Microstructural Characterization of the As-Cast and Homogenized Al-Cu-Mg-Ag Alloy
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. As-Cast Microstructure
3.2. Homogenization Heat Treatment of the Large-Scale Al-Cu-Mg-Ag Alloy Ingots
3.3. Optimization for the Al-Cu-Mg-Ag Alloy Homogenization Heat Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Yang, L.; Zhan, L.; Yu, H.; Huang, M. Creep Mechanisms of an Al-Cu-g Alloy at the Macro-and Micro-Scale: Effect of the S′/S Precipitate. Materials 2019, 12, 2907. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Liu, Z.; Zhao, Q.; Bai, S.; Liu, F. P-Texture effect on the fatigue crack propagation resistance in an Al-Cu-Mg alloy bearing a small amount of silver. Materials 2018, 11, 2481. [Google Scholar] [CrossRef] [Green Version]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminum alloys. Mater. Des. (1980–2015) 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Sakai, Y. The current state and future of aluminum alloy applications for railway rolling stock. Light Met. Age 2007, 65, 14–17. [Google Scholar]
- Zhang, X.; Chen, Y.; Hu, J. Recent advances in the development of aerospace materials. Prog. Aerosp. Sci. 2018, 97, 22–34. [Google Scholar] [CrossRef]
- Williams, J.C.; Starke, E.A., Jr. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, H. Precipitation Behavior during Aging Operations in an Ultrafine-Grained Al–Cu–Mg Alloy Produced by High-Strain-Rate Processing. Materials 2022, 15, 8687. [Google Scholar] [CrossRef]
