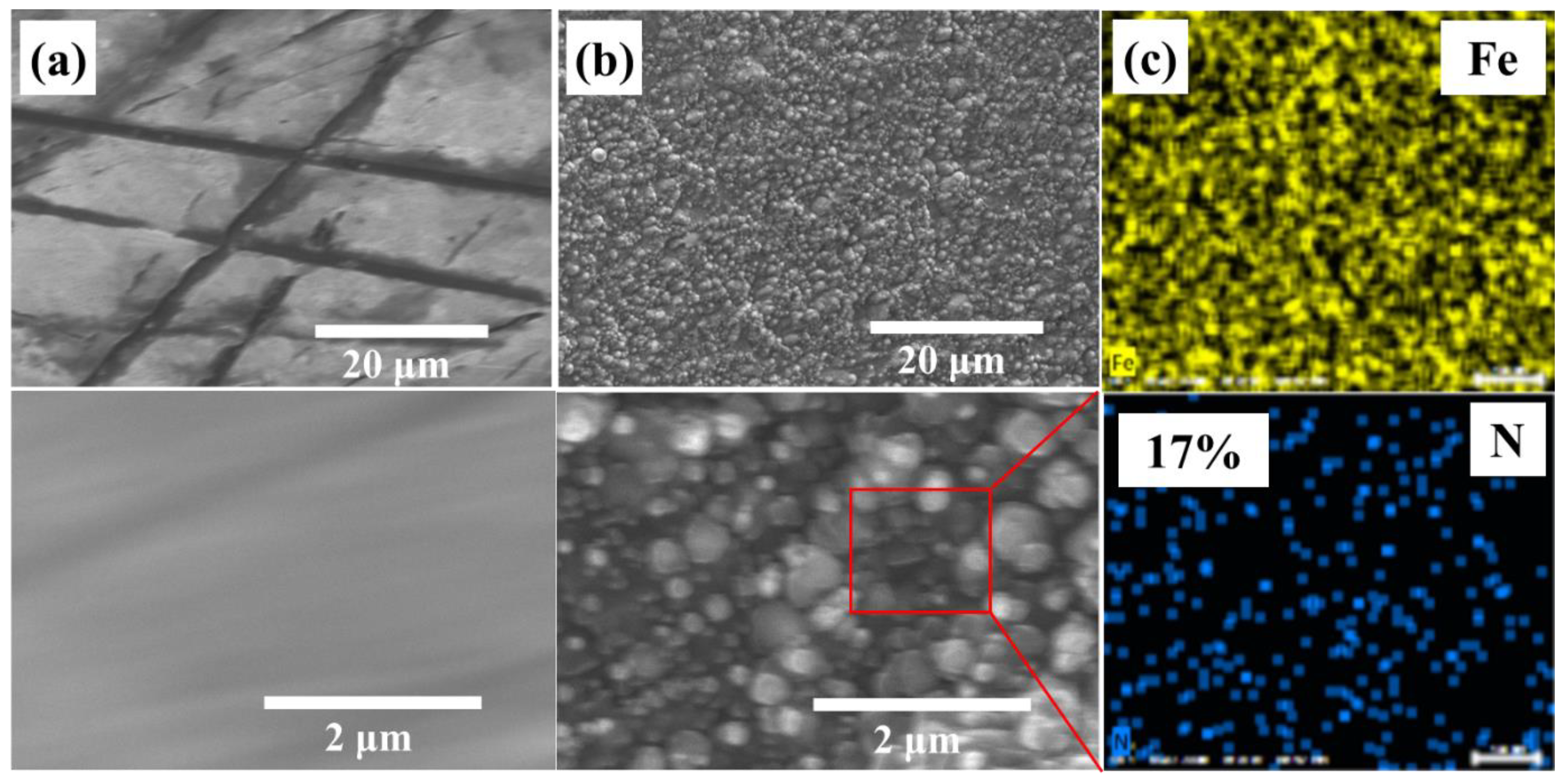
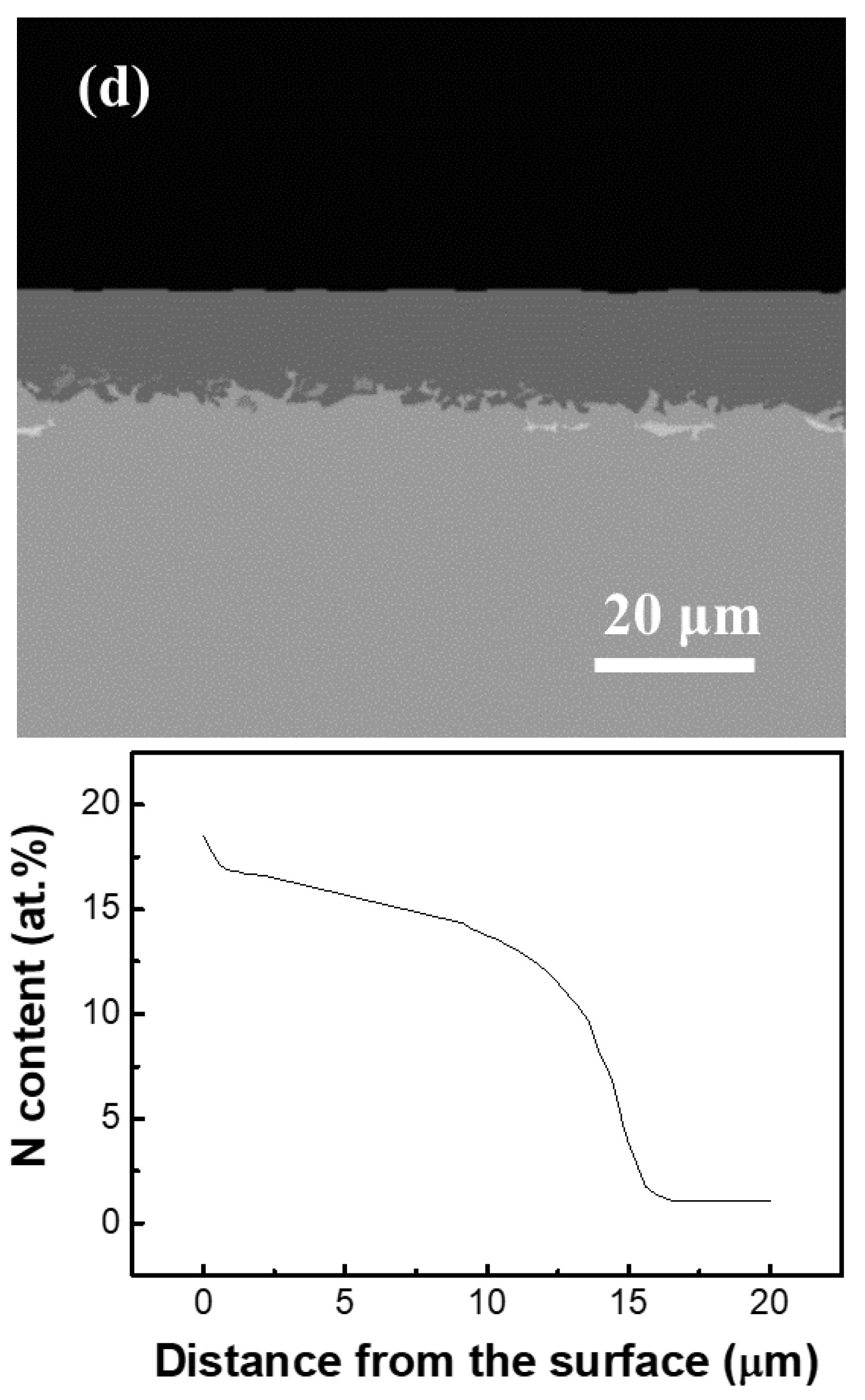
3.1. Phase and Microstructure Analysis
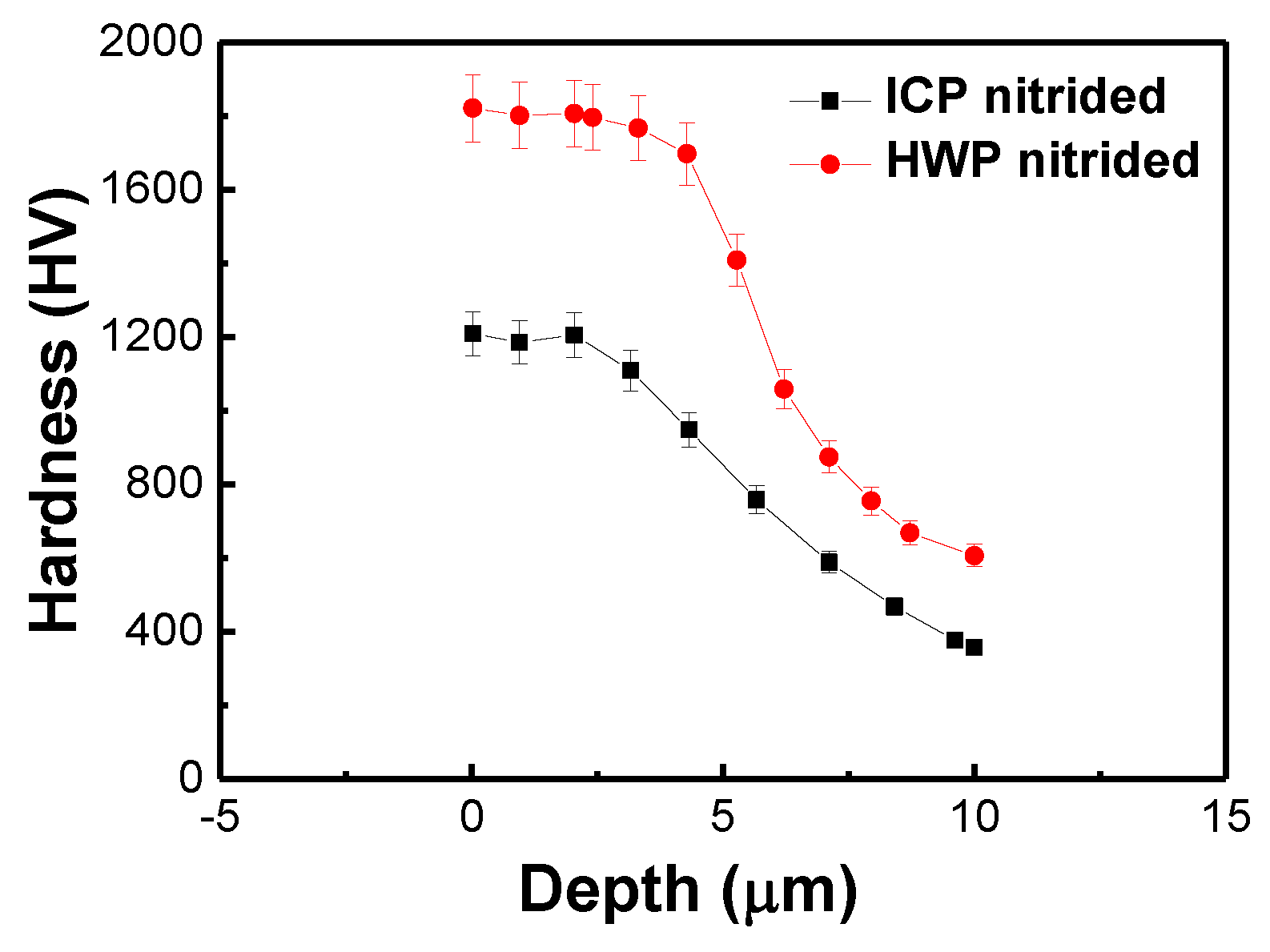
3.2. Hardness Measurements
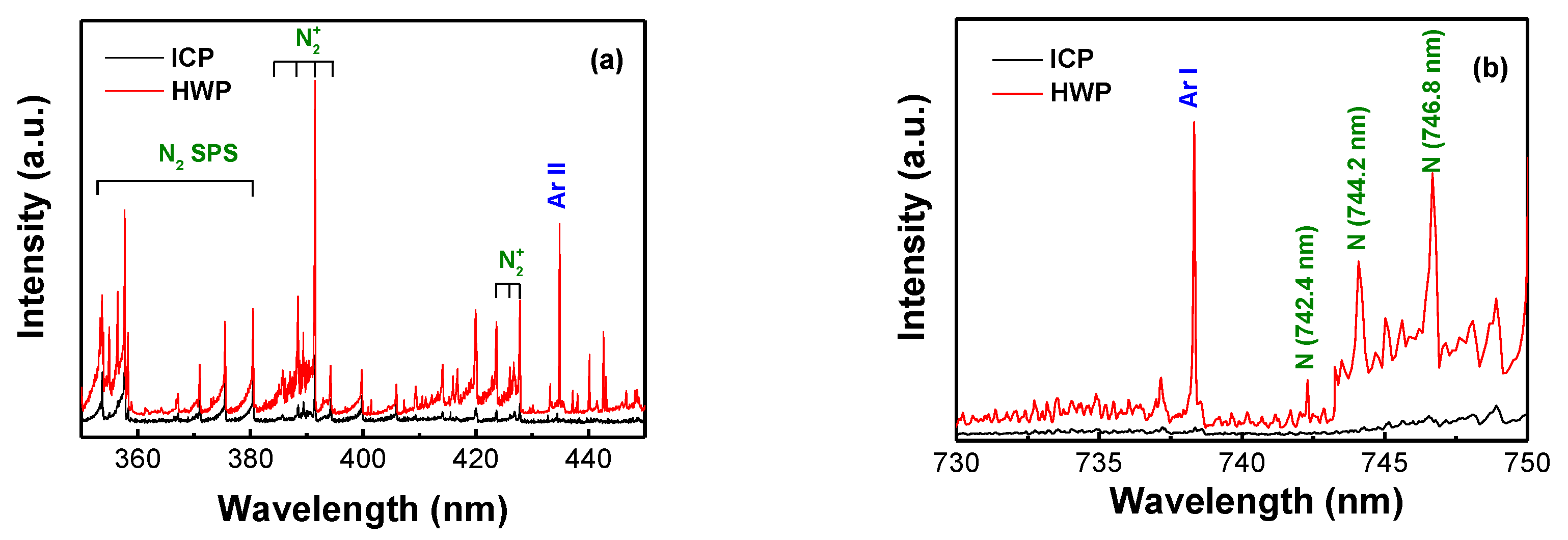
3.3. Plasma Diagnostics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, L.H.; Chen, S.C.; Wu, C.Z.; Hung, J.M.; Ou, K.L. Microstructure and antibacterial properties of microwave plasma nitrided layers on biomedical stainless steels. Appl. Surf. Sci. 2011, 257, 7375–7380. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Peng, J.; Li, K.; Dai, Q.; Gao, G.; Zhang, Y.; Cao, W. Estimation of mechanical strength for pre-strained 316L austenitic stainless steel by small punch test. Vacuum 2019, 160, 37–53. [Google Scholar] [CrossRef]
- Gallo, S.C.; Dong, H. EBSD and AFM observations of the microstructural changes induced by low temperature plasma carburising on AISI 316. Appl. Surf. Sci. 2011, 258, 608–613. [Google Scholar] [CrossRef]
- Zeng, D.; Yang, S.; Xiang, Z.D. Formation of hard surface layer on austenitic stainless steels via simultaneous chromising and nitriding by pack cementation process. Appl. Surf. Sci. 2012, 258, 5175–5178. [Google Scholar] [CrossRef]
