ITO Thin Films for Low-Resistance Gas Sensors
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Structural Properties of ITO Thin Films
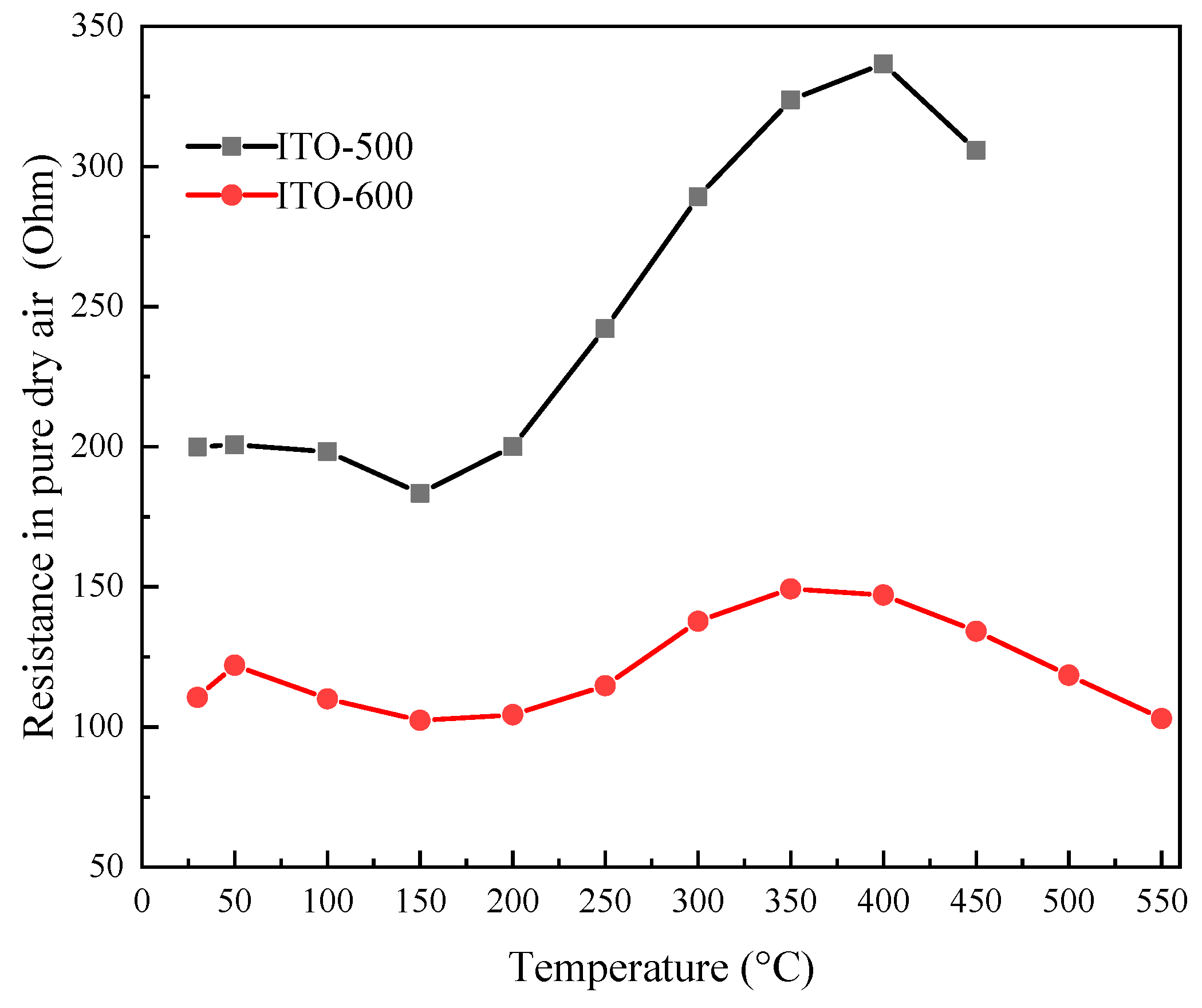
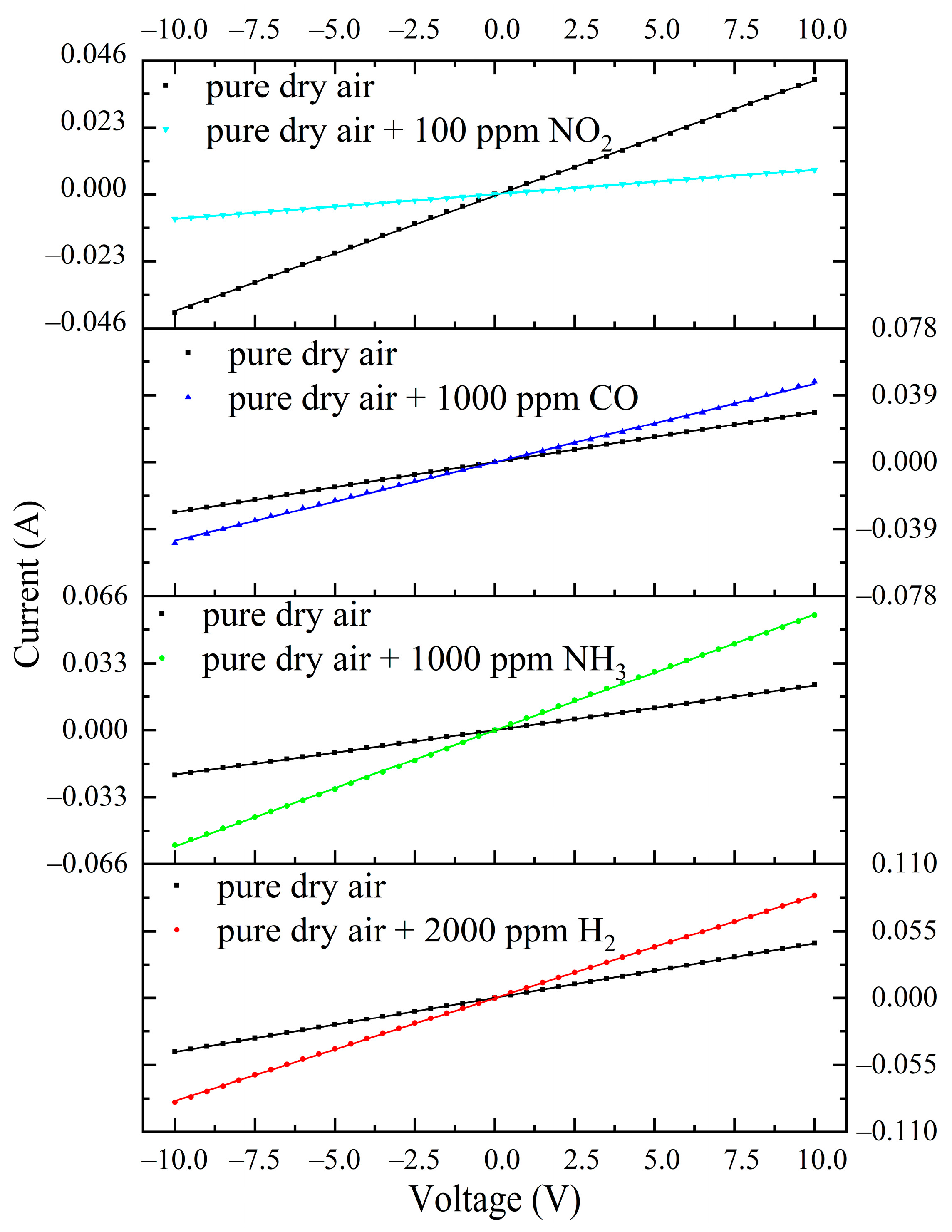
3.2. The Electrically Conductive Properties of ITO Thin Films in Pure Dry Air
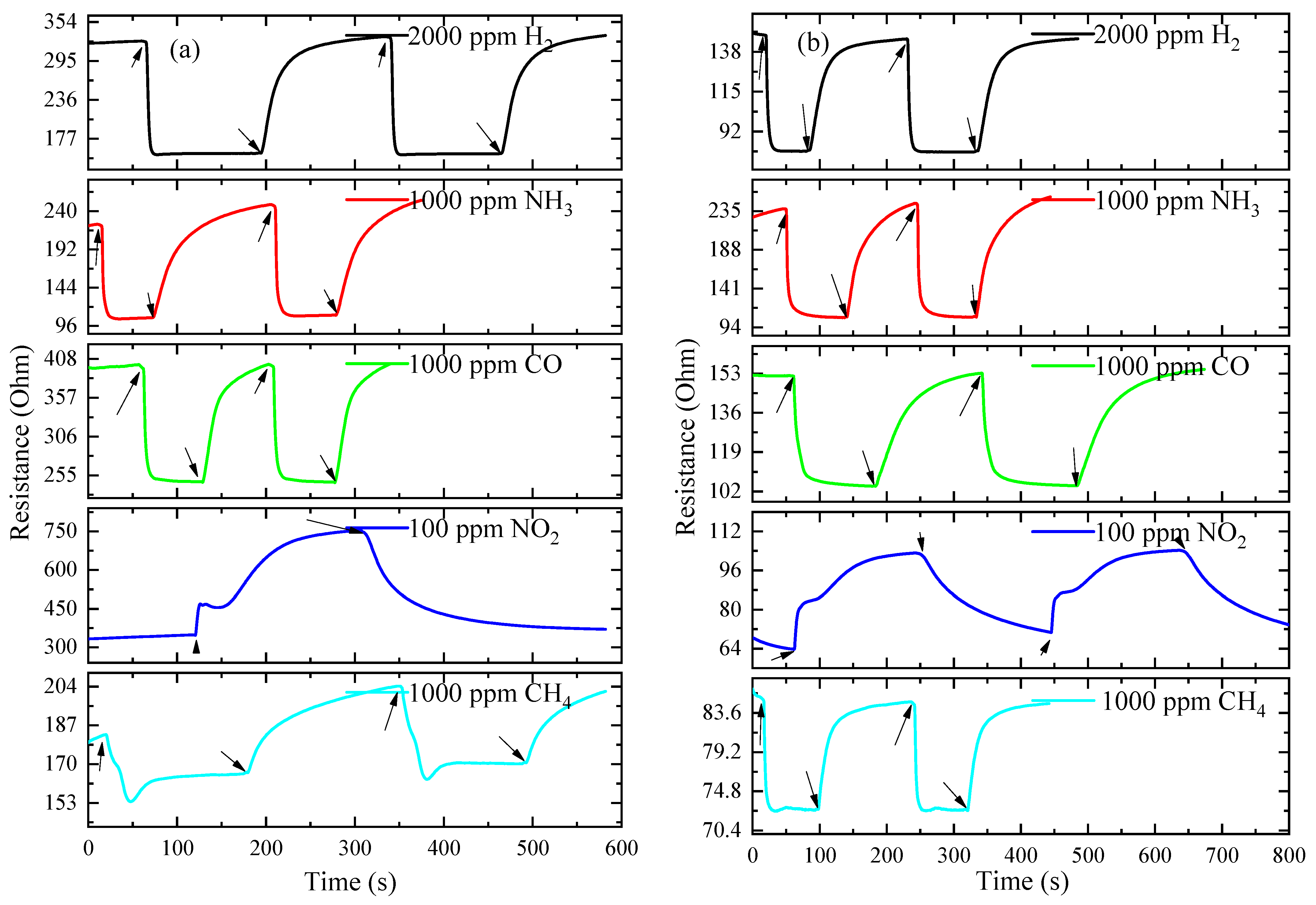
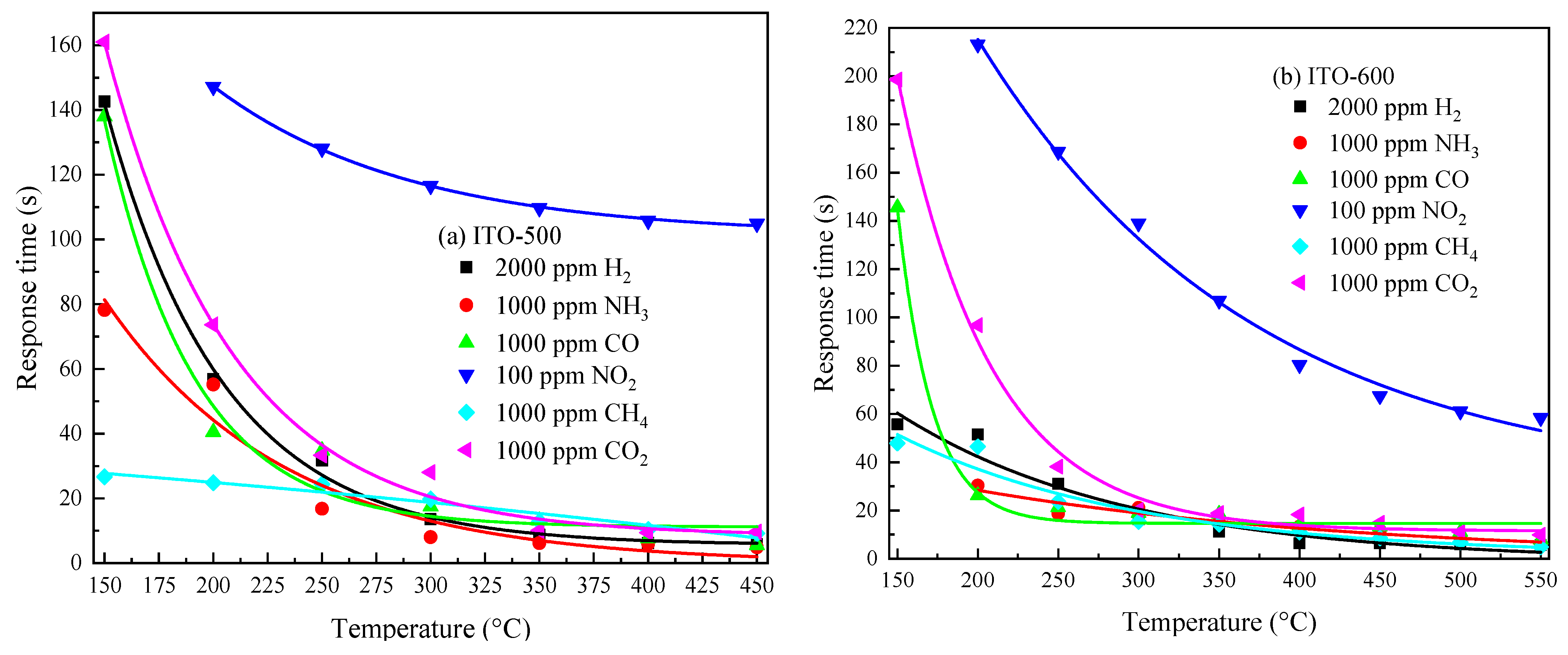
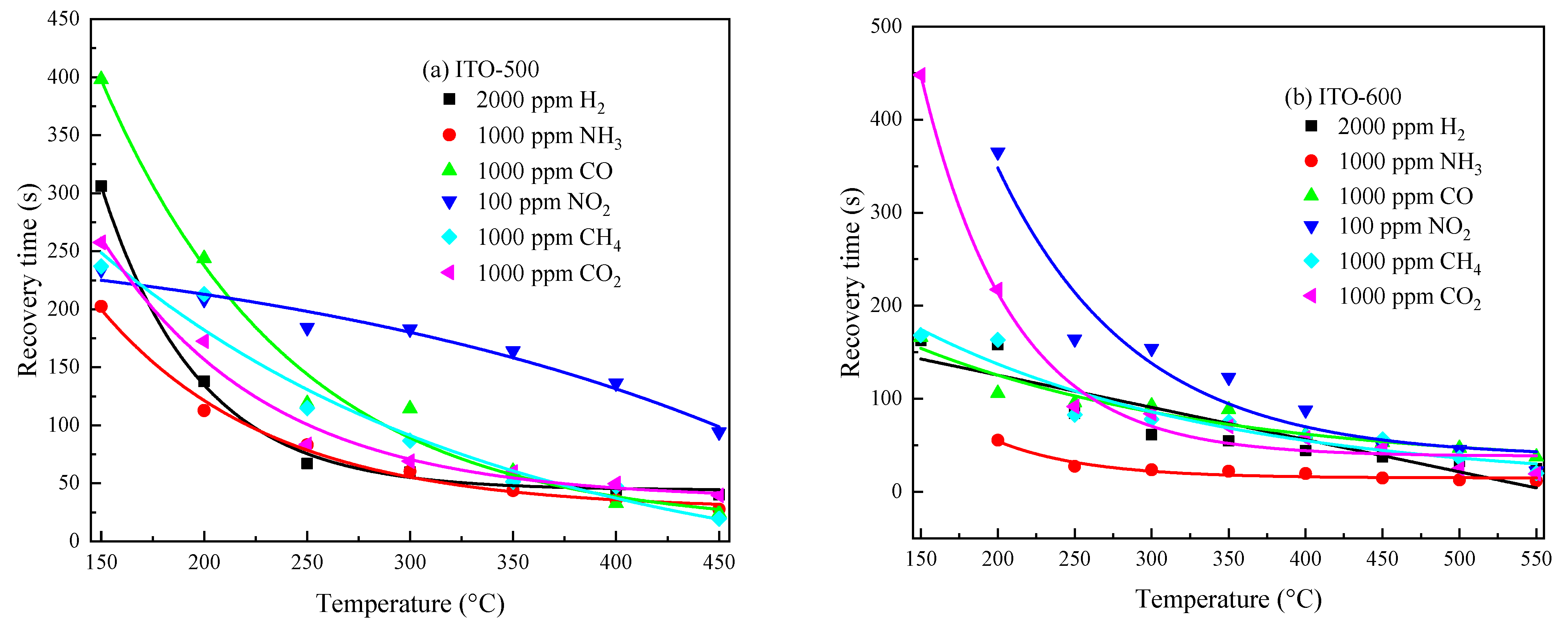
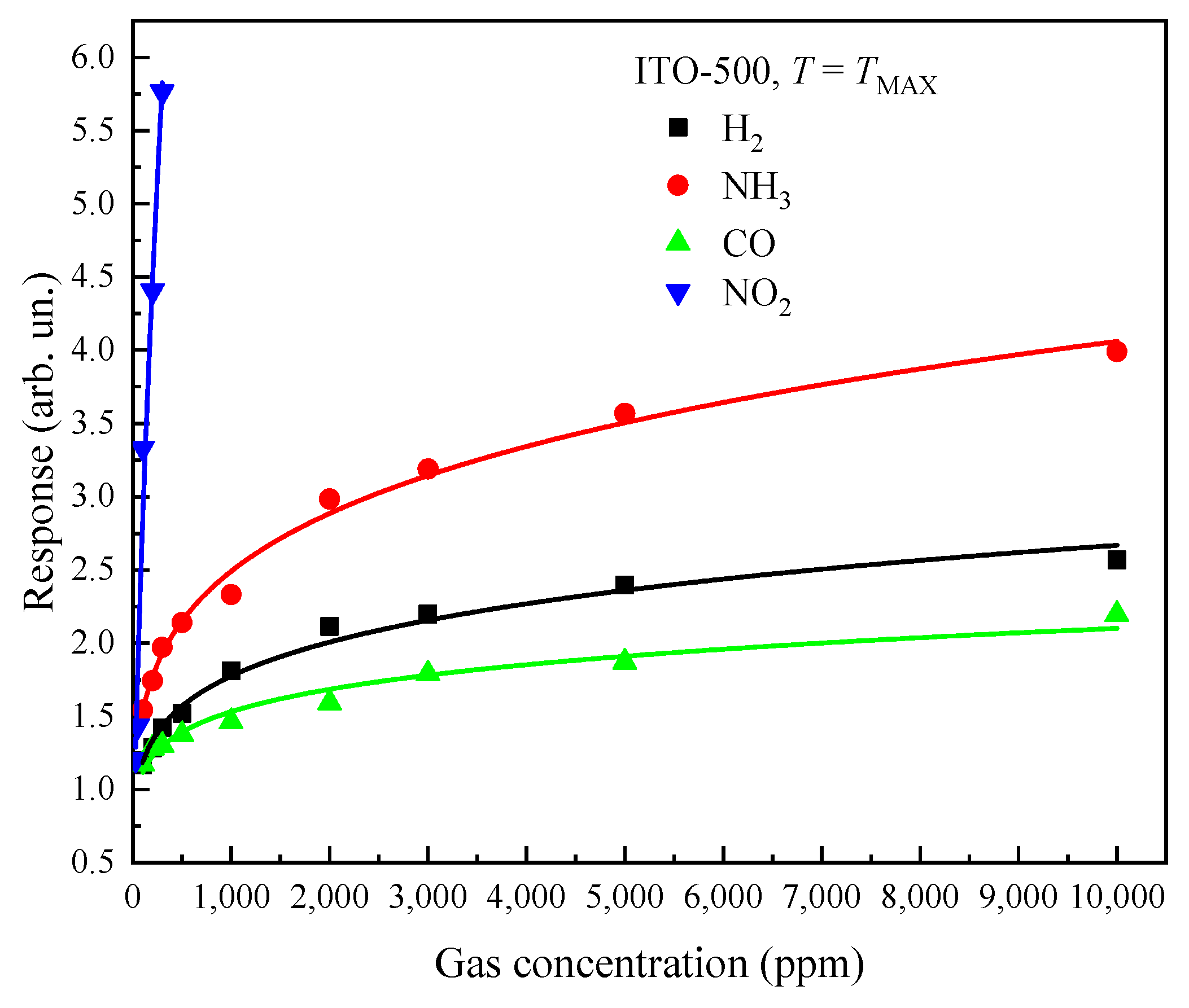
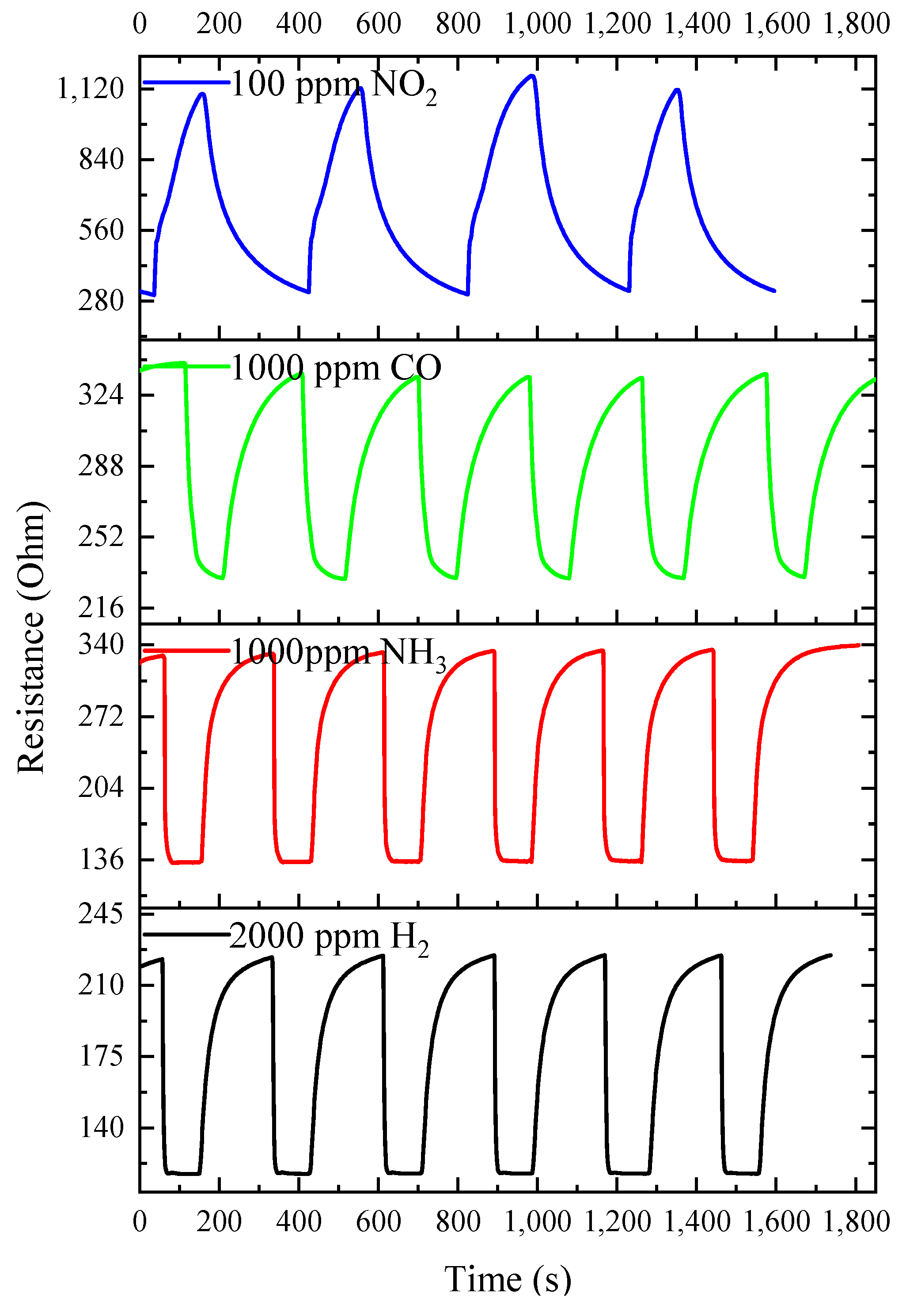
3.3. Gas-Sensitive Properties of ITO Thin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Shah, V.; Bhaliya, J.; Patel, G.M.; Joshi, P. Room-Temperature Chemiresistive Gas Sensing of SnO2 Nanowires: A Review. J. Inorg. Organomet. Polym. 2022, 32, 741–772. [Google Scholar] [CrossRef]
