In-Situ Growth of ZnO Whiskers on Ti2ZnC MAX Phases
Abstract
:1. Introduction
2. Materials and Methods
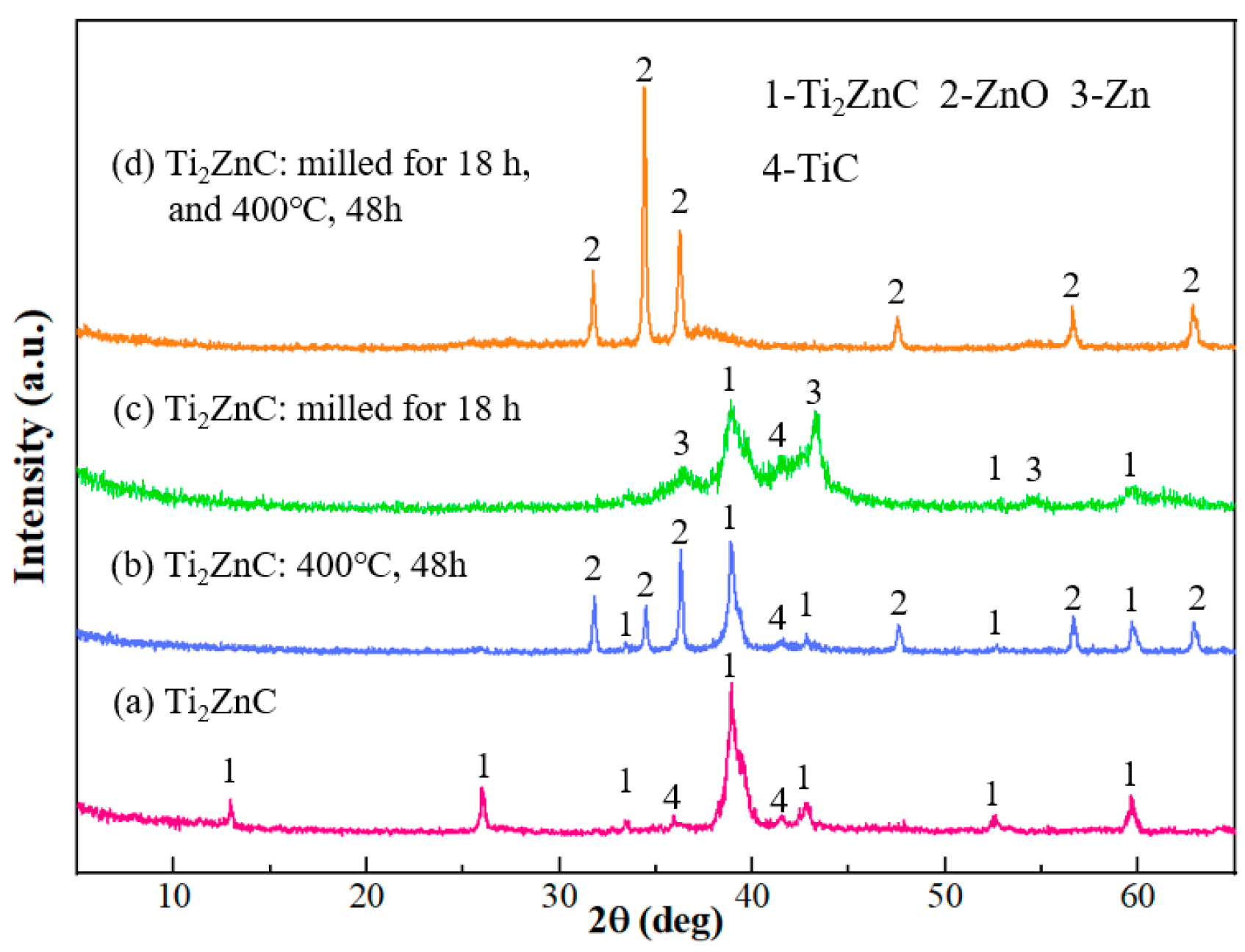
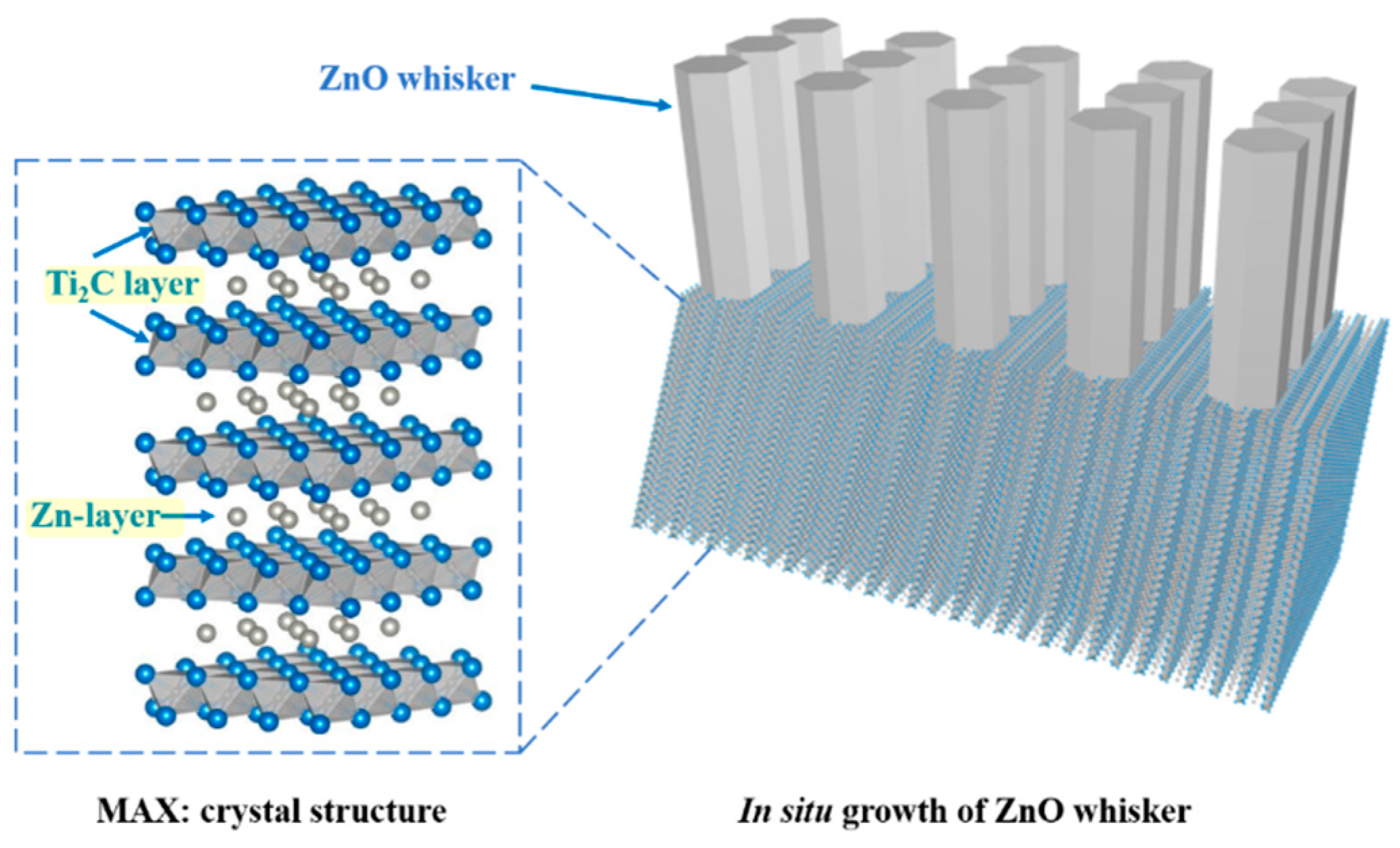
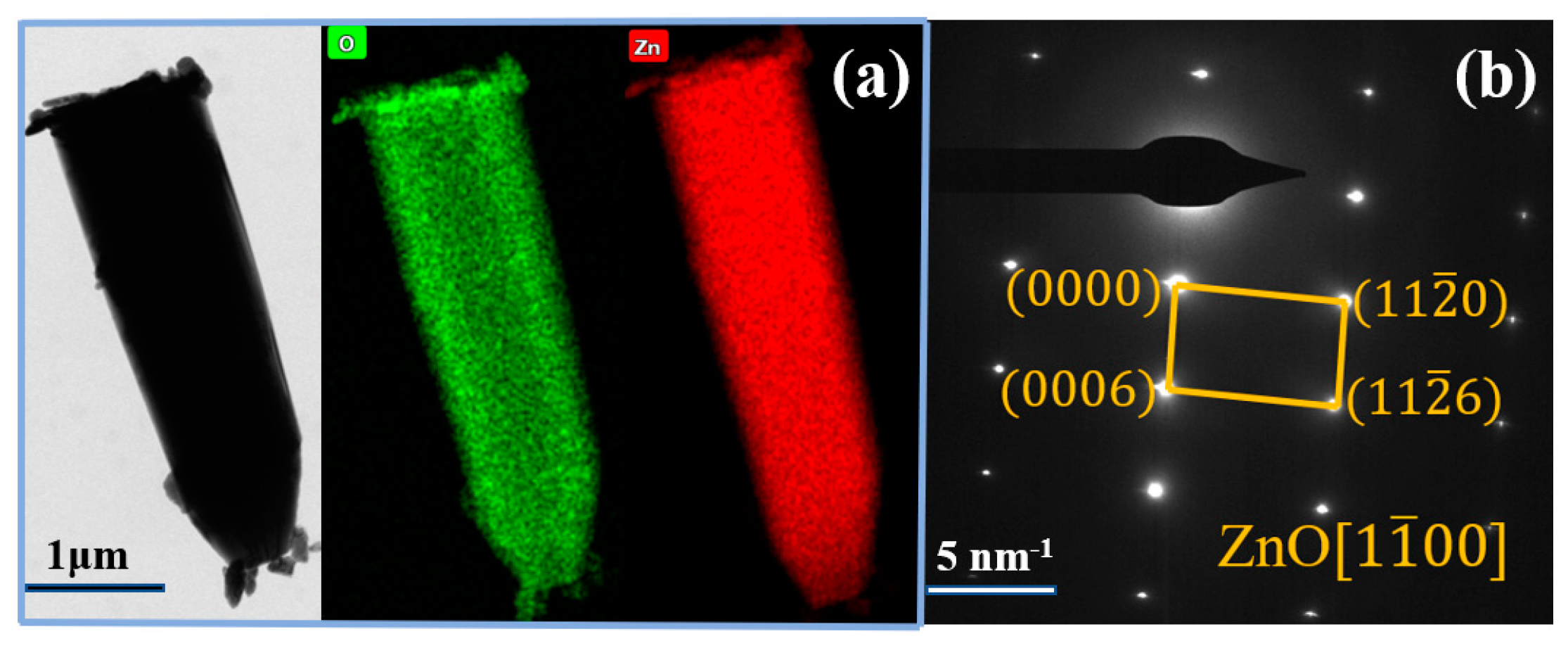
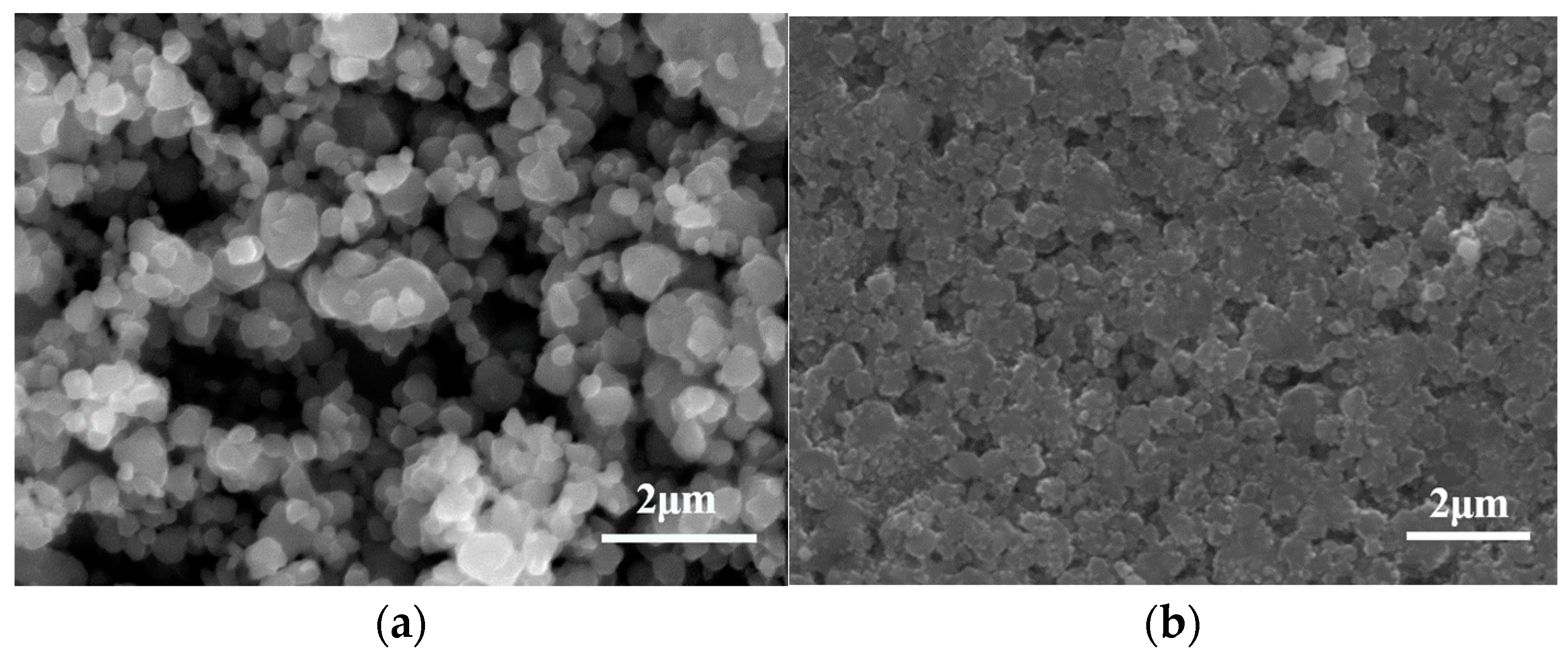
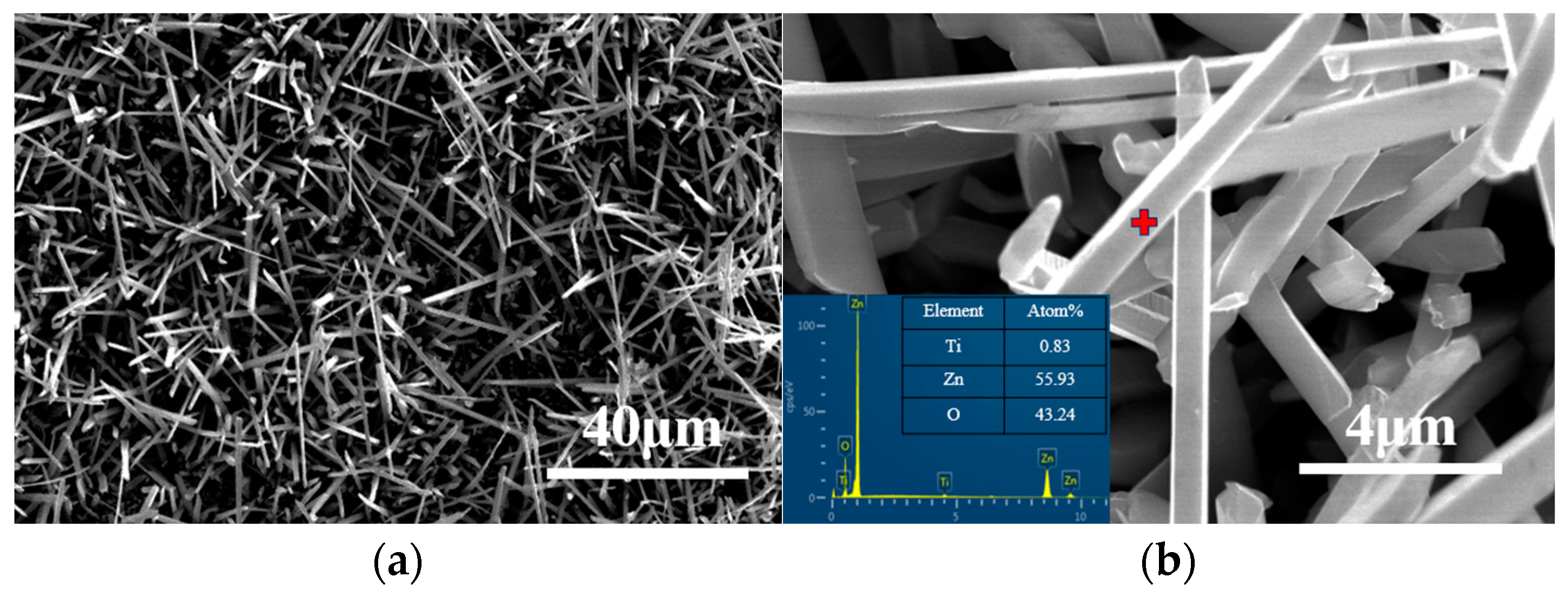
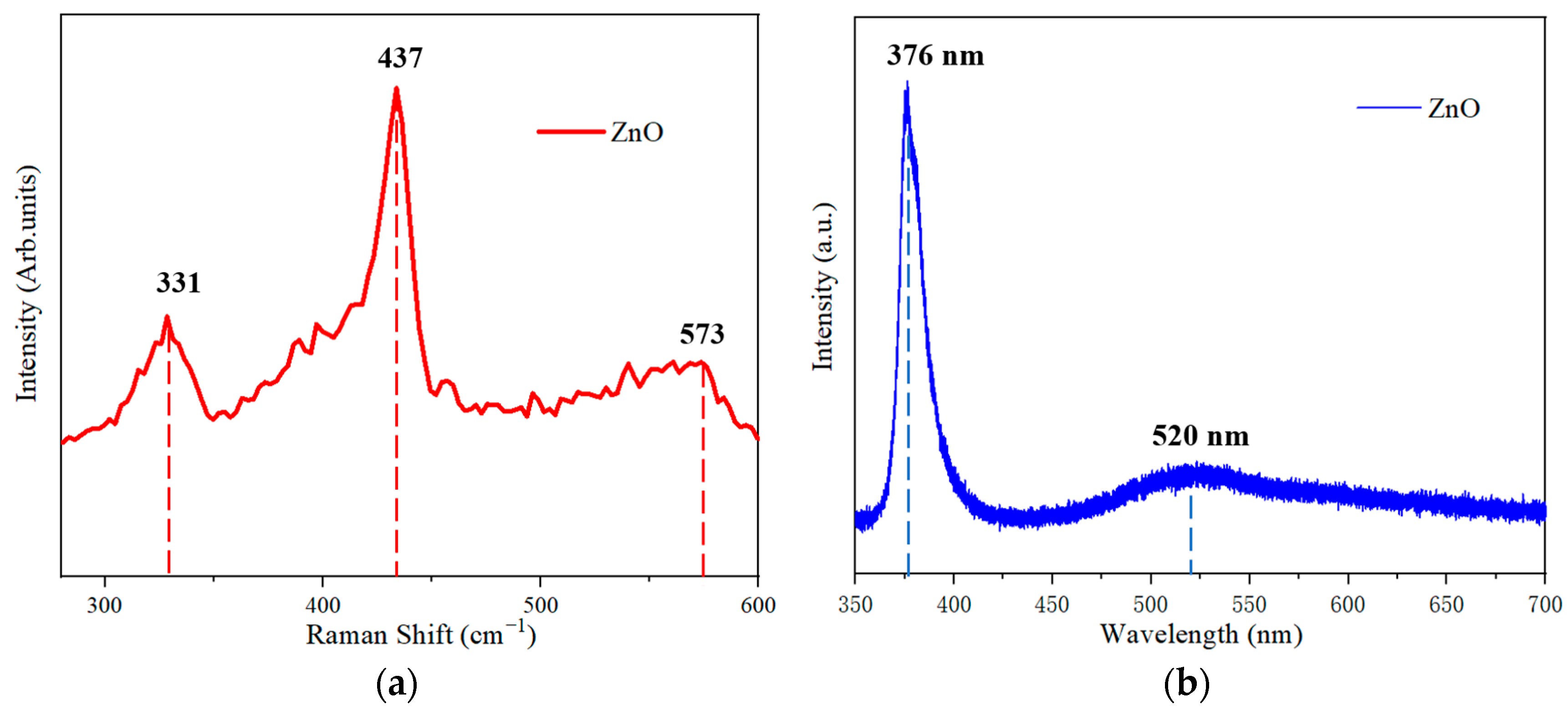
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wlazlo, M.; Haras, M.; Kolodziej, G.; Szawcow, O.; Ostapko, J.; Andrysiewicz, W.; Kharytonau, D.S.; Skotnicki, T. Piezoelectric Response and Substrate Effect of ZnO Nanowires for Mechanical Energy Harvesting in Internet-of-Things Applications. Materials 2022, 15, 6767. [Google Scholar] [CrossRef] [PubMed]
- Peksu, E.; Karaagac, H. Doping and annealing effects on structural, electrical and optical properties of tin-doped zinc-oxide thin films. J. Alloys Compd. 2018, 764, 616–625. [Google Scholar] [CrossRef]
- Qin, L.; Mawignon, F.J.; Hussain, M.; Ange, N.K.; Lu, S.; Hafezi, M.; Dong, G. Economic Friendly ZnO-Based UV Sensors Using Hydrothermal Growth: A Review. Materials 2021, 14, 4083. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ionics 2021, 360, 115544. