Establish TiNb2O7@C as Fast-Charging Anode for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of TNO
2.2. Synthesis of TNO@C
2.3. Material Characterization
2.4. Electrochemical Measurements
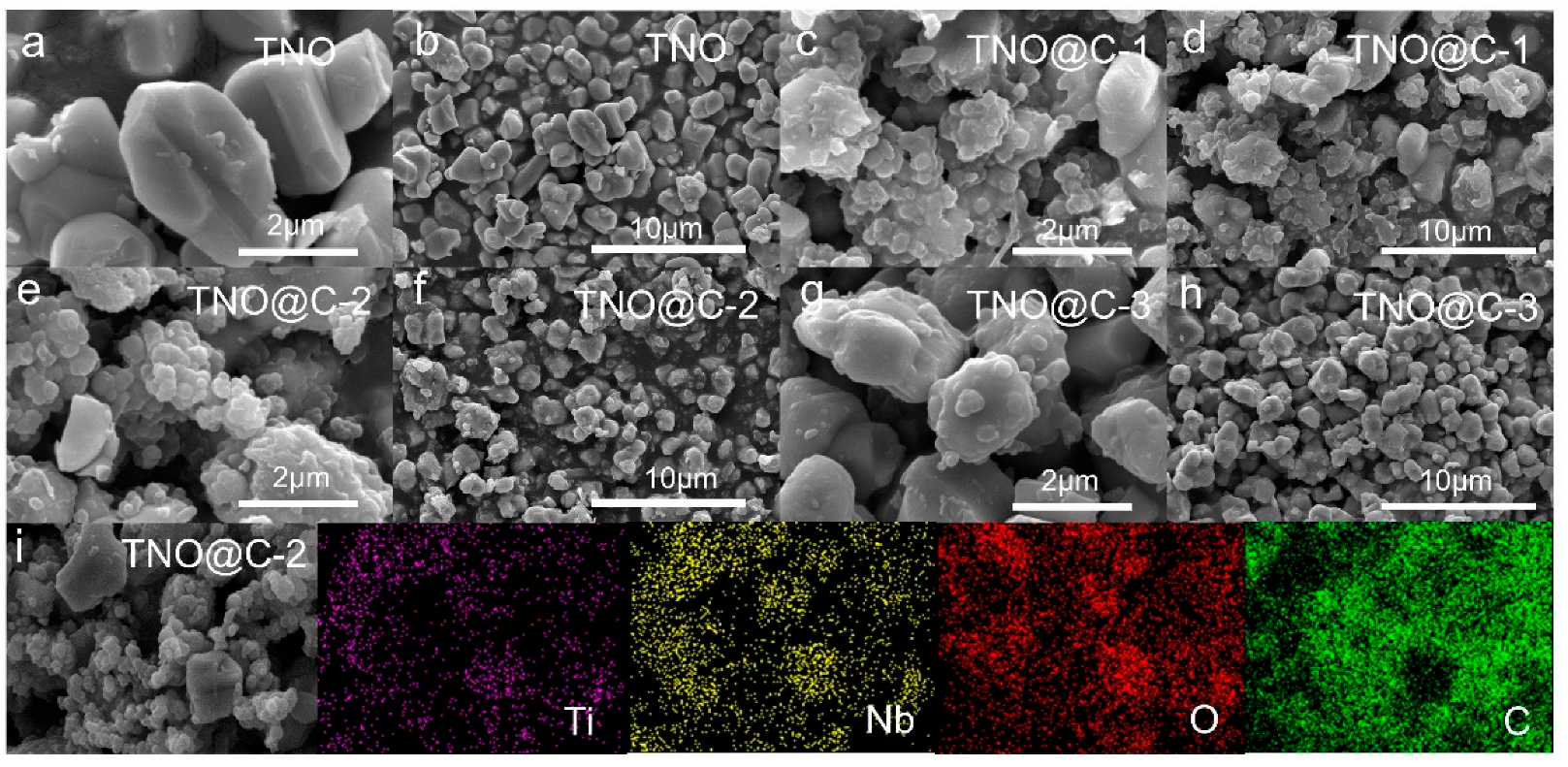
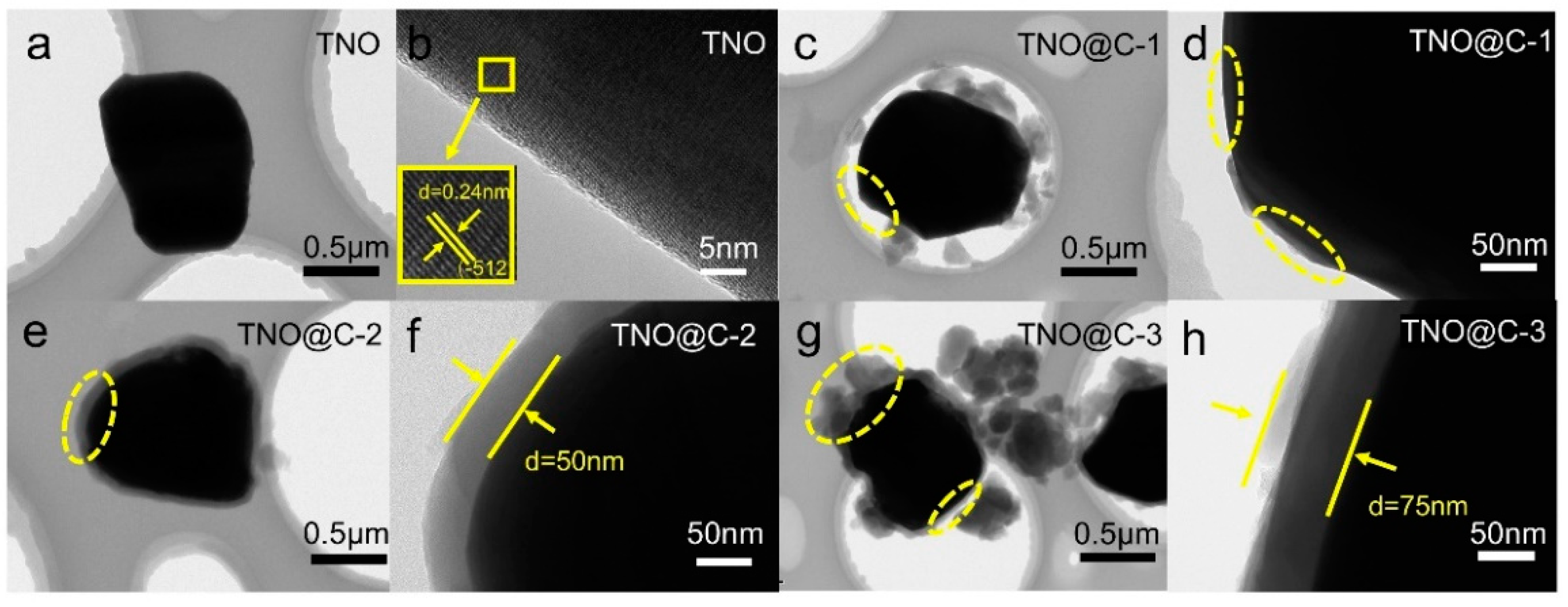
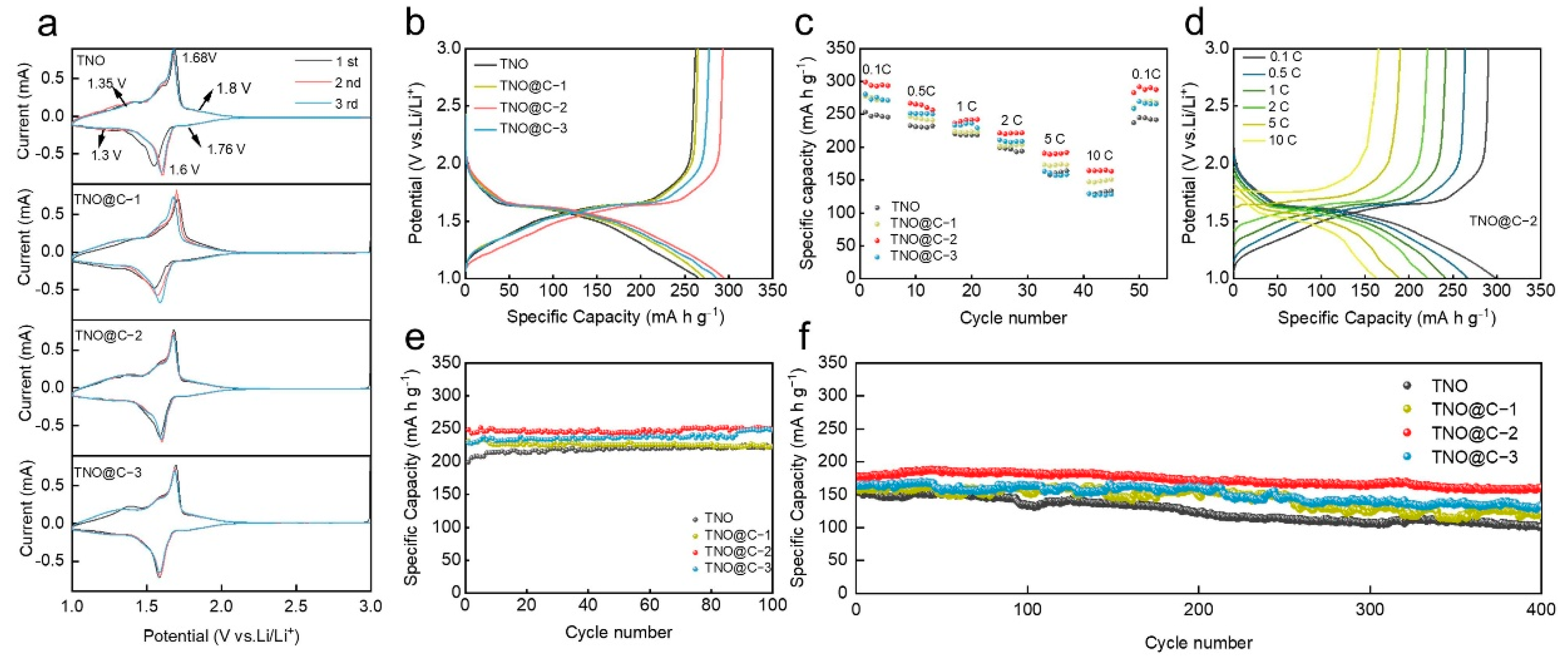
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Q.; Cao, H.; Liang, G.; Luo, L.; Chen, Y.; Murugadoss, V.; Wu, S.; Ding, T.; Lin, C.; Guo, Z. A highly Li+-conductive HfNb24O62 anode material for superior Li+ storage. Chem. Commun. 2020, 56, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Xin, Y.; Zhou, Y.; Ma, Y.; Zhou, H.; Qi, L. Self-supported Li4Ti5O12 nanosheet arrays for lithium ion batteries with excellent rate capability and ultralong cycle life. Energy Environ. Sci. 2014, 7, 1924–1930. [Google Scholar] [CrossRef]
- Liu, W.; Song, M.-S.; Kong, B.; Cui, Y. Flexible and Stretchable Energy Storage: Recent Advances and Future Perspectives. Adv. Mater. 2017, 29, 1603436. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Liang, G.; Wu, Z.; Didier, C.; Zhang, W.; Cuan, J.; Li, B.; Ko, K.; Hung, P.; Lu, C.; Chen, Y.; et al. A Long Cycle-Life High-Voltage Spinel Lithium-Ion Battery Electrode Achieved by Site-Selective Doping. Angew. Chem. Int. Ed. 2020, 59, 10594–10602. [Google Scholar] [CrossRef]
- Lu, X.; Jian, Z.; Fang, Z.; Gu, L.; Hu, Y.-S.; Chen, W.; Wang, Z.; Chen, L. Atomic-scale investigation on lithium storage mechanism in TiNb2O7. Energy Environ. Sci. 2011, 4, 2638–2644. [Google Scholar] [CrossRef]
- Lv, Z.; Zhu, H.; Meng, W.; Wei, L.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Cation mixing in Wadsley-Roth phase anode of lithium-ion battery improves cycling stability and fast Li+ storage. Appl. Phys. Rev. 2021, 8, 031404. [Google Scholar] [CrossRef]
