Review of Potential Drug-Eluting Contact Lens Technologies
Abstract
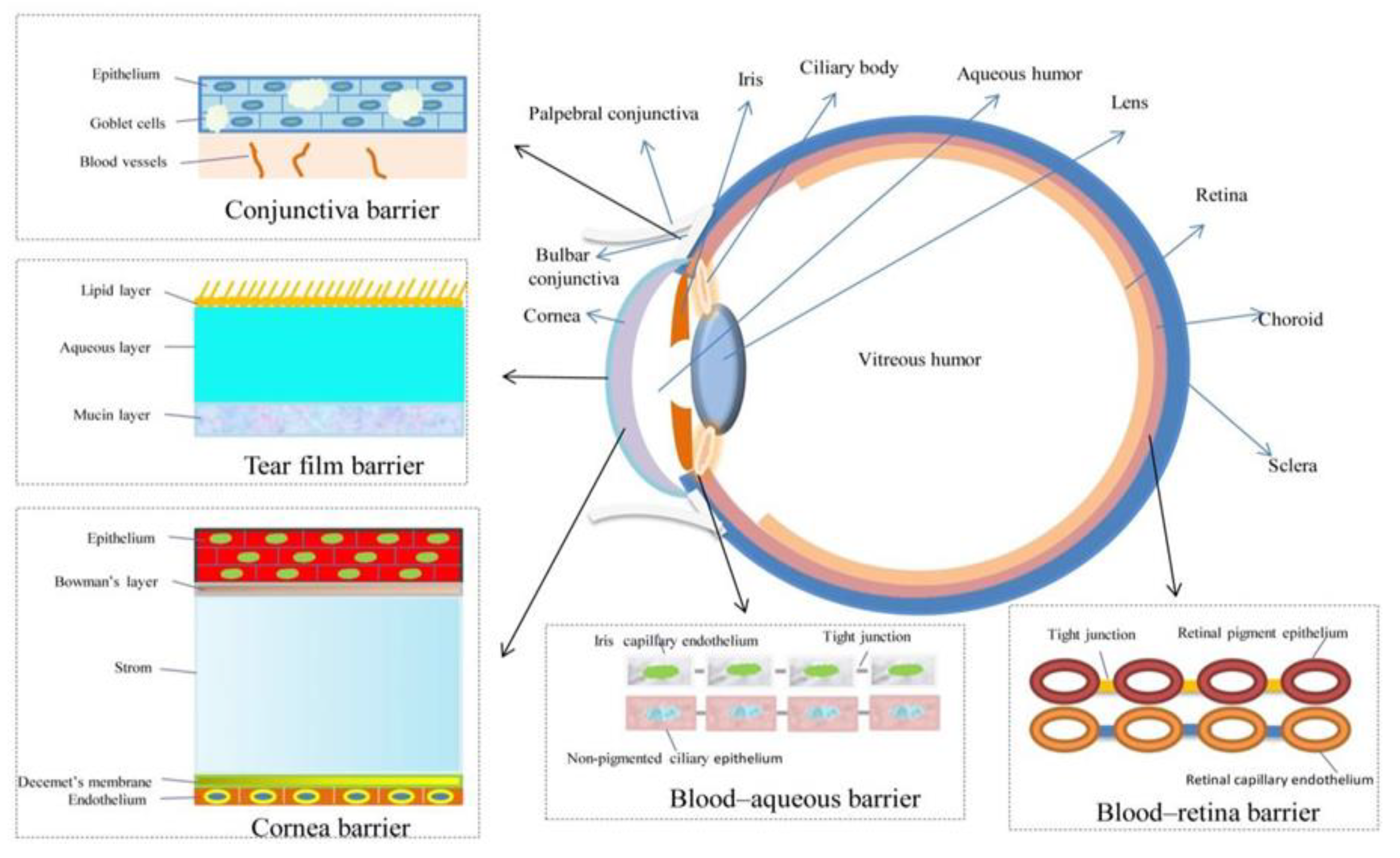
:1. Introduction
2. Lenses
2.1. Initial Considerations
2.2. Lens Materials
2.2.1. “Classical” Hydrogels
2.2.2. Silicone Hydrogels
2.2.3. pHEMA
2.2.4. PLGA
3. Methods of Binding Drugs into/to Lenses
3.1. Layered Contact Lenses
3.2. Surface-Modified Contact Lenses
3.3. Soaking Method
3.4. Molecular Imprinting
3.5. Colloidal Nanoparticles
3.5.1. Polymeric Nanoparticles
3.5.2. Cyclodextrins
3.5.3. Liposomes
3.5.4. Microemulsions and Micelles

3.6. Supercritical Fluid Technology (SCF)
3.7. Vitamin E as a Release-Modifying Additive
3.8. Other Solutions
4. Contact Lenses Preparation Methods for Use in Drug-Delivery
4.1. Cast-Moulding Method
4.2. Lathe-Cutting Method
4.3. Spin-Casting Method
5. Future Prospects

6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mitra, A.K. Role of Transporters in Ocular Drug Delivery System. Pharm. Res. 2009, 26, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A Comprehensive Review on Contact Lens for Ophthalmic Drug Delivery. J Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Lim, L.; Lim, E.W.L. Therapeutic Contact Lenses in the Treatment of Corneal and Ocular Surface Diseases—A Review. Asia-Pac. J. Ophthalmol. 2020, 9, 524–532. [Google Scholar] [CrossRef]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Aghabegi Moghanjoughi, A.; Khoshnevis, D.; Zarrabi, A. A Concise Review on Smart Polymers for Controlled Drug Release. Drug Deliv. Transl. Res. 2016, 6, 333–340. [Google Scholar] [CrossRef]
- Stapleton, F.; Stretton, S.; Papas, E.; Skotnitsky, C.; Sweeney, D.F. Silicone Hydrogel Contact Lenses and the Ocular Surface. Ocul. Surf. 2006, 4, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Aranguez, A.; Colligris, B.; Pintor, J. Contact Lenses: Promising Devices for Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Auffarth, G.U.; Apple, D.J. Zur Entwicklungsgeschichte Der Intraokularlinsen. Der Ophthalmol. 2001, 98, 1017–1031. [Google Scholar] [CrossRef]
- Nicolson, P.C.; Vogt, J. Soft Contact Lens Polymers: An Evolution. Biomaterials 2001, 22, 3273–3283. [Google Scholar] [CrossRef] [PubMed]
- Center for Devices and Radiological Health. Class II Daily Wear Contact Lenses—Premarket Notification [510(k)] Guidance Document; FDA: Silver Spring, MD, USA, 2020.
- Gonzalez-Chomon, C.; Concheiro, A.; Alvarez-Lorenzo, C. Soft Contact Lenses for Controlled Ocular Delivery: 50 Years in the Making. Ther. Deliv. 2013, 4, 1141–1161. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, Z.; Shams Es-Haghi, S.; Cakmak, M. Recent Trends in Advanced Contact Lenses. Adv. Health Mater. 2019, 8, e1801390. [Google Scholar] [CrossRef] [PubMed]
- Donshik, P.C. Extended Wear Contact Lenses. Ophthalmol. Clin. N. Am. 2003, 16, 305–309. [Google Scholar] [CrossRef]
- The Editors of Encyclopaedia Britannica. PolyHEMA. Available online: https://www.britannica.com/science/polyHEMA (accessed on 10 May 2023).
- Dixon, P.; Shafor, C.; Gause, S.; Hsu, K.H.; Powell, K.C.; Chauhan, A. Therapeutic Contact Lenses: A Patent Review. Expert Opin. Ther. Pat. 2015, 25, 1117–1129. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymer 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Ciolino, J.B.; Hudson, S.P.; Mobbs, A.N.; Hoare, T.R.; Iwata, N.G.; Fink, G.R.; Kohane, D.S. A Prototype Antifungal Contact Lens. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6286–6291. [Google Scholar] [CrossRef]
