Uniaxial Rotational Molding of Bio-Based Low-Density Polyethylene Filled with Black Tea Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
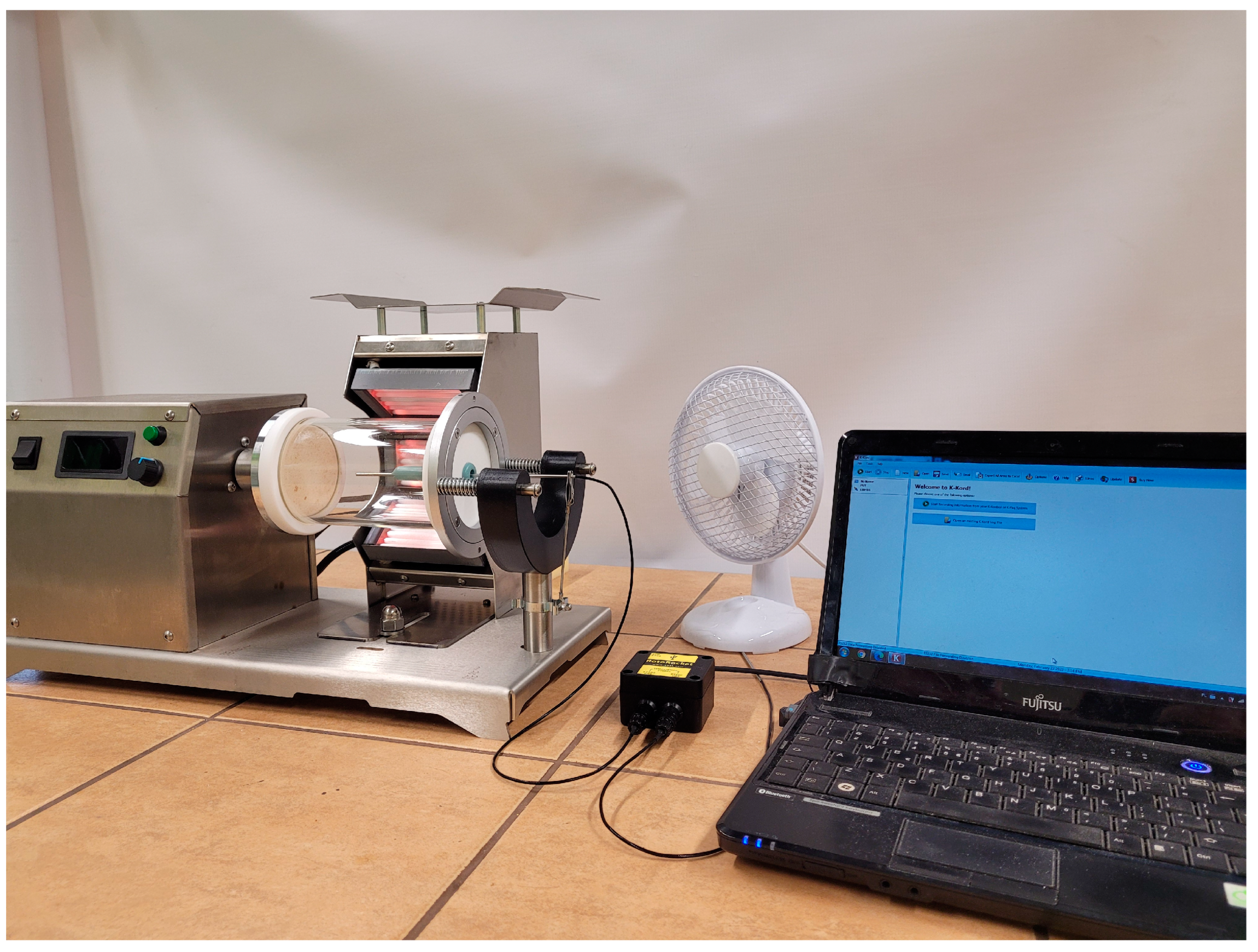
2.2. Preparation of Composites
2.3. Characterization of Fillers
2.4. Characterization of Composites
3. Results and Discussion
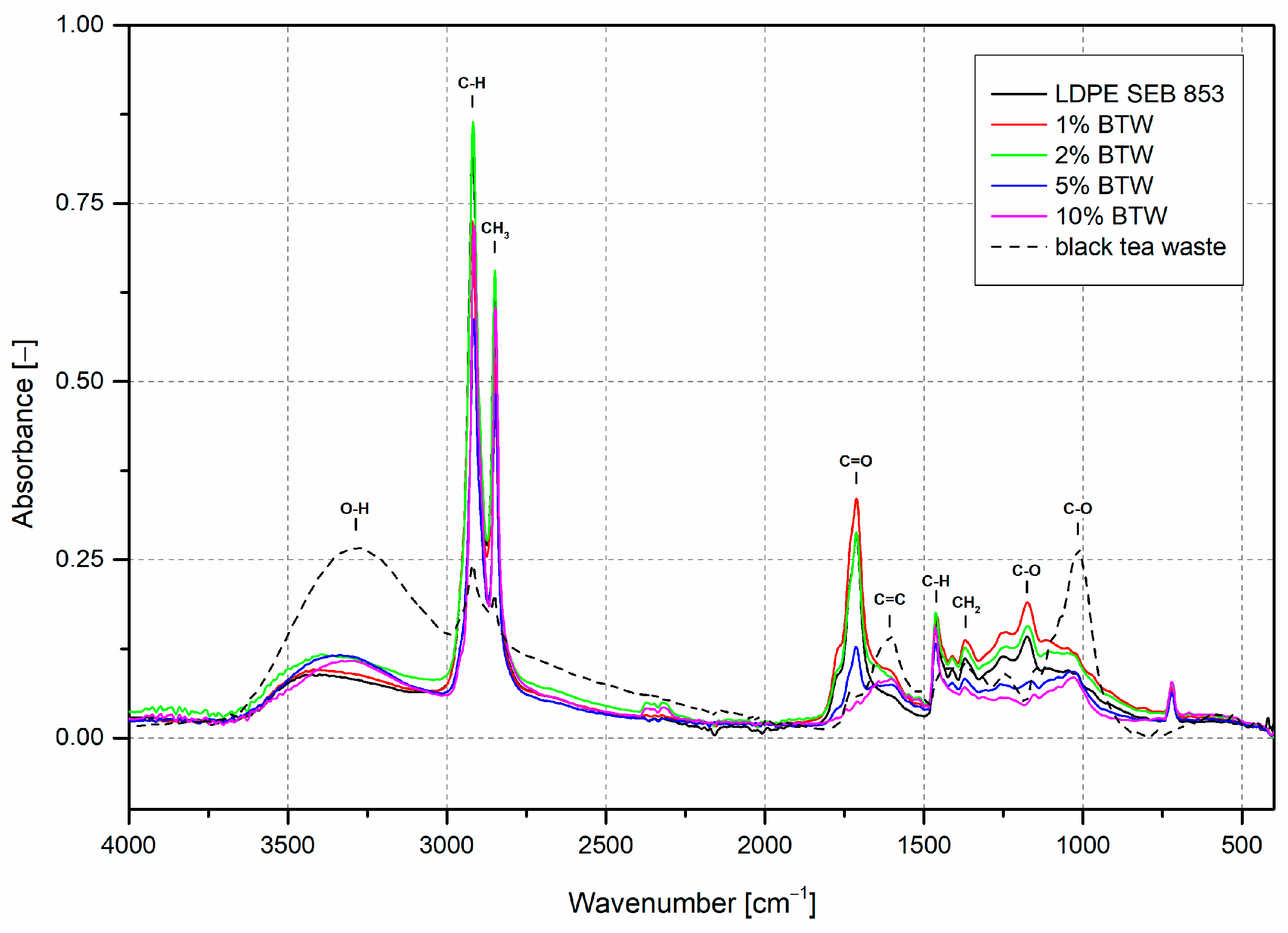
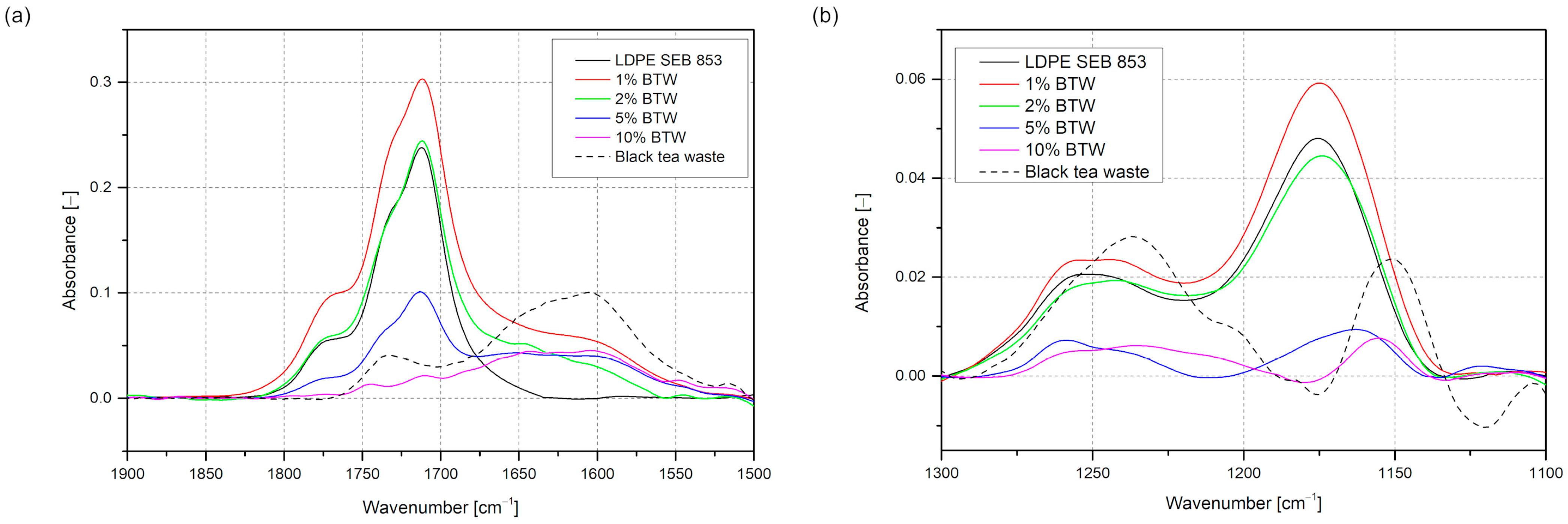
3.1. Characterization of Filler
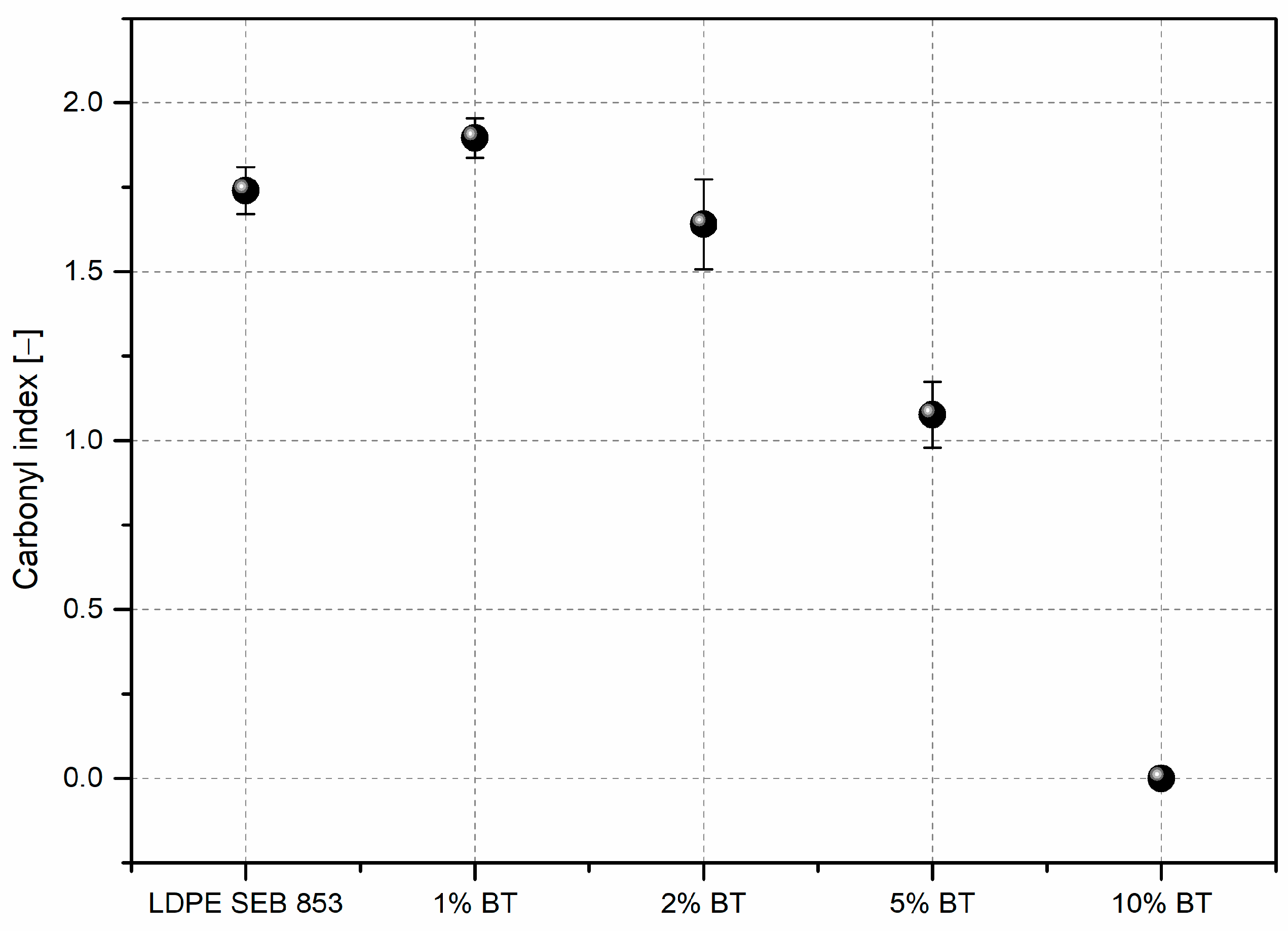
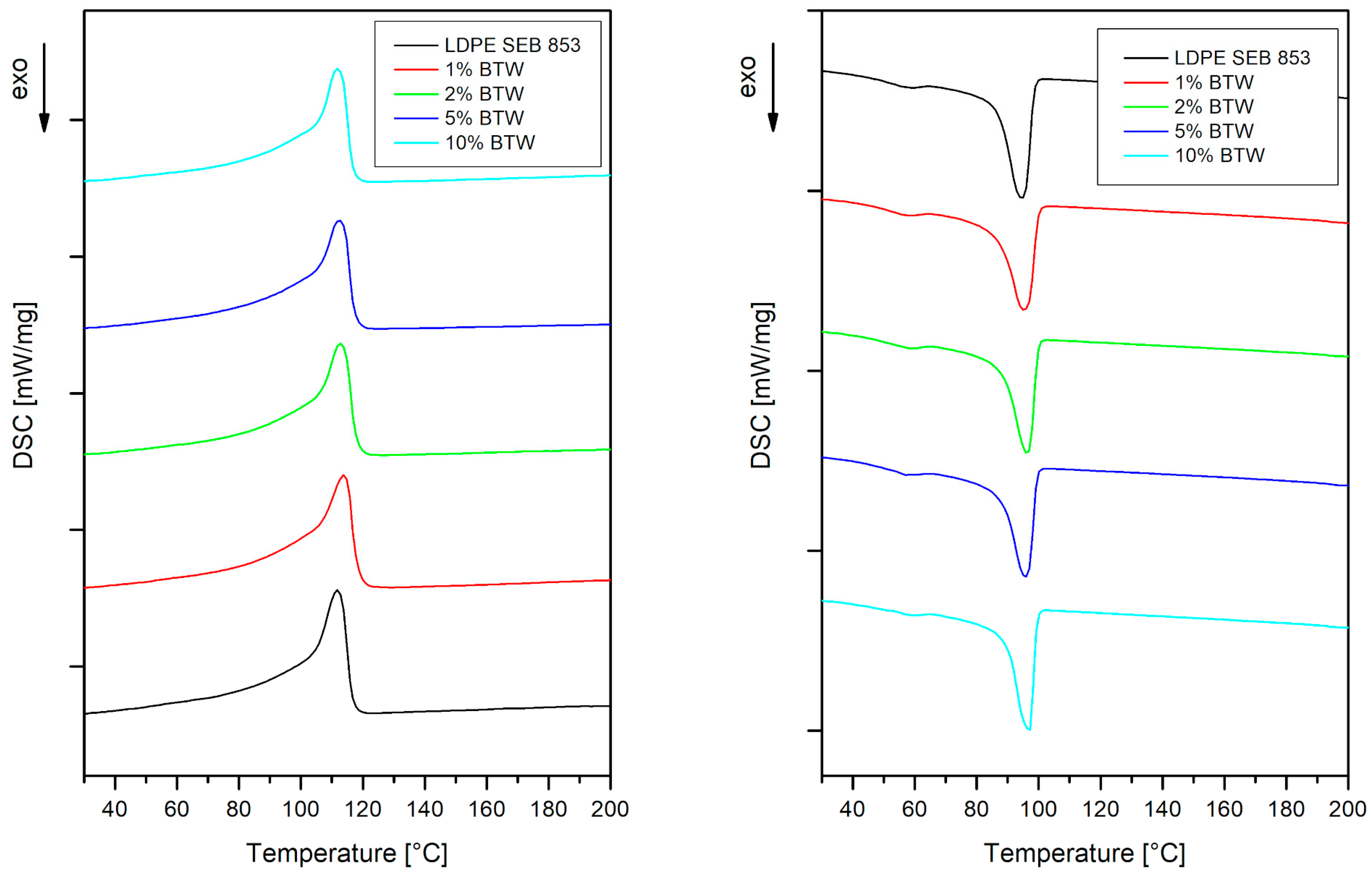
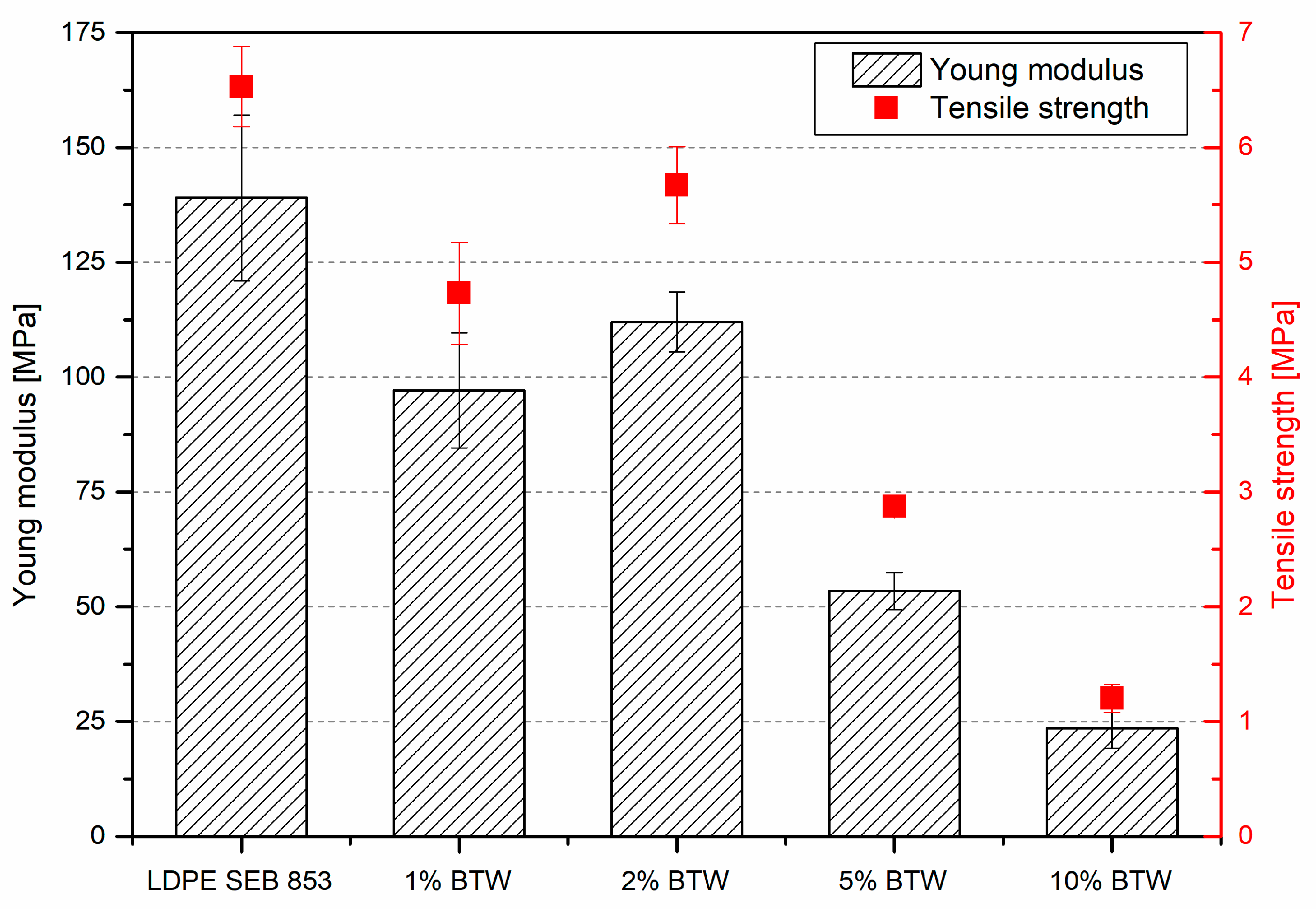
3.2. Characterization of Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hejna, A.; Barczewski, M.; Andrzejewski, J.; Kosmela, P.; Piasecki, A.; Szostak, M.; Kuang, T. Rotational Molding of Linear Low-Density Polyethylene Composites Filled with Wheat Bran. Polymers 2020, 12, 1004. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Krawczak, A.; Wesoły, K.; Szostak, M. Rotational Molding of Biocomposites with Addition of Buckwheat Husk Filler. Structure-Property Correlation Assessment for Materials Based on Polyethylene (PE) and Poly(Lactic Acid) PLA. Compos. Part B Eng. 2020, 202, 108410. [Google Scholar] [CrossRef]
- Crawford, R.J.; Throne, J.L. Rotational Molding Technology; Elsevier: Amsterdam, The Netherlands, 2002; ISBN 1884207855. [Google Scholar]
- Olinek, J.; Anand, C.; Bellehumeur, C.T. Experimental Study on the Flow and Deposition of Powder Particles in Rotational Molding. Polym. Eng. Sci. 2005, 45, 62–73. [Google Scholar] [CrossRef]
- Ogila, K.O.; Shao, M.; Yang, W.; Tan, J. Rotational Molding: A Review of the Models and Materials. Express Polym. Lett. 2017, 11, 778–798. [Google Scholar] [CrossRef]
- Aissa, A.A.; Duchesne, C.; Rodrigue, D. Characterization of Polymer Powder Motion in a Spherical Mold in Biaxial Rotation. Polym. Eng. Sci. 2012, 52, 953–963. [Google Scholar] [CrossRef]
- Chaudhary, B.I.; Takács, E.; Vlachopoulos, J. Processing Enhancers for Rotational Molding of Polyethylene. Polym. Eng. Sci. 2001, 41, 1731–1742. [Google Scholar] [CrossRef]
- Ortega, Z.; Romero, F.; Paz, R.; Suárez, L.; Benítez, A.N.; Marrero, M.D. Valorization of Invasive Plants from Macaronesia as Filler Materials in the Production of Natural Fiber Composites by Rotational Molding. Polymers 2021, 13, 2220. [Google Scholar] [CrossRef]
