Novel Functional Soft Magnetic CoFe2O4/Fe Composites: Preparation, Characterization, and Low Core Loss
Abstract
:1. Introduction
2. Materials and Methods
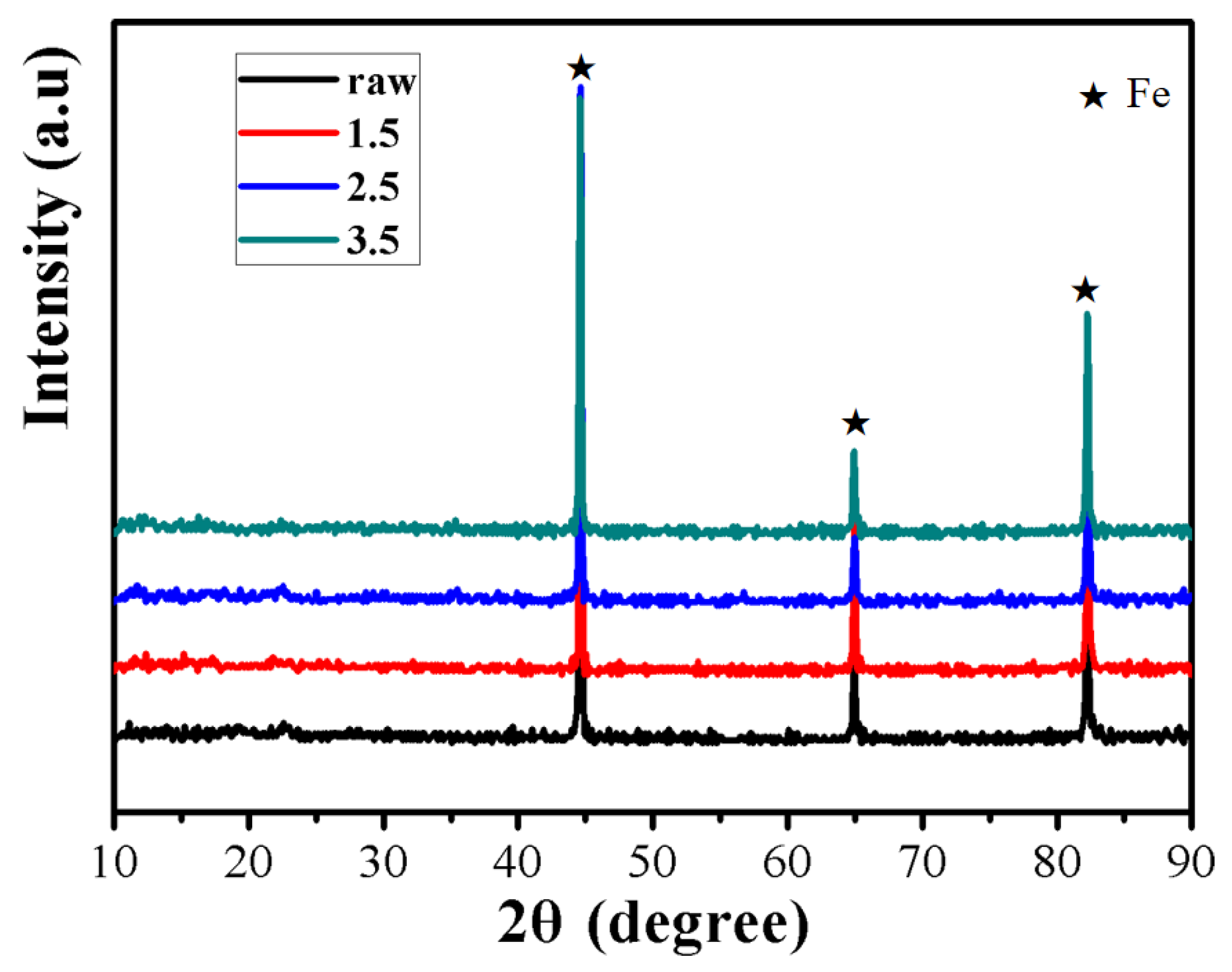