- Nakada, K.; Sugiyama, A. Process for Producing Controlled Drug-Release Contact Lens, and Controlled Drug-Release Contact Lens Thereby Produced. U.S. Patent US6027745A, 29 May 1998. [Google Scholar]
- Danion, A.; Brochu, H.; Martin, Y.; Vermette, P. Fabrication and Characterization of Contact Lenses Bearing Surface-Immobilized Layers of Intact Liposomes. J. Biomed. Mater. Res. A 2007, 82, 41–51. [Google Scholar] [CrossRef]
- Danion, A.; Arsenault, I.; Vermette, P. Antibacterial Activity of Contact Lenses Bearing Surface-Immobilized Layers of Intact Liposomes Loaded with Levofloxacin. J. Pharm. Sci. 2007, 96, 2350–2363. [Google Scholar] [CrossRef]
- Qiu, Y. Silicone Hydrogel Lens with a Crosslinked Hydrophilic Coating. U.S. Patent No. 9,708,087, 18 July 2017. [Google Scholar]
- Li, J.; Zhang, Z.; Loose, C.R.; Coury, A. Silicone Hydrogel Contact Lens Modified Using Lanthanide or Transition Metal Oxidants. U.S. Patent No. 8,870,372, 28 October 2014. [Google Scholar]
- Korogiannaki, M.; Samsom, M.; Schmidt, T.A.; Sheardown, H. Surface-Functionalized Model Contact Lenses with a Bioinspired Proteoglycan 4 (PRG4)-Grafted Layer. ACS Appl. Mater. Interfaces 2018, 10, 30125–30136. [Google Scholar] [CrossRef]
- Winterton, L.C. Method of Making Silicone Hydrogel Contact Lenses. U.S. Patent No. 7,858,000, 28 December 2010. [Google Scholar]
- Li, C.C.; Chauhan, A. Ocular Transport Model for Ophthalmic Delivery of Timolol through P-HEMA Contact Lenses. J. Drug Deliv. Sci. Technol. 2007, 17, 69–79. [Google Scholar] [CrossRef]
- Kim, J.; Chauhan, A. Dexamethasone Transport and Ocular Delivery from Poly(Hydroxyethyl Methacrylate) Gels. Int. J. Pharm. 2008, 353, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. Extended Release of Hyaluronic Acid from Hydrogel Contact Lenses for Dry Eye Syndrome. J. Biomater. Sci. Polym. Ed. 2015, 26, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Ruben, M.; Watkins, R. Pilocarpine Dispensation for the Soft Hydrophilic Contact Lens. Br. J. Ophthalmol. 1975, 59, 455–458. [Google Scholar] [CrossRef]
- Soluri, A.; Hui, A.; Jones, L. Delivery of Ketotifen Fumarate by Commercial Contact Lens Materials. Optom. Vis. Sci. 2012, 89, 1140–1149. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Sun, F. In Vitro and in Vivo Evaluation of Ketotifen Fumarate-Loaded Silicone Hydrogel Contact Lenses for Ocular Drug Delivery. Drug Deliv. 2011, 18, 150–158. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A Review on Therapeutic Contact Lenses for Ocular Drug Delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Schultz, C.L.; Poling, T.R.; Mint, J.O. A Medical Device/Drug Delivery System for Treatment of Glaucoma. Clin. Exp. Optom. 2009, 92, 343–348. [Google Scholar] [CrossRef]
- Xinming, L.; Yingde, C.; Lloyd, A.W.; Mikhalovsky, S.V.; Sandeman, S.R.; Howel, C.A.; Liewen, L. Polymeric Hydrogels for Novel Contact Lens-Based Ophthalmic Drug Delivery Systems: A Review. Contact Lens. Anterior Eye 2008, 31, 57–64. [Google Scholar] [CrossRef]
- Maulvi, D.F. Effect of Timolol Maleate Concentration on Uptake and Release from Hydrogel Contact Lenses Using Soaking Method. J. Pharm. Appl. Sci. 2014, 1, 17–23. [Google Scholar]