- Robledo-Ortíz, J.R.; González-López, M.E.; Rodrigue, D.; Gutiérrez-Ruiz, J.F.; Prezas-Lara, F.; Pérez-Fonseca, A.A. Improving the Compatibility and Mechanical Properties of Natural Fibers/Green Polyethylene Biocomposites Produced by Rotational Molding. J. Polym. Environ. 2020, 28, 1040–1049. [Google Scholar] [CrossRef]
- Stagnaro, P.; Utzeri, R.; Vignali, A.; Falcone, G.; Iannace, S.; Bertini, F. Lightweight Polyethylene-Hollow Glass Microspheres Composites for Rotational Molding Technology. J. Appl. Polym. Sci. 2021, 138, 49766. [Google Scholar] [CrossRef]
- Vignali, A.; Iannace, S.; Falcone, G.; Utzeri, R.; Stagnaro, P.; Bertini, F. Lightweight Poly(ε-Caprolactone) Composites with Surface Modified Hollow Glass Microspheres for Use in Rotational Molding: Thermal, Rheological and Mechanical Properties. Polymers 2019, 11, 624. [Google Scholar] [CrossRef]
- Barczewski, M.; Szostak, M.; Nowak, D.; Piasecki, A. Effect of Wood Flour Addition and Modification of Its Surface on the Properties of Rotationally Molded Polypropylene Composites. Polimery 2018, 63, 772–784. [Google Scholar] [CrossRef]
- Aniśko, J.; Barczewski, M.; Piasecki, A.; Skórczewska, K.; Szulc, J.; Szostak, M. The Relationship between a Rotational Molding Processing Procedure and the Structure and Properties of Biobased Polyethylene Composites Filled with Expanded Vermiculite. Materials 2022, 15, 5903. [Google Scholar] [CrossRef]
- Gugumus, F. Thermooxidative Degradation of Polyolefins in the Solid State. Part 2: Homogeneous and Heterogeneous Aspects of Thermal Oxidation. Polym. Degrad. Stab. 1996, 52, 145–157. [Google Scholar] [CrossRef]
- Dopico-García, M.S.; López-Vilariñó, J.M.; González-Rodríguez, M.V. Antioxidant Content of and Migration from Commercial Polyethylene, Polypropylene, and Polyvinyl Chloride Packages. J. Agric. Food Chem. 2007, 55, 3225–3231. [Google Scholar] [CrossRef]
- Al-Malaika, S. Perspectives in Stabilisation of Polyolefins. Adv. Polym. Sci. 2004, 169, 121–150. [Google Scholar] [CrossRef]