- Afzal, A. β-Ga2O3 nanowires and thin films for metal oxide semiconductor gas sensors: Sensing mechanisms and performance enhancement strategies. J. Mater. 2019, 5, 542–557. [Google Scholar] [CrossRef]
- Gu, H.; Wang, Z.; Hu, Y. Hydrogen Gas Sensors Based on Semiconductor Oxide Nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Xu, H.; Liu, T.; Zeb, S.; Nie, Y.; Zhao, Y.; Qin, C.; Jiang, X. Advanced development of metal oxide nanomaterials for H2 gas sensing applications. Mater. Adv. 2021, 2, 1530. [Google Scholar] [CrossRef]
- Koo, W.-T.; Cho, H.-J.; Kim, D.-H.; Kim, Y.H.; Shin, H.; Penner, R.M.; Kim, I.D. Chemiresistive Hydrogen Sensors: Fundamentals, Recent Advances, and Challenges. ACS Nano 2020, 14, 14284–14322. [Google Scholar] [CrossRef]
- Padvi, M.N.; Moholkar, A.V.; Prasad, S.R.; Prasad, N.R. A Critical Review on Design and Development of Gas Sensing Materials. Eng. Sci. 2021, 15, 20–37. [Google Scholar] [CrossRef]
- Comini, E. Metal oxides nanowires chemical/gas sensors: Recent advances. Mater. Today Adv. 2020, 7, 100099. [Google Scholar] [CrossRef]
- Grassi, M.; Malcovati, P.; Baschirotto, A. Fundamental Limitations in Resistive Wide-Range Gas-Sensor Interface Circuits Design. In Sensors and Microsystems. Lecture Notes in Electrical Engineering; Springer: Dordrecht, The Netherlands, 2010; pp. 25–29. [Google Scholar]
- Jang, H.; Kim, M.; Kim, Y. Batch-processed semiconductor gas sensor array for the selective detection of NOx in automotive exhaust gas. Micro Nano Syst. Lett. 2016, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Merino, J.L.; Bota, S.A.; Casanova, R.; Diéguez, A.; Cané, C.; Samitier, J. A Reusable Smart Interface for Gas Sensor Resistance Measurement. IEEE Trans. Instrum. Meas. 2004, 53, 1173–1178. [Google Scholar] [CrossRef]
- Grassi, M.; Malcovati, P.; Baschirotto, A. Wide-range integrated gas sensor interface based on a resistance-to-number converter technique with the oscillator decoupled from the input device. In Proceedings of the IEEE International Symposium on Circuits and Systems, Island of Kos, Greece, 21–24 May 2006. [Google Scholar]
- Barrettino, D.; Graf, M.; Taschini, S.; Hafizovic, S.; Hagleitner, C.; Hierlemann, A. CMOS Monolithic Metal–Oxide Gas Sensor Microsystems. IEEE Sens. J. 2006, 6, 276–286. [Google Scholar] [CrossRef]
- Figaro USA, Inc. Available online: https://www.figarosensor.com/ (accessed on 30 October 2022).