- Shih, T.S.; Huang, Y.S.; Chen, C.F. Constituted oxides/nitrides on nitriding 304, 430 and 17-4 PH stainless steel in salt baths over the temperature range 723 to 923 K. Appl. Surf. Sci. 2011, 258, 81–88. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Wang, L. Surface properties of nitrided layer on AISI 316L austenitic stainless steel produced by high temperature plasma nitriding in short time. Appl. Surf. Sci. 2014, 298, 243–250. [Google Scholar] [CrossRef]
- Ohtsu, N.; Miura, K.; Hirano, M.; Kodama, K. Investigation of admixed gas effect on plasma nitriding of AISI316L austenitic stainless steel. Vacuum 2021, 193, 110545. [Google Scholar] [CrossRef]
- Galeano-Osorio, D.S.; Vargas, S.; Vélez, J.M.; Mello, A.; Tanaka, M.N.; Castano, C.E. Hemocompatibility of plasma nitrided 316L stainless steel: Effect of processing temperature. Appl. Surf. Sci. 2020, 509, 144704. [Google Scholar] [CrossRef]
- Tang, L.; Sun, F.; Miao, X.; Li, J.; Hu, J. Evolution of pre-oxide layer during subsequent plasma nitriding. Vacuum 2018, 152, 337–339. [Google Scholar] [CrossRef]
