Mechanisms of Growth and Hydrogen Permeation of Zirconium Nitride Film on Zirconium Hydride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Hydrogen Permeation Barrier
2.2. Film Characterization
2.3. Diffusion Coefficient Calculation
2.4. Evaluation of Hydrogen Permeation Performance
3. Results and Discussion
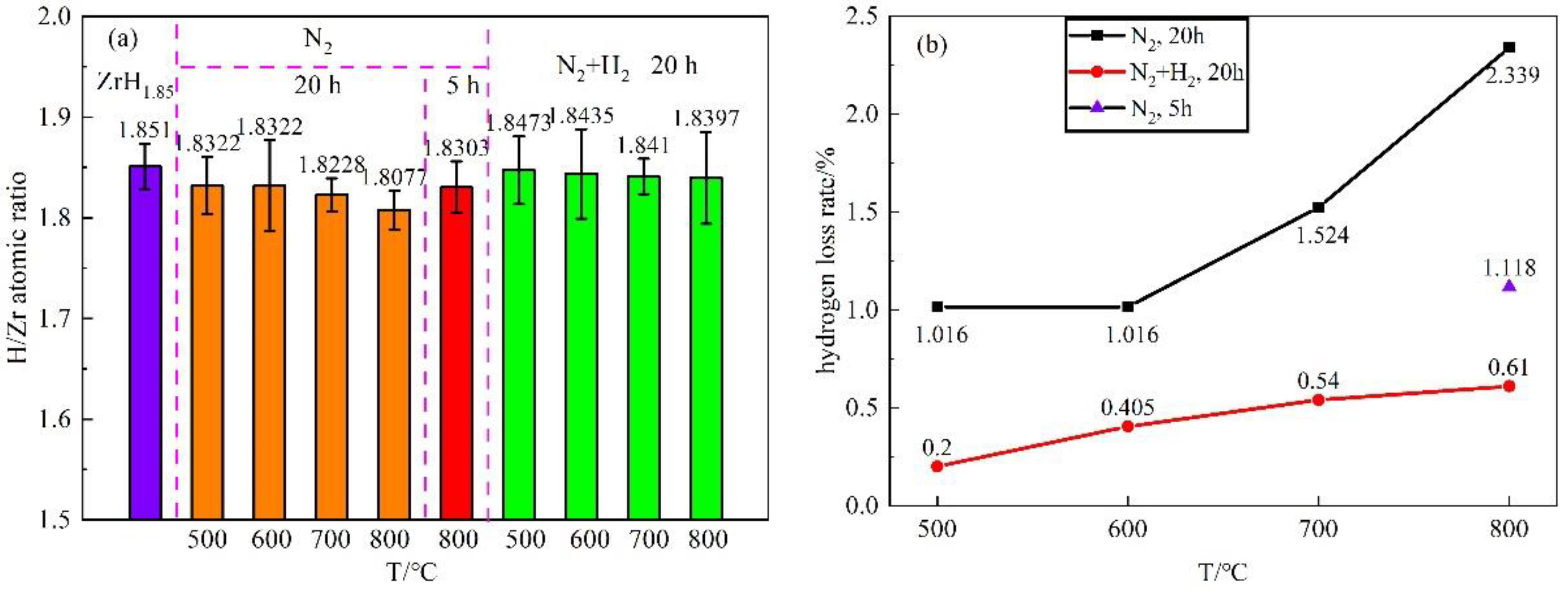
3.1. Hydrogen Loss of Zirconium Hydride Matrix after Film Preparation
3.2. Morphologies and Chemical Composition of Nitride Film
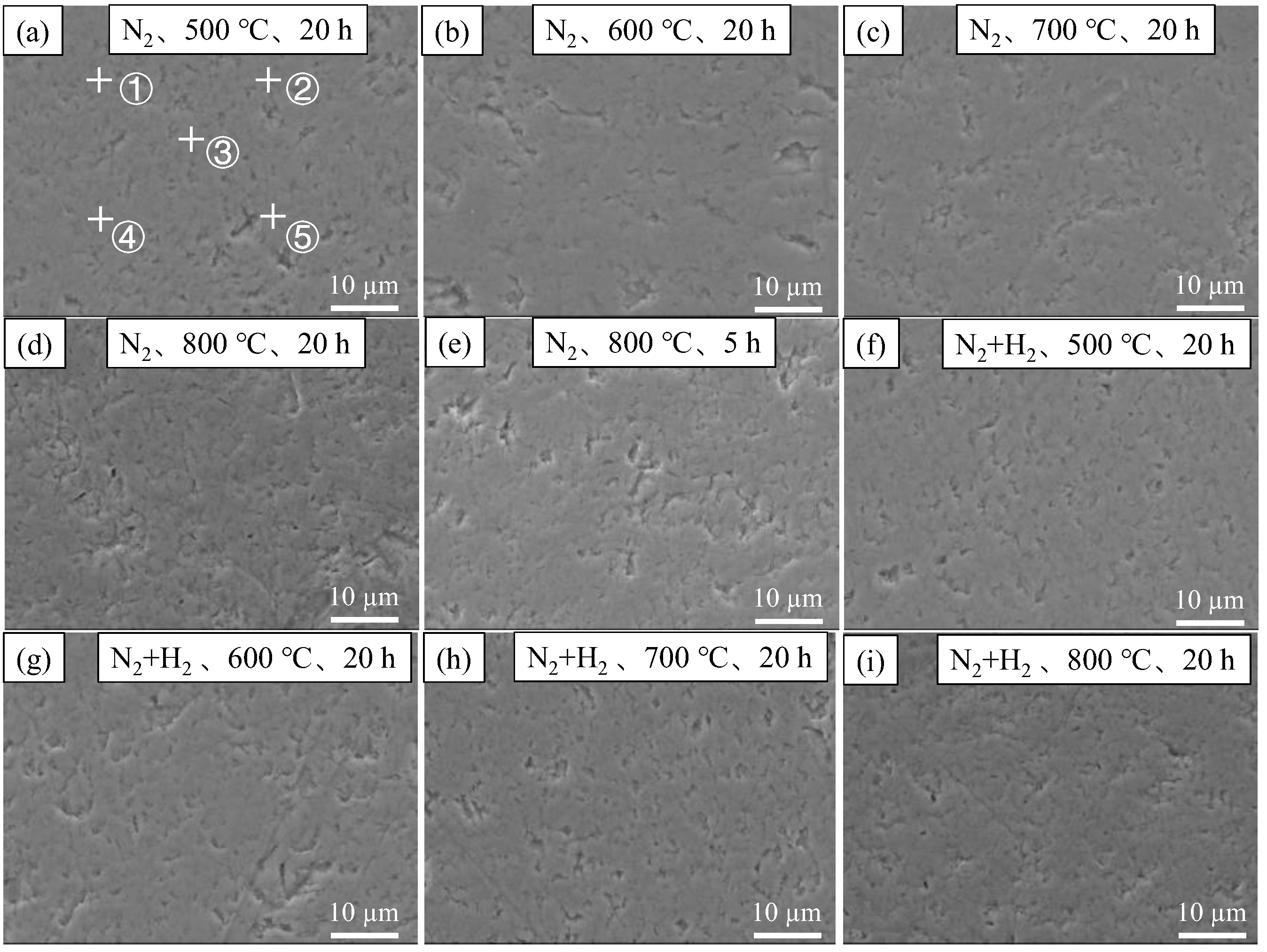
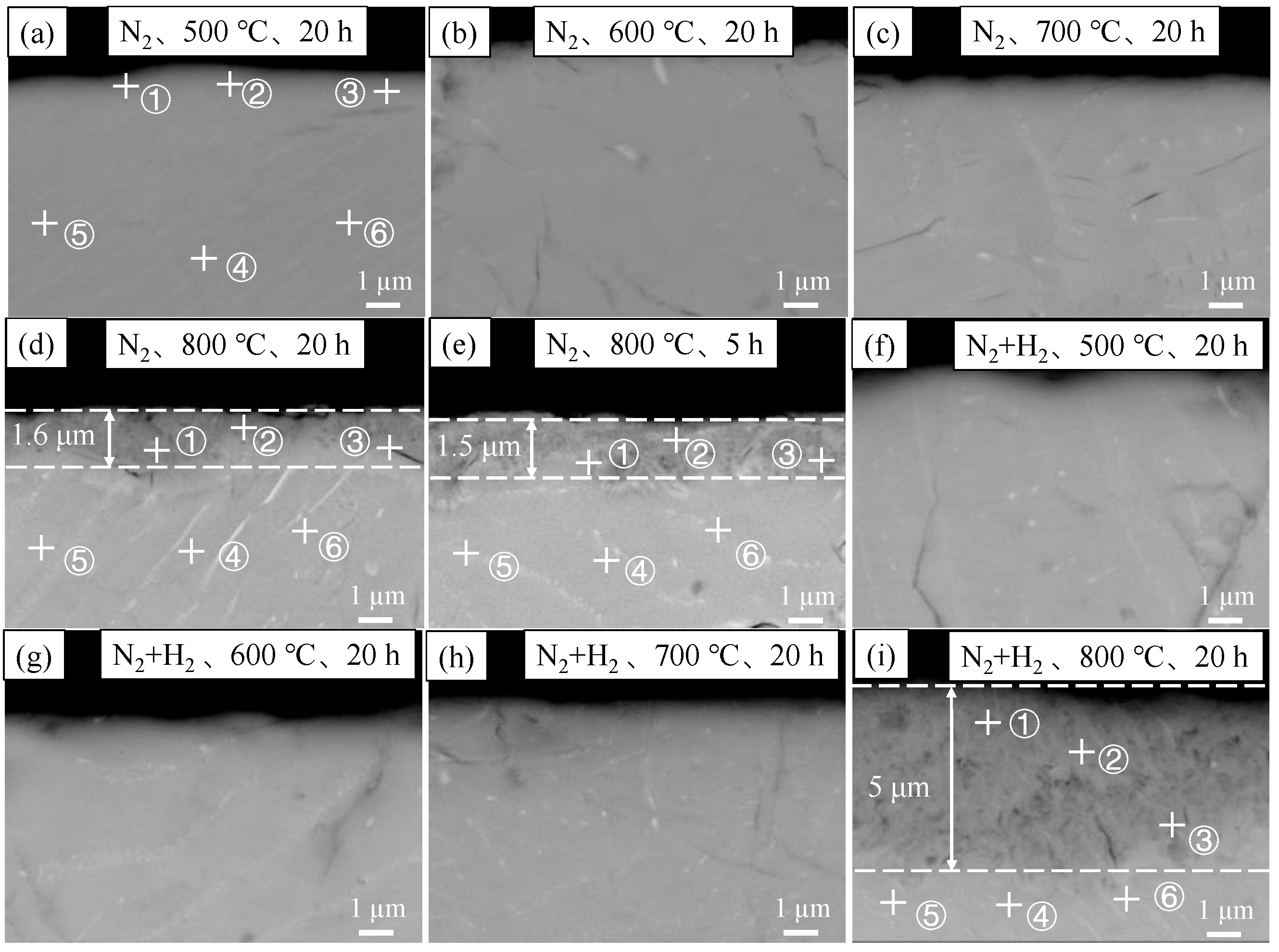
3.2.1. Film Thicknesses and Morphologies
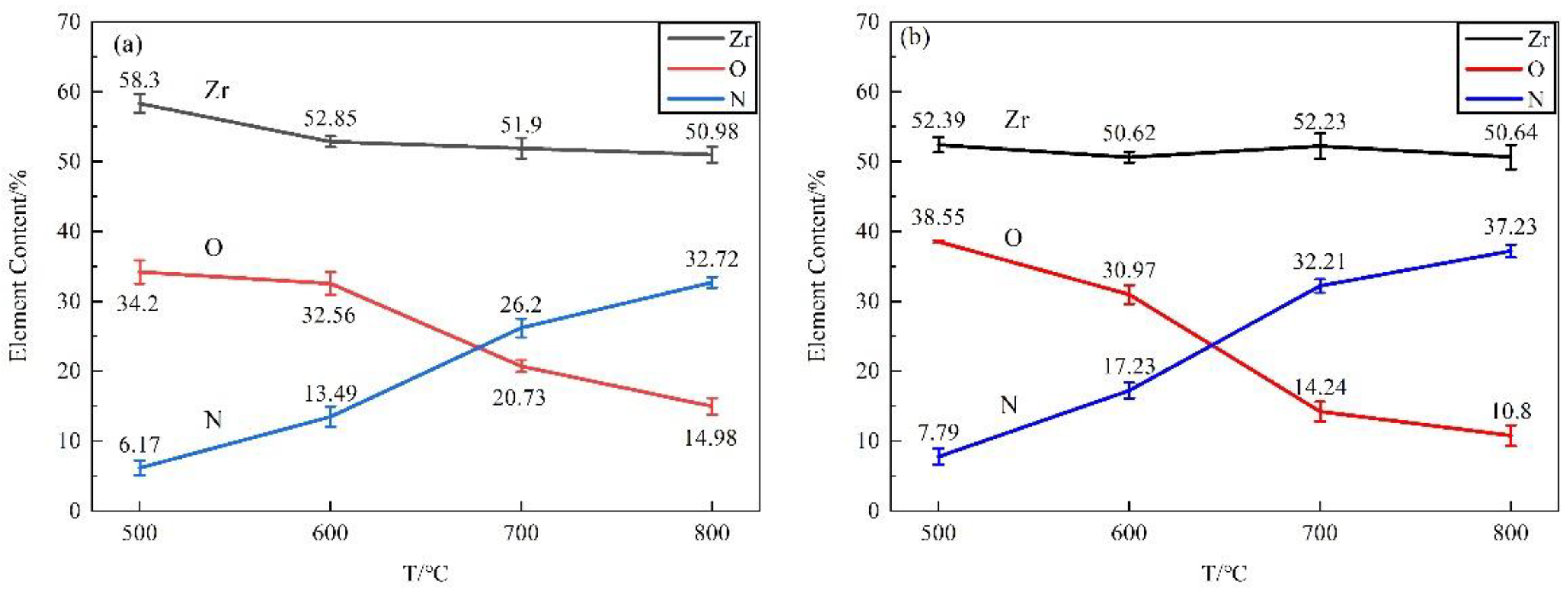