- Moumen, A.; Kumarage, G.C.W.; Comini, E. P-Type Metal Oxide Semiconductor Thin Films: Synthesis and Chemical Sensor Applications. Sensors 2022, 22, 1359. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, M.; Meixner, H. Thin-film gas sensors based on high-temperature-operated metal oxides. J. Vac. Sci. Technol. A 1991, 17, 1866. [Google Scholar] [CrossRef]
- Soonmin, H.; Vanalakar, S.A.; Galal, A.; Singh, V.N. A review of nanostructured thin films for gas sensing and corrosion protection. Mediterr. J. Chem. 2019, 7, 433–451. [Google Scholar] [CrossRef]
- Rydosz, A.; Brudnik, A.; Staszek, K. Metal Oxide Thin Films Prepared by Magnetron Sputtering Technology for Volatile Organic Compound Detection in the Microwave Frequency Range. Materials 2019, 12, 877. [Google Scholar] [CrossRef] [Green Version]
- Maksimova, N.K.; Almaev, A.V.; Sevastyanov, E.Y.; Potekaev, A.I.; Chernikov, E.V.; Sergeychenko, N.V.; Korusenko, P.M.; Nesov, S.N. Effect of Additives Ag and Rare-Earth Elements Y and Sc on the Properties of Hydrogen Sensors Based on Thin SnO2 Films during Long-Term Testing. Coatings 2019, 9, 423. [Google Scholar] [CrossRef] [Green Version]
- Gaman, V.I. Basic physics of semiconductor hydrogen sensors. Russ. Phys. J. 2008, 51, 425–441. [Google Scholar] [CrossRef]
- Sahm, T.; Gurlo, A.; Bârsan, N.; Weimar, U.; Mädler, L. Fundamental studies on SnO2 by means of simultaneous work function change and conduction measurements. Thin Solid Films 2005, 490, 43–47. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Receptor Function and Response of Semiconductor Gas Sensor. J. Sens. 2009, 2009, 875704. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Engineering approaches to improvement of conductometric gas sensor parameters. Part 2: Decrease of dissipated (consumable) power and improvement stability and reliability. Sens. Actuators B Chem. 2014, 198, 316–341. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Sisman, O.; Comini, E. Metal oxide -based heterostructures for gas sensors—A review. Anal. Chim. Acta 2018, 1, 1039. [Google Scholar] [CrossRef] [PubMed]
- Mokrushin, A.S.; Fisenko, N.A.; Gorobtsov, P.Y.; Simonenko, T.L.; Glumov, O.V.; Melnikova, N.A.; Simonenko, N.P.; Bukunov, K.A.; Simonenko, E.P.; Sevastyanov, V.G.; et al. Pen plotter printing of ITO thin film as a highly CO sensitive component of a resistive gas sensor. Talanta 2021, 221, 121455. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, G.N.; Gromov, V.F.; Ikim, M.I.; Ilegbusi, O.J.; Ozerin, S.A.; Trakhtenberg, L.I. Structure and gas-sensing properties of SnO2-In2O3 nanocomposites synthesized by impregnation method. Sens. Actuators B Chem. 2020, 320, 128406. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chen, H.-I.; Chen, T.-Y.; Huang, C.-C.; Hsu, C.-S.; Liu, R.-C.; Liu, W.-C. On an indium–tin-oxide thin film based ammonia gas sensor. Sens. Actuators B Chem. 2011, 160, 1481–1484. [Google Scholar] [CrossRef]