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Petru, M.; Mahmood, A.; Kejzlar, P. Photocatalytic Behaviour of Zinc Oxide Nanostructures on Surface Activation of Polymeric Fibres. Polymers 2021, 13, 1227. [Google Scholar] [CrossRef]
- Gao, C.; Zhong, K.; Fang, X.; Fang, D.; Zhao, H.; Wang, D.; Li, B.; Zhai, Y.; Chu, X.; Li, J.; et al. Brief Review of Photocatalysis and Photoresponse Properties of ZnO–Graphene Nanocomposites. Energies 2021, 14, 6403. [Google Scholar] [CrossRef]
- Li, H.; Ding, J.; Cai, S.; Zhang, W.; Zhang, X.; Wu, T.; Wang, C.; Foss, M.; Yang, R. Plasmon-enhanced photocatalytic properties of Au/ZnO nanowires. Appl. Surf. Sci. 2022, 583, 152539. [Google Scholar] [CrossRef]
- Ma, W.; Xu, L.; Tian, Z.; Zang, A. Changes in photocatalytic activity and optical properties of ZnO whiskers induced by UV irradiation. J. Lumin. 2022, 249, 119015. [Google Scholar] [CrossRef]
- Shekofteh-Gohari, M.; Habibi-Yangjeh, A.; Abitorabi, M.; Rouhi, A. Magnetically separable nanocomposites based on ZnO and their applications in photocatalytic processes: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 806–857. [Google Scholar] [CrossRef]
