Fucoidan-Containing, Low-Adhesive Siloxane Coatings for Medical Applications: Inhibition of Bacterial Growth and Biofilm Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Compositions
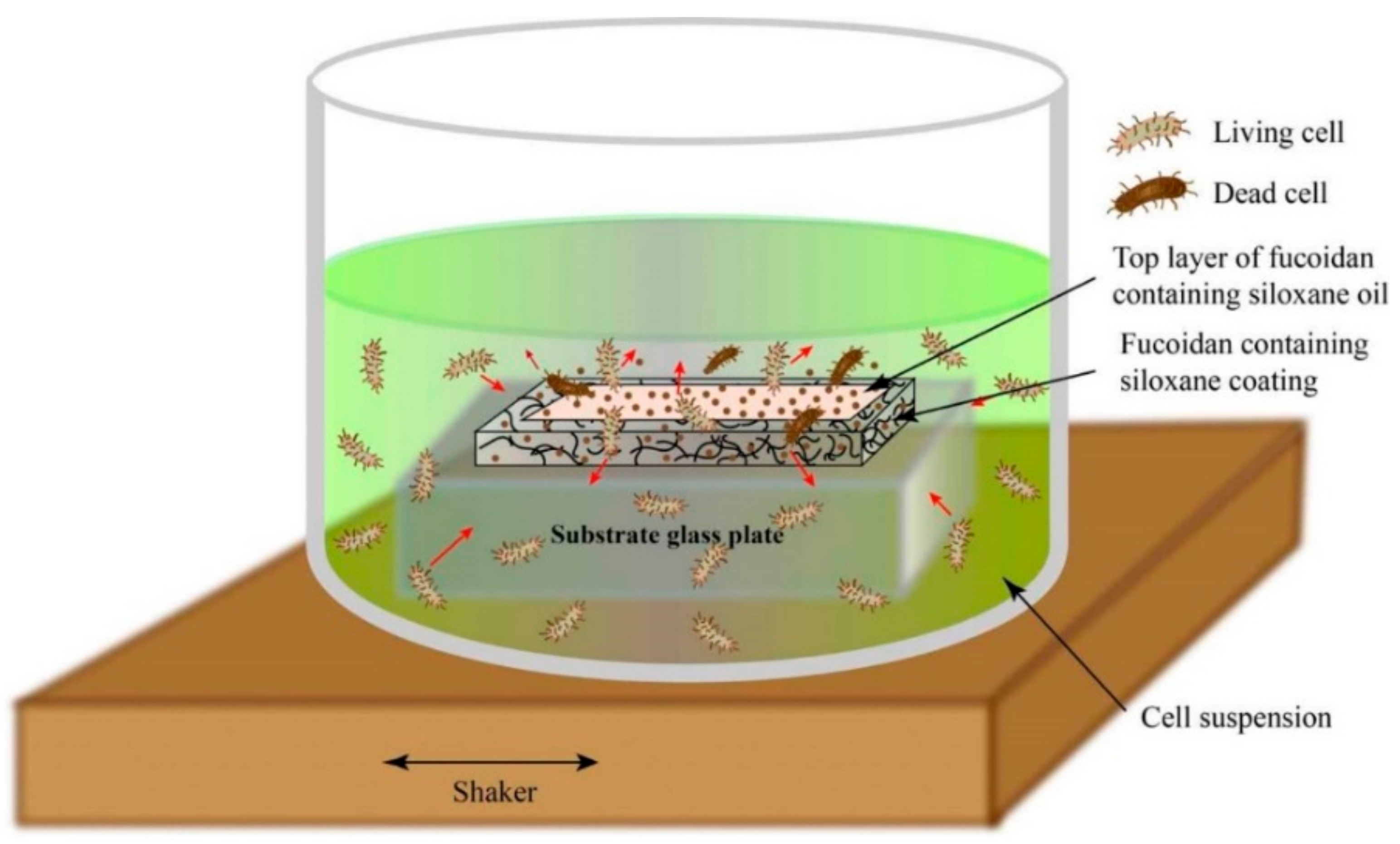
2.2. Coated Test Samples
- Sample (1)—Control: glass, covered with siloxane coating, does not contain fucoidan;
- Sample (2)—Glass, covered with siloxane coating, containing 1 wt.% fucoidan;
- Sample (3)—Glass, covered with siloxane coating, containing 2 wt.% fucoidan;
- Sample (4)—Glass, covered with siloxane coating, containing 3 wt.% fucoidan;
- Sample (5)—Glass, covered with siloxane coating, containing 5 wt.% fucoidan;
- Sample (6)—Glass, covered with siloxane coating, containing 8 wt.% fucoidan.