- Yang, M.; Hong, S.H. Orientation and thickness dependence of electrical and gas sensing properties in heteroepitaxial indium tin oxide films. Sens. Actuators B Chem. 2011, 160, 490–498. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chen, H.-I.; Chen, T.-Y.; Huang, C.-C.; Hsu, C.-S.; Liu, W.-C. Ammonia Sensing Characteristics of Sputtered Indium Tin Oxide (ITO) Thin Films on Quartz and Sapphire Substrates. IEEE Trans. Electron Devices 2011, 58, 4407–4412. [Google Scholar] [CrossRef]
- Yang, M.; Hong, S.-H. Fabrication of ITO / SnO2 Two-Layer Thin Films and Their Gas Sensing Properties. J. Electrochem. Soc. 2010, 157, J392. [Google Scholar] [CrossRef]
- Murali, A.; Sohn, H.Y. Plasma-assisted chemical vapor synthesis of indium tin oxide (ITO) nanopowder and hydrogen-sensing property of ITO thin film. Mater. Res. Express 2018, 5, 065045. [Google Scholar] [CrossRef]
- Nicolescu, M.; Mitrea, D.; Hornoiu, C.; Preda, S.; Stroescu, H.; Anastasescu, M.; Calderon-Moreno, J.M.; Predoana, L.; Teodorescu, V.S.; Maraloiu, V.-A.; et al. Structural, Optical, and Sensing Properties of Nb-Doped ITO Thin Films Deposited by the Sol–Gel Method. Gels 2022, 8, 717. [Google Scholar] [CrossRef]
- Lopez-Santos, C.; Puerto, D.; Siegel, J.; Macias-Montero, M.; Florian, C.; Gil-Rostra, J.; López-Flores, V.; Borras, A.; González-Elipe, A.R.; Solis, J. Anisotropic Resistivity Surfaces Produced in ITO Films by Laser-Induced Nanoscale Self-organization. Adv. Opt. Mater. 2021, 9, 2001086. [Google Scholar] [CrossRef]
- Thirumoorthi, M.; Thomas Joseph Prakash, J. Structure, optical and electrical properties of indium tin oxide ultra thin films prepared by jet nebulizer spray pyrolysis technique. J. Asian Ceram. Soc. 2016, 4, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Jafan, M.-H.; Zamani-Meymian, M.-R.; Rahimi, R.; Rabbani, M. The effect of solvents and the thickness on structural, optical and electrical properties of ITO thin films prepared by a sol–gel spin-coating process. J. Nanostruct. Chem. 2014, 4, 89. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.F.; Chiou, B.-S. Effect of annealing on electrical and optical properties of RF magnetron sputtered indium tin oxide films. Appl. Surf. Sci. 1993, 68, 497–504. [Google Scholar] [CrossRef]
- Krylov, P.N.; Zakirova, R.M.; Fedotova, I.V. Optical properties of ITO films obtained by high-frequency magnetron sputtering with accompanying ion treatment. Semiconductors 2013, 47, 1412–1415. [Google Scholar] [CrossRef]