- Franco, P.; De Marco, I. Contact Lenses as Ophthalmic Drug Delivery Systems: A Review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef] [PubMed]
- Topete, A.; Oliveira, A.S.; Fernandes, A.; Nunes, T.G.; Serro, A.P.; Saramago, B. Improving Sustained Drug Delivery from Ophthalmic Lens Materials through the Control of Temperature and Time of Loading. Eur. J. Pharm. Sci. 2018, 117, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Ishida, W.; Kishimoto, T.; Nakajima, I.; Hino, S.; Arai, R.; Matsunaga, T.; Fukushima, A.; Yamagami, S. In Vitro and in Vivo Performance of Epinastine Hydrochloride-Releasing Contact Lenses. PLoS ONE 2019, 14, e0210362. [Google Scholar] [CrossRef] [PubMed]
- Zidarič, T.; Finšgar, M.; Maver, U.; Maver, T. Artificial Biomimetic Electrochemical Assemblies. Biosensors 2022, 12, 44. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Hiratani, H.; Gomez-Amoza, J.L.; Martinez-Pacheco, R.; Souto, C.; Concheiro, A. Soft Contact Lenses Capable of Sustained Delivery of Timolol. J. Pharm. Sci. 2002, 91, 2182–2192. [Google Scholar] [CrossRef]
- Hiratani, H.; Mizutani, Y.; Alvarez-Lorenzo, C. Controlling Drug Release from Imprinted Hydrogels by Modifying the Characteristics of the Imprinted Cavities. Macromol. Biosci. 2005, 5, 728–733. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Yanez, F.; Barreiro-Iglesias, R.; Concheiro, A. Imprinted Soft Contact Lenses as Norfloxacin Delivery Systems. J. Control. Release 2006, 113, 236–244. [Google Scholar] [CrossRef]
- Tieppo, A.; Pate, K.M.; Byrne, M.E. In Vitro Controlled Release of an Anti-Inflammatory from Daily Disposable Therapeutic Contact Lenses under Physiological Ocular Tear Flow. Eur. J. Pharm. Biopharm. 2012, 81, 170–177. [Google Scholar] [CrossRef]
- Venkatesh, S.; Saha, J.; Pass, S.; Byrne, M.E. Transport and Structural Analysis of Molecular Imprinted Hydrogels for Controlled Drug Delivery. Eur. J. Pharm. Biopharm. 2008, 69, 852–860. [Google Scholar] [CrossRef]
- Hiratani, H.; Fujiwara, A.; Tamiya, Y.; Mizutani, Y.; Alvarez-Lorenzo, C. Ocular Release of Timolol from Molecularly Imprinted Soft Contact Lenses. Biomaterials 2005, 26, 1293–1298. [Google Scholar] [CrossRef]
- White, C.J.; McBride, M.K.; Pate, K.M.; Tieppo, A.; Byrne, M.E. Extended Release of High Molecular Weight Hydroxypropyl Methylcellulose from Molecularly Imprinted, Extended Wear Silicone Hydrogel Contact Lenses. Biomaterials 2011, 32, 5698–5705. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.; Wedel, T.; Moll, R.; Geerling, G. Combination of Serum Eye Drops with Hydrogel Bandage Contact Lenses in the Treatment of Persistent Epithelial Defects. Graefes. Arch. Clin. Exp. Ophthalmol. 2006, 244, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.E.; Salian, V. Molecular Imprinting within Hydrogels II: Progress and Analysis of the Field. Int. J. Pharm. 2008, 364, 188–212. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, C.Y.; Wang, X.H.; Li, R.S.; Yang, J.R.; Huang, Y.P.; Liu, Z.S. Macromolecular Crowding of Molecular Imprinting: A Facile Pathway to Produce Drug Delivery Devices for Zero-Order Sustained Release. Int. J. Pharm. 2015, 496, 822–833. [Google Scholar] [CrossRef]