- Till, D.E.; Ehntholt, D.J.; Reid, R.C.; Schwartz, P.S.; Sldman, K.R.; Schwope, A.D.; Whelan, R.H. Migration of BHT Antioxidant from High Density Polyethylene to Foods and Food Simulants. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 106–113. [Google Scholar] [CrossRef]
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Natural Antioxidants as Stabilizers for Polymers. Polym. Degrad. Stab. 2017, 145, 25–40. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the Use of Natural Antioxidants in Active Food Packaging: A Review. Food Addit. Contam. Part A 2014, 31, 374–395. [Google Scholar] [CrossRef]
- Tátraaljai, D.; Földes, E.; Pukánszky, B. Efficient Melt Stabilization of Polyethylene with Quercetin, a Flavonoid Type Natural Antioxidant. Polym. Degrad. Stab. 2014, 102, 41–48. [Google Scholar] [CrossRef]
- Tátraaljai, D.; Major, L.; Földes, E.; Pukánszky, B. Study of the Effect of Natural Antioxidants in Polyethylene: Performance of β-Carotene. Polym. Degrad. Stab. 2014, 102, 33–40. [Google Scholar] [CrossRef]
- Kirschweng, B.; Vörös, B.; Arroussi, M.; Tátraaljai, D.; Zsuga, M. Melt Stabilization of Polyethylene with Natural Antioxidants: Comparison of a Natural Extract and Its Main Component. J. Therm. Anal. Calorim. 2021, 145, 67–75. [Google Scholar] [CrossRef]
- Doudin, K.; Al-Malaika, S.; Sheena, H.H.; Tverezovskiy, V.; Fowler, P. New Genre of Antioxidants from Renewable Natural Resources: Synthesis and Characterisation of Rosemary Plant-Derived Antioxidants and Their Performance in Polyolefins. Polym. Degrad. Stab. 2016, 130, 126–134. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Mysiukiewicz, O.; Kuzmin, A. Coffee Silverskin as a Multifunctional Waste Filler for High-Density Polyethylene Green Composites. J. Compos. Sci. 2021, 5, 44. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Mysiukiewicz, O. Comparative Analysis of the Coffee and Cocoa Industry By-Products on the Performance of Polyethylene-Based Composites. Waste Biomass Valorization 2023, 1–6. [Google Scholar] [CrossRef]
- Iyer, K.A.; Zhang, L.; Torkelson, J.M. Direct Use of Natural Antioxidant-Rich Agro-Wastes as Thermal Stabilizer for Polymer: Processing and Recycling. ACS Sustain. Chem. Eng. 2016, 4, 881–889. [Google Scholar] [CrossRef]