- Bochenkov, V.E.; Sergeev, G.B. Adsorption, catalysis, and reactions on the surfaces of metal nano-oxides. Catal. Ind. 2010, 2, 1–10. [Google Scholar] [CrossRef]
- Hernández-Gutiérrez, C.A.; Kudriavtsev, Y.; Cardona, D.; Hernández, A.G.; Camas-Anzueto, J.L. Optical, electrical, and chemical characterization of nanostructured InxGa1-xN formed by high fluence In+ ion implantation into GaN. Opt. Mat. 2021, 111, 110541. [Google Scholar] [CrossRef]
- Hernández-Gutiérrez, C.A.; Casallas-Moreno, Y.L.; Rangel-Kuoppa, V.-T.; Cardona, D.; Hu, Y.; Kudriavtsev, Y.; Zambrano-Serrano, M.A.; Gallardo-Hernandez, S.; Lopez-Lopez, M. Study of the heavily p-type doping of cubic GaN with Mg. Sci. Rep. 2020, 10, 16858. [Google Scholar] [CrossRef]
- Hohmann, M.V.; Wachau, A.; Klein, A. In situ Hall effect and conductivity measurements of ITO thin films. Solid State Ionics 2014, 262, 636–639. [Google Scholar] [CrossRef]
- Hamzah, N.A.; Asri, R.I.M.; Ahmad, M.A.; Md Sahar, M.A.A.Z.; Waheeda, S.N.; Hassan, Z. Effect of post-annealing in oxygen environment on ITO thin films deposited using RF magnetron sputtering. J. Phys. Conf. Ser. 2020, 1535, 012036. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Golovanov, V.; Blinov, Y. Kinetics of gas response to reducing gases of SnO2 films, deposited by spray pyrolysis. Sens. Actuators B Chem. 2004, 98, 41–45. [Google Scholar] [CrossRef]
- Almaev, A.V.; Yakovlev, N.N.; Chernikov, E.V.; Tolbanov, O.P. Selective Sensors of Nitrogen Dioxide Based on Thin Tungsten Oxide Films under Optical Irradiation. Tech. Phys. Lett. 2019, 45, 1016–1019. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen Ionosorption and Spectroscopic Evidence for Adsorbed Oxygen. ChemPhysChem 2006, 7, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Forleo, A.; Francioso, L.; Capone, S.; Casino, F.; Siciliano, P.; Tan, O.K.; Hui, H. Fabrication at wafer level of miniaturized gas sensors based on SnO2 nanorods deposited by PECVD and gas sensing characteristics. Sens. Actuators B Chem. 2011, 154, 283–287. [Google Scholar] [CrossRef]
- Vorobyeva, N.; Rumyantseva, M.; Platonov, V.; Filatova, D.; Chizhov, A.; Marikutsa, A.; Bozhev, I.; Gaskov, A. Ga2O3(Sn) Oxides for High-Temperature Gas Sensors. Nanomaterials 2021, 11, 2938. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Basu, P.K. Nanocrystalline Metal Oxides for Methane Sensors: Role of Noble Metals. J. Sens. 2009, 2009, 861968. [Google Scholar] [CrossRef]
- Jiao, Z.; Wu, M.; Qin, Z.; Lu, M.; Gu, J. The NO2 sensing ITO thin films prepared by ultrasonic spray pyrolysis. Sensors 2003, 3, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Bundesmann, C.; Neumann, H. Tutorial: The systematics of ion beam sputtering for deposition of thin films with tailored properties. J. Appl. Phys. 2018, 124, 231102. [Google Scholar] [CrossRef]