- Malakooti, N.; Alexander, C.; Alvarez-Lorenzo, C. Imprinted Contact Lenses for Sustained Release of Polymyxin B and Related Antimicrobial Peptides. J. Pharm. Sci. 2015, 104, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, F.; Concheiro, A.; Alvarez-Lorenzo, C. Epalrestat-Loaded Silicone Hydrogels as Contact Lenses to Address Diabetic-Eye Complications. Eur. J. Pharm. Biopharm. 2018, 122, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Nair, A.S.; Parvathy, J. Extended Wear Therapeutic Contact Lens Fabricated from Timolol Imprinted Carboxymethyl Chitosan-g-Hydroxy Ethyl Methacrylate-g-Poly Acrylamide as a Onetime Medication for Glaucoma. Eur. J. Pharm. Biopharm. 2016, 109, 61–71. [Google Scholar] [CrossRef]
- Gupta, C.; Chauhan, A. Drug Transport in HEMA Conjunctival Inserts Containing Precipitated Drug Particles. J. Colloid Interface Sci. 2010, 347, 31–42. [Google Scholar] [CrossRef]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma Therapy by Extended Release of Timolol from Nanoparticle Loaded Silicone-Hydrogel Contact Lenses. J. Control. Release 2013, 165, 82–89. [Google Scholar] [CrossRef]
- Hsu, K.H.; Gause, S.; Chauhan, A. Review of Ophthalmic Drug Delivery by Contact Lenses. J. Drug Deliv. Sci. Technol. 2014, 24, 123–135. [Google Scholar] [CrossRef]
- Fazly Bazzaz, B.S.; Khameneh, B.; Jalili-Behabadi, M.M.; Malaekeh-Nikouei, B.; Mohajeri, S.A. Preparation, Characterization and Antimicrobial Study of a Hydrogel (Soft Contact Lens) Material Impregnated with Silver Nanoparticles. Contact Lens. Anterior Eye 2014, 37, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Gulsen, D.; Chauhan, A. Ophthalmic Drug Delivery through Contact Lenses. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Gulsen, D.; Li, C.C.; Chauhan, A. Dispersion of DMPC Liposomes in Contact Lenses for Ophthalmic Drug Delivery. Curr. Eye Res. 2005, 30, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Gulsen, D.; Chauhan, A. Dispersion of Microemulsion Drops in HEMA Hydrogel: A Potential Ophthalmic Drug Delivery Vehicle. Int. J. Pharm. 2005, 292, 95–117. [Google Scholar] [CrossRef]
- Jung, H.J.; Chauhan, A. Temperature Sensitive Contact Lenses for Triggered Ophthalmic Drug Delivery. Biomaterials 2012, 33, 2289–2300. [Google Scholar] [CrossRef]
- Zhang, W.; Zu, D.; Chen, J.; Peng, J.; Liu, Y.; Zhang, H.; Li, S.; Pan, W. Bovine Serum Albumin-Meloxicam Nanoaggregates Laden Contact Lenses for Ophthalmic Drug Delivery in Treatment of Postcataract Endophthalmitis. Int. J. Pharm. 2014, 475, 25–34. [Google Scholar] [CrossRef]
- Loftsson, T.; Masson, M.; Brewster, M.E. Self-Association of Cyclodextrins and Cyclodextrin Complexes. J. Pharm. Sci. 2004, 93, 1091–1099. [Google Scholar] [CrossRef]
- dos Santos, J.F.; Couceiro, R.; Concheiro, A.; Torres-Labandeira, J.J.; Alvarez-Lorenzo, C. Poly(Hydroxyethyl Methacrylate-Co-Methacrylated-Beta-Cyclodextrin) Hydrogels: Synthesis, Cytocompatibility, Mechanical Properties and Drug Loading/Release Properties. Acta Biomater. 2008, 4, 745–755. [Google Scholar] [CrossRef]
- dos Santos, J.F.; Alvarez-Lorenzo, C.; Silva, M.; Balsa, L.; Couceiro, J.; Torres-Labandeira, J.J.; Concheiro, A. Soft Contact Lenses Functionalized with Pendant Cyclodextrins for Controlled Drug Delivery. Biomaterials 2009, 30, 1348–1355. [Google Scholar] [CrossRef]