- Van Doan, T.T.; Kusmič, D.; Pospíchal, M.; Tran, Q.D.; Nguyen, V.T. Friction and wear behaviour of 42CrMo4 steel treated by tenifer hard chorme and plasma nitriding Technologies. Manuf. Technol. 2017, 17, 168–174. [Google Scholar]
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, C.; Yang, W.; Chen, Y.; Yu, B. TiN films deposition inside stainless-steel tubes using magnetic field-enhanced arc ion plating. Vacuum 2015, 112, 46–54. [Google Scholar] [CrossRef]
- Ueda, M.; Silva, A.R.; Pillaca, E.J.D.M.; Mariano, S.F.M.; Rossi, J.O.; Oliveira, R.M.; Pichon, L.; Reuther, H. New possibilities of plasma immersion ion implantation (PIII) and deposition (PIII&D) in industrial components using metal tube fixtures. Surf. Coat. Technol. 2017, 312, 37–46. [Google Scholar]
- Sousa, F.A.; da Costa, J.A.P.; de Sousa, R.R.M.; Barbosa, J.C.P.; de Araújo, F.O. Internal coating of pipes using the cathodic cage plasma nitriding technique. Surf. Interfaces 2020, 21, 100691. [Google Scholar] [CrossRef]
- Silva, C.; Ueda, M.; Mello, C.B. Metal Cylindrical Sieve (MCS) for plasma confinement and low sputtering nitrogen plasma immersion ion implantation. Appl. Surf. Sci. 2020, 509, 145232. [Google Scholar] [CrossRef]
