In Situ Synthesis of Bi2MoO6/Bi2SiO5 Heterojunction for Efficient Degrading of Persistent Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
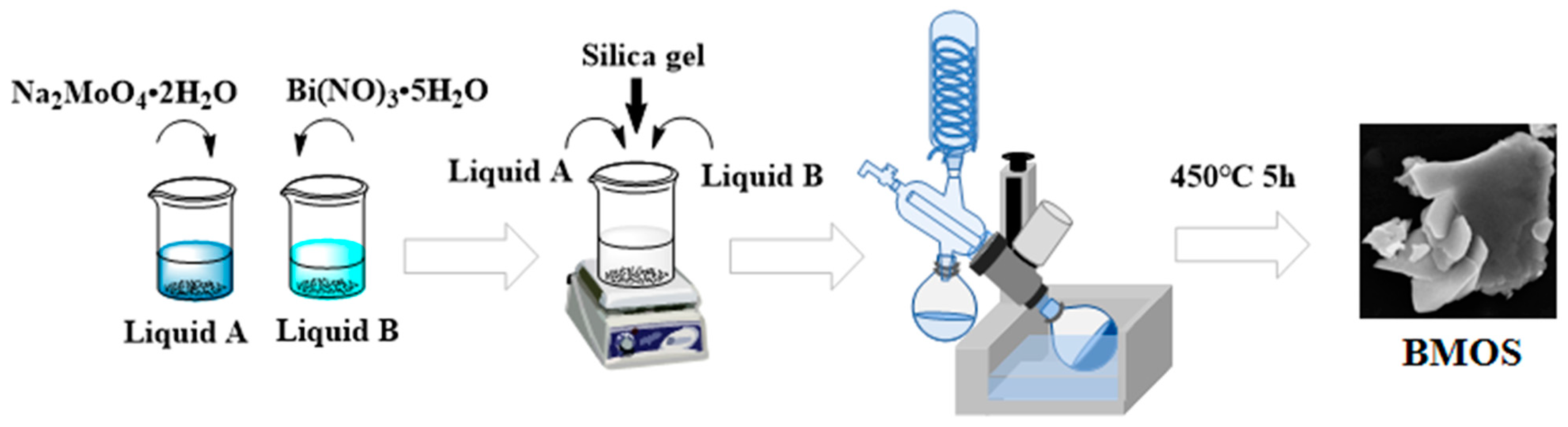
2.2. Preparation of Catalysts
2.3. Characterization
2.4. Photocatalytic Degradation Experiment
2.5. Photoelectrochemical Measurements
3. Results and Discussion
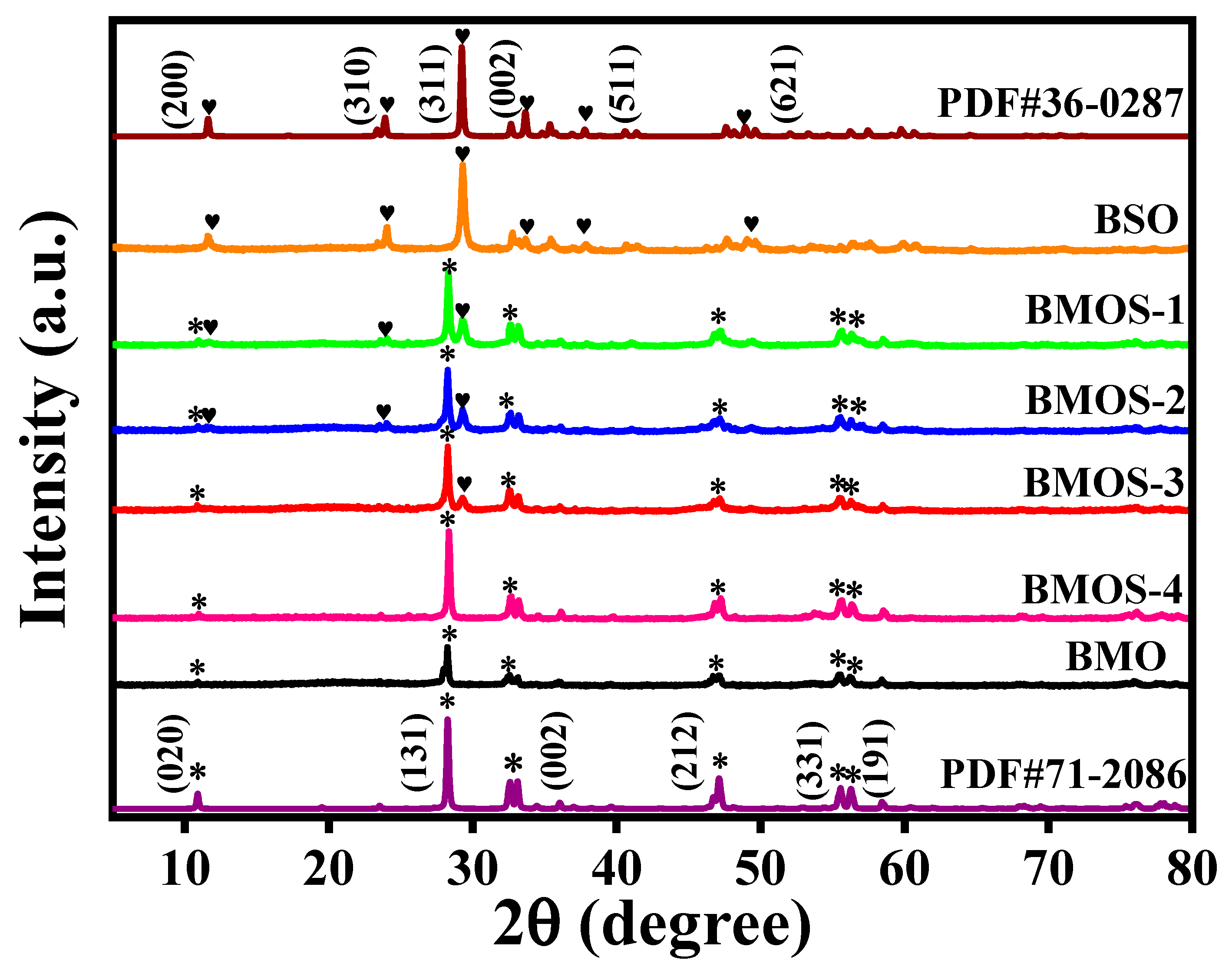
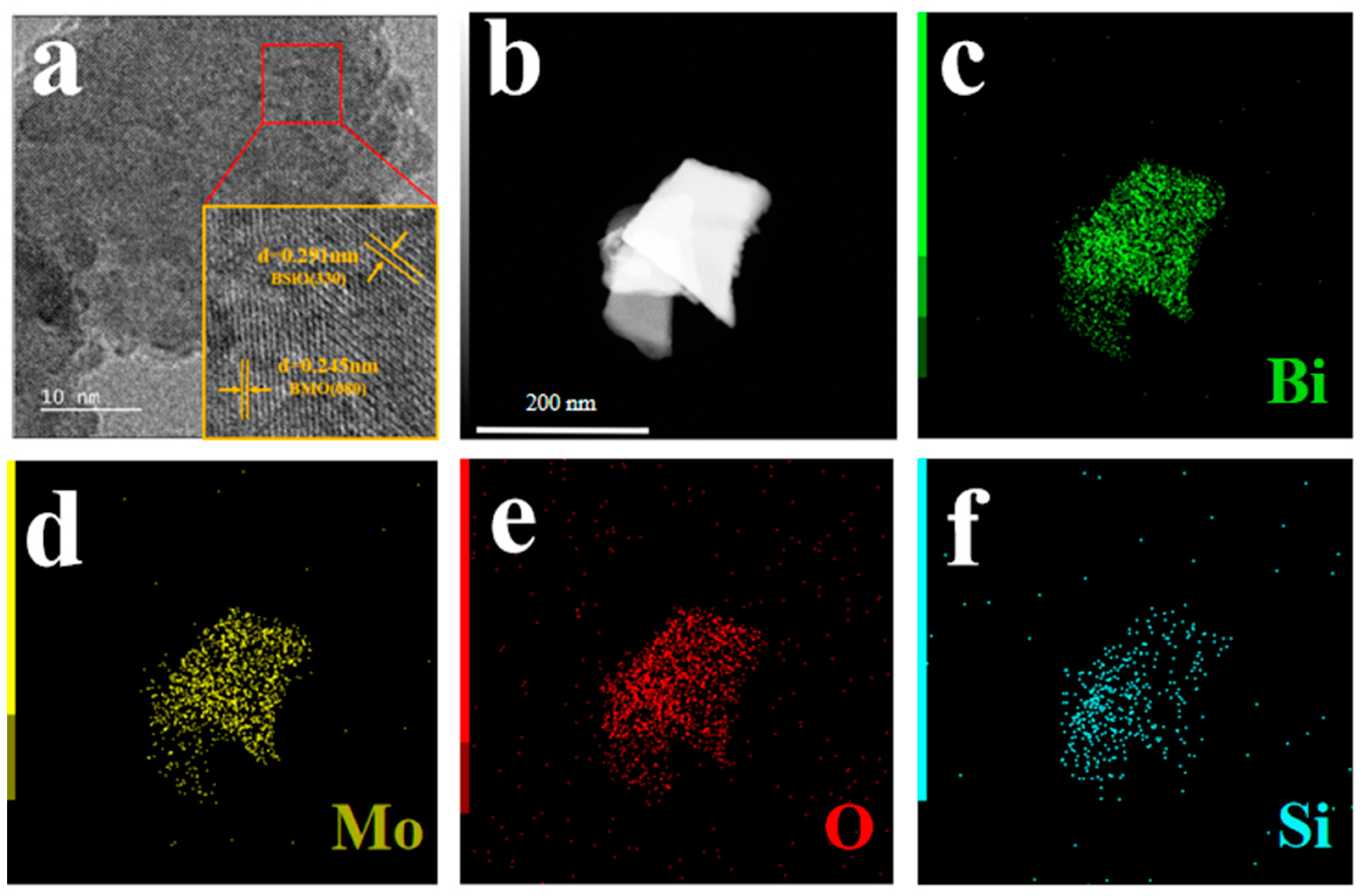
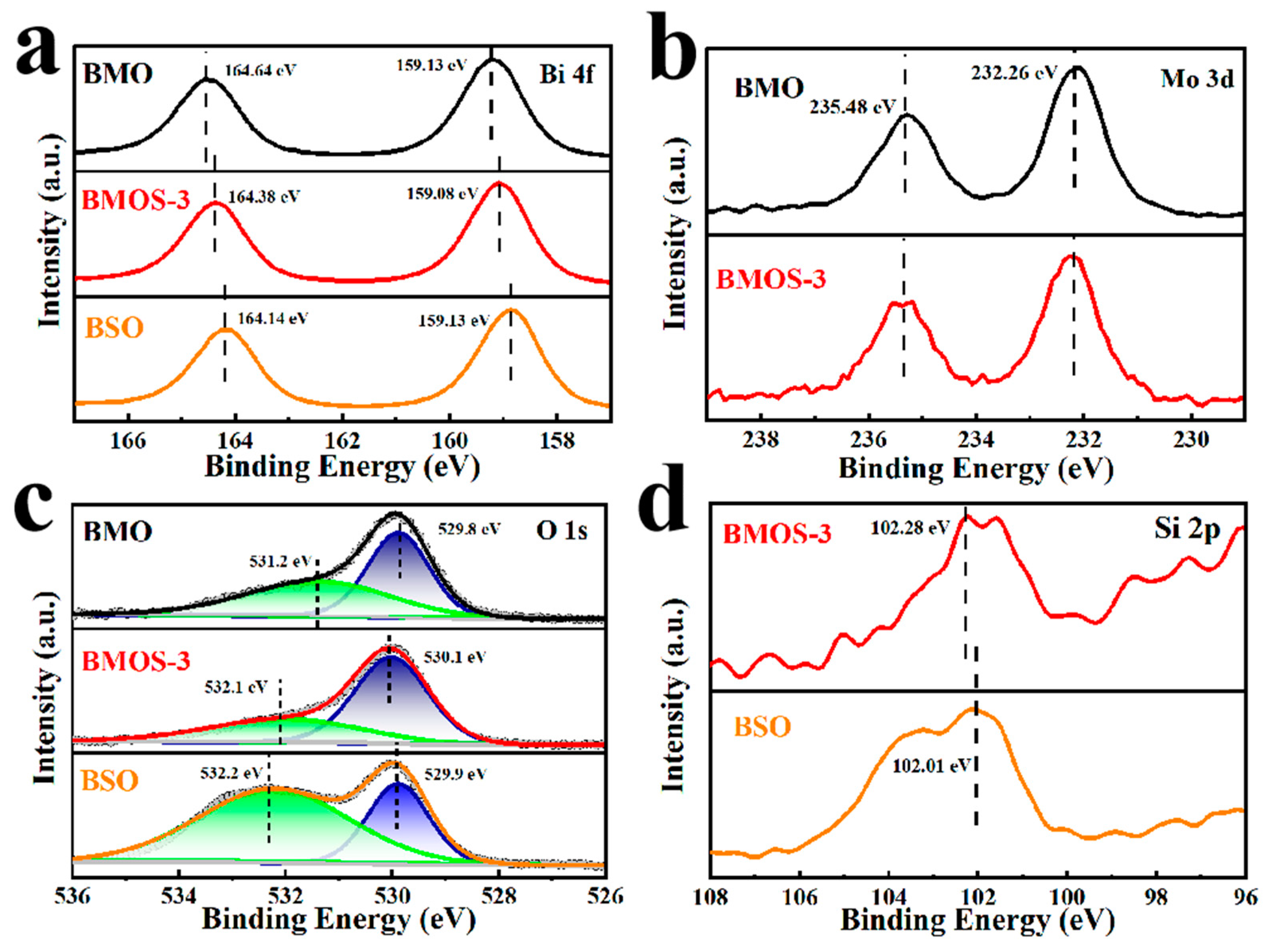
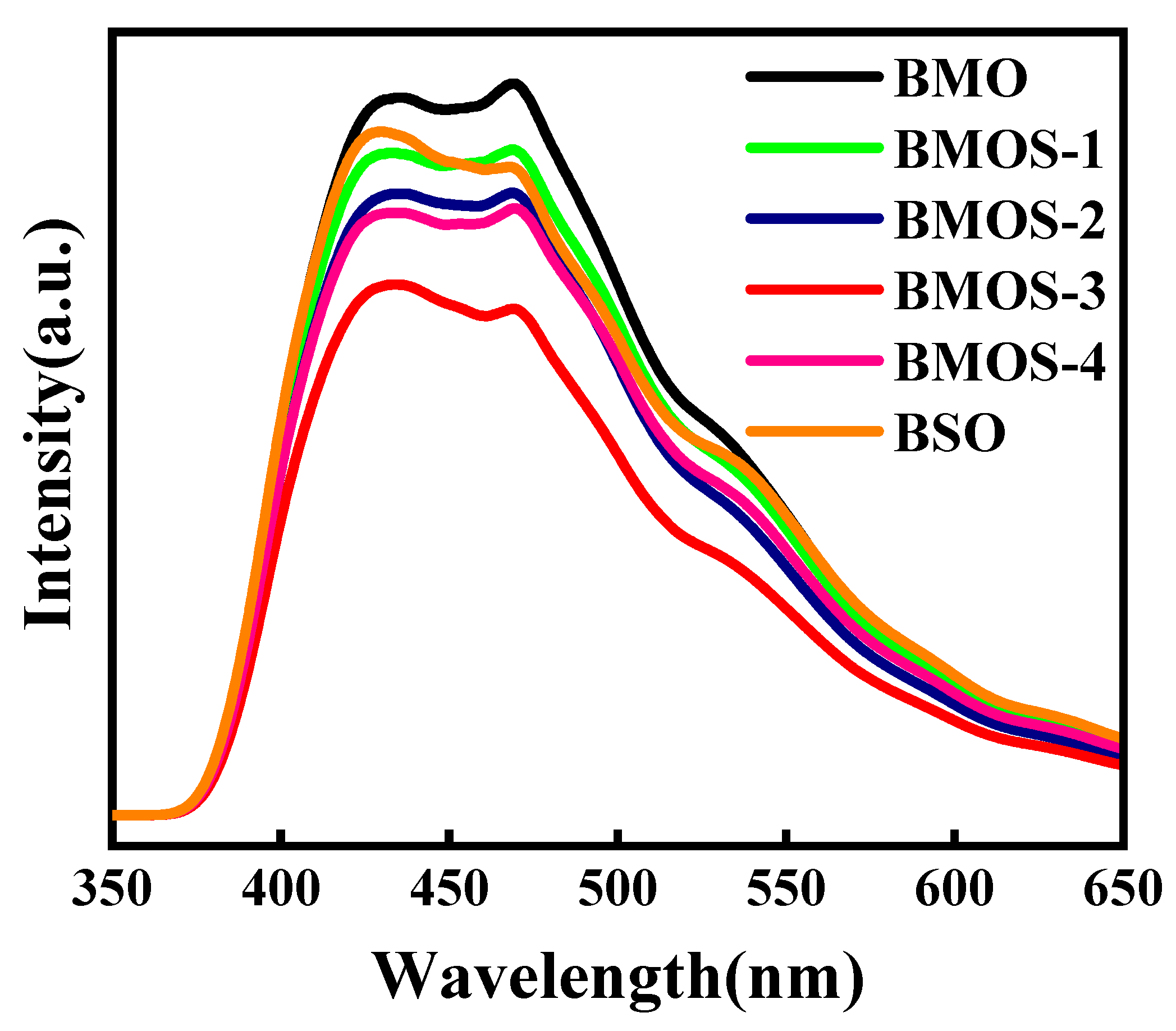
3.1. Material Characterization
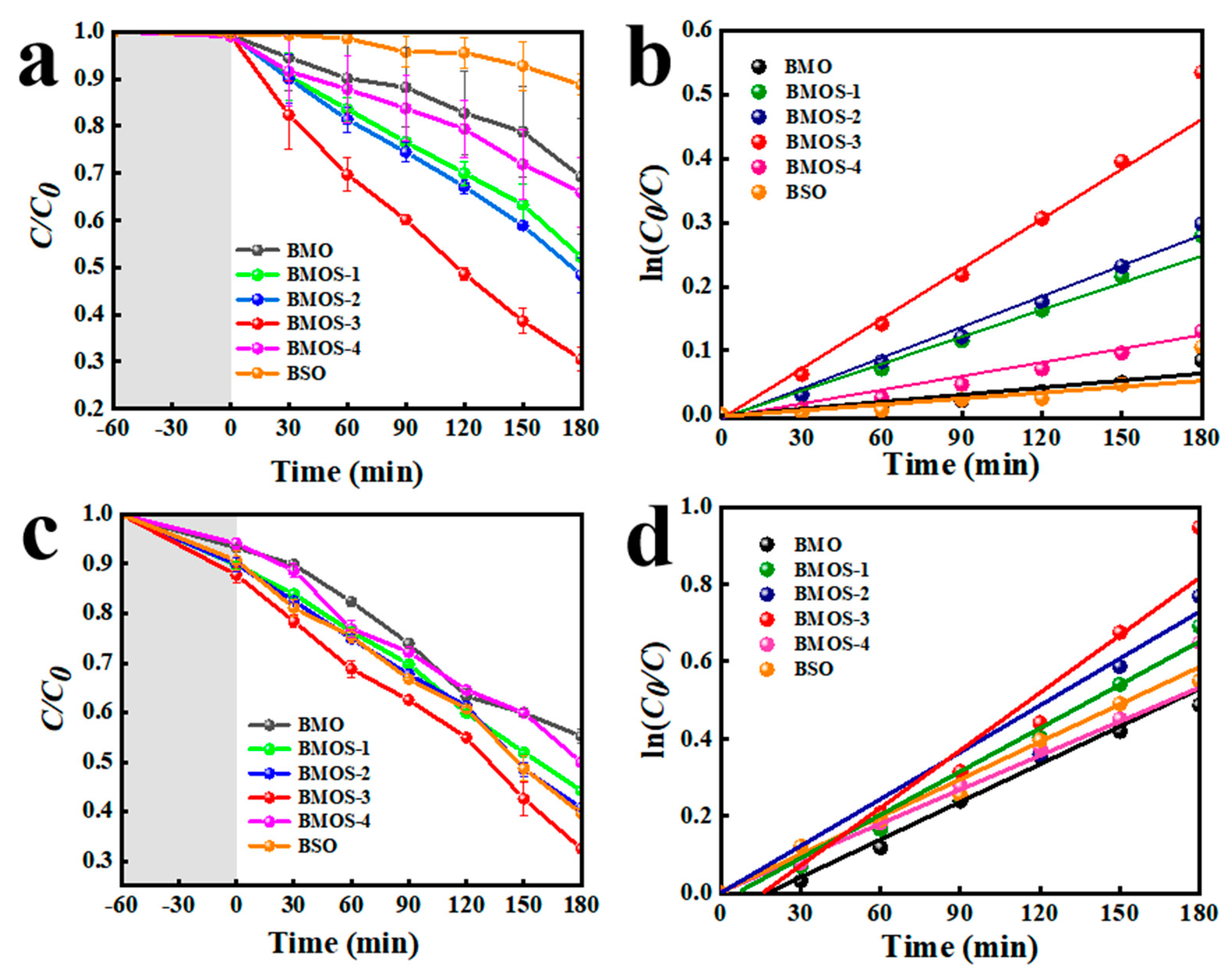
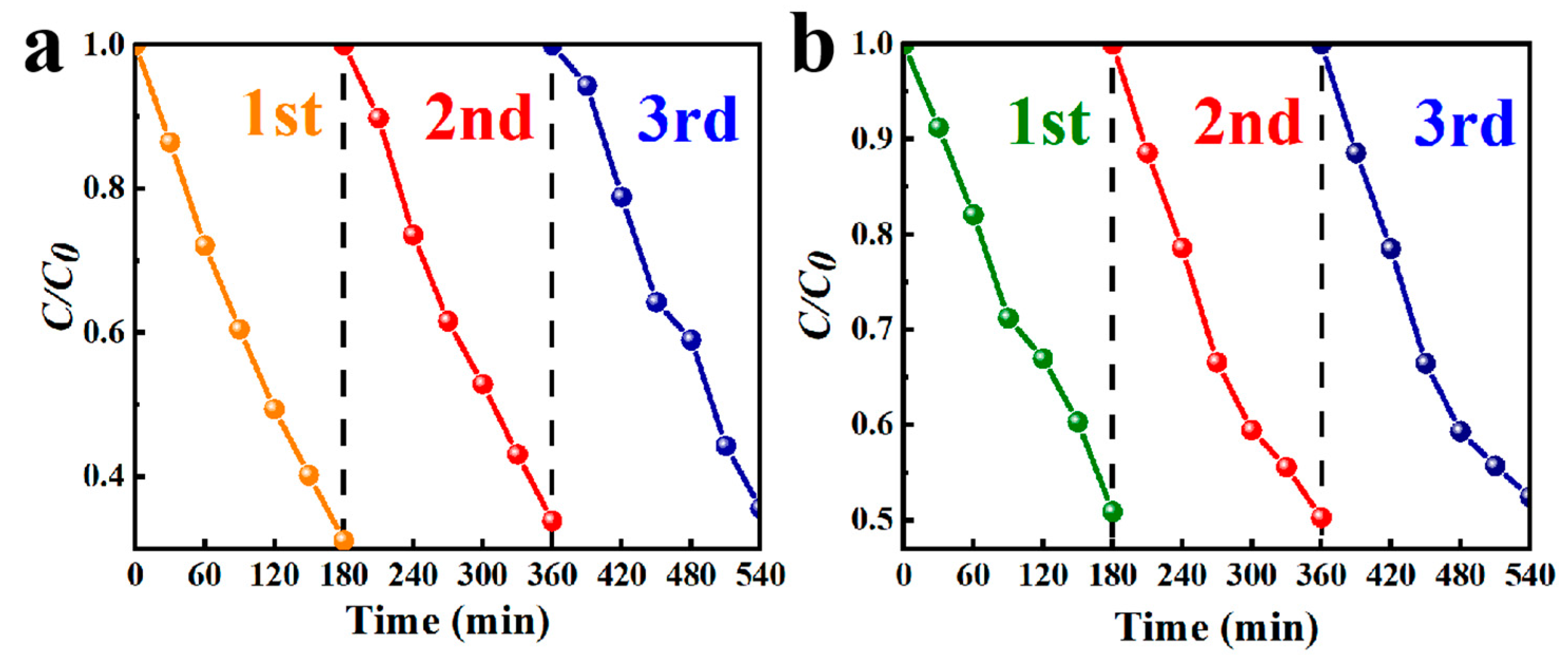
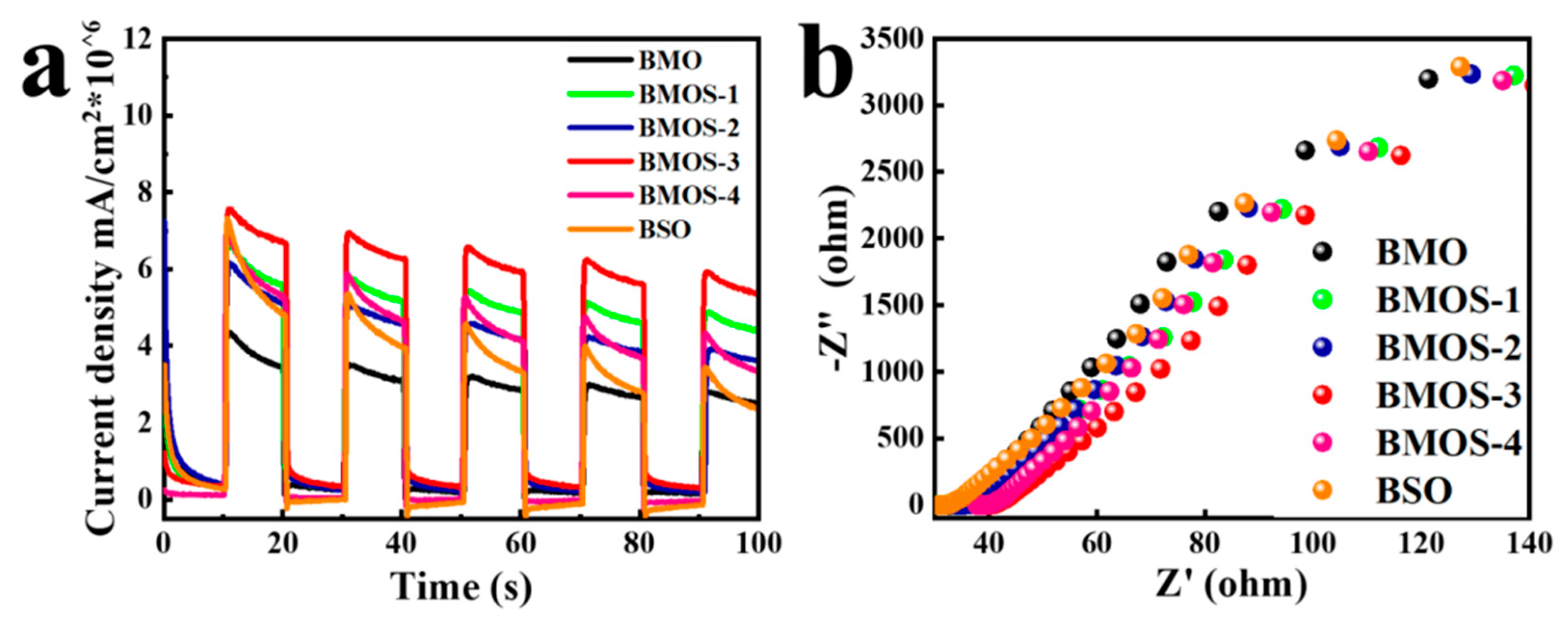
3.2. Photocatalytic Activity
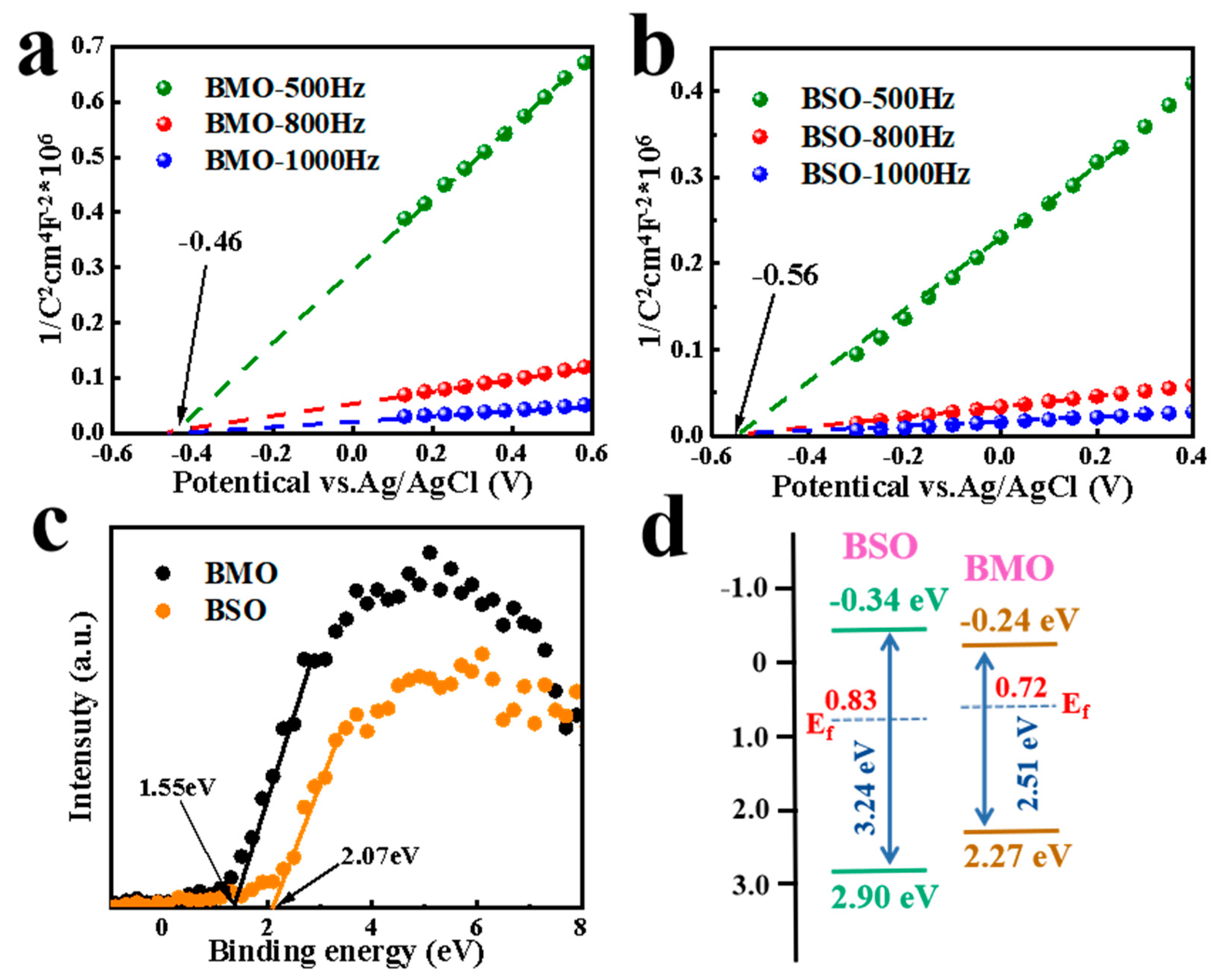
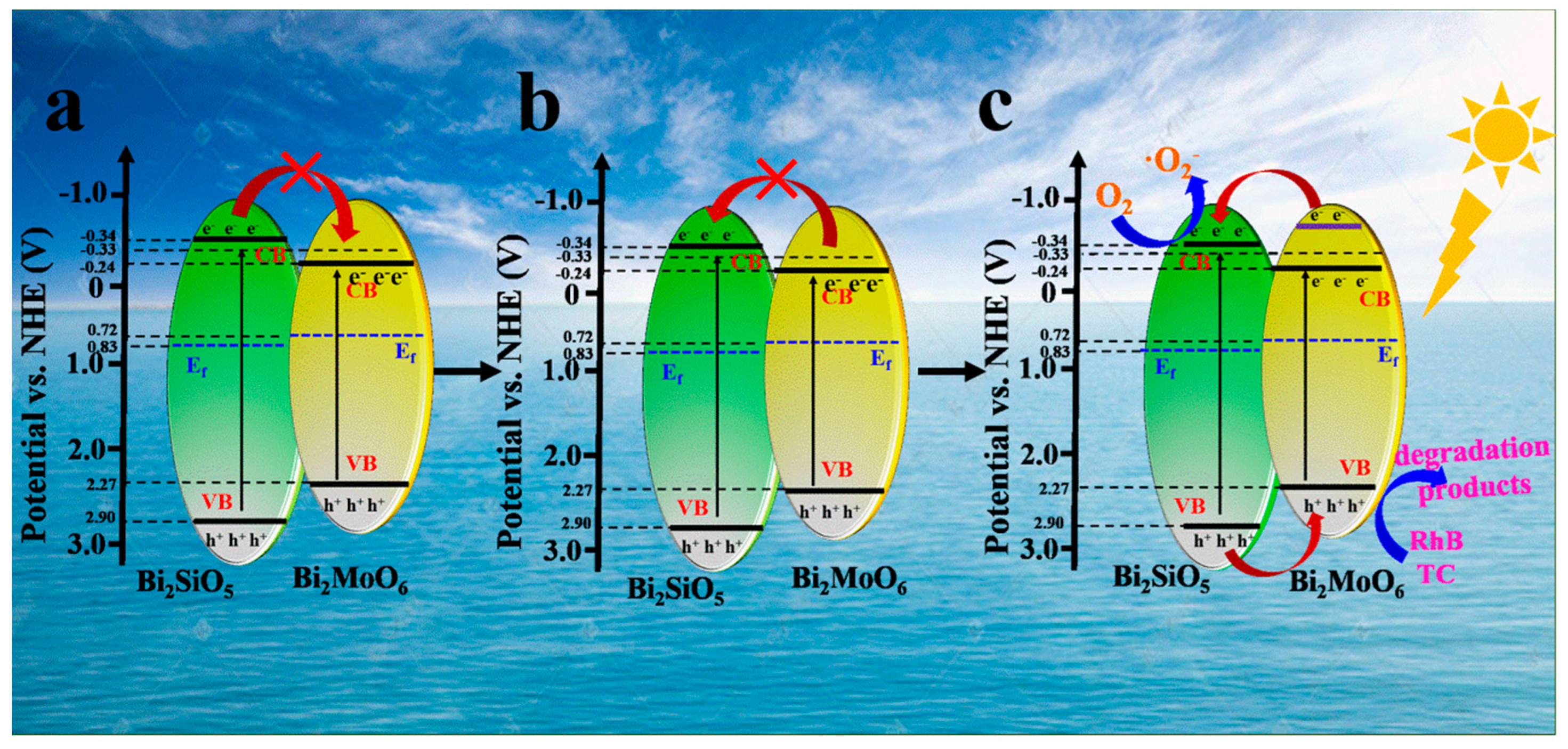
3.3. Possible Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bose, S.; Kumar, P.S.; Vo, D.-V.N.; Rajamohan, N.; Saravanan, R. Microbial degradation of recalcitrant pesticides: A review. Environ. Chem. Lett. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; ur Rehman, M.N.; Batool, S.; Hasan, M.; Riaz, M.; ur Rehman, K.; Iqbal, F. Highly efficient tri-phase TiO2–Y2O3–V2O5 nanocomposite: Structural, optical, photocatalyst, and antibacterial studies. J. Nanostruct. Chem. 2021, 12, 547–564. [Google Scholar] [CrossRef]