2.3. Water Contact Angle (WCA) and Surface Energy (Ec)
2.4. Atom Force Microscopy (AFM)
2.5. Depth-Sensing Indentation (DSI)
2.6. X-ray Photoelectron Spectroscopy (XPS)
2.7. In Vitro Antibacterial Activity
2.7.1. Test Bacterial Strains
2.7.2. Cell Growth Inhibition
- N is the number of bacteria in the rinse liquid;
- A is the number of counted colonies in a Petri dish from a decimal dilution;
- n is the relevant number of decimal dilutions used to inoculate the Petri dish; and
- 0.1 is the quantity of inoculum in every Petri dish.
2.7.3. Fluorescent Microscopy Observation
3. Experimental Results and Discussion
3.1. Surface Characteristics
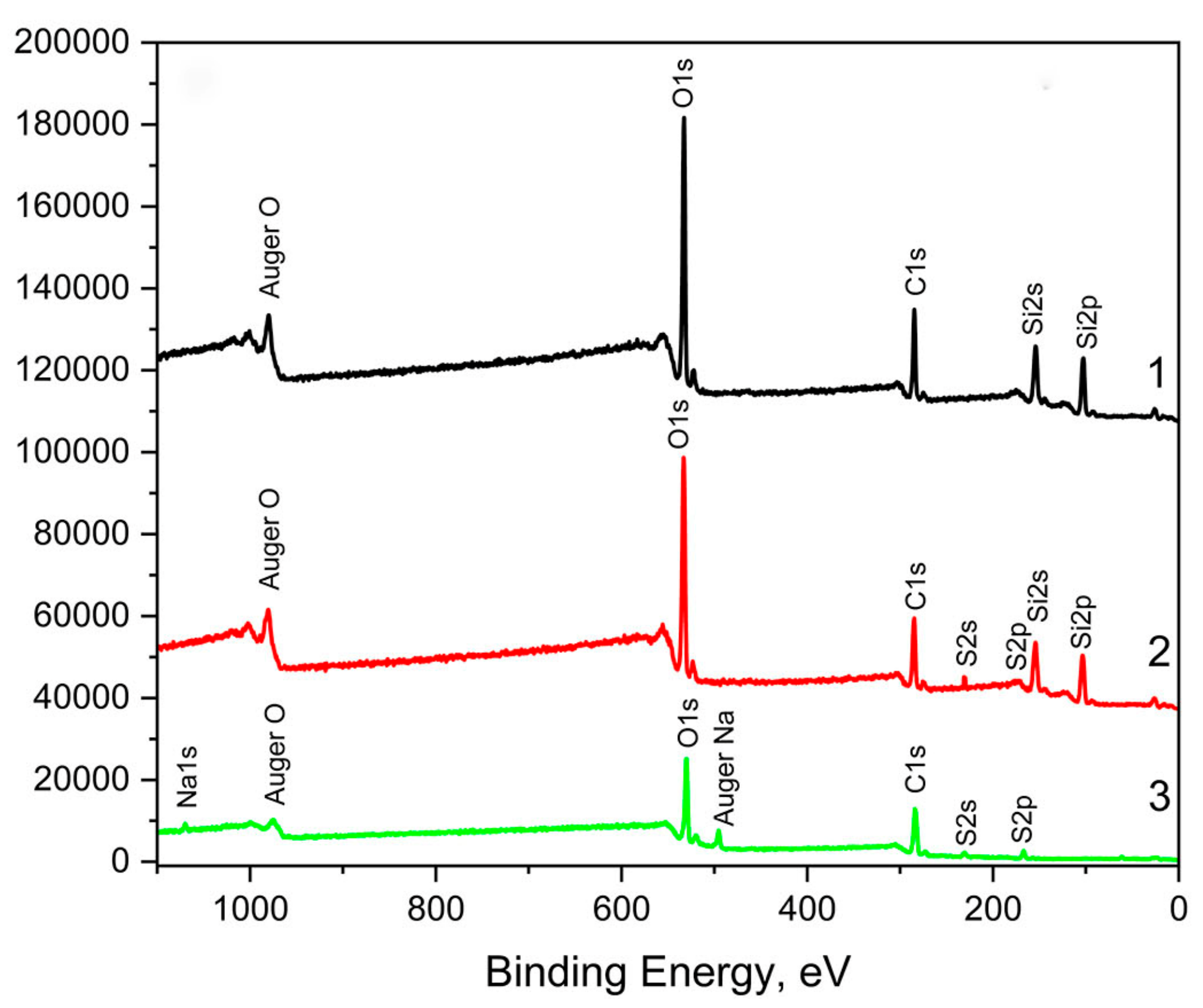
3.2. XPS
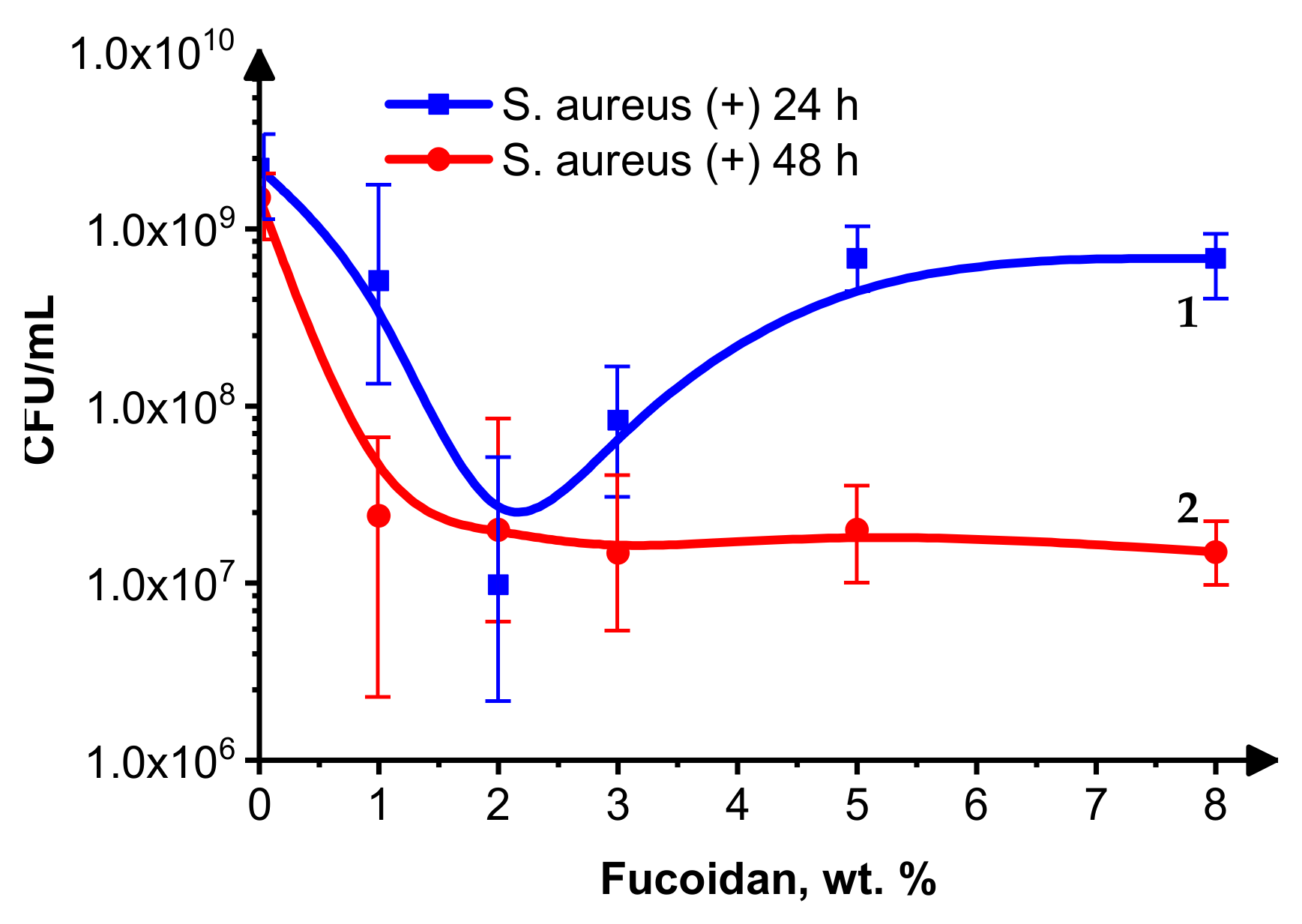
3.3. Cell Growth Inhibition
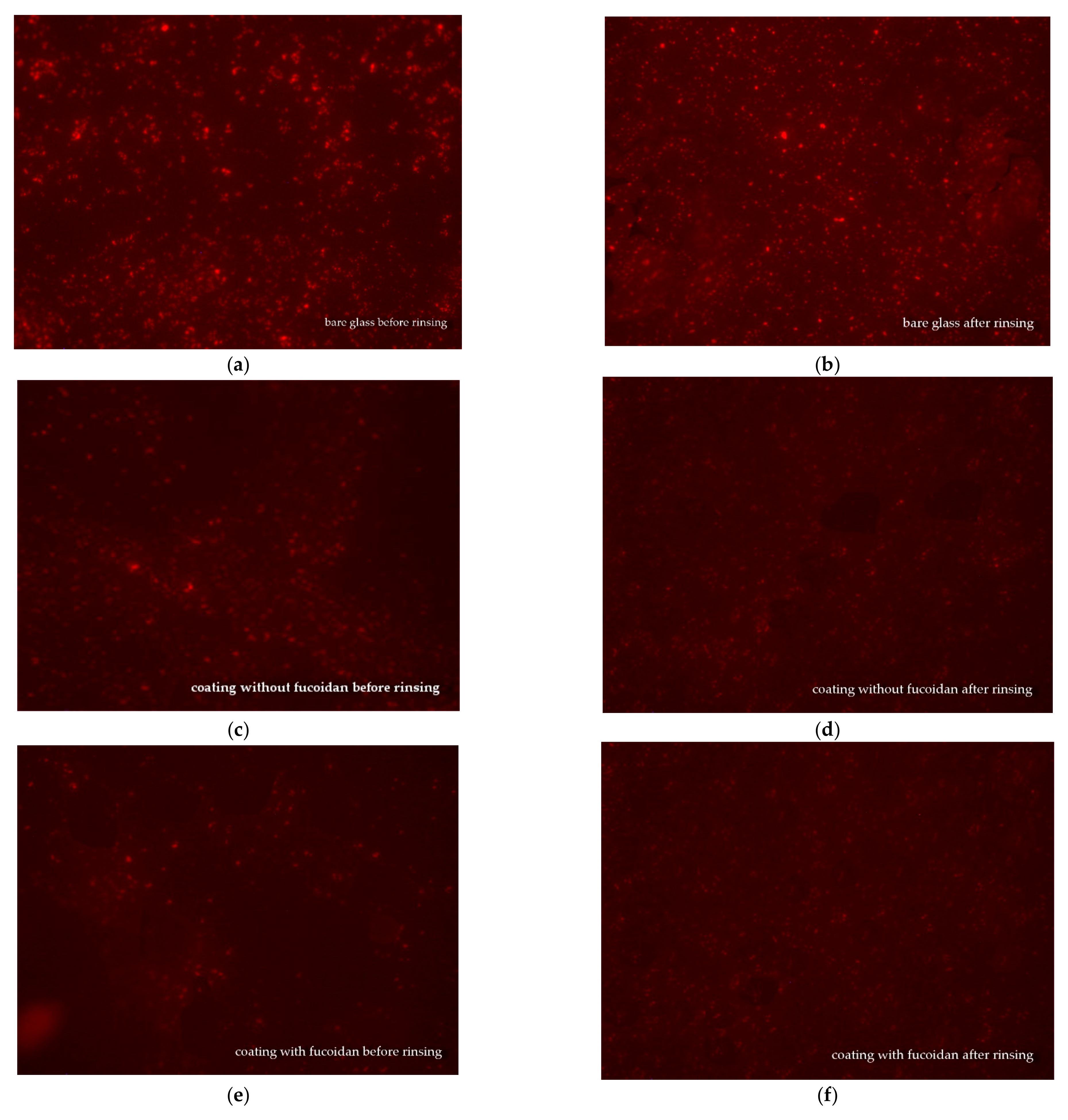
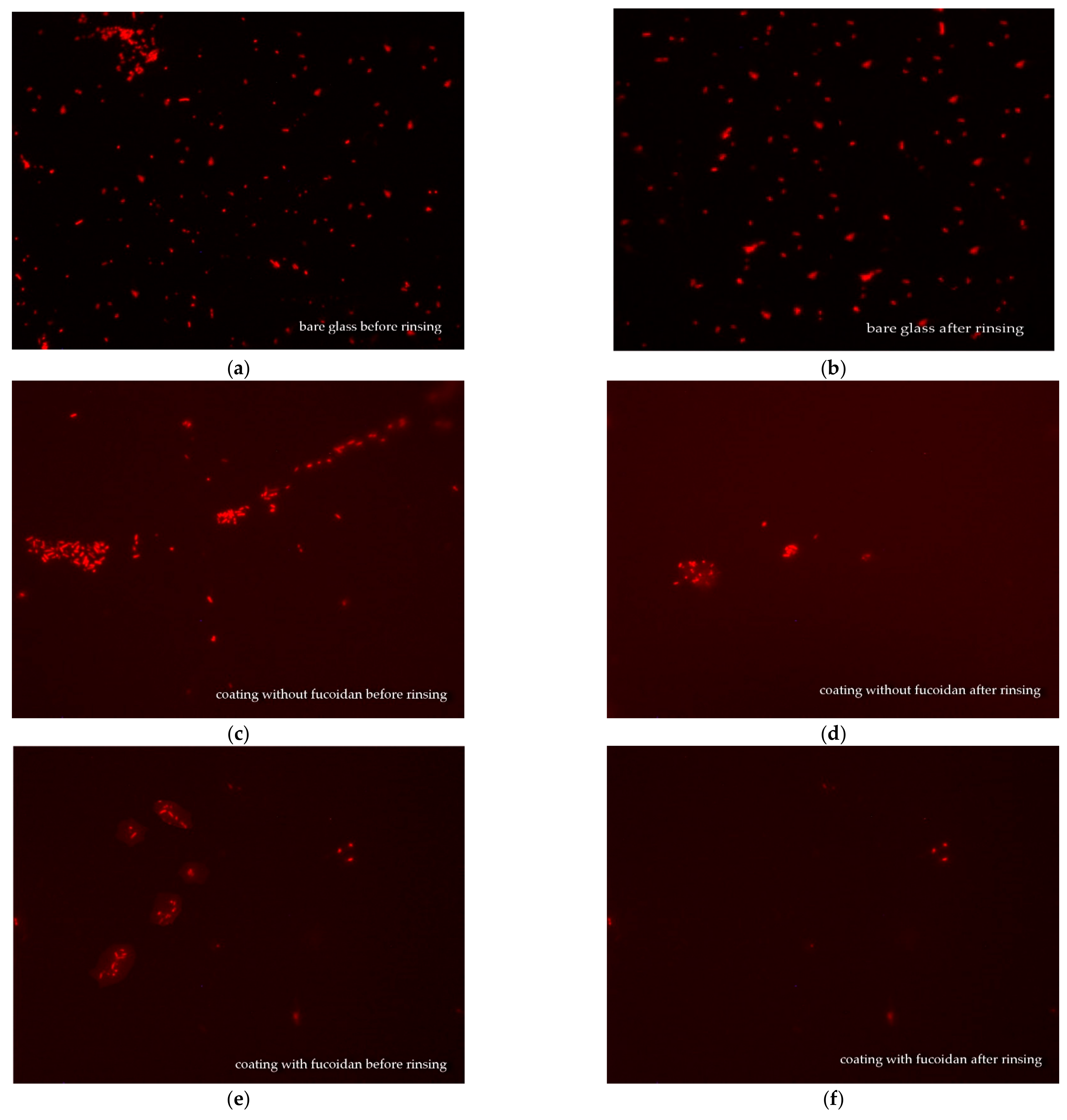
3.4. Fluorescence Microscopy
3.5. Mode of Antimicrobial Action of the Studied Fucoidan-Containing, Low-Adhesive Siloxane Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, R.; Dempsey-Hibbert, N.C.; Whitehead, K.A. Antimicrobial Strategies to Reduce Polymer Biomaterial Infections and Their Economic Implications and Considerations. Int. Biodeterior. Biodegrad. 2019, 136, 1–14. [Google Scholar] [CrossRef]
- Olivares, E.; Badel-Berchoux, S.; Provot, C.; Prévost, G.; Bernardi, T.; Jehl, F. Clinical Impact of Antibiotics for the Treatment of Pseudomonas Aeruginosa Biofilm Infections. Front. Microbiol. 2020, 10, 2894. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Raju, S.; Thiyagarajan, V. (Eds.) Biofilms Control: Biomedical and Industrial Environments; Alpha Science International Ltd.: Oxford, UK, 2018; ISBN 978-1-78332-393-7. [Google Scholar]