- Xiong, B. The 4th chapter of the report on the development of advanced materials industries of China. In Proceedings of the Forum on Science and Technology in China, Beijing, China, 30 June 2010; pp. 40–51. [Google Scholar]
- Liu, X.; Pan, Q.; Lu, Z.; Cao, S.; He, Y.; Li, W. Thermal stability of Al-Cu-Mg-Ag heat resistant alloy. Chin. J. Nonferrous Met. 2011, 21, 1244–1251. [Google Scholar]
- Zhang, K.; Dai, S.; Yang, S.; Huang, M.; Yan, M. Research progress of Al-Cu-Mg-Ag new heat-resistant aluminum alloys. J. Aeronaut. Mater. 2006, 3, 251–257. [Google Scholar]
- Liu, X.; Pan, Q.; Fan, X.; He, Y.; Li, W.; Liang, W. Microstructural evolution of Al-Cu-Mg-Ag alloy during homogenization. J. Alloys Compd. 2009, 484, 790–794. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, B.; Zhang, Y.; Li, Z.; Li, P. Microstructural characterization of as-cast and homogenized 2D70 aluminum alloy. Int. J. Miner. Metall. Mater. 2009, 16, 427–431. [Google Scholar] [CrossRef]
- Polmear, I.J.; Couper, M.J. Design and development of an experimental wrought aluminum alloy for use at elevated temperatures. Metall. Trans. A 1988, 19, 1027–1035. [Google Scholar] [CrossRef]
- Polmear, I.J.; Chester, R.J. Abnormal age hardening in an Al-Cu-Mg alloy containing silver and lithium. Scr. Metall. 1989, 23, 1213–1217. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Liu, Y.; Xia, Q. Research progress of Al-Cu-Mg-Ag alloy precipitates. Chin. J. Nonferrous Met. 2007, 12, 1905–1915. [Google Scholar]