- Huang, H.; Guo, T.; Wang, K.; Li, Y.; Zhang, G. Efficient activation of persulfate by a magnetic recyclable rape straw biochar catalyst for the degradation of tetracycline hydrochloride in water. Sci. Total Environ. 2021, 758, 143957. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-X.; Wang, C.-C.; Du, X.; Li, Y.; Wang, F.; Wang, P. Efficient removal of emerging organic contaminants via photo-Fenton process over micron-sized Fe-MOF sheet. Chem. Eng. J. 2022, 429, 132495. [Google Scholar] [CrossRef]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Thurston, T.; Wilcoxon, J. Photooxidation of organic chemicals catalyzed by nanoscale MoS2. J. Phys. Chem. B 1999, 103, 11–17. [Google Scholar] [CrossRef]
- Zhao, X.; Qu, J.; Liu, H.; Hu, C. Photoelectrocatalytic degradation of triazine-containing azo dyes at γ-Bi2MoO6 film electrode under visible light irradiation (λ > 420 nm). Environ. Sci. Technol. 2007, 41, 6802–6807. [Google Scholar] [CrossRef]
- Tian, G.; Chen, Y.; Zhou, W.; Pan, K.; Dong, Y.; Tian, C.; Fu, H. Facile solvothermal synthesis of hierarchical flower-like Bi2MoO6 hollow spheres as high performance visible-light driven photocatalysts. J. Mater. Chem. 2011, 21, 887–892. [Google Scholar] [CrossRef]
- Ding, X.; Ho, W.; Shang, J.; Zhang, L. Self doping promoted photocatalytic removal of no under visible light with Bi2MoO6: Indispensable role of superoxide ions. Appl. Catal. B Environ. 2016, 182, 316–325. [Google Scholar] [CrossRef]