- Flemming, H.-C. Biofouling and Me: My Stockholm Syndrome with Biofilms. Water Res. 2020, 173, 115576. [Google Scholar] [CrossRef]
- Vladkova, T.G. Surface Engineering of Polymeric Biomaterials; Smithers Rapra Technology: Shrewsbury, UK, 2013; ISBN 978-1-84735-659-8. [Google Scholar]
- Vladkova, T.G.; Dineff, P.D.; Zlatanov, I.Y.; Kathioli, S.; Ramasammy, V.; Vasudeva, P.S.M. Composition Coating for Biofouling Protection. WO2008074102A1, 26 June 2008. [Google Scholar]
- Akuzov, D.; Brümmer, F.; Vladkova, T. Some Possibilities to Reduce the Biofilm Formation on Transparent Siloxane Coatings. Colloids Surf. B Biointerfaces 2013, 104, 303–310. [Google Scholar] [CrossRef]
- Akuzov, D.; Franca, L.; Grunwald, I.; Vladkova, T. Sharply Reduced Biofilm Formation from Cobetia Marina and in Black Sea Water on Modified Siloxane Coatings. Coatings 2018, 8, 136. [Google Scholar] [CrossRef]
- Akuzov, D.; Vladkova, T.; Zamfirova, G.; Gaydarov, V.; Nascimento, M.V.; Szeglat, N.; Grunwald, I. Polydimethyl Siloxane Coatings with Superior Antibiofouling Efficiency in Laboratory and Marine Conditions. Prog. Org. Coat. 2017, 103, 126–134. [Google Scholar] [CrossRef]
- Vladkova, T.G.; Martinov, B.L.; Gospodinova, D.N. Anti-Biofilm Agents from Marine Biota. J. Chem. Technol. Metall. 2023, in press. [Google Scholar]