- Voskanyan, A.A.; Abramchuk, M.; Navrotsky, A. Entropy Stabilization of TiO2–Nb2O5 Wadsley–Roth Shear Phases and Their Prospects for Lithium-Ion Battery Anode Materials. Chem. Mater. 2020, 32, 5301–5308. [Google Scholar] [CrossRef]
- Lou, S.; Ma, Y.; Cheng, X.; Gao, J.; Gao, Y.; Zuo, P.; Du, C.; Yin, G. Facile synthesis of nanostructured TiNb2O7 anode materials with superior performance for high-rate lithium ion batteries. Chem. Commun. 2015, 51, 17293–17296. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198 Pt 2, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Yu, S.; Zhao, H.; Wu, S.; Wang, G.; Yu, L.; Li, Y.; Zhu, Z.Z.; Li, J.; Lin, S. Defective Ti2Nb10O27.1: An advanced anode material for lithium-ion batteries. Sci. Rep. 2015, 5, 17836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Cheng, X.; Zhu, H.; Zheng, R.; Liu, T.; Zhang, J.; Shui, M.; Xie, Y.; Shu, J. Deep insights into kinetics and structural evolution of nitrogen-doped carbon coated TiNb24O62 nanowires as high-performance lithium container. Nano Energy 2018, 54, 227–237. [Google Scholar] [CrossRef]
- Lyu, H.; Li, J.; Wang, T.; Thapaliya, B.P.; Men, S.; Jafta, C.J.; Tao, R.; Sun, X.-G.; Dai, S. Carbon Coated Porous Titanium Niobium Oxides as Anode Materials of Lithium-Ion Batteries for Extreme Fast Charge Applications. ACS Appl. Energy Mater. 2020, 3, 5657–5665. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Zhang, S.; Liu, G.; Xie, H.; Ma, J. Design of well-defined porous Ti2Nb10O29/C microspheres assembled from nanoparticles as anode materials for high-rate lithium ion batteries. Electrochim. Acta 2018, 292, 759–768. [Google Scholar] [CrossRef]
- Wang, X.; Shen, G. Intercalation pseudo-capacitive TiNb2O7@carbon electrode for high-performance lithium ion hybrid electrochemical supercapacitors with ultrahigh energy density. Nano Energy 2015, 15, 104–115. [Google Scholar] [CrossRef]