- Jain, R.L.; Shastri, J.P. Study of Ocular Drug Delivery System Using Drug-Loaded Liposomes. Int. J. Pharm. Investig. 2011, 1, 35–41. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ghate, V.M.; Lewis, S.A. Next-Generation Contact Lenses: Towards Bioresponsive Drug Delivery and Smart Technologies in Ocular Therapeutics. Eur. J. Pharm. Biopharm. 2021, 161, 80–99. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Abrahamson, M.; Kapoor, Y.; Chauhan, A. Timolol Transport from Microemulsions Trapped in HEMA Gels. J. Colloid Interface Sci. 2007, 315, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Chauhan, A. Ophthalmic Delivery of Cyclosporine A from Brij-97 Microemulsion and Surfactant-Laden p-HEMA Hydrogels. Int. J. Pharm. 2008, 361, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Yoganathan, R.B.; Kociolek, M.; Allen, C. Hydrogel Containing Silica Shell Cross-Linked Micelles for Ocular Drug Delivery. J. Pharm. Sci. 2013, 102, 627–637. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Mangukiya, M.A.; Patel, P.A.; Vaidya, R.J.; Koli, A.R.; Ranch, K.M.; Shah, D.O. Extended Release of Ketotifen from Silica Shell Nanoparticle-Laden Hydrogel Contact Lenses: In Vitro and in Vivo Evaluation. J. Mater. Sci. Mater. Med. 2016, 27, 113. [Google Scholar] [CrossRef]
- Shayani Rad, M.; Khameneh, B.; Sabeti, Z.; Mohajeri, S.A.; Fazly Bazzaz, B.S. Antibacterial Activity of Silver Nanoparticle-Loaded Soft Contact Lens Materials: The Effect of Monomer Composition. Curr. Eye Res. 2016, 41, 1286–1293. [Google Scholar] [CrossRef]
- Chandasana, H.; Prasad, Y.D.; Chhonker, Y.S.; Chaitanya, T.K.; Mishra, N.N.; Mitra, K.; Shukla, P.K.; Bhatta, R.S. Corneal Targeted Nanoparticles for Sustained Natamycin Delivery and Their PK/PD Indices: An Approach to Reduce Dose and Dosing Frequency. Int. J. Pharm. 2014, 477, 317–325. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Sun, F. Cyclodextrin-Containing Hydrogels for Contact Lenses as a Platform for Drug Incorporation and Release. Acta Biomater. 2010, 6, 486–493. [Google Scholar] [CrossRef]
- Garcia-Fernandez, M.J.; Tabary, N.; Martel, B.; Cazaux, F.; Oliva, A.; Taboada, P.; Concheiro, A.; Alvarez-Lorenzo, C. Poly-(Cyclo)Dextrins as Ethoxzolamide Carriers in Ophthalmic Solutions and in Contact Lenses. Carbohydr. Polym. 2013, 98, 1343–1352. [Google Scholar] [CrossRef]
- Glisoni, R.J.; Garcia-Fernandez, M.J.; Pino, M.; Gutkind, G.; Moglioni, A.G.; Alvarez-Lorenzo, C.; Concheiro, A.; Sosnik, A. Beta-Cyclodextrin Hydrogels for the Ocular Release of Antibacterial Thiosemicarbazones. Carbohydr. Polym. 2013, 93, 449–457. [Google Scholar] [CrossRef]
- Arslan, M.; Gevrek, T.N.; Sanyal, R.; Sanyal, A. Fabrication of Poly(Ethylene Glycol)-Based Cyclodextrin Containing Hydrogels via Thiol-Ene Click Reaction. Eur. Polym. J. 2015, 62, 426–434. [Google Scholar] [CrossRef]
- Hu, X.; Tan, H.; Wang, X.; Chen, P. Surface Functionalization of Hydrogel by Thiol-Yne Click Chemistry for Drug Delivery. Colloids Surf. Physicochem. Eng. Asp. 2016, 489, 297–304. [Google Scholar] [CrossRef]
- Prakash, M.; Dhesingh, R.S. Nanoparticle Modified Drug Loaded Biodegradable Polymeric Contact Lenses for Sustainable Ocular Drug Delivery. Curr. Drug Deliv. 2017, 14, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Danion, A.; Doillon, C.J.; Giasson, C.J.; Djouahra, S.; Sauvageau, P.; Paradis, R.; Vermette, P. Biocompatibility and Light Transmission of Liposomal Lenses. Optom. Vis. Sci. 2007, 84, 954–961. [Google Scholar] [CrossRef]