- Danquah, C.A.; Minkah, P.A.B.; Agana, T.A.; Moyo, P.; Tetteh, M.; Junior, I.O.D.; Amankwah, K.B.; Somuah, S.O.; Ofori, M.; Maharaj, V.J.; et al. Natural Products as Antibiofilm Agents; IntechOpen: London, UK, 2022; ISBN 978-1-80355-796-0. [Google Scholar]
- Shannon, E.; Abu-Ghannam, N. Antibacterial Derivatives of Marine Algae: An Overview of Pharmacological Mechanisms and Applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Shen, P.; Yin, Z.; Qu, G.; Wang, C. 11—Fucoidan and Its Health Benefits. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 223–238. ISBN 978-0-12-813312-5. [Google Scholar]
- Nutra Ingredients. Fucoidan: A New Wave in Digestive Health. Available online: https://www.nutraingredients-usa.com/News/Promotional-Features/Fucoidan-A-new-wave-in-digestive-health (accessed on 9 February 2023).
- Sanjeewa, K.K.A.; Jeon, Y.-J. Fucoidans as Scientifically and Commercially Important Algal Polysaccharides. Mar. Drugs 2021, 19, 284. [Google Scholar] [CrossRef]
- Xiao, M.; Ren, X.; Yu, Y.; Gao, W.; Zhu, C.; Sun, H.; Kong, Q.; Fu, X.; Mou, H. Fucose-Containing Bacterial Exopolysaccharides: Sources, Biological Activities, and Food Applications. Food Chem. X 2022, 13, 100233. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Dawood, M.A.O.; Alagawany, M.; Faggio, C.; Nowosad, J.; Kucharczyk, D. Health Benefits and Potential Applications of Fucoidan (FCD) Extracted from Brown Seaweeds in Aquaculture: An Updated Review. Fish Shellfish Immunol. 2022, 122, 115–130. [Google Scholar] [CrossRef]