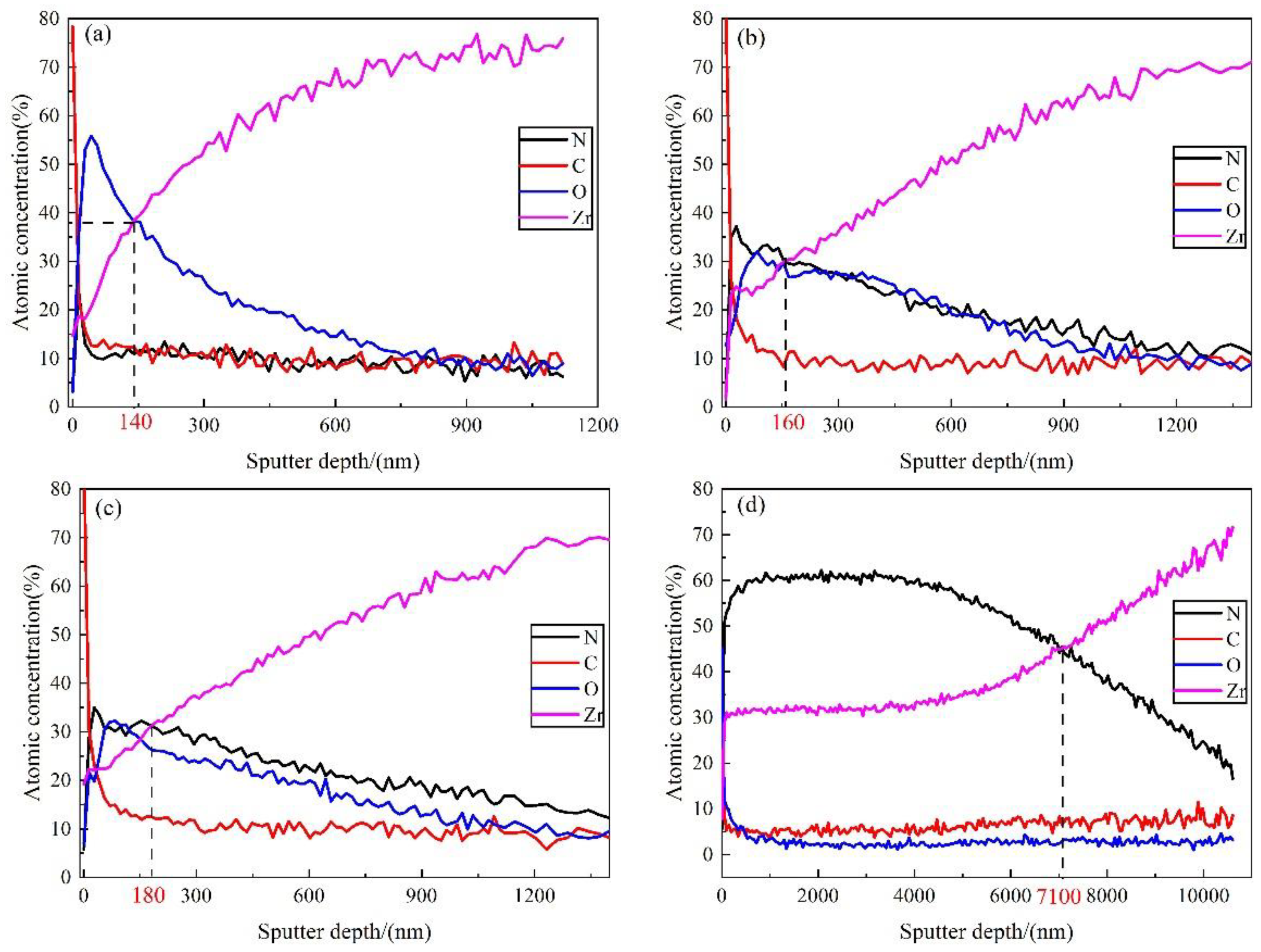
3.2.2. Elemental Composition and Distribution of the Nitride Films
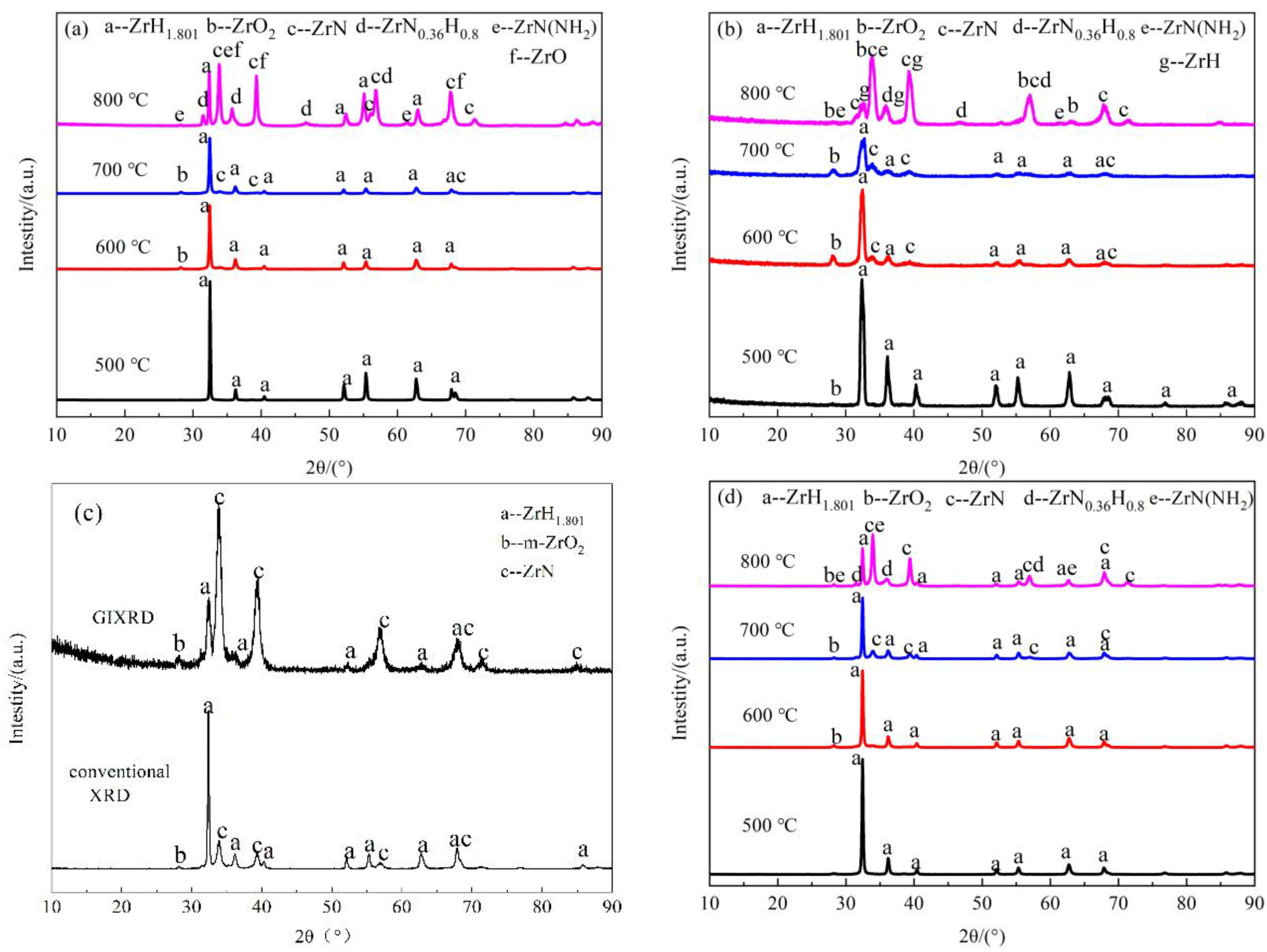
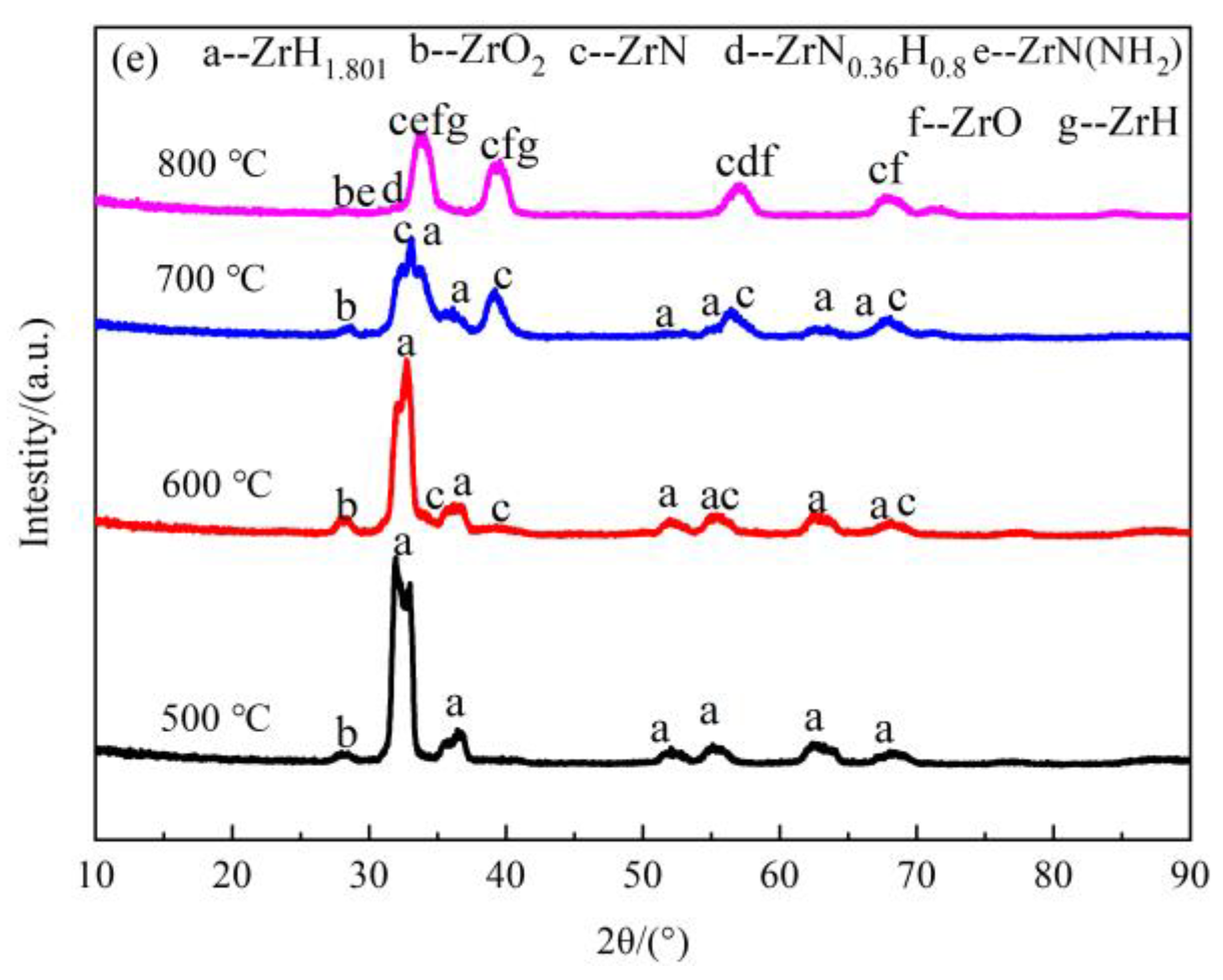
3.2.3. Phase Compositions of the Nitride Films
3.3. Growth Kinetics of Nitride Film
3.4. Hydrogen Permeation Performance and Mechanism
3.4.1. Hydrogen Permeation Performance
3.4.2. Hydrogen Permeation Mechanisms of Nitride Film
4. Conclusions
- (1)
- The growth of nitride films on zirconium hydride was mainly determined by temperature, followed by reaction atmosphere, and holding time. The film preparation was optimized at 800 °C in N2 + H2 atmosphere.