- Ortiz-Casas, B.; Galdámez-Martínez, A.; Gutiérrez-Flores, J.; Baca Ibañez, A.; Kumar Panda, P.; Santana, G.; de la Vega, H.A.; Suar, M.; Gutiérrez Rodelo, C.; Kaushik, A.; et al. Bio-acceptable 0D and 1D ZnO nanostructures for cancer diagnostics and treatment. Mater. Today 2021, 50, 533–569. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J.J.S. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef]
- Le, A.T.; Le, T.D.H.; Cheong, K.-Y.; Pung, S.-Y. Immobilization of zinc oxide-based photocatalysts for organic pollutant degradation: A review. J. Environ. Chem. Eng. 2022, 10, 108505. [Google Scholar] [CrossRef]
- Bi, N.; Zhang, L.; Zheng, Q.; Zhuge, F.; Li, J.; Gao, X.P.A.; Du, J. Control of ZnO nanowire growth and optical properties in a vapor deposition process. J. Mater. Sci. Technol. 2017, 33, 850–855. [Google Scholar] [CrossRef]
- Guniat, L.; Caroff, P.; Fontcuberta, I.M.A. Vapor Phase Growth of Semiconductor Nanowires: Key Developments and Open Questions. Chem. Rev. 2019, 119, 8958–8971. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, P.C.; Fontcuberta i Morral, A. Semiconductor nanowires: To grow or not to grow? Mater. Today Nano 2020, 9, 100058. [Google Scholar] [CrossRef]