- Kwok, D.K.; Zeng, X.; Chen, Q.; Chu, P.K.; Sheridan, T.E. Effects of tube length and radius for inner surface plasma immersion ion implantation using an auxiliary electrode. IEEE Trans. Plasma Sci. 1999, 27, 225–238. [Google Scholar] [CrossRef]
- Shinohara, S. Helicon high-density plasma sources: Physics and applications. Adv. Phys. X 2018, 3, 1420424. [Google Scholar] [CrossRef]
- Caneses, J.F.; Blackwell, B.D.; Piotrowicz, P. Helicon antenna radiation patterns in a high-density hydrogen linear plasma device. Phys. Plasmas 2017, 24, 113513. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Reliable determination of chemical state in x-ray photoelectron spectroscopy based on sample-work-function referencing to adventitious carbon: Resolving the myth of apparent constant binding energy of the C 1s peak. Appl. Surf. Sci. 2018, 451, 99–103. [Google Scholar] [CrossRef]
- Mändl, S.; Rauschenbach, B. Concentration dependent nitrogen diffusion coefficient in expanded austenite formed by ion implantation. J. Appl. Phys. 2002, 91, 9737–9742. [Google Scholar] [CrossRef]
- Blawert, C.; Weisheit, A.; Mordike, B.L.; Knoop, R.M. Plasma immersion ion implantation of stainless steel: Austenitic stainless steel in comparison to austenitic-ferritic stainless steel. Surf. Coat. Technol. 1996, 85, 15–27. [Google Scholar] [CrossRef]