- Bengani, L.C.; Chauhan, A. Extended Delivery of an Anionic Drug by Contact Lens Loaded with a Cationic Surfactant. Biomaterials 2013, 34, 2814–2821. [Google Scholar] [CrossRef]
- Kapoor, Y.; Thomas, J.C.; Tan, G.; John, V.T.; Chauhan, A. Surfactant-Laden Soft Contact Lenses for Extended Delivery of Ophthalmic Drugs. Biomaterials 2009, 30, 867–878. [Google Scholar] [CrossRef]
- Costa, V.P.; Braga, M.E.M.; Duarte, C.M.M.; Alvarez-Lorenzo, C.; Concheiro, A.; Gil, M.H.; de Sousa, H.C. Anti-Glaucoma Drug-Loaded Contact Lenses Prepared Using Supercritical Solvent Impregnation. J. Supercrit. Fluids 2010, 53, 165–173. [Google Scholar] [CrossRef]
- Yanez, F.; Martikainen, L.; Braga, M.E.; Alvarez-Lorenzo, C.; Concheiro, A.; Duarte, C.M.; Gil, M.H.; de Sousa, H.C. Supercritical Fluid-Assisted Preparation of Imprinted Contact Lenses for Drug Delivery. Acta Biomater. 2011, 7, 1019–1030. [Google Scholar] [CrossRef]
- Zaidi, S.A. Molecular Imprinting: A Useful Approach for Drug Delivery. Mater. Sci. Energy Technol. 2020, 3, 72–77. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Simplicio, A.L.; Vega-González, A.; Subra-Paternault, P.; Coimbra, P.; Gil, M.H.; de Sousa, H.C.; Duarte, C.M.M. Supercritical Fluid Impregnation of a Biocompatible Polymer for Ophthalmic Drug Delivery. J. Supercrit. Fluids 2007, 42, 373–377. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Casimiro, T.; Aguiar-Ricardo, A.; Simplício, A.L.; Duarte, C.M.M. Supercritical Fluid Polymerisation and Impregnation of Molecularly Imprinted Polymers for Drug Delivery. J. Supercrit. Fluids 2006, 39, 102–106. [Google Scholar] [CrossRef]
- Yokozaki, Y.; Sakabe, J.; Ng, B.; Shimoyama, Y. Effect of Temperature, Pressure and Depressurization Rate on Release Profile of Salicylic Acid from Contact Lenses Prepared by Supercritical Carbon Dioxide Impregnation. Chem. Eng. Res. Des. 2015, 100, 89–94. [Google Scholar] [CrossRef]
- Peng, C.C.; Kim, J.; Chauhan, A. Extended Delivery of Hydrophilic Drugs from Silicone-Hydrogel Contact Lenses Containing Vitamin E Diffusion Barriers. Biomaterials 2010, 31, 4032–4047. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Peng, C.C.; Chauhan, A. Extended Release of Dexamethasone from Silicone-Hydrogel Contact Lenses Containing Vitamin E. J. Control. Release 2010, 148, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Chauhan, A. Extended Cyclosporine Delivery by Silicone-Hydrogel Contact Lenses. J. Control. Release 2011, 154, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.H.; Fentzke, R.C.; Chauhan, A. Feasibility of Corneal Drug Delivery of Cysteamine Using Vitamin E Modified Silicone Hydrogel Contact Lenses. Eur. J. Pharm. Biopharm. 2013, 85, 531–540. [Google Scholar] [CrossRef]
- Peng, C.C.; Burke, M.T.; Chauhan, A. Transport of Topical Anesthetics in Vitamin E Loaded Silicone Hydrogel Contact Lenses. Langmuir 2012, 28, 1478–1487. [Google Scholar] [CrossRef]
- Peng, C.C.; Burke, M.T.; Carbia, B.E.; Plummer, C.; Chauhan, A. Extended Drug Delivery by Contact Lenses for Glaucoma Therapy. J. Control. Release 2012, 162, 152–158. [Google Scholar] [CrossRef]