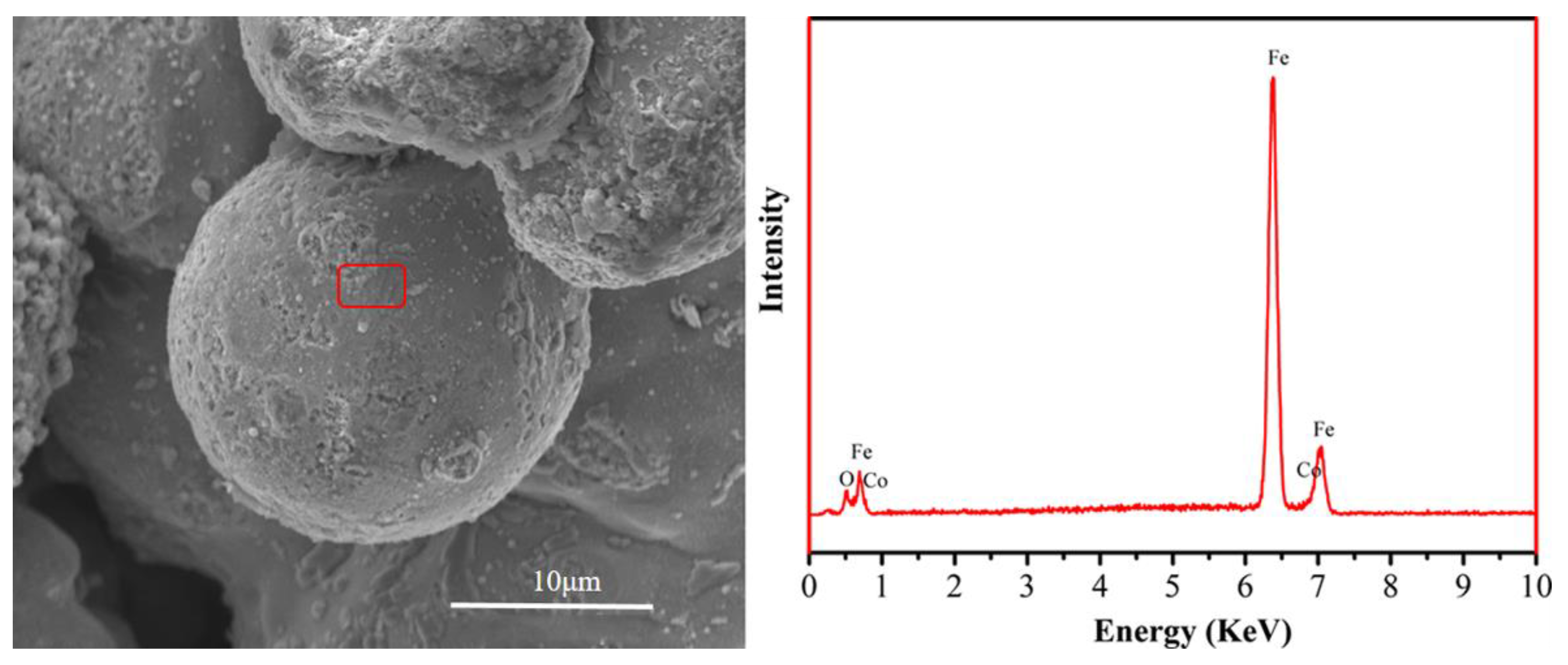
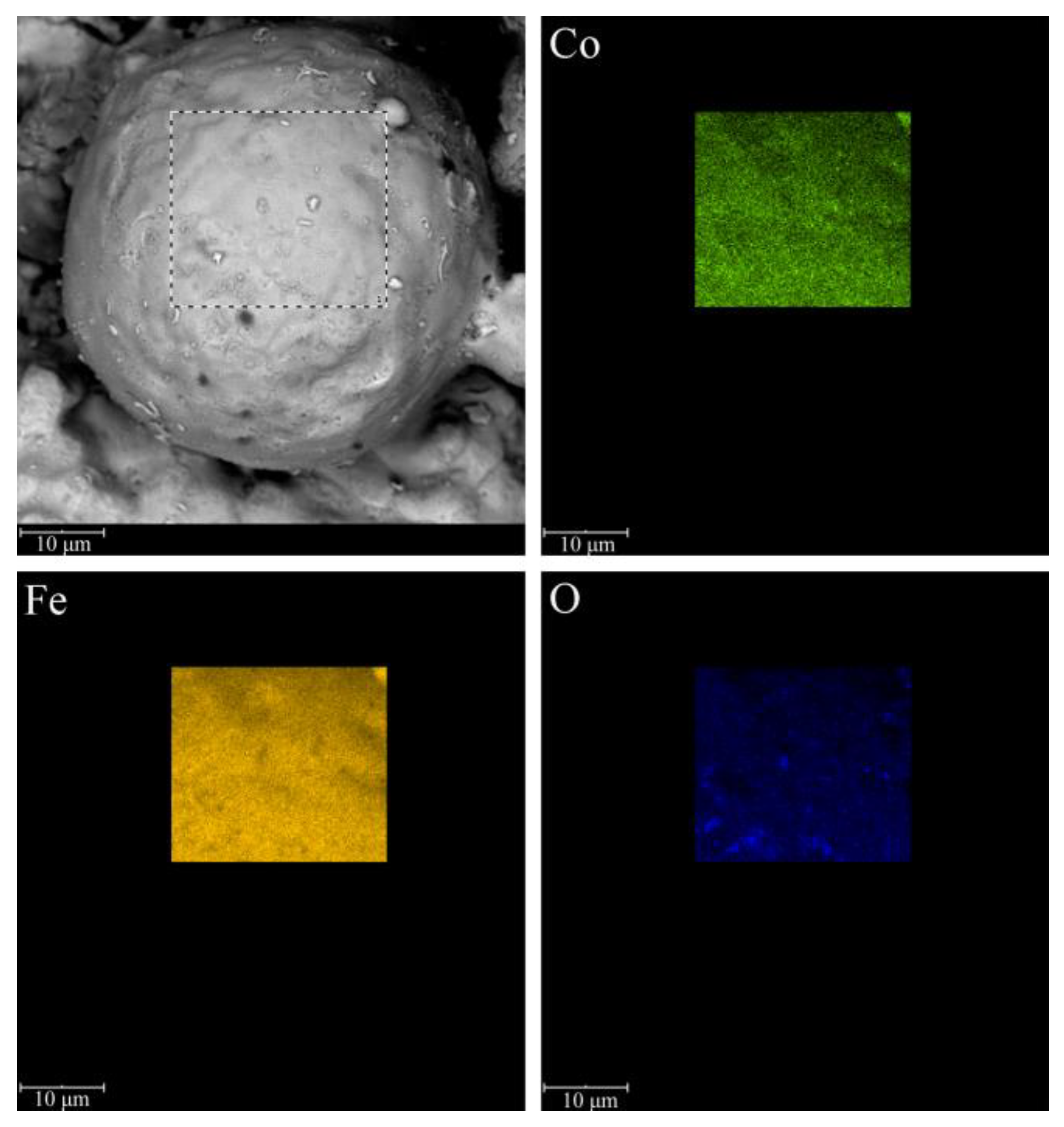
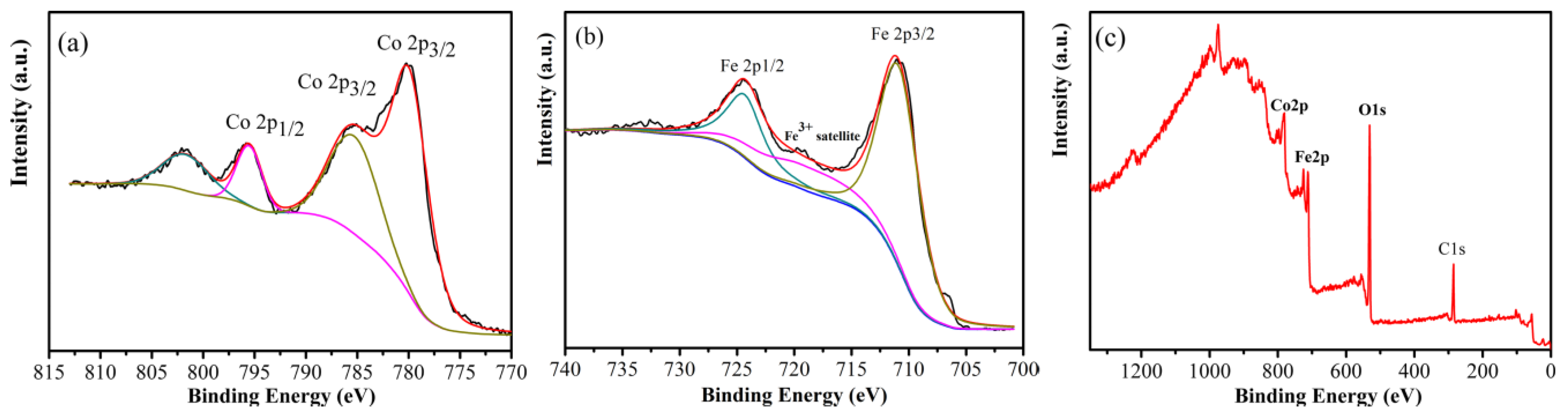