- Feugeas, J.; Gomez, B.; Craievich, A. Ion nitriding of stainless steels. Real time surface characterization by synchrotron X-ray diffraction. Surf. Coat. Technol. 2002, 154, 167–175. [Google Scholar] [CrossRef]
- Moskalioviene, T.; Galdikas, A.; Riviere, J.P.; Pichon, L. Modeling of nitrogen penetration in polycrystalline AISI 316L austenitic stainless steel during plasma nitriding. Surf. Coat. Technol. 2011, 205, 3301–3306. [Google Scholar] [CrossRef]
- Mani, S.P.; Rajendran, N. Corrosion and interfacial contact resistance behavior of electrochemically nitrided 316L SS bipolar plates for proton exchange membrane fuel cells. Energy 2017, 133, 1050–1062. [Google Scholar] [CrossRef]
- Zhou, Z.; Xia, L.; Sun, M. The carbon nitride films prepared at various substrate temperatures by vacuum cathodic arc method. Diam. Relat. Mater. 2004, 13, 14–21. [Google Scholar] [CrossRef]
- Marcus, P.; Bussell, M.E. XPS study of the passive films formed on nitrogen-implanted austenitic stainless steels. Appl. Surf. Sci. 1992, 59, 7–21. [Google Scholar] [CrossRef]


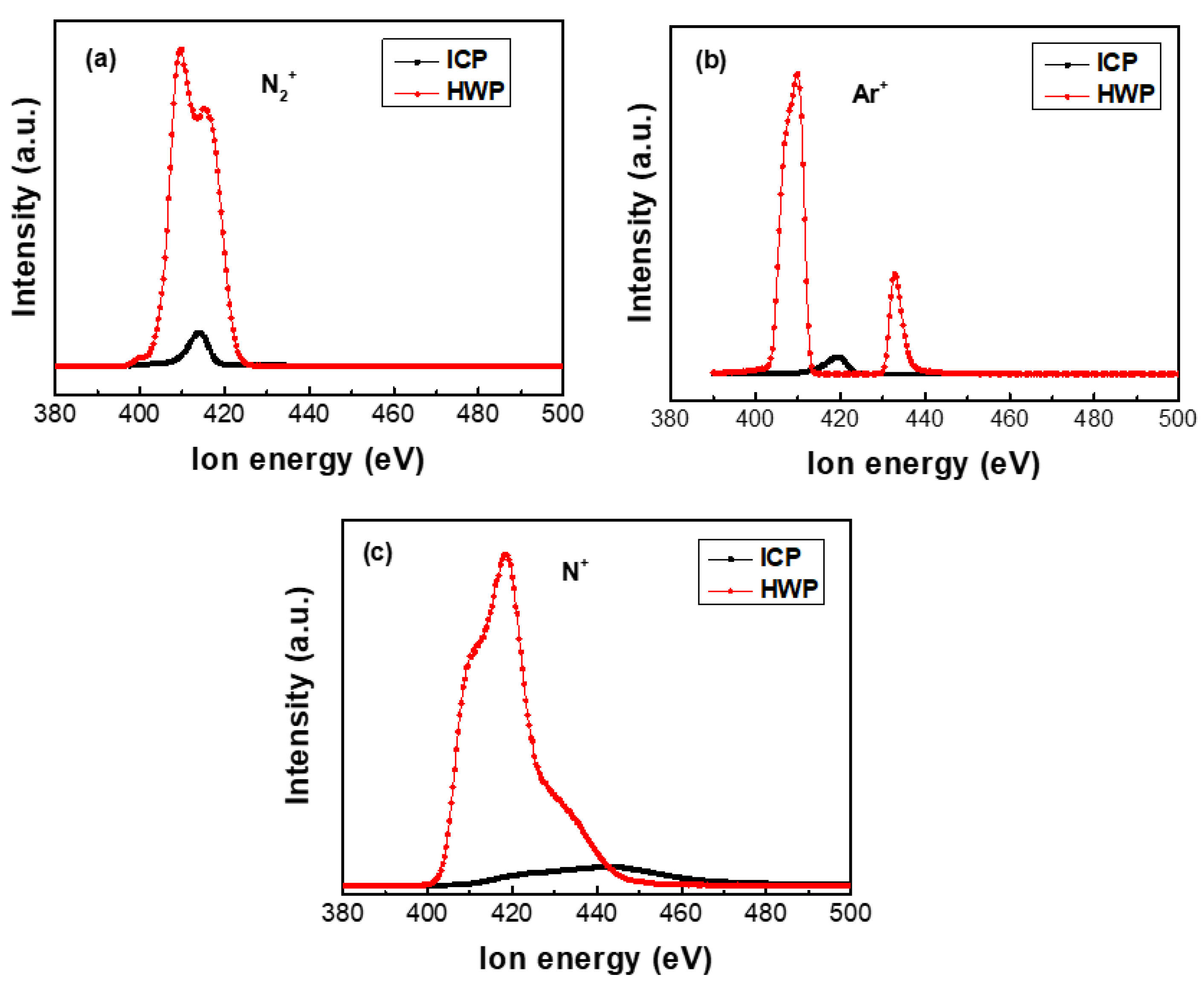