- Łuczaj, W.; Skrzydlewska, E. Antioxidative Properties of Black Tea. Prev. Med. 2005, 40, 910–918. [Google Scholar] [CrossRef]
- Henning, S.M.; Niu, Y.; Lee, N.H.; Thames, G.D.; Minutti, R.R.; Wang, H.; Go, V.L.W.; Heber, D. Bioavailability and Antioxidant Activity of Tea Flavanols after Consumption of Green Tea, Black Tea, or a Green Tea Extract Supplement. Am. J. Clin. Nutr. 2004, 80, 1558–1564. [Google Scholar] [CrossRef]
- Chen, C.-W.; Ho, C.-T. Antioxidant Properties of Polyphenols Extracted From Green and Black Teas. J. Food Lipids 1995, 2, 35–46. [Google Scholar] [CrossRef]
- Shon, M.-Y.; Park, S.-K.; Nam, S.-H. Antioxidant Activity of Theaflavin and Thearubigin Separated from Korean Microbially Fermented Tea. Prev. Nutr. Food Sci. 2007, 12, 7–10. [Google Scholar] [CrossRef]
- Sinha, S.K.; Ghaskadbi, S.S. Thearubigins Rich Black Tea Fraction Reveals Strong Antioxidant Activity. Int. J. Green Pharm. 2013, 7, 336–344. [Google Scholar] [CrossRef]
- International Commission on Illumination. Recommendations on Uniform Color Spaces, Color-Difference Equations, Psychometric Color Terms; CIE: Vienna, Austria, 1978. [Google Scholar]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Aniśko, J.; Mysiukiewicz, O.; Marć, M. Mandarin Peel As an Auspicious Functional Filler for Polymer Composites. Maced. J. Chem. Chem. Eng. 2021, 40, 89–106. [Google Scholar] [CrossRef]
- Kasim, R.; Kasim, M.U. Biochemical Changes and Color Properties of Fresh-Cut Green Bean (Phaseolus vulgaris L. Cv.Gina) Treated with Calcium Chloride during Storage. Food Sci. Technol. 2015, 35, 266–272. [Google Scholar] [CrossRef]
- Rojas-Lema, S.; Torres-Giner, S.; Quiles-Carrillo, L.; Gomez-Caturla, J.; Garcia-Garcia, D.; Balart, R. On the Use of Phenolic Compounds Present in Citrus Fruits and Grapes as Natural Antioxidants for Thermo-Compressed Bio-Based High-Density Polyethylene Films. Antioxidants 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Morais, S.; Carvalho, D.O.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. Brewer’s Spent Grain from Different Types of Malt: Evaluation of the Antioxidant Activity and Identification of the Major Phenolic Compounds. Food Res. Int. 2013, 54, 382–388. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Yu, J.; Wang, K. Thermal Oxidation Products and Kinetics of Polyethylene Composites. Polym. Degrad. Stab. 2006, 91, 1651–1657. [Google Scholar] [CrossRef]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the Carbonyl Index of Polyethylene and Polypropylene Using Specified Area under Band Methodology with ATR-FTIR Spectroscopy. e-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Yagoubi, W.; Abdelhafidi, A.; Sebaa, M.; Chabira, S.F. Identification of Carbonyl Species of Weathered LDPE Films by Curve Fitting and Derivative Analysis of IR Spectra. Polym. Test. 2015, 44, 37–48. [Google Scholar] [CrossRef]
- Aniśko, J.; Barczewski, M.; Mietliński, P.; Piasecki, A.; Szulc, J. Valorization of Disposable Polylactide (PLA) Cups by Rotational Molding Technology: The Influence of Pre-Processing Grinding and Thermal Treatment. Polym. Test. 2022, 107, 107481. [Google Scholar] [CrossRef]
- Thummajitsakul, S.; Samaikam, S.; Tacha, S.; Silprasit, K. Study on FTIR Spectroscopy, Total Phenolic Content, Antioxidant Activity and Anti-Amylase Activity of Extracts and Different Tea Forms of Garcinia Schomburgkiana Leaves. LWT 2020, 134, 110005. [Google Scholar] [CrossRef]
- Thirumal, S. FT-IR Analysis and Correlation Studies on the Antioxidant Activity, Total Phenolics and Total Flavonoids of Indian Commercial Teas (Camellia sinensis L.)—A Novel Approach. Int. Res. J. Biol. Sci. 2017, 6, 1–7. [Google Scholar]
- Volli, V.; Gollakota, A.R.K.; Shu, C.M. Comparative Studies on Thermochemical Behavior and Kinetics of Lignocellulosic Biomass Residues Using TG-FTIR and Py-GC/MS. Sci. Total Environ. 2021, 792, 148392. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Durrani, A.I.; Farooq, U.; Tariq, M. Efficacy of Spent Black Tea for the Removal of Nitrobenzene from Aqueous Media. J. Environ. Manag. 2018, 223, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Heryanto, R.; Pradono, D.I.; Marlina, E.; Darusman, L.K. Classification of Java Tea (Orthosiphon Aristatus) Quality Using FTIR Spectroscopy and Chemometrics. J. Phys. Conf. Ser. 2017, 835, 012012. [Google Scholar] [CrossRef]