- Tian, J.; Hao, P.; Wei, N.; Cui, H.; Liu, H. 3D Bi2MoO6 nanosheet/TiO2 nanobelt heterostructure: Enhanced photocatalytic activities and photoelectochemistry performance. ACS Catal. 2015, 5, 4530–4536. [Google Scholar] [CrossRef]
- Zhang, M.; Shao, C.; Mu, J.; Zhang, Z.; Guo, Z.; Zhang, P.; Liu, Y. One-dimensional Bi2MoO6/TiO2 hierarchical heterostructures with enhanced photocatalytic activity. CrystEngComm 2012, 14, 605–612. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Hou, W.; Du, N.; Zhang, R.; Tao, X. Synthesis and characterization of g-C3N4/Bi2MoO6 heterojunctions with enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2014, 160, 89–97. [Google Scholar] [CrossRef]
- Yan, T.; Yan, Q.; Wang, X.; Liu, H.; Li, M.; Lu, S.; Xu, W.; Sun, M. Facile fabrication of heterostructured g-C3N4/Bi2MoO6 microspheres with highly efficient activity under visible light irradiation. Dalton Trans. 2015, 44, 1601–1611. [Google Scholar] [CrossRef]
- Chawla, H.; Saha, M.; Upadhyay, S.; Rohilla, J.; Ingole, P.P.; Sapi, A.; Szenti, I.; Yadav, M.; Lebedev, V.T.; Chandra, A. Enhanced photocatalytic activity and easy recovery of visible light active MoSe2/BiVO4 heterojunction immobilized on Luffa cylindrica–experimental and DFT study. Environ. Sci. Nano 2021, 8, 3028–3041. [Google Scholar] [CrossRef]
- Lin, X.; Guo, X.Y.; Wang, Q.W.; Chang, L.M.; Zhai, H.J. Hydrothermal synthesis and efficient visible light photocatalytic activity of Bi2MoO6/BiVO4 heterojunction. Acta Phys. Chim. Sin. 2014, 30, 2113–2120. [Google Scholar]