- Hsu, K.H.; Carbia, B.E.; Plummer, C.; Chauhan, A. Dual Drug Delivery from Vitamin E Loaded Contact Lenses for Glaucoma Therapy. Eur. J. Pharm. Biopharm. 2015, 94, 312–321. [Google Scholar] [CrossRef]
- Dixon, P.; Fentzke, R.C.; Bhattacharya, A.; Konar, A.; Hazra, S.; Chauhan, A. In Vitro Drug Release and in Vivo Safety of Vitamin E and Cysteamine Loaded Contact Lenses. Int. J. Pharm. 2018, 544, 380–391. [Google Scholar] [CrossRef]
- Dixon, P.; Chauhan, A. Carbon Black Tinted Contact Lenses for Reduction of Photophobia in Cystinosis Patients. Curr. Eye Res. 2019, 44, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Sekar, P.; Chauhan, A. Effect of Vitamin-E Integration on Delivery of Prostaglandin Analogs from Therapeutic Lenses. J. Colloid Interface Sci. 2019, 539, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Torres-Luna, C.; Hu, N.; Tammareddy, T.; Domszy, R.; Yang, J.; Wang, N.S.; Yang, A. Extended Delivery of Non-Steroidal Anti-Inflammatory Drugs through Contact Lenses Loaded with Vitamin E and Cationic Surfactants. Contact Lens Anterior Eye 2019, 42, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II Pathophysiology Report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Li, P.; Beachley, V.; McDonnell, P.; Elisseeff, J.H. A Hyaluronic Acid-Binding Contact Lens with Enhanced Water Retention. Contact Lens Anterior Eye 2015, 38, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Codina, C.; Efron, N. Impact of Manufacturing Technology and Material Composition on the Mechanical Properties of Hydrogel Contact Lenses. Ophthalmic. Physiol. Opt. 2004, 24, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Singhania, S.S.; Desai, A.R.; Shukla, M.R.; Tannk, A.S.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. Contact Lenses with Dual Drug Delivery for the Treatment of Bacterial Conjunctivitis. Int. J. Pharm. 2018, 548, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Sammons, W.A. The Nissel Memorial LectureManufacturing—Materials, Methods and Measurements. J. Br. Contact Lens Assoc. 1989, 12, 12–19. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical Properties of Hydrogels and Their Experimental Determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Baker, J.; Blanch, H.; Prausnitz, J. Equilibrium Swelling Properties of Weakly Lonizable 2-Hydroxyethyl Methacrylate (HEMA)-Based Hydrogels. J. Appl. Polym. Sci. 1994, 52, 783–788. [Google Scholar] [CrossRef]
- Milojević, M.; Gradišnik, L.; Stergar, J.; Skelin Klemen, M.; Stožer, A.; Vesenjak, M.; Dobnik Dubrovski, P.; Maver, T.; Mohan, T.; Stana Kleinschek, K.; et al. Development of Multifunctional 3D Printed Bioscaffolds from Polysaccharides and NiCu Nanoparticles and Their Application. Appl. Surf. Sci. 2019, 488, 836–852. [Google Scholar] [CrossRef]
- Milojević, M.; Harih, G.; Vihar, B.; Vajda, J.; Gradišnik, L.; Zidarič, T.; Stana Kleinschek, K.; Maver, U.; Maver, T. Hybrid 3D Printing of Advanced Hydrogel-Based Wound Dressings with Tailorable Properties. Pharmaceutics 2021, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Štiglic, A.D.; Gürer, F.; Lackner, F.; Bračič, D.; Winter, A.; Gradišnik, L.; Makuc, D.; Kargl, R.; Duarte, I.; Plavec, J.; et al. Organic Acid Cross-Linked 3D Printed Cellulose Nanocomposite Bioscaffolds with Controlled Porosity, Mechanical Strength, and Biocompatibility. iScience 2022, 25, 104263. [Google Scholar] [CrossRef] [PubMed]