- Nugrahani, I.; Sundalian, M. Chemometrical Analysis of Fourier Transform Infrared Spectrum Profile of Indonesia’s Black Tea Products (Camellia sinensis L.). Biointerface Res. Appl. Chem. 2019, 10, 4721–4727. [Google Scholar] [CrossRef]
- Reyes-Rivera, J.; Terrazas, T. Lignin Analysis by HPLC and FTIR. Methods Mol. Biol. 2017, 1544, 193–299. [Google Scholar] [CrossRef]
- Graham, H.N. Green Tea Composition, Consumption, and Polyphenol Chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Rosu, D.; Teaca, C.A.; Bodirlau, R.; Rosu, L. FTIR and Color Change of the Modified Wood as a Result of Artificial Light Irradiation. J. Photochem. Photobiol. B Biol. 2010, 99, 144–149. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Nugent, P. Rotational Molding; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9781437735147. [Google Scholar]
- Rodriguez, A.K.; Mansoor, B.; Ayoub, G.; Colin, X.; Benzerga, A.A. Effect of UV-Aging on the Mechanical and Fracture Behavior of Low Density Polyethylene. Polym. Degrad. Stab. 2020, 180, 109185. [Google Scholar] [CrossRef]
- Salvalaggio, M.; Bagatin, R.; Fornaroli, M.; Fanutti, S.; Palmery, S.; Battistel, E. Multi-Component Analysis of Low-Density Polyethylene Oxidative Degradation. Polym. Degrad. Stab. 2006, 91, 2775–2785. [Google Scholar] [CrossRef]
- Lomonte, J.N. Integrated Absorption Standardization for Infrared Determination of Ester Content in Oxidized Polyethylene. Anal. Chem. 1964, 36, 192–194. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts; Wiley: Hoboken, NJ, USA, 2001; ISBN 978-0-470-09307-8. [Google Scholar]
- Guilhen, A.; Gadioli, R.; Fernandes, F.C.; Waldman, W.R.; Aurelio De Paoli, M. High-Density Green Polyethylene Biocomposite Reinforced with Cellulose Fibers and Using Lignin as Antioxidant. J. Appl. Polym. Sci. 2017, 134, 45219. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, S.H.; Zhang, Z.Y.; Yang, X.L.; Yang, Z.G.; Yang, H.G. Degradation of Sunlight Exposure on the High-Density Polyethylene (HDPE) Pipes for Transportation of Natural Gases. Polym. Degrad. Stab. 2021, 194, 109752. [Google Scholar] [CrossRef]
- Chabira, S.F.; Sebaa, M.; G’sell, C. Oxidation and Crosslinking Processes during Thermal Aging of Low-Density Polyethylene Films. J. Appl. Polym. Sci. 2011, 124, 5200–5208. [Google Scholar] [CrossRef]