- Liu, T.; Li, B.; Hao, Y.; Yao, Z. MoO3-nanowire membrane and Bi2Mo3O12/MoO3 nano-heterostructural photocatalyst for wastewater treatment. Chem. Eng. J. 2014, 244, 382–390. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2019, 250, 313–324. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Ma, Z. Flower-like Ag2O/Bi2MoO6 p-n heterojunction with enhanced photocatalytic activity under visible light irradiation. J. Mol. Catal. A Chem. 2016, 424, 37–44. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Wang, Y.; Zhu, Y. An anion exchange strategy for construction of a novel Bi2SiO5/Bi2MoO6 heterostructure with enhanced photocatalytic performance. Catal. Sci. Technol. 2018, 8, 3278–3285. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Y.; Li, K.; Yang, G.; Yin, S. One-step low-temperature synthesis of 0D CeO2 quantum dots/2D BiOX (X = Cl, Br) nanoplates heterojunctions for highly boosting photo-oxidation and reduction ability. Appl. Catal. B Environ. 2019, 250, 17–30. [Google Scholar] [CrossRef]
- He, C.; Tao, J. Transition metal carbides coupled with nitrogen-doped carbon as efficient and stable Bi-functional catalysts for oxygen reduction reaction and hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 13240–13250. [Google Scholar] [CrossRef]