- Lin, C.; Li, Q.; Guang, H.; An, M. Electrodeposited Zn: A promising alternative to ZnO seed layer for hydrothermal growth of ZnO nanowire array. Mater. Lett. 2022, 314, 131848. [Google Scholar] [CrossRef]
- Almamari, M.R.; Ahmed, N.M.; Holi, A.M.; Yam, F.K.; Kyaw, H.H.; Almessiere, M.A.; Al-Abri, M.Z. Some Distinct Attributes of ZnO Nanorods Arrays: Effects of Varying Hydrothermal Growth Time. Materials 2022, 15, 5827. [Google Scholar] [CrossRef]
- Podrezova, L.V.; Porro, S.; Cauda, V.; Fontana, M.; Cicero, G. Comparison between ZnO nanowires grown by chemical vapor deposition and hydrothermal synthesis. Appl. Phys. A 2013, 113, 623–632. [Google Scholar] [CrossRef]
- Cui, J. Zinc oxide nanowires. Mater. Charact. 2012, 64, 43–52. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, D.; Mo, Y.; Song, L.; Brewer, E.; Huang, X.; Xiong, Y. Photocatalytic activity of polymer-modified ZnO under visible light irradiation. J. Hazard. Mater. 2008, 156, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Dutta, J. Photocatalytic degradation of organic dyes with manganese-doped ZnO nanoparticles. J. Hazard. Mater. 