- Vajda, J.; Vihar, B.; Ćurić, L.Č.; Maver, U.; Vesenjak, M.; Dubrovski, P.D.; Milojević, M. Sr2+ vs. Ca2+ as Post-Processing Ionic Crosslinkers: Implications for 3D Bioprinting of Polysaccharide Hydrogels in Tissue Engineering. J. Mater. Res. Technol. 2023, 23, 1805–1820. [Google Scholar] [CrossRef]
- Tan, G.; Ioannou, N.; Mathew, E.; Tagalakis, A.D.; Lamprou, D.A.; Yu-Wai-Man, C. 3D Printing in Ophthalmology: From Medical Implants to Personalised Medicine. Int. J. Pharm. 2022, 625, 122094. [Google Scholar] [CrossRef] [PubMed]
- Mohamdeen, Y.M.G.; Tabriz, A.G.; Tighsazzadeh, M.; Nandi, U.; Khalaj, R.; Andreadis, I.; Boateng, J.S.; Douroumis, D. Development of 3D Printed Drug-Eluting Contact Lenses. J. Pharm. Pharmacol. 2022, 74, 1467–1476. [Google Scholar] [CrossRef]
- Bandari, S.; Nyavanandi, D.; Dumpa, N.; Repka, M.A. Coupling Hot Melt Extrusion and Fused Deposition Modeling: Critical Properties for Successful Performance. Adv. Drug Deliv. Rev. 2021, 172, 52–63. [Google Scholar] [CrossRef]
- Alam, F.; Elsherif, M.; AlQattan, B.; Salih, A.; Lee, S.M.; Yetisen, A.K.; Park, S.; Butt, H. 3D Printed Contact Lenses. ACS Biomater. Sci. Eng. 2021, 7, 794–803. [Google Scholar] [CrossRef]
- Gruene, M.; Pflaum, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Wilhelmi, M.; Haverich, A.; Chichkov, B.N. Adipogenic Differentiation of Laser-Printed 3D Tissue Grafts Consisting of Human Adipose-Derived Stem Cells. Biofabrication 2011, 3, 015005. [Google Scholar] [CrossRef]
- Phan, C.-M.; Bajgrowicz, M.; Gao, H.; Subbaraman, L.N.; Jones, L.W. Release of Fluconazole from Contact Lenses Using a Novel In Vitro Eye Model. Optom. Vis. Sci. 2016, 93, 387–394. [Google Scholar] [CrossRef]











| Colloidal Nanoparticles | Examples | Characteristics | References |
|---|---|---|---|
| Polymeric nanoparticles |
|
| [55,61,62] |
| Cyclodextrins |
|
| [63,64,65] |
| Liposomes |
|
| [9,21,33,59,66] |
| Microemulsions and micelles |
|
| [60,67,68,69,70,71] |
| Method | Mechanism | Advantages | Limitations | References |
|---|---|---|---|---|
| Layered contact lenses | Approaches:
|
|
| [17,22,33] |
| Surface-modified contact lenses |
|
|
| [21,25] |
| Soaking method |
|
|
| [27,28,29,30,33,34,35,36] |
| Molecular imprinting |
|
|
| [33,40,41,48,49] |
| Colloidal nanoparticles |
|
|
| [33,54,55,56,59,60] |
| Supercritical fluid technology |
|
|
| [33,83,84] |
| Vitamin E as a release-modifying additive |
|
|
| [17,33,89,92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lovrec-Krstič, T.; Orthaber, K.; Maver, U.; Sarenac, T. Review of Potential Drug-Eluting Contact Lens Technologies. Materials 2023, 16, 3653. https://doi.org/10.3390/ma16103653
Lovrec-Krstič T, Orthaber K, Maver U, Sarenac T. Review of Potential Drug-Eluting Contact Lens Technologies. Materials. 2023; 16(10):3653. https://doi.org/10.3390/ma16103653
Chicago/Turabian StyleLovrec-Krstič, Tina, Kristjan Orthaber, Uroš Maver, and Tomislav Sarenac. 2023. "Review of Potential Drug-Eluting Contact Lens Technologies" Materials 16, no. 10: 3653. https://doi.org/10.3390/ma16103653
APA StyleLovrec-Krstič, T., Orthaber, K., Maver, U., & Sarenac, T. (2023). Review of Potential Drug-Eluting Contact Lens Technologies. Materials, 16(10), 3653. https://doi.org/10.3390/ma16103653