- Chen, R.; Bi, J.; Wu, L.; Wang, W.; Li, Z.; Fu, X. Template-free hydrothermal synthesis and photocatalytic performances of novel Bi2SiO5 nanosheets. Inorg. Chem. 2009, 48, 9072–9076. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; ur Rehman, M.N.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Dual Z-scheme core-shell PANI-CeO2-Fe2O3-NiO heterostructured nanocomposite for dyes remediation under sunlight and bacterial disinfection. Environ. Res. 2022, 215, 114140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Yu, N.; Xu, K.; Li, S.; Wang, H.; Liu, J. Flower-like Bi2S3/Bi2MoO6 heterojunction superstructures with enhanced visible-light-driven photocatalytic activity. RSC Adv. 2015, 5, 75081–75088. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Yi, Z.; Wang, S.; Ma, J.; Gao, H.; Wu, X.; Liu, G.; Yang, H. PH-induced structural evolution, photodegradation mechanism and application of bismuth molybdate photocatalyst. Adv. Powder Technol. 2022, 33, 103858. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Sun, S.; Jiang, D.; Gao, E. Solar light photocatalysis using Bi2O3/Bi2SiO5 nanoheterostructures formed in mesoporous SiO2 microspheres. CrystEngComm 2013, 15, 10043–10048. [Google Scholar] [CrossRef]
- Wang, D.; Shen, H.; Guo, L.; Wang, C.; Fu, F.; Liang, Y. Ag/Bi2MoO6-x with enhanced visible-light-responsive photocatalytic activities via the synergistic effect of surface oxygen vacancies and surface plasmon. Appl. Surf. Sci. 2018, 436, 536–547. [Google Scholar] [CrossRef]