| Sample | C | O | N | Fe | Cr | Ni |
|---|---|---|---|---|---|---|
| ICP nitride | 2.7 | 2.9 | 16.5 | 52.9 | 15.2 | 9.8 |
| HWP nitride | 2.2 | 2.1 | 20.6 | 50.8 | 14.6 | 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Zhang, Y.; Wang, C.; Liu, M.; Ling, W.; He, L.; Yang, Y.; E, P. Plasma Nitriding of Inner Surface of Slender Tubes using Small Diameter Helicon Plasma. Materials 2023, 16, 311. https://doi.org/10.3390/ma16010311
Jin C, Zhang Y, Wang C, Liu M, Ling W, He L, Yang Y, E P. Plasma Nitriding of Inner Surface of Slender Tubes using Small Diameter Helicon Plasma. Materials. 2023; 16(1):311. https://doi.org/10.3390/ma16010311
Chicago/Turabian StyleJin, Chenggang, Yongqi Zhang, Chen Wang, Manxing Liu, Wenbin Ling, Liang He, Yan Yang, and Peng E. 2023. "Plasma Nitriding of Inner Surface of Slender Tubes using Small Diameter Helicon Plasma" Materials 16, no. 1: 311. https://doi.org/10.3390/ma16010311
APA StyleJin, C., Zhang, Y., Wang, C., Liu, M., Ling, W., He, L., Yang, Y., & E, P. (2023). Plasma Nitriding of Inner Surface of Slender Tubes using Small Diameter Helicon Plasma. Materials, 16(1), 311. https://doi.org/10.3390/ma16010311