- Park, H.; Bin Wu, H.; Song, T.; Lou, X.W.D.; Paik, U. Porosity-Controlled TiNb2O7 Microspheres with Partial Nitridation as A Practical Negative Electrode for High-Power Lithium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1401945. [Google Scholar] [CrossRef]
- Liu, M.; Dong, H.; Zhang, S.; Chen, X.; Sun, Y.; Gao, S.; Xu, J.; Wu, X.; Yuan, A.; Lu, W. Three-Dimensional Porous TiNb2O7/CNT-KB Composite Microspheres as Lithium-Ion Battery Anode Material. ChemElectroChem 2019, 6, 3959–3965. [Google Scholar] [CrossRef]
- Hsiao, Y.-S.; Chang-Jian, C.-W.; Weng, H.C.; Chiang, H.-H.; Lu, C.-Z.; Pang, W.K.; Peterson, V.K.; Jiang, X.-C.; Wu, P.-I.; Chen, C.-P.; et al. Doping with W6+ ions enhances the performance of TiNb2O7 as an anode material for lithium-ion batteries. Appl. Surf. Sci. 2022, 573, 151517. [Google Scholar] [CrossRef]
- Shang, B.; Peng, Q.; Jiao, X.; Xi, G.; Hu, X. TiNb2O7/carbon nanotube composites as long cycle life anode for sodium-ion batteries. Ionics 2018, 25, 1679–1688. [Google Scholar] [CrossRef]
- Lin, C.; Hu, L.; Cheng, C.; Sun, K.; Guo, X.; Shao, Q.; Li, J.; Wang, N.; Guo, Z. Nano-TiNb2O7/carbon nanotubes composite anode for enhanced lithium-ion storage. Electrochim. Acta 2018, 260, 65–72. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Q.; Che, R. Hollow TiNb2O7@C Spheres with Superior Rate Capability and Excellent Cycle Performance as Anode Material for Lithium-Ion Batteries. Chem.–A Eur. J. 2018, 24, 12932–12937. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yang, L.; Wei, X.; Ding, J.; Zhong, J.; Chu, P. Highly-crystalline ultrathin gadolinium doped and carbon-coated Li4Ti5O12 nanosheets for enhanced lithium storage. J. Power Sources 2015, 295, 305–313. [Google Scholar] [CrossRef]