- Koike, T.; Sugimoto, A.; Kosono, S.; Komaba, S.; Kanno, Y.; Kitamura, T.; Anzai, I.; Watanabe, T.; Takahashi, D.; Toshima, K. Synthesis of Low-Molecular Weight Fucoidan Derivatives and Their Binding Abilities to SARS-CoV-2 Spike Proteins. RSC Med. Chem. 2021, 12, 2016–2021. [Google Scholar] [CrossRef]
- Chmit, M.; Kanaan, H.; Habib, J.; Abbass, M.; Mcheik, A.; Chokr, A. Antibacterial and Antibiofilm Activities of Polysaccharides, Essential Oil, and Fatty Oil Extracted from Laurus Nobilis Growing in Lebanon. Asian Pac. J. Trop. Med. 2014, 7, S546–S552. [Google Scholar] [CrossRef]
- Jun, J.-Y.; Jung, M.-J.; Jeong, I.-H.; Yamazaki, K.; Kawai, Y.; Kim, B.-M. Antimicrobial and Antibiofilm Activities of Sulfated Polysaccharides from Marine Algae against Dental Plaque Bacteria. Mar. Drugs 2018, 16, 301. [Google Scholar] [CrossRef]
- Mohan, T.; Čas, A.; Bračič, M.; Plohl, O.; Vesel, A.; Rupnik, M.; Zemljič, L.F.; Rebol, J. Highly Protein Repellent and Antiadhesive Polysaccharide Biomaterial Coating for Urinary Catheter Applications. ACS Biomater. Sci. Eng. 2019, 5, 5825–5832. [Google Scholar] [CrossRef]
- Achmad, H.; Huldani; Ramadhany, Y.F. Antimicrobial Activity and Sulfated Polysaccharides Antibiofilms in Marine Algae Against Dental Plaque Bacteria: A Literature Review. Syst. Rev. Pharm. 2020, 11, 459–465. [Google Scholar]
- Raju, P.; Natarajan, S. Anticancer, Anti-Biofilm and Antimicrobial Activity of Fucoidan-Loaded Zeolitic Imidazole Framework Fabricated by One-Pot Synthesis Method. Appl. Nanosci. 2021, 13, 1919–1937. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, H.; Mei, L.; Dou, K.; Jiang, Y.; Sun, Z.; Wang, S.; Hasanin, M.S.; Deng, J.; Zhou, Q. Fucoidan-Derived Carbon Dots against Enterococcus Faecalis Biofilm and Infected Dentinal Tubules for the Treatment of Persistent Endodontic Infections. J. Nanobiotechnol. 2022, 20, 321. [Google Scholar] [CrossRef]
- Vladkova, T.; Georgieva, N.; Staneva, A.; Gospodinova, D. Recent Progress in Antioxidant Active Substances from Marine Biota. Antioxidants 2022, 11, 439. [Google Scholar] [CrossRef]
- Chan, C.-M. Polymer Surface Modification and Characterization; Wiley: Hoboken, NJ, USA, 1993; ISBN 978-1-56990-158-8. [Google Scholar]
- Rijal, N. Most Probable Number (MPN) Test: Principle, Procedure, Results. Microbe Online. Available online: https://microbeonline.com/probable-number-mpn-test-principle-procedure-results/ (accessed on 9 February 2023).
- Stoyanova, D.; Ivanova, I.; Angelov, O.; Vladkova, T. Antibacterial Activity of Thin Films TiO2 Doped with Ag and Cu on Gracilicutes and Firmicutes Bacteria. BioDiscovery 2017, 20, e15076. [Google Scholar] [CrossRef]
- Vladkova, T. Surface Engineering for Non-Toxic Biofouling Control (Review). J. Univ. Chem. Technol. Metall. 2007, 42, 239–256. [Google Scholar]
- Bos, R.; van der Mei, H.C.; Gold, J.; Busscher, H.J. Retention of Bacteria on a Substratum Surface with Micro-Patterned Hydrophobicity. FEMS Microbiol. Lett. 2000, 189, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.B.; Kogoma, T. Oxidative Stress Responses in Escherichia Coli and Salmonella Typhimurium. Microbiol. Rev. 1991, 55, 561–585. [Google Scholar] [CrossRef]