- (2)
- The hydrogen content of the zirconium hydride matrix was reduced during the film preparation, and the hydrogen loss increased with the increase of temperature. In addition, the hydrogen loss was obviously reduced after shortening the preparation time and adding hydrogen to nitrogen. When the film preparation time was shortened from 20 h to 5 h in pure N2 atmosphere at 800 °C, the hydrogen loss rate was reduced from 2.339% to 1.118%; substituting N2 + H2 atmosphere for pure N2, the hydrogen loss rate was reduced from 2.339% to 0.610%.
- (3)
- The ZrN-based films prepared at 800 °C was mainly composed of Zr, N, and O; the major phase of the film was ZrN, and the secondary phases was ZrO2, ZrO, ZrN (NH2), and ZrN0.36H0.8. The diffusion activation energies of N and O for film growth were 205.6052 KJ/mol and 68.5074 KJ/mol, respectively.
- (4)
- Three mechanisms of hydrogen permeation resistance of nitride film were revealed, including the capture of the diffusing hydrogen, the continuous and dense structure of ZrN-based film, and the film preparation at a higher temperature than the usage.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.W.; Chen, R.H.; Tian, W.X.; Qiu, S.Z.; Su, G.H. Start-up Simulation of TOPAZ-II System. At. Energy Sci. Technol. 2016, 7, 55–62. [Google Scholar]
- Wang, C.L.; Liu, T.C.; Tang, S.M.; Tian, W.X.; Qiu, S.Z.; Su, G.H. Thermal hydraulic analysis of space nuclear reactor TOPAZ-II with modified RELAP5. Nucl. Sci. Tech. 2019, 30, 117–127. [Google Scholar] [CrossRef]
- Chernikov, A.S.; Syasin, V.A.; Kostin, V.M.; Boiko, E.B. Influence of hydrogen content on the strength and the presence of defects in zirconium hydride. J. Alloys Compd. 2002, 330–332, 393–395. [Google Scholar] [CrossRef]
- Konashi, K.; Ikeshoji, T.; Kawazoe, Y.; Matsui, H. A molecular dynamics study of thermal conductivity of zirconium hydride. J. Alloys Compd. 2003, 356–357, 279–282. [Google Scholar] [CrossRef]
- Tsuchiya, B.; Teshigawara, M.; Konashi, K.; Yamawaki, M. Thermal diffusivity and electrical resistivity of zirconium hydride. J. Alloys Compd. 2002, 330, 357–360. [Google Scholar] [CrossRef]
- Tsuchiya, B.; Huang, J.; Konashi, K.; Teshigawara, K.; Yamawaki, M. Thermophysical properties of zirconium hydride and uranium–zirconium hydride. J. Nucl. Mater. 2001, 289, 329–333. [Google Scholar] [CrossRef]
- Shoji, T.; Inoue, A. Hydrogen absorption and desorption behavior of Zr-based amorphous alloys with a large structurally relaxed amorphous region. J. Alloys Compd. 1999, 292, 275–280. [Google Scholar] [CrossRef]
- Zhang, H.F.; Yang, Q.F.; Wang, Z.D.; Liu, X.Z. Study on hydrogen permeation barrier on zirconium hydride. At. Energy Sci. Technol. 2005, 39, 83–87. [Google Scholar]
- Li, Q.; Liu, J.; Lv, W.L.; Mo, L.B.; Duan, D.W.; Gu, H.W.; Ding, F.Z.; Tang, T.; Luo, D.L.; Cao, J.L. Stability of Y2O3 hydrogen isotope permeation barriers in hydrogen at high temperatures. Int. J. Hydrogen Energy 2013, 38, 4266–4271. [Google Scholar] [CrossRef]
- He, D.; Li, S.; Liu, X.P.; Zhang, C.; Yu, Q.H.; Wang, S.M.; Jiang, L.J. Preparation of Cr2O3 film by MOCVD as hydrogen permeation barrier. Fusion Eng. Des. 2014, 89, 35–39. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Xiang, Q.Y.; Yan, K.; Yao, W.Q.; Cao, J.L. Study on influence factors of permeation reduction factor of Al2O3 hydrogen isotopes permeation barriers. Int. J. Hydrogen Energy 2016, 41, 4326–4331. [Google Scholar] [CrossRef]
- Yin, R.; Hu, L.L.; Tang, J.; Cheng, T.; Zhang, D.X.; Zhang, G.K.; Chen, Z.Q.; Hong, M.Q. In-Situ oxidation of aluminized stainless-steel to form alumina as tritium permeation barrier coating. Fusion Eng. Des. 2021, 163, 112154. [Google Scholar] [CrossRef]
- Huang, J.; Xie, H.; Luo, L.M.; Zan, X.; Liu, D.G.; Wu, Y.C. Preparation and properties of FeAl/Al2O3 composite tritium permeation barrier coating on surface of 316L stainless steel. Surf. Coat. Technol. 2020, 383, 125382. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yang, T.T.; Zhu, L.; Luo, L.M.; Liu, D.G.; Wu, Y.C. Preparation and properties of Al2O3/SiO2 composite tritium permeation barrier and its effects on the inner walls of pipelines. Fusion Eng. Des. 2022, 179, 113134. [Google Scholar] [CrossRef]