- Aravindan, V.; Sundaramurthy, J.; Jain, A.; Kumar, P.S.; Ling, W.C.; Ramakrishna, S.; Srinivasan, M.P.; Madhavi, S. Unveiling TiNb2O7 as an Insertion Anode for Lithium Ion Capacitors with High Energy and Power Density. ChemSusChem 2014, 7, 1858–1863. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Wang, J.; Fang, S.; Zhang, Y.; Dou, H.; Zhang, X. Three-dimensionally ordered porous TiNb2O7 nanotubes: A superior anode material for next generation hybrid supercapacitors. J. Mater. Chem. A 2015, 3, 16785–16790. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, C.; Zhao, W.; Sun, B.; Xiao, X.; Huo, H.; Ma, Y.; Zuo, P.; Lou, S.; Yin, G. Crystallographic engineering to reduce diffusion barrier for enhanced intercalation pseudocapacitance of TiNb2O7 in fast-charging batteries. Energy Storage Mater. 2022, 47, 178–186. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Q.; Zhao, Y.; Che, R. Nanoporous TiNb2O7/C Composite Microspheres with Three-Dimensional Conductive Network for Long-Cycle-Life and High-Rate-Capability Anode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 41258–41264. [Google Scholar] [CrossRef] [PubMed]
- Inada, R.; Kumasaka, R.; Inabe, S.; Tojo, T.; Sakurai, Y. Li+Insertion/Extraction Properties for TiNb2O7 Single Particle Characterized by a Particle-Current Collector Integrated Microelectrode. J. Electrochem. Soc. 2018, 166, A5157–A5162. [Google Scholar] [CrossRef]
- Ise, K.; Morimoto, S.; Harada, Y.; Takami, N. Large lithium storage in highly crystalline TiNb2O7 nanoparticles synthesized by a hydrothermal method as anodes for lithium-ion batteries. Solid State Ionics 2018, 320, 7–15. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, H.; Liang, Q.; Yuan, D.; Xi, S.; Wu, C.; Manalastas, W.; Ma, J.; Fang, W.; Zheng, Y.; et al. High-performance flexible quasi-solid-state zinc-ion batteries with layer-expanded vanadium oxide cathode and zinc/stainless steel mesh composite anode. Nano Energy 2019, 62, 94–102. [Google Scholar] [CrossRef]