2008, 156, 194–200. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, Z.; Zhang, P.; Zhang, Y.; Liu, Y.; He, W.; Pan, L.; Liu, Y.; Sun, Z. Rapid and massive growth of tin whisker on mechanochemically decomposed Ti2SnC. Mater. Today Commun. 2022, 31, 103466. [Google Scholar] [CrossRef]
- Tian, Z.; Xu, X.; Tang, J.; Zhang, Q.; Wu, F.; Zhang, P.; Liu, J.; Sun, Z. Large-scale preparation of nano-sized carbides and metal whiskers via mechanochemical decomposition of MAX phases. Int. J. Appl. Ceram. Technol. 2022, 20, 823–832. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Zhang, Y.; Ding, J.; Shi, J.; Sun, Z.J.M.L. Spontaneous growth of Sn whiskers and a new formation mechanism. Mater. Lett. 2016, 178, 111–114. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, J.; Tang, H.; Tian, Z.; Zhang, P.; Zhang, Y.; Liu, J.; Sun, Z.M. Method for inhibiting Sn whisker growth on Ti2SnC. J. Mater. Sci. 2022, 57, 20462–20471. [Google Scholar] [CrossRef]
- Zhang, P.G.; Ding, J.X.; Liu, Y.S.; Yang, L.; Tian, W.B.; Ouyang, J.; Zhang, Y.M.; Sun, Z.M. Mechanism and mitigation of spontaneous Ga whisker growth on Cr2GaC. Sci. China Technol. Sci. 2020, 63, 440–445. [Google Scholar] [CrossRef]