- Mukhopadhyay, A.K.; Eggeler, G.; Skrotzki, B. Nucleation of ω phase in an Al-Cu-Mg-Mn-Ag alloy aged at temperatures below 200 °C. Scr. Mater. 2001, 44, 545–551. [Google Scholar] [CrossRef]
- Fan, J.; Yang, B.; Wang, Y.; Gao, M.; Guan, R. Enhancing the tensile strength and heat resistance induced by high-density Ω phases in an Al-Cu-Mg-Ag alloy. J. Mater. Res. Technol. 2022, 18, 3347–3357. [Google Scholar] [CrossRef]
- Zhang, W.L.; Xiao, D.H.; Li, T.; Du, J.; Ding, D. Microstructure and mechanical properties of two-stage aged Al-Cu-Mg-Ag-Sm alloy. Rare Met. 2019, 38, 42–51. [Google Scholar] [CrossRef]
- Mei, Z.; Liu, Z.; Bai, S.; Wang, J.; Gao, T. Effects of yttrium additions on microstructures and mechanical properties of cast Al-Cu-Mg-Ag alloys. J. Alloys Compd. 2021, 870, 159435. [Google Scholar] [CrossRef]
- Li, B.; Liang, S.; Wen, S.; Zhao, Z.; Wu, X.; Wang, W.; Gao, K.; Huang, H.; Nie, Z. Competition between precipitation and segregation of Sc and its effects on thermal stability of Al-Cu-Mg-Ag alloys. Mater. Lett. 2021, 297, 129927. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, J.; Cao, J.; Luo, L.; Guo, S.; Ou, L.; Liu, Z.; Bai, S. Effect of Minor Er Additions on the Microstructures and Mechanical Properties of Cast Al-Cu-Mg-Ag Alloys. Materials 2021, 14, 4212. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Z.; Yu, R.; Li, Y. Effects of homogenization annealing on the microstructure and properties of Al-Cu-Mg-Ag alloys. Hot Work. Technol. 2006, 7, 8–10. [Google Scholar]