- Ramstedt, M.; Ribeiro, I.A.C.; Bujdakova, H.; Mergulhão, F.J.M.; Jordao, L.; Thomsen, P.; Alm, M.; Burmølle, M.; Vladkova, T.; Can, F.; et al. Evaluating Efficacy of Antimicrobial and Antifouling Materials for Urinary Tract Medical Devices: Challenges and Recommendations. Macromol. Biosci. 2019, 19, 1800384. [Google Scholar] [CrossRef]

| Parameter | Coated Glass Sample, No | |||||
|---|---|---|---|---|---|---|
| 1 Control | 2 1 wt.% Fucoidan | 3 2 wt.% Fucoidan | 4 3 wt.% Fucoidan | 5 5 wt.% Fucoidan | 6 8 wt.% Fucoidan | |
| WCA, ° | ||||||
| at initial time | 106.5 ± 0.21 | 116.9 ± 0.14 | 120.2 ± 0.22 | 117.9 ± 0.70 | 104.7 ± 4.17 | 99.7 ± 2.28 |
| after 120 s | 100.8 ± 0.35 | 100.9 ± 0.14 | 101.4 ± 1.83 | 103.5 ± 0.21 | 98.3 ± 4.45 | 98.3 ± 4.45 |
| E, mN/m | ||||||
| at initial time | 20.0 ± 0.72 | 19.34 ± 0.15 | 18.59 ± 0.30 | 19.39 ± 0.32 | 20.45 ± 0.50 | 22.05 ± 0.54 |
| after 120 s | 23.31 ± 0.13 | 23.30 ± 0.31 | 23.78 ± 0.86 | 22.19 ± 0.36 | 24.36 ± 0.74 | 22.27 ± 2.02 |
| Ed, mN/m, | ||||||
| at initial time | 20.45 ± 0.02 | 19.33 ± 0.14 | 18.42 ± 0.28 | 19.04 ± 0.26 | 20.03 ± 0.14 | 20.91 ± 0.96 |
| after 120 s | 22.79 ± 0.10 | 23.06 ± 0.29 | 22.89 ± 0.59 | 16.83 ± 0.28 | 23.39 ± 0. 12 | 21.98 ± 1.77 |
| Ep, mN/m | ||||||
| at initial time | 0.26 ± 0.02 | 0.01 ± 0.00 | 0.17 ± 0.02 | 0.35 ± 0.06 | 0.41 ± 0.36 | 1.14 ± 0.54 |
| after 120 s | 0.53 ± 0.03 | 0.23 ± 0.02 | 0.89 ± 0.26 | 1.36 ± 0.09 | 0.98 ± 0.62 | 5.61 ± 0.02 |
| Ra, nm | 0.139 | 0.163 | 0.291 | 0.918 | 22.316 | 35.911 |
| Rq, nm | 0.217 | 0.226 | 0.312 | 1.316 | 18.112 | 40.213 |