- Wu, Y.P.; Zhu, S.F.; Zhang, Y.P.; Liu, T.W.; Rao, Y.C.; Luo, L.Z.; Wang, Q.G. The adhesion strength and deuterium permeation property of SiC films synthesized by magnetron sputtering. Int. J. Hydrogen Energy 2016, 41, 10827–10832. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Xiang, Q.Y.; Cao, J.L. Performances of AlN coatings as hydrogen isotopes permeation barriers. Fusion Eng. Des. 2016, 102, 94–98. [Google Scholar] [CrossRef]
- Nemanič, V.; McGuiness, P.; Daneu, N.; Zajec, B.; Siketić, Z.; Waldhauser, W. Hydrogen permeation through silicon nitride films. J. Alloys Compd. 2012, 539, 184–189. [Google Scholar] [CrossRef]
- Tamura, M.; Noma, M.; Yamashita, M. Characteristic change of hydrogen permeation in stainless steel plate by BN coating. Surf. Coat. Technol. 2014, 260, 148–154. [Google Scholar] [CrossRef]
- Bazzanella, N.; Checchetto, R.; Miotello, A.; Patton, B.; Kale, A.N.; Kothari, D.C. High temperature efficient deuterium permeation and oxidation (Al,Ti)N barriers deposited on stainless steel. Appl. Phys. Lett. 2002, 81, 3762–3764. [Google Scholar] [CrossRef]
- Mcguiness, P.J.; Čekada, M.; Nemanič, V.; Zajec, B.; Recnik, A. Hydrogen permeation through TiAlN-coated Eurofer’97 steel. Surf. Coat. Technol. 2011, 205, 2709–2713. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.W.; Feng, S.J.; Zhang, Y.; Ouyang, T.Y.; Suo, J.P. Preparation of tritium permeation barrier consisting of titanium by the pack cementation method. Surf. Coat. Technol. 2016, 307, 271–277. [Google Scholar] [CrossRef]
- Checchetto, R.; Bonelli, M.; Gratton, L.M.; Miotelloa, A.; Sabbionia, A.; Guzmanb, L.; Horinoc, Y.; Benamatid, G. Analysis of the hydrogen permeation properties of TiN-TiC bilayers deposited on martensitic stainless steel. Surf. Coat. Technol. 1996, 83, 40–44. [Google Scholar] [CrossRef]
- Chen, W.D.; Wang, L.J.; Chen, S.; Luo, Y.H.; Han, L.; Zhang, J.D.; Yin, Y.X. Hydrogen Permeation Barrier of Chromium Coatings Electrodeposited on Zirconium Hydride. Rare Met. 2007, 31, 102–104. [Google Scholar]
- Zhong, X.K.; Yan, G.Q.; Chen, W.D. Effect of Na5P3O10 Addition on the MAO Coating on the Surface of ZrH1.8. Rare Met. Mater. Eng. 2012, 41, 541–544. [Google Scholar]
- Yan, S.F.; Liu, X.D.; Chen, W.D.; Wang, Z.G.; Fan, X.J.; Xu, Z.G. Influence of Oxidation time on MAO Film on the Surface of ZrH1.8 in a Phosphate System. Rare Met. Mater. Eng. 2014, 43, 1717–1721. [Google Scholar]
- Wu, M.; Chen, Y.; Yan, G.Q.; Peng, J.Q.; Sun, Y.P.; Wang, L.J. Structure and Performance of KH570/A171 Modified Silica Sol Film on Zirconium Hydride Surface. Rare Met. Mater. Eng. 2018, 47, 630–635. [Google Scholar]
- Wu, M.; Chen, Y.; Peng, J.Q.; Yan, G.Q.; Sun, Y.P.; Zhang, J.D.; Zhang, S.L.; Wang, L.J. Hydrogen permeation resistance and characterization of Si-Al and Si-Zr composite sol oxide coating on surface of zirconium hydride. Rare Met. 2017, 36, 55–60. [Google Scholar] [CrossRef]
- Wu, M.; Peng, J.Q.; Yan, G.Q.; Chen, Y.; He, Z.Y.; Xu, Z.G.; Wang, L.J. Preparation and properties of composite hydrogen permeation barrier on ZrH1.8 by sol–gel technique. Surf. Coat. Technol. 2018, 352, 159–165. [Google Scholar] [CrossRef]
- Chen, W.D.; Wang, L.J.; Wang, J.W.; Yan, S.F. Oxidation behavior of zirconium hydride in O2 and CO2. Rare Met. Mater. Eng. 2008, 37, 1970–1972. [Google Scholar]
- Chen, W.D.; Wang, L.J.; Lu, S.G. Influence of oxide layer on hydrogen desorption from zirconium hydride. J. Alloys Compd. 2009, 469, 142–145. [Google Scholar] [CrossRef]
- Peng, J.Q.; Gu, Z.C.; Chen, Y.; Yan, G.Q.; Wang, L.J.; Wu, M. Hydrogen desorption behavior of the hydrides of Zr-Y alloys under Ar and CO2 atmosphere. J. Alloys Compd. 2017, 693, 103–109. [Google Scholar] [CrossRef]
- Yan, G.Q.; Chen, Y.; Wu, M.; Peng, J.Q.; Wang, L.J. AES/XPS analysis of in-situ reaction layer between urea and zirconium hydride at high temperature. Rare Met. Mater. Eng. 2016, 45, 3302–3305. [Google Scholar]
- Yan, G.Q.; Chen, Y.; Peng, J.Q.; Wu, M.; Zhang, J.D.; Zhang, S.L.; Wang, L.J. Preparation of Multi-Component Coating on the Surface of Zirconium Hydride. Rare Met. Mater. Eng. 2017, 46, 3838–3842. [Google Scholar]
- Bai, S.; Yuan, X.M.; Yan, G.Q.; Liu, Z.X.; Yang, H.G.; Wang, L.J. Hydrogen permeation rate and stability of in-situ oxidation layer on surface of ZrH1.85 at 600 °C. Rare Met. 2020, 44, 41–47. [Google Scholar]