- Qiu, Y.; Lu, J.; Yan, Y.; Niu, J.; Duan, Y. Bismuth molybdate photocatalyst for the efficient photocatalytic degradation of tetracycline in water under visible-light irradiation. Surf. Interfaces 2022, 31, 102009. [Google Scholar] [CrossRef]
- Li, H.; Hu, T.; Zhang, R.; Liu, J.; Hou, W. Preparation of solid-state Z-scheme Bi2MoO6/MO (M = Cu, Co3/4, or Ni) heterojunctions with internal electric field-improved performance in photocatalysis. Appl. Catal. B Environ. 2016, 188, 313–323. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, F.; Cai, W.; Liu, C.; Wang, Y.; Meng, Z.; Mi, C. A novel I-type 0D/0D ZnS/Ag6Si2O7 heterojunction for photocatalytic hydrogen evolution. J. Phys. Chem. Solids 2023, 175, 111206. [Google Scholar] [CrossRef]
- Kandi, D.; Martha, S.; Thirumurugan, A.; Parida, K. CdS QDs-decorated self-doped γ- Bi2MoO6: A sustainable and versatile photocatalyst toward photoreduction of Cr (VI) and degradation of phenol. ACS Omega 2017, 2, 9040–9056. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Li, J.; Wang, B.; Li, Y. Direct Z-scheme Bi2MoO6/UiO-66-NH2 heterojunctions for enhanced photocatalytic degradation of ofloxacin and ciprofloxacin under visible light. Appl. Catal. B Environ. 2022, 318, 121820. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, Q.; Zhang, Y.; Meng, L.; Wang, X. In situ construction of S-scheme AgBr/BiOBr heterojunction with surface oxygen vacancy for boosting photocatalytic CO2 reduction with H2O. Appl. Catal. B Environ. 2022, 301, 120802. [Google Scholar] [CrossRef]