- Li, Y.; Liu, Z.; Xia, Q.; Yu, R. Existing form of Er in Al-Cu-Mg-Ag alloy and its homogenization process. J. Cent. South Univ. (Nat. Sci. Ed.) 2006, 6, 1043–1047. [Google Scholar]
- Ma, F.; Liu, Z.; Deng, C.; Bai, S.; Luo, H.; Li, Y. Microstructure evolution of Al-Cu-Mg-Ag alloy containing trace Zr during multi-stage homogenization heat treatment. J. Mater. Heat Treat. 2009, 30, 96–101. [Google Scholar]
- Li, Y.; Liu, Z.; Xia, Q.; Zhou, J.; Yu, R. Study on the homogenization process of cerium-containing Al-Cu-Mg-Ag-Mn-Zr aluminum alloy. Met. Heat Treat. 2006, 10, 55–57. [Google Scholar]
- Liu, Q.; Li, X.; Li, Z.; Chen, L.; Lin, H. Effect of Cu Content on the Microstructures of As-Cast and Homogenized Al-Cu-Mg-Ag Alloys. Key Eng. Mater. 2022, 921, 59–64. [Google Scholar] [CrossRef]


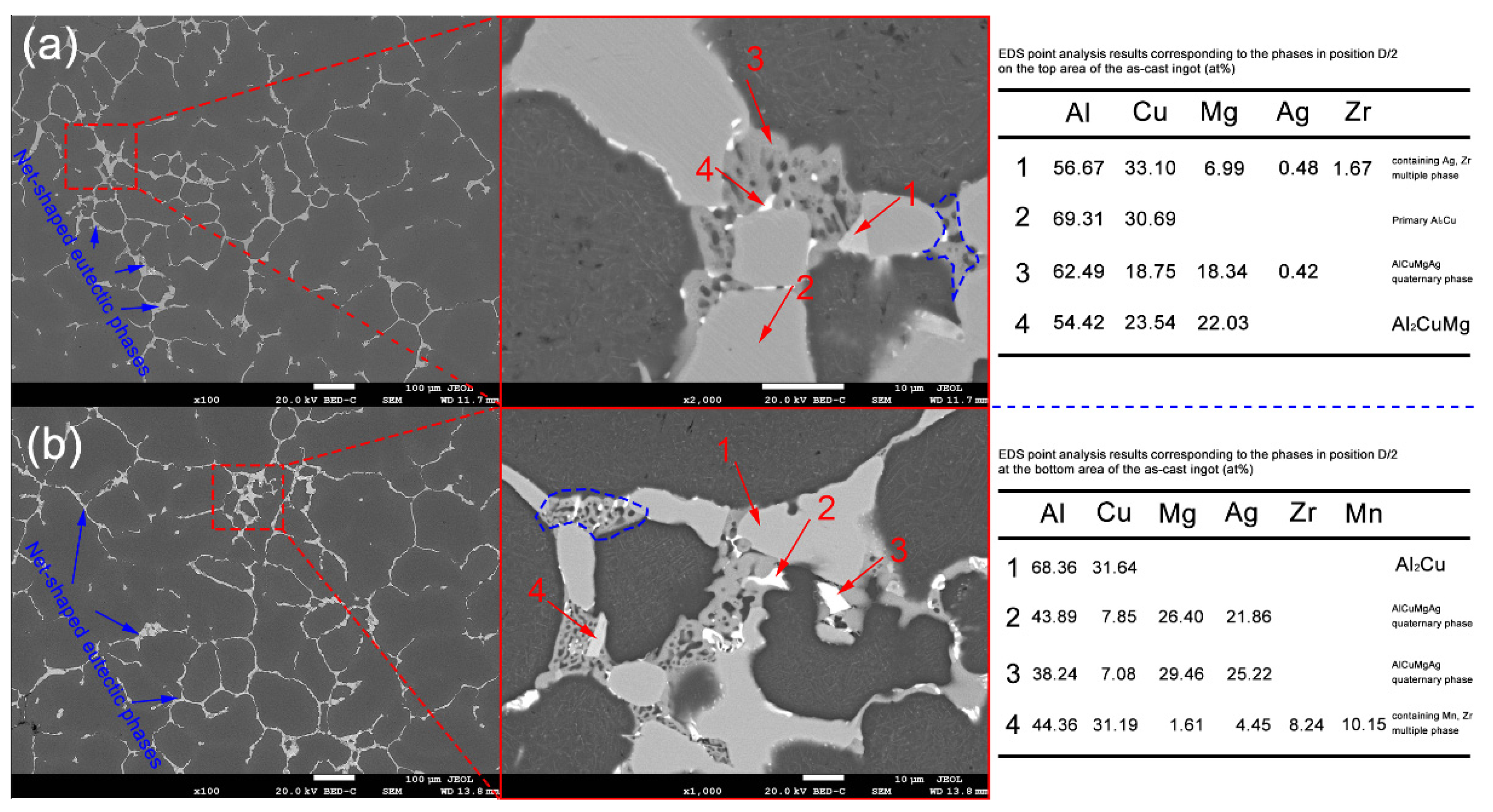
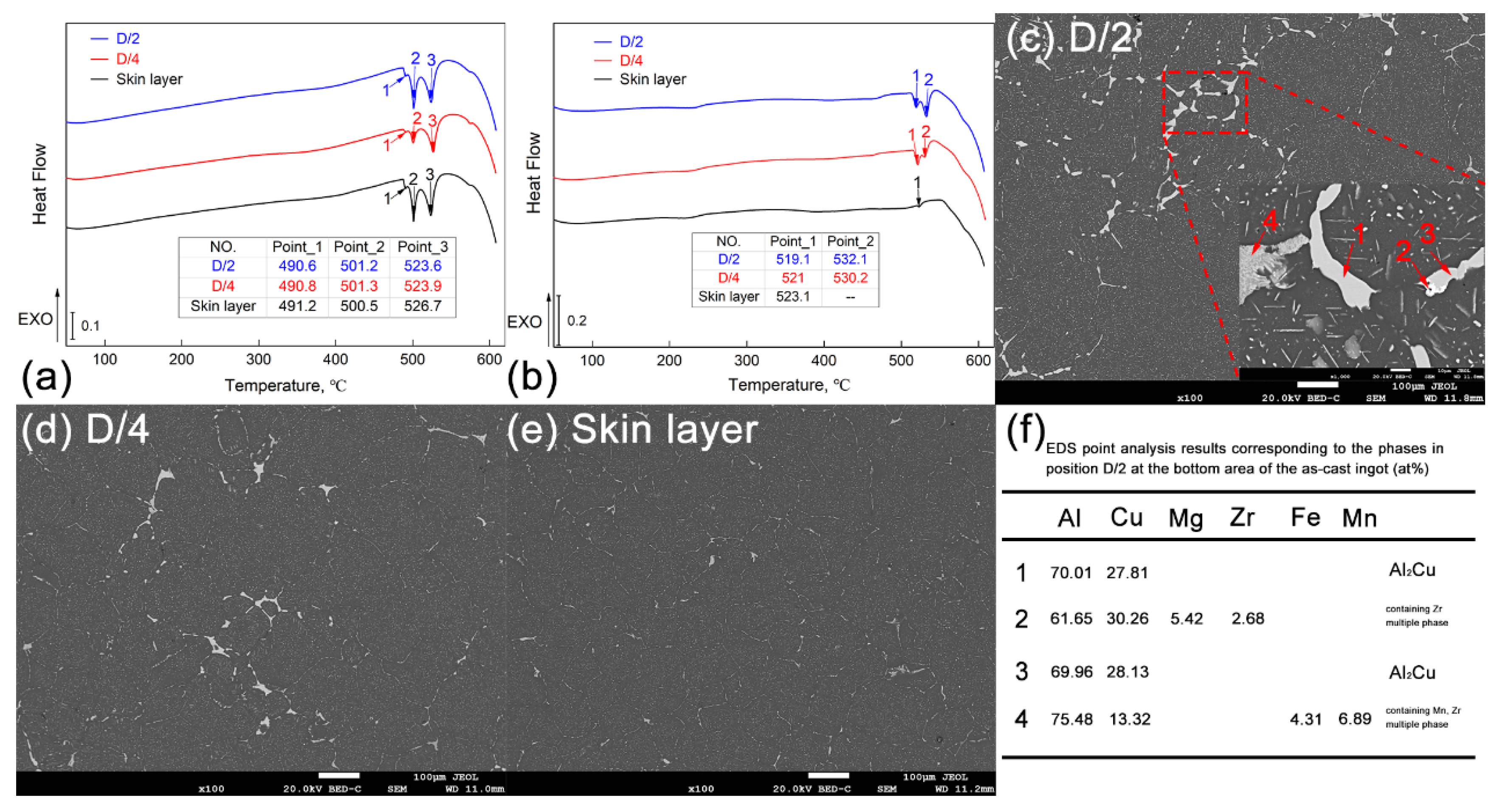
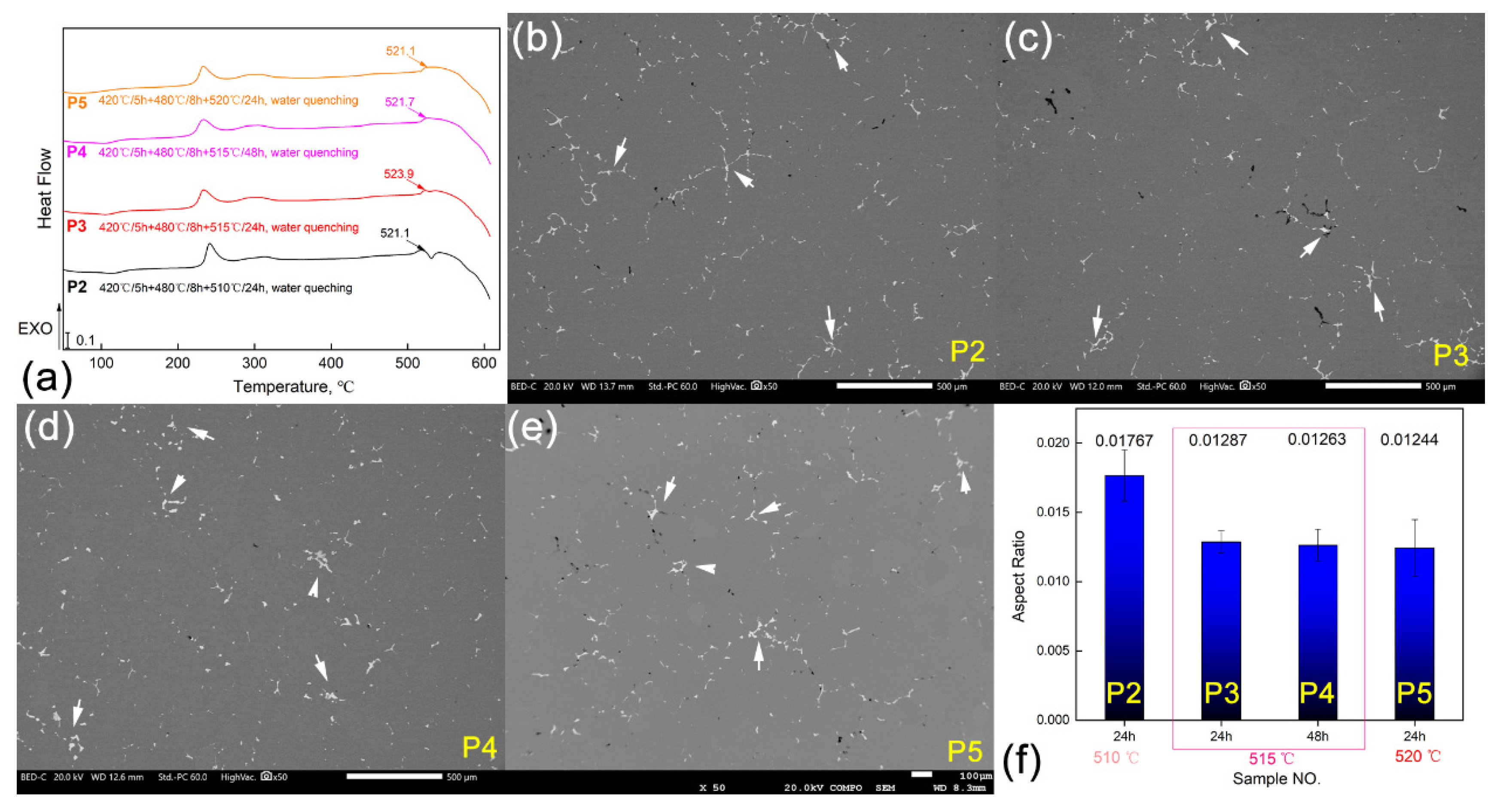
| Positions | NO. | Element Type (w/%) | |||||
|---|---|---|---|---|---|---|---|
| Mg | Mn | Cu | Ag | Zr | Al | ||
| Top area | D/2 | 0.96 | 0.62 | 5.30 | 0.62 | 0.11 | Bal |
| D/4 | 0.96 | 0.66 | 5.24 | 0.62 | 0.12 | Bal | |
| Skin layer | 0.80 | 0.60 | 4.14 | 0.53 | 0.13 | Bal | |
| Bottom area | D/2 | 0.92 | 0.61 | 5.20 | 0.60 | 0.11 | Bal |