- Sokol, M.; Natu, V.; Kota, S.; Barsoum, M.W. On the Chemical Diversity of the MAX Phases. Trends Chem. 2019, 1, 210–223. [Google Scholar] [CrossRef]
- Tian, Z.; Wu, F.; Hu, P.; Ding, J.; Zhang, Y.; Zhang, P.; Sun, Z. Synthesis of Ti3(SnxAl1−x)C2 solid solutions over the whole composition range. J. Alloys Compd. 2022, 894, 162429. [Google Scholar] [CrossRef]
- Fu, L.; Xia, W. MAX Phases as Nanolaminate Materials: Chemical Composition, Microstructure, Synthesis, Properties, and Applications. Adv. Eng. Mater. 2021, 23, 2001191. [Google Scholar] [CrossRef]
- Xu, X.; Yang, L.; Zheng, W.; Zhang, H.; Wu, F.; Tian, Z.; Zhang, P.; Sun, Z. MXenes with applications in supercapacitors and secondary batteries: A comprehensive review. Mater. Rep. Energy 2022, 2, 100080. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, X.; Jia, D.; Zhou, Y.; van der Zwaag, S. On the formation mechanisms and properties of MAX phases: A review. J. Eur. Ceram. Soc. 2021, 41, 3851–3878. [Google Scholar] [CrossRef]