- Arif, M.; Zhang, M.; Qiu, B.; Yao, J.; Bu, Q.; Ali, A.; Muhmood, T.; Hussian, I.; Liu, X.; Zhou, B. Synergistic effect of ultrathin thickness and surface oxygen vacancies in high-efficiency Ti-mediated Bi2MoO6 for immense photocatalytic nitrofurantoin degradation and Cr (VI) reduction. Appl. Surf. Sci. 2021, 543, 148816. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, X.; Yu, X.-F.; Xia, L.; Peng, Y.; Li, Z.; Zhang, Y.; Cheng, J.; Zhang, K.; Yu, J. Surface oxygen vacancies of Pd/Bi2MoO6-x acts as “Electron Bridge” to promote photocatalytic selective oxidation of alcohol. Appl. Catal. B Environ. 2021, 285, 119790. [Google Scholar] [CrossRef]
- Fu, X.; Xie, M.; Luan, P.; Jing, L. Effective visible-excited charge separation in silicate-bridged ZnO/BiVO4 nanocomposite and its contribution to enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2014, 6, 18550–18557. [Google Scholar] [CrossRef]
- Ju, P.; Wang, P.; Li, B.; Fan, H.; Ai, S.; Zhang, D.; Wang, Y. A novel calcined Bi2WO6/BiVO4 heterojunction photocatalyst with highly enhanced photocatalytic activity. Chem. Eng. J. 2014, 236, 430–437. [Google Scholar] [CrossRef]