- Hoàng, E.M.; Allen, N.S.; Liauw, C.M.; Fontán, E.; Lafuente, P. The Thermo-Oxidative Degradation of Metallocene Polyethylenes: Part 2: Thermal Oxidation in the Melt State. Polym. Degrad. Stab. 2006, 91, 1363–1372. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Kosmela, P.; Barczewski, M.; Hejna, A. Mechanical, Thermal and Rheological Properties of Polyethylene-Based Composites Filled with Micrometric Aluminum Powder. Materials 2020, 13, 1242. [Google Scholar] [CrossRef]
- Arrigo, R.; Malucelli, G. Rheological Behavior of Polymer/Carbon Nanotube Composites: An Overview. Materials 2020, 13, 2771. [Google Scholar] [CrossRef]
- Patti, A.; Acierno, D.; Lecocq, H.; Serghei, A.; Cassagnau, P. Viscoelastic Behaviour of Highly Filled Polypropylene with Solid and Liquid Tin Microparticles: Influence of the Stearic Acid Additive. Rheol. Acta 2021, 60, 661–673. [Google Scholar] [CrossRef]
- Zia, J.; Paul, U.C.; Heredia-Guerrero, J.A.; Athanassiou, A.; Fragouli, D. Low-Density Polyethylene/Curcumin Melt Extruded Composites with Enhanced Water Vapor Barrier and Antioxidant Properties for Active Food Packaging. Polymer 2019, 175, 137–145. [Google Scholar] [CrossRef]
- Babaghayou, M.I.; Mourad, A.H.I.; Ochoa, A.; Beltrán, F.; Cherupurakal, N. Study on the Thermal Stability of Stabilized and Unstabilized Low-Density Polyethylene Films. Polym. Bull. 2021, 78, 5225–5241. [Google Scholar] [CrossRef]
- Weon, J. Il Effects of Thermal Ageing on Mechanical and Thermal Behaviors of Linear Low Density Polyethylene Pipe. Polym. Degrad. Stab. 2010, 95, 14–20. [Google Scholar] [CrossRef]
- Jose, A.S.; Athijayamani, A.; Jani, S.P. A Review on the Mechanical Properties of Bio Waste Particulate Reinforced Polymer Composites. Mater. Today Proc. 2020, 37, 1757–1760. [Google Scholar] [CrossRef]
- Tian, L.; Shen, B.; Xu, H.; Li, F.; Wang, Y.; Singh, S. Thermal Behavior of Waste Tea Pyrolysis by TG-FTIR Analysis. Energy 2016, 103, 533–542. [Google Scholar] [CrossRef]
- Gogos, G. Bubble Removal in Rotational Molding. Polym. Eng. Sci. 2004, 44, 388–394. [Google Scholar] [CrossRef]



| L* | a* | b* | BI | ΔE | |