- Qi, S.; Ma, Z.H.; Yan, G.Q.; Wang, Z.H.; Wang, L.J. Hydrogen permeation rate of coating zirconium hydride moderator-a prediction model. Int. J. Hydrog. Energy 2021, 45, 14710–14719. [Google Scholar] [CrossRef]
- Wolowiec, E.; Michalski, J.; Kucharska, B. Kinetic aspects of low-pressure nitriding process. Vacuum 2018, 155, 292–299. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, H.F.; Ren, Z.C.; Zhao, J.Y.; Hou, X.N.; Doll, G.L.; Donga, Y.L.; Ye, C. Low-temperature nitriding of nanocrystalline Inconel 718 alloy. Surf. Coat. Technol. 2017, 330, 10–16. [Google Scholar] [CrossRef]
- Chen, X.; Bao, X.Y.; Xiao, Y.; Zhang, C.S.; Tang, L.N.; Yao, L.; Cui, G.D.; Yang, Y. Low-temperature gas nitriding of AISI 4140 steel accelerated by LaFeO3 perovskite oxide. Appl. Surf. Sci. 2019, 466, 989–999. [Google Scholar] [CrossRef]
- Kucharska, B.; Michalski, J.; Wójcik, G. Mechanical and microstructural aspects of C20-steel blades subjected to gas nitriding. Arch. Civ. Mech. Eng. 2019, 19, 147–156. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Ali, H.; Karatas, C.; Al-Sharafi, A. Laser gas assisted nitriding and characterization of tungsten surface. Opt. Laser Technol. 2018, 107, 274–280. [Google Scholar] [CrossRef]
- Qin, J.W.; Wang, X.F.; Zhang, Y.B.; Hu, Y.; Lu, L.; Zhou, P.; Li, F.F.; Zhang, Y.Z.; Liu, K.Z.; Shuai, M.B. Oxidation kinetics of uranium treated by pulsed laser nitriding in air. Surf. Coat. Technol. 2019, 357, 864–869. [Google Scholar] [CrossRef]
- Wang, W.H.; Xue, B.J.; Guo, L.T.; Fan, Y.; Li, B.; Ling, Y.H.; Qiang, Y.H. Laser nitriding and fusion of bonding porcelain coating on Ti surface. J. Alloys Compd. 2019, 777, 392–396. [Google Scholar] [CrossRef]
- Nishimoto, A.; Fukube, T.; Maruyama, T. Microstructural, mechanical, and corrosion properties of plasma-nitrided CoCrFeMnNi high-entropy alloys. Surf. Coat. Technol. 2019, 376, 55–58. [Google Scholar] [CrossRef]
- Kurelo, B.S.; Oliveira, W.R.; Serbena, F.C. Surface mechanics and wear resistance of supermartensitic stainless steel nitrided by plasma immersion ion implantation. Surf. Coat. Technol. 2018, 353, 199–209. [Google Scholar] [CrossRef]
- Kadri, E.; Dhahri, K.; Zaafouri, A.; Krichen, M. Ac conductivity and dielectric behavior of a−Si: H/c−Si Ge/p−Si thin films synthesized by Molecular Beam Epitaxial method. J. Alloys Compd. 2017, 705, 708–713. [Google Scholar] [CrossRef]
- Kadri, E.; Krichen, M.; Mohammed, R.; Zaafouri, A.; Khirouni, K. Electrical transport mechanisms in amorphous silicon/crystalline silicon germanium heterojunction solar cell: Impact of passivation layer in conversion efficiency. Opt. Quant. Electron. 2016, 48, 546.1–546.15. [Google Scholar] [CrossRef]
- Rasheed, M.; Shihab, S.; Sabah, O.W. An investigation of the structural, electrical and optical properties of graphene-oxide thin films using different solvents. J. Phys. Conf. Ser. 2021, 1795, 012052. [Google Scholar] [CrossRef]
- Abbas, M.M.; Rasheed, M. Solid state reaction synthesis and characterization of Cu doped TiO2 nanomaterials. J. Phys. Conf. Ser. 2021, 1795, 012059. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Qin, Y.C. Metallurgy and Heat Treatment; China Machine Press: Beijing, China, 2007; pp. 299–302. [Google Scholar]
- Mike, M.; Florian, S.; Uwe, G. On the estimation of the diffusion coefficient and distribution of hydrogen in stainless steel. Surf. Coat. Technol. 2018, 339, 139–146. [Google Scholar]
- Zhang, J.S.; Liao, J.J.; Wei, T.G.; Long, C.S. Oxygen diffusion behaviors during vacuum annealing treatment of oxidized zirconium. Rare Met. Mater. Eng. 2021, 50, 1590–1595. [Google Scholar]
- Fang, J.L.; Liu, Q.; Han, K.P.; Chen, Y.H. Study of the black molybdate conversion film on stainless steel. Electrochemistry 1995, 1, 193–197. [Google Scholar]
- Wang, W.K.; Yan, G.Q.; Zhang, J.D.; Ma, Z.H.; Wang, L.J.; Guo, Z.C.; Zhang, S.L.; Wu, Y.K. Hydrogen permeation behavior of zirconium nitride film on zirconium hydride. Materials 2022, 15, 550. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, X.; Xiong, Y.; Shi, Y.; Song, J.F.; Luo, D.L. Mechanism for adsorption, dissociation and diffusion of hydrogen in hydrogen permeation barrier of α-Al2O3: A density functional theory study. Int. J. Hydrogen Energy 2013, 38, 1157–1165. [Google Scholar] [CrossRef]