- Huang, H.; Liu, L.; Zhang, Y.; Tian, N. One pot hydrothermal synthesis of a novel BiIO4/Bi2MoO6 heterojunction photocatalyst with enhanced visible-light-driven photocatalytic activity for rhodamine B degradation and photocurrent generation. J. Alloys Compd. 2015, 619, 807–811. [Google Scholar] [CrossRef]
- Pascariu, P.; Airinei, A.; Olaru, N.; Olaru, L.; Nica, V. Photocatalytic degradation of Rhodamine B dye using ZnO–SnO2 electrospun ceramic nanofibers. Ceram. Int. 2016, 42, 6775–6781. [Google Scholar] [CrossRef]
- Shang, J.; Chen, H.; Chen, T.; Wang, X.; Feng, G.; Zhu, M.; Yang, Y.; Jia, X. Photocatalytic degradation of rhodamine B and phenol over BiFeO3/BiOCl nanocomposite. Appl. Phys. A 2019, 125, 133. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, K.; Jia, H.; Chen, D.; Feng, Y.; Liang, Y.; Chen, K.; Hao, D. In Situ Synthesis of Bi2MoO6/Bi2SiO5 Heterojunction for Efficient Degrading of Persistent Pollutants. Materials 2023, 16, 3631. https://doi.org/10.3390/ma16103631
Yuan K, Jia H, Chen D, Feng Y, Liang Y, Chen K, Hao D. In Situ Synthesis of Bi2MoO6/Bi2SiO5 Heterojunction for Efficient Degrading of Persistent Pollutants. Materials. 2023; 16(10):3631. https://doi.org/10.3390/ma16103631
Chicago/Turabian StyleYuan, Kaiwen, Hailong Jia, Daimei Chen, Yanmei Feng, Yu Liang, Kai Chen, and Derek Hao. 2023. "In Situ Synthesis of Bi2MoO6/Bi2SiO5 Heterojunction for Efficient Degrading of Persistent Pollutants" Materials 16, no. 10: 3631. https://doi.org/10.3390/ma16103631