- Zhou, A.G.; Liu, Y.; Li, S.B.; Wang, X.H.; Ying, G.B.; Xia, Q.X.; Zhang, P.G. From structural ceramics to 2D materials with multi-applications: A review on the development from MAX phases to MXenes. J. Adv. Ceram. 2021, 10, 1194–1242. [Google Scholar] [CrossRef]
- Zhang, P.; Shen, L.; Ouyang, J.; Zhang, Y.; Wu, S.; Sun, Z.J. Room temperature mushrooming of gallium wires and its growth mechanism. J. Alloy Compd. 2015, 619, 488–497. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.S.; Zhang, P.G.; Zhang, Y.; Lu, C.J.; Pan, L.; Ding, J.X.; Sun, Z.M. Interface energy-driven indium whisker growth on ceramic substrates. J. Mater. Sci. Mater. Electron. 2021, 32, 16881–16888. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Hashimoto, S.; Takeuchi, M.; Inoue, K.; Honda, S.; Awaji, H.; Fukuda, K.; Zhang, S. Pressureless sintering and mechanical properties of titanium aluminum carbide. Mater. Lett. 2008, 62, 1480–1483. [Google Scholar] [CrossRef]
- Zheng-Ming, S.U.N.; Wu-Bian, T.; Pei-Gen, Z.; Dan-Dan, W.; Yu-Hui, Z.H.A.; Pei-Yan, H.; Jian-Xiang, D. High-purity Ti2AlC Powder: Preparation and Application in Ag-based Electrical Contact Materials. J. Inorg. Mater. 2019, 35, 729–734. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Wang, J.Y.; Zhou, Y.C. Low-temperature instability of Ti2SnC: A combined transmission electron microscopy, differential scanning calorimetry, and X-ray diffraction investigations. J. Mater. Res. 2009, 24, 39–49. [Google Scholar] [CrossRef]
- Rajalakshmi, M.; Arora, A.K.; Bendre, B.S.; Mahamuni, S. Optical phonon confinement in zinc oxide nanoparticles. J. Appl. Phys. 2000, 87, 2445–2448. [Google Scholar] [CrossRef]
- Karthik, K.V.; Raghu, A.V.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Tian, Z.; Zhang, Y.; Wu, F.; Xie, H.; Zhang, Q.; Zhang, P.; Sun, Z. In-Situ Growth of ZnO Whiskers on Ti2ZnC MAX Phases. Materials 2023, 16, 3610. https://doi.org/10.3390/ma16103610
Ren Y, Tian Z, Zhang Y, Wu F, Xie H, Zhang Q, Zhang P, Sun Z. In-Situ Growth of ZnO Whiskers on Ti2ZnC MAX Phases. Materials. 2023; 16(10):3610. https://doi.org/10.3390/ma16103610
Chicago/Turabian StyleRen, Yinan, Zhihua Tian, Yan Zhang, Fushuo Wu, Hao Xie, Qianqian Zhang, Peigen Zhang, and Zhengming Sun. 2023. "In-Situ Growth of ZnO Whiskers on Ti2ZnC MAX Phases" Materials 16, no. 10: 3610. https://doi.org/10.3390/ma16103610