- Wang, J.P.; Lu, Z.X.; Ling, Y.H.; Wang, R.G.; Li, Y.H.; Zhou, Q.Y.; Zhang, Z.J. Hydrogen permeation properties of CrxCy/Cr2O3/Al2O3 composite coating derived from selective oxidation of a Cr-C alloy and atomic layer deposition. Int. J. Hydrogen Energy 2018, 43, 21133–21141. [Google Scholar] [CrossRef]
- Deminsky, M.; Knizhnik, A.; Belov, I.; Umanskii, S.; Rykova, E.; Bagatur’yants, A.; Potapkin, B.; Stoker, M.; Korkin, A. Mechanism and kinetics of thin zirconium and hafnium oxide film growth in an ALD reactor. Surf. Sci. 2004, 549, 67–86. [Google Scholar] [CrossRef]


| No. | Atmosphere | t/h | T/°C | Test Content |
|---|---|---|---|---|
| 1 | N2 | 20 | 500 | SEM, XRD, H content |
| 2 | 600 | SEM, XRD, H content | ||
| 3 | 700 | SEM, XRD, H content | ||
| 4 | 800 | SEM, XRD, H content | ||
| 5 | 5 | 800 | SEM, XRD, H content | |
| 6 | N2 + H2 | 20 | 500 | SEM, XRD, H content, AES |
| 7 | 600 | SEM, XRD, H content, AES | ||
| 8 | 700 | SEM, XRD, H content, AES | ||
| 9 | 800 | SEM, XRD, H content, AES |
| Films | Atmosphere | T/°C | t/h |
|---|---|---|---|
| nitride film 1 | N2 + H2 | 800 | 20 |
| nitride film 2 | N2 | 800 | 20 |
| oxide film | CO2 | 600 | 20 |
| Atmosphere | T/°C | t/h | H/Zr Atomic Ratio | Standard Deviation σ | Hydrogen Loss Rate/% |
|---|---|---|---|---|---|
| zirconium hydride | 1.8510 | 0.0338 | - | ||
| N2 | 500 | 20 | 1.8322 | 0.0283 | 1.016 |
| 600 | 1.8322 | 0.0449 | 1.016 | ||
| 700 | 1.8228 | 0.0164 | 1.524 | ||
| 800 | 1.8077 | 0.0192 | 2.339 | ||
| 800 | 5 | 1.8303 | 0.0256 | 1.118 | |
| N2 + H2 | 500 | 20 | 1.8473 | 0.0337 | 0.200 |
| 600 | 1.8435 | 0.0444 | 0.405 | ||
| 700 | 1.8410 | 0.0175 | 0.540 | ||
| 800 | 1.8397 | 0.0456 | 0.610 | ||
| Atmosphere | t/h | T/°C | XRD | Phase | ||||
|---|---|---|---|---|---|---|---|---|
| N2 | 20 | 500 | XRD | ZrH1.801 | - | - | - | - |
| GIXRD | ZrH1.801 | m-ZrO2 | - | - | - | |||
| 600 | XRD | ZrH1.801 | m-ZrO2 | - | - | - | ||
| GIXRD | ZrH1.801 | m-ZrO2 | ZrN | - | - | |||
| 700 | XRD | ZrH1.801 | m-ZrO2 | ZrN | - | - | ||
| GIXRD | ZrH1.801 | m-ZrO2 | ZrN | - | - | |||
| 800 | XRD | ZrH1.801 | ZrO | ZrN | ZrN(NH2) | ZrN0.36H0.8 | ||
| GIXRD | ZrH | m-ZrO2 | ZrN | ZrN(NH2) | ZrN0.36H0.8 | |||
| 5 | 800 | XRD | ZrH1.801 | m-ZrO2 | ZrN | - | - | |
| GIXRD | ZrH1.801 | m-ZrO2 | ZrN | - | - | |||
| N2 + H2 | 20 | 500 | XRD | ZrH1.801 | - | - | - | - |
| GIXRD | ZrH1.801 | m-ZrO2 | - | - | - | |||
| 600 | XRD | ZrH1.801 | m-ZrO2 | - | - | - | ||
| GIXRD | ZrH1.801 | m-ZrO2 | ZrN | - | - | |||
| 700 | XRD | ZrH1.801 | m-ZrO2 | ZrN | - | - | ||
| GIXRD | ZrH1.801 | m-ZrO2 | ZrN | - | - | |||
| 800 | XRD | ZrH1.801 | m-ZrO2 | ZrN | ZrN(NH2) | ZrN0.36H0.8 | ||
| GIXRD | ZrH | ZrO/m-ZrO2 | ZrN | ZrN(NH2) | ZrN0.36H0.8 | |||
| Elements | T/°C | x1/nm | x2/nm | c1/at% | cx/at% | k/(cm2/s) |
|---|---|---|---|---|---|---|
| N | 500 | 14 | 70 | 23.5314 | 9.9047 | 3.36 × 10−16 |
| 600 | 28 | 1428 | 37.2274 | 8.5421 | 9.42 × 10−14 | |
| 700 | 28 | 1484 | 35.0039 | 11.4784 | 1.54 × 10−13 | |
| 800 | 912 | 10,608 | 61.7605 | 16.6006 | 5.34 × 10−12 | |
| O | 500 | 42 | 896 | 55.8545 | 5.2977 | 1.82 × 10−14 |
| 600 | 84 | 1540 | 32.0525 | 5.5136 | 7.55 × 10−14 | |
| 700 | 84 | 1568 | 32.1972 | 4.9837 | 7.89 × 10−14 | |
| 800 | 48 | 1680 | 12.0662 | 1.5902 | 8.14 × 10−14 |
| Film | Hydrogen Loss Rate of Hydrogen Determinator Method/% | Hydrogen Loss Rate of Gas Chromatography Method/% |
|---|---|---|
| Nitride film 1 | 0.497 | 0.576 |
| Nitride film 2 | 1.796 | 1.204 |
| Oxide film | 3.248 | 2.319 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yan, G.; Ma, Z.; Zhang, J.; Wang, L.; Guo, Z. Mechanisms of Growth and Hydrogen Permeation of Zirconium Nitride Film on Zirconium Hydride. Materials 2023, 16, 349. https://doi.org/10.3390/ma16010349
Wang W, Yan G, Ma Z, Zhang J, Wang L, Guo Z. Mechanisms of Growth and Hydrogen Permeation of Zirconium Nitride Film on Zirconium Hydride. Materials. 2023; 16(1):349. https://doi.org/10.3390/ma16010349
Chicago/Turabian StyleWang, Wenke, Guoqing Yan, Zhaohui Ma, Jiandong Zhang, Lijun Wang, and Zhancheng Guo. 2023. "Mechanisms of Growth and Hydrogen Permeation of Zirconium Nitride Film on Zirconium Hydride" Materials 16, no. 1: 349. https://doi.org/10.3390/ma16010349
APA StyleWang, W., Yan, G., Ma, Z., Zhang, J., Wang, L., & Guo, Z. (2023). Mechanisms of Growth and Hydrogen Permeation of Zirconium Nitride Film on Zirconium Hydride. Materials, 16(1), 349. https://doi.org/10.3390/ma16010349