|---|---|---|---|---|---|
| BTW before thermal treatment | 54.65 | 7.58 | 23.10 | 9.91 | 25.98 |
| BTW after thermal treatment | 29.83 | 8.21 | 15.42 | 19.10 |
| Antioxidant Activity [%] | DRSC [mg/g Dry Mass] | |
|---|---|---|
| BTW before thermal treatment | 32.20 | 46.60 |
| BTW after thermal treatment | 11.50 | 9.16 |
| Outer Surface | Inner Surface | ΔE2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | BI | ΔE1 | Picture | L* | a* | b* | BI | ΔE1 | Picture | ||
| LDPE SEB 853 | 67.08 ± 1.82 | 0.57 ± 0.26 | 3.03 ± 0.88 | 0.62 ± 0.25 |  | 67.86 ± 1.46 | 0.62 ± 0.25 | 2.73 ± 0.83 | 0.63 ± 0.24 |  | 0.84 | ||
| 1% BTW | 34.67 ± 1.25 | 7.83 ± 1.41 | 11.00 ± 2.20 | 15.80 ± 3.11 | 34.16 |  | 29.79 ± 3.72 | 10.99 ± 0.25 | 18.50 ± 0.99 | 25.43 ± 2.62 | 42.50 |  | 9.49 |
| 2% BTW | 30.54 ± 0.77 | 6.61 ± 0.38 | 9.05 ± 0.34 | 15.12 ± 0.41 | 37.52 |  | 21.62 ± 1.68 | 9.43 ± 2.11 | 12.85 ± 1.95 | 29.06 ± 3.91 | 48.15 |  | 10.10 |
| 5% BTW | 31.40 ± 1.63 | 6.26 ± 0.33 | 9.00 ± 0.41 | 14.02 ± 1.34 | 36.62 |  | 17.59 ± 0.85 | 6.11 ± 0.35 | 5.55 ± 0.43 | 23.72 ± 1.87 | 50.65 |  | 14.23 |
| 10% BTW | 24.86 ± 1.65 | 3.71 ± 0.69 | 4.59 ± 0.83 | 10.62 ± 2.22 | 42.37 |  | 21.72 ± 1.41 | 4.17 ± 0.25 | 4.27 ± 0.38 | 13.49 ± 1.22 | 46.31 |  | 3.19 |
| Tm [°C] | Tc [°C] | ΔHm [J/g] | Xc [%] | |
|---|---|---|---|---|
| LDPE SEB 853 | 111.8 | 94.4 | 69.55 | 23.74 |
| 1% BTW | 113.8 | 95.2 | 65.31 | 22.52 |
| 2% BTW | 112.7 | 96.3 | 65.05 | 22.65 |
| 5% BTW | 112.4 | 95.8 | 64.16 | 23.05 |
| 10% BTW | 111.8 | 97.0 | 64.08 | 24.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniśko, J.; Barczewski, M. Uniaxial Rotational Molding of Bio-Based Low-Density Polyethylene Filled with Black Tea Waste. Materials 2023, 16, 3641. https://doi.org/10.3390/ma16103641
Aniśko J, Barczewski M. Uniaxial Rotational Molding of Bio-Based Low-Density Polyethylene Filled with Black Tea Waste. Materials. 2023; 16(10):3641. https://doi.org/10.3390/ma16103641
Chicago/Turabian StyleAniśko, Joanna, and Mateusz Barczewski. 2023. "Uniaxial Rotational Molding of Bio-Based Low-Density Polyethylene Filled with Black Tea Waste" Materials 16, no. 10: 3641. https://doi.org/10.3390/ma16103641