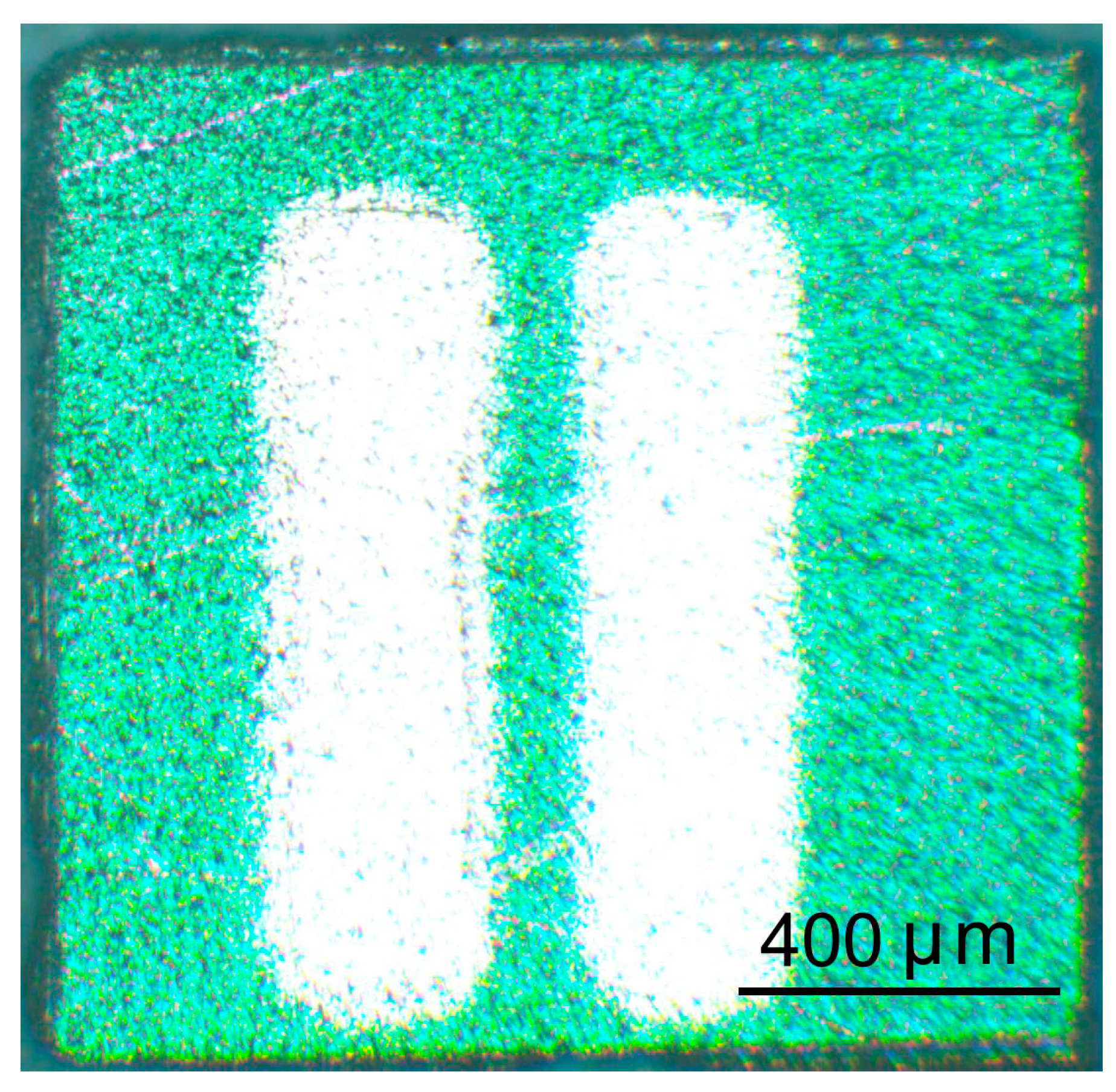

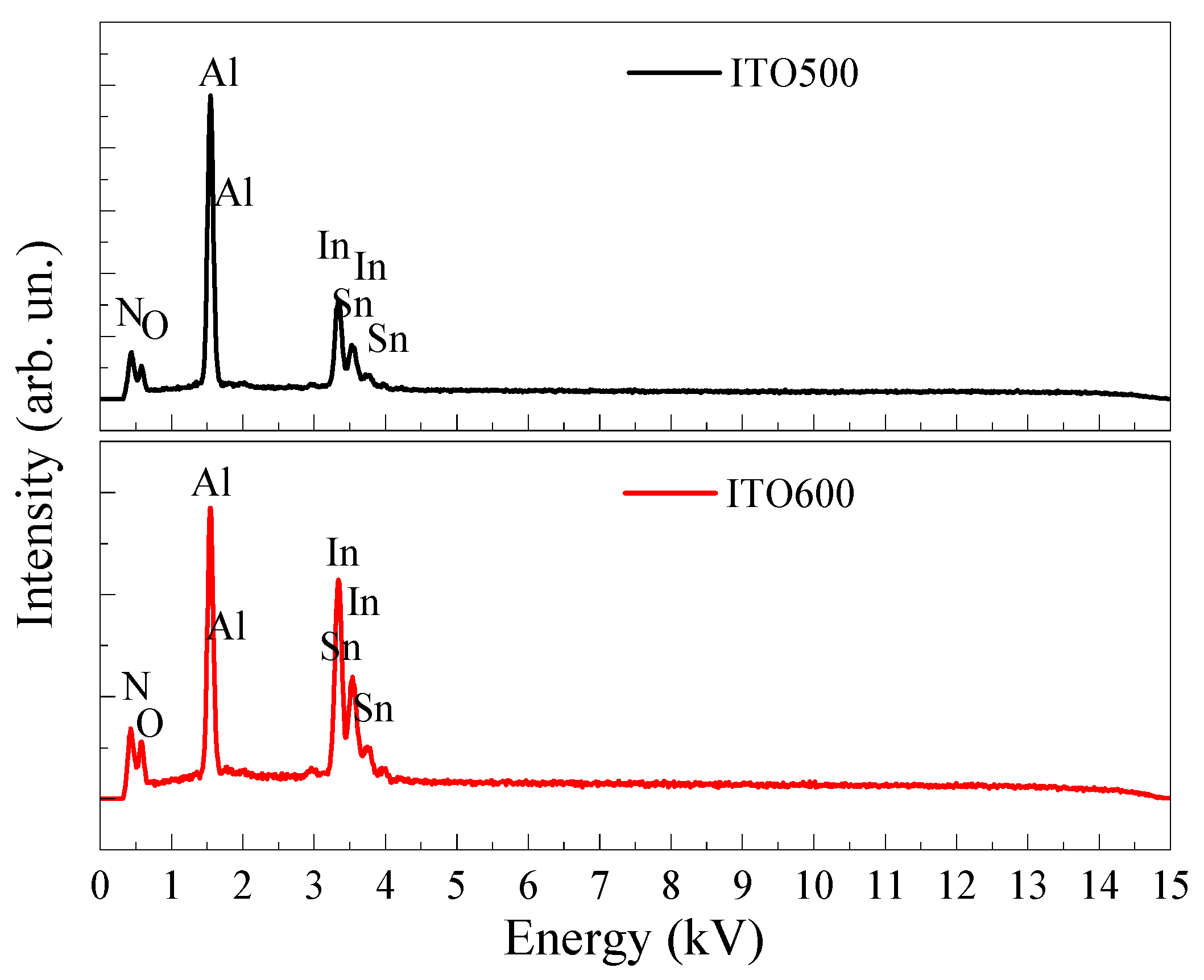
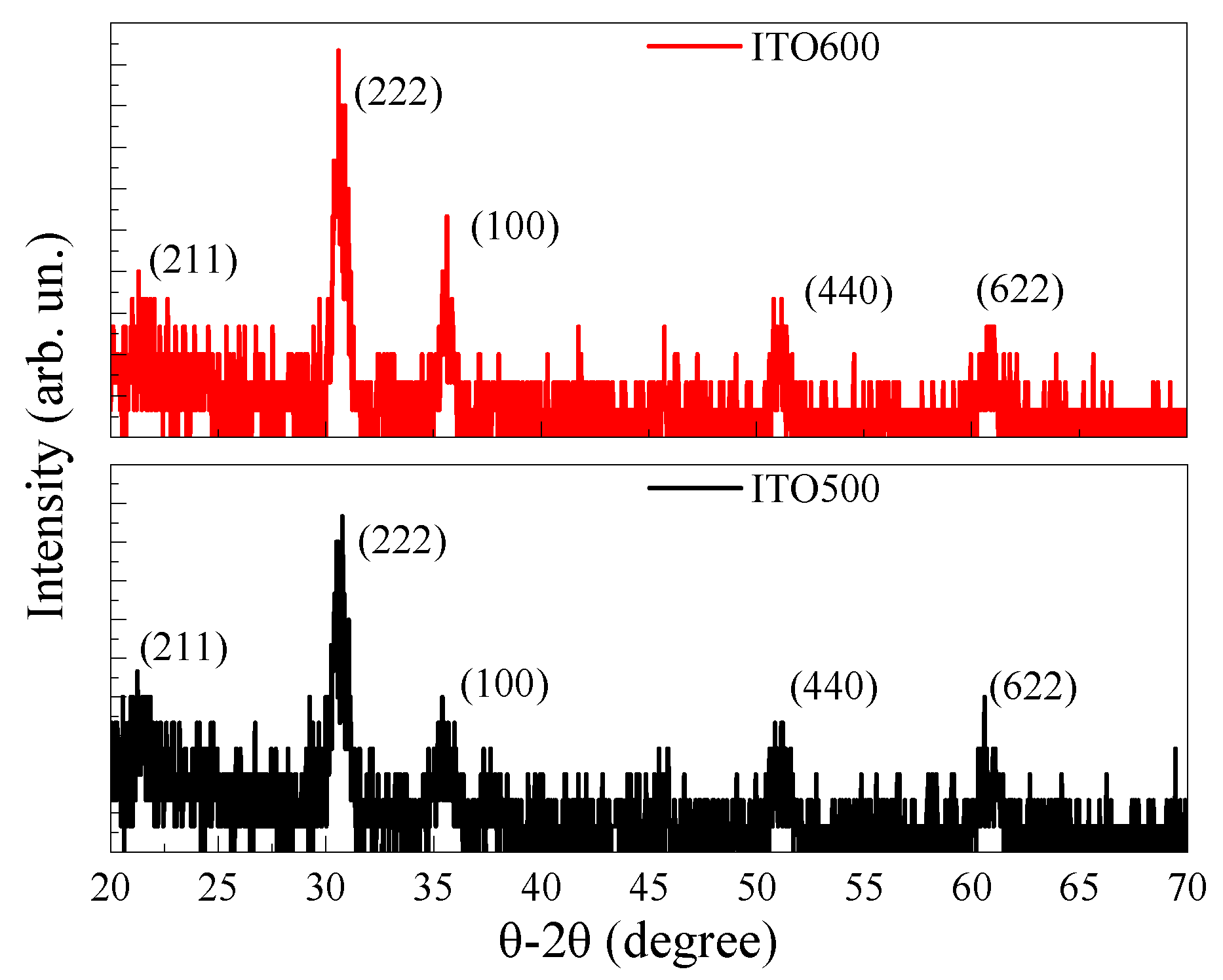
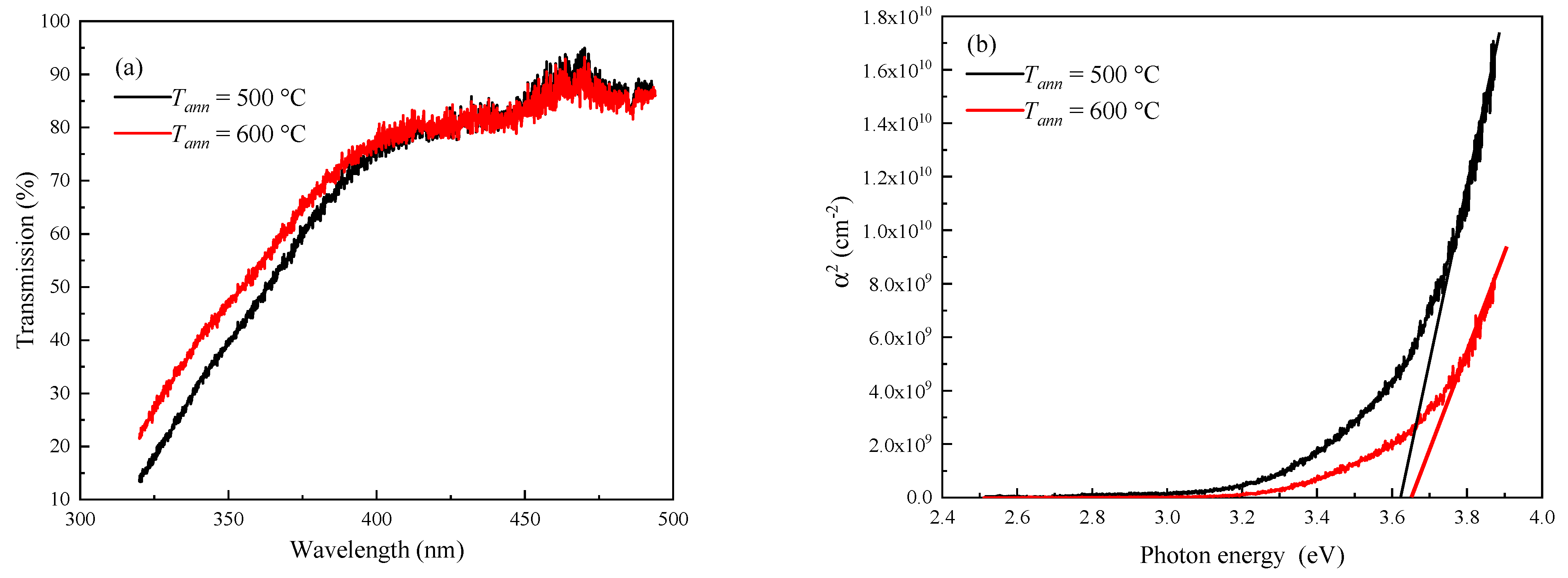

| Tann (°C) | Element Content (wt.%) | ||
|---|---|---|---|
| In | Sn | O | |
| 500 | 55.26–56.93 | 4.10–5.15 | 38.82–39.72 |
| 600 | 58.54–60.65 | 4.97–6.15 | 34.13–36.12 |
| Gas | ng (ppm) | ITO-500 | ITO-600 | ||||
|---|---|---|---|---|---|---|---|
| TMAX (°C) | Rair (Ohm) | SMAX (arb. un.) | TMAX (°C) | Rair (Ohm) | SMAX (arb. un.) | ||
| H2 | 2000 | 400 | 336.69 | 2.21 | 400 | 144.14 | 1.80 |
| NH3 | 1000 | 350 | 259.87 | 2.39 | 350 | 210.01 | 2.40 |
| CO | 1000 | 400 | 370.14 | 1.91 | 350 | 154.17 | 1.46 |
| NO2 | 100 | 350 | 337.55 | 2.14 | 350 | 62.40 | 1.68 |
| CH4 | 1000 | 300 | 211.98 | 1.25 | 500 | 94.87 | 1.16 |
| Gas | H2 | NH3 | CO | NO2 |
|---|---|---|---|---|
| m | 0.19 ± 0.01 | 0.21 ± 0.01 | 0.14 ± 0.01 | 0.72 ± 0.07 |
| Rair (Ohm) | ρair (Ohm × cm) | T (°C) | ng (ppm) | S (arb. un.) | Refs. |
|---|---|---|---|---|---|
| H2 | |||||
| - | 7.5 | 250 | 100 | 1.1 | [25] |
| ~104 | - | 150 | 1000 | 11 | [29] |
| 105–106 | 103 | 100 | 100 | 6 | [28] |
| - | 2.47 × 10−3 | 350 | 400 | 1.55–1.7 | [31] |
| 103 | - | 320 | 1100 | 25 | [26] |
| 200 | 3.6 × 10−6 | 400 | 2000 | 2.21 | This work |
| NH3 | |||||
| - | ~3 | 150 | 100 | 2 | [25] |
| ~104 | 150 | 1000 | 24 | [29] | |
| 105–106 | 103 | 100 | 100 | 2 | [28] |
| 200 | 3.6 × 10−6 | 350 | 1000 | 2.39 | This work |
| NO2 | |||||
| 105–106 | 103 | 100 | 200 | 80 | [28] |
| 106 | - | 300 | 100 | 18 | [49] |
| 200 | 3.6 × 10−6 | 350 | 100 | 2.14 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almaev, A.V.; Kopyev, V.V.; Novikov, V.A.; Chikiryaka, A.V.; Yakovlev, N.N.; Usseinov, A.B.; Karipbayev, Z.T.; Akilbekov, A.T.; Koishybayeva, Z.K.; Popov, A.I. ITO Thin Films for Low-Resistance Gas Sensors. Materials 2023, 16, 342. https://doi.org/10.3390/ma16010342
Almaev AV, Kopyev VV, Novikov VA, Chikiryaka AV, Yakovlev NN, Usseinov AB, Karipbayev ZT, Akilbekov AT, Koishybayeva ZK, Popov AI. ITO Thin Films for Low-Resistance Gas Sensors. Materials. 2023; 16(1):342. https://doi.org/10.3390/ma16010342
Chicago/Turabian StyleAlmaev, Aleksei V., Viktor V. Kopyev, Vadim A. Novikov, Andrei V. Chikiryaka, Nikita N. Yakovlev, Abay B. Usseinov, Zhakyp T. Karipbayev, Abdirash T. Akilbekov, Zhanymgul K. Koishybayeva, and Anatoli I. Popov. 2023. "ITO Thin Films for Low-Resistance Gas Sensors" Materials 16, no. 1: 342. https://doi.org/10.3390/ma16010342