- Lou, S.; Cheng, X.; Gao, J.; Li, Q.; Wang, L.; Cao, Y.; Ma, Y.; Zuo, P.; Gao, Y.; Du, C.; et al. Pseudocapacitive Li+ intercalation in porous Ti2Nb10O29 nanospheres enables ultra-fast lithium storage. Energy Storage Mater. 2018, 11, 57–66. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, H.; Xing, C.; Wang, Y.; Zhang, J.; Zhang, X.; Zhang, S. Intensified Energy Storage in High-Voltage Nanohybrid Supercapacitors via the Efficient Coupling between TiNb2O7/Holey-rGO Nanoarchitectures and Ionic Liquid-Based Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 21349–21361. [Google Scholar] [CrossRef] [PubMed]



| Material | Reversible Specific Capacity (mA h g−1) | High-Rate Capability (mA h g−1) | Mass Loading (mg.cm−2) |
|---|---|---|---|
| Porous TNO@C [14] | ~280 at 0.1 C | 211 at 10 C | 2.3 |
| Ti2Nb10O29/C microspheres [15] | 275 at 1 C | 214 at 30 C | 1.5 |
| TNO@C [16] | 265 at 0.5 A g−1 | 75 at 6 A g−1 | 1.5–2.0 |
| NPTNO MS-3 [17] | 265 at 0.1 C | ~120 at 30 C | --- |
| TiNb2O7/CNT-KB [18] | 327 at 0.1 C | 151 at 20 C | 1.0 |
| TNO/CNT [21] | 346 at 0.1 C | 163 at 30 C | 1.4 |
| Hollow TNO@C spheres [22] | 283 at 0.25 C | 157 at 10 C | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any in-jury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, S.; Wang, Y.; Li, M.; Wen, Y.; Xu, B.; Wang, H.; Qiu, J.; Li, B. Establish TiNb2O7@C as Fast-Charging Anode for Lithium-Ion Batteries. Materials 2023, 16, 333. https://doi.org/10.3390/ma16010333
Gong S, Wang Y, Li M, Wen Y, Xu B, Wang H, Qiu J, Li B. Establish TiNb2O7@C as Fast-Charging Anode for Lithium-Ion Batteries. Materials. 2023; 16(1):333. https://doi.org/10.3390/ma16010333
Chicago/Turabian StyleGong, Shuya, Yue Wang, Meng Li, Yuehua Wen, Bin Xu, Hong Wang, Jingyi Qiu, and Bin Li. 2023. "Establish TiNb2O7@C as Fast-Charging Anode for Lithium-Ion Batteries" Materials 16, no. 1: 333. https://doi.org/10.3390/ma16010333