| HMV, N/mm2 | 0.120 ± 0.02 | 0.100 ± 0.02 | 0.112 ± 0.02 | 0.117 ± 0.03 | 0.61 ± 0.02 | 0.96 ± 0.02 |
| HIT, N/mm2 | 0.361 ± 0.01 | 0.318 ± 0.02 | 0.319 ± 0.01 | 0.422 ± 0.04 | 0.939 ± 0.02 | 1.830 ± 0.04 |
| EIT, N/mm2 | 1.66 ± 0.07 | 1.69 ± 0.04 | 1.61 ± 0.06 | 1.90 ± 0.05 | 9.66 ± 0.09 | 12.93 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladkova, T.G.; Staneva, A.D.; Avramova, I.A.; Ivanova, I.A.; Gospodinova, D.N. Fucoidan-Containing, Low-Adhesive Siloxane Coatings for Medical Applications: Inhibition of Bacterial Growth and Biofilm Development. Materials 2023, 16, 3651. https://doi.org/10.3390/ma16103651
Vladkova TG, Staneva AD, Avramova IA, Ivanova IA, Gospodinova DN. Fucoidan-Containing, Low-Adhesive Siloxane Coatings for Medical Applications: Inhibition of Bacterial Growth and Biofilm Development. Materials. 2023; 16(10):3651. https://doi.org/10.3390/ma16103651
Chicago/Turabian StyleVladkova, Todorka G., Anna D. Staneva, Ivalina A. Avramova, Iliana A. Ivanova, and Dilyana N. Gospodinova. 2023. "Fucoidan-Containing, Low-Adhesive Siloxane Coatings for Medical Applications: Inhibition of Bacterial Growth and Biofilm Development" Materials 16, no. 10: 3651. https://doi.org/10.3390/ma16103651
APA StyleVladkova, T. G., Staneva, A. D., Avramova, I. A., Ivanova, I. A., & Gospodinova, D. N. (2023). Fucoidan-Containing, Low-Adhesive Siloxane Coatings for Medical Applications: Inhibition of Bacterial Growth and Biofilm Development. Materials, 16(10), 3651. https://doi.org/10.3390/ma16103651