| D/4 | 0.94 | 0.66 | 5.23 | 0.62 | 0.12 | Bal | |
| Skin layer | 0.82 | 0.60 | 4.43 | 0.54 | 0.12 | Bal | |
| NO. | Heat Treatment Parameters | Position |
|---|---|---|
| P1 | 420 °C/5 h + 480 °C/8 h + 510 °C/24 h, air cooling | D/2, D/4, Skin layer |
| P2 | 420 °C/5 h + 480 °C/8 h + 510 °C/24 h, water quenching | D/4 |
| P3 | 420 °C/5 h + 480 °C/8 h + 515 °C/24 h, water quenching | |
| P4 | 420 °C/5 h + 480 °C/8 h + 515 °C/48 h, water quenching | |
| P5 | 420 °C/5 h + 480 °C/8 h + 520 °C/24 h, water quenching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Zhu, K.; Liu, Q.; Chen, L.; Wang, Z.; Li, X. Microstructural Characterization of the As-Cast and Homogenized Al-Cu-Mg-Ag Alloy. Materials 2023, 16, 433. https://doi.org/10.3390/ma16010433
Lin H, Zhu K, Liu Q, Chen L, Wang Z, Li X. Microstructural Characterization of the As-Cast and Homogenized Al-Cu-Mg-Ag Alloy. Materials. 2023; 16(1):433. https://doi.org/10.3390/ma16010433
Chicago/Turabian StyleLin, Haitao, Kai Zhu, Qilong Liu, Lifang Chen, Zhengan Wang, and Xiwu Li. 2023. "Microstructural Characterization of the As-Cast and Homogenized Al-Cu-Mg-Ag Alloy" Materials 16, no. 1: 433. https://doi.org/10.3390/ma16010433
APA StyleLin, H., Zhu, K., Liu, Q., Chen, L., Wang, Z., & Li, X. (2023). Microstructural Characterization of the As-Cast and Homogenized Al-Cu-Mg-Ag Alloy. Materials, 16(1), 433. https://doi.org/10.3390/ma16010433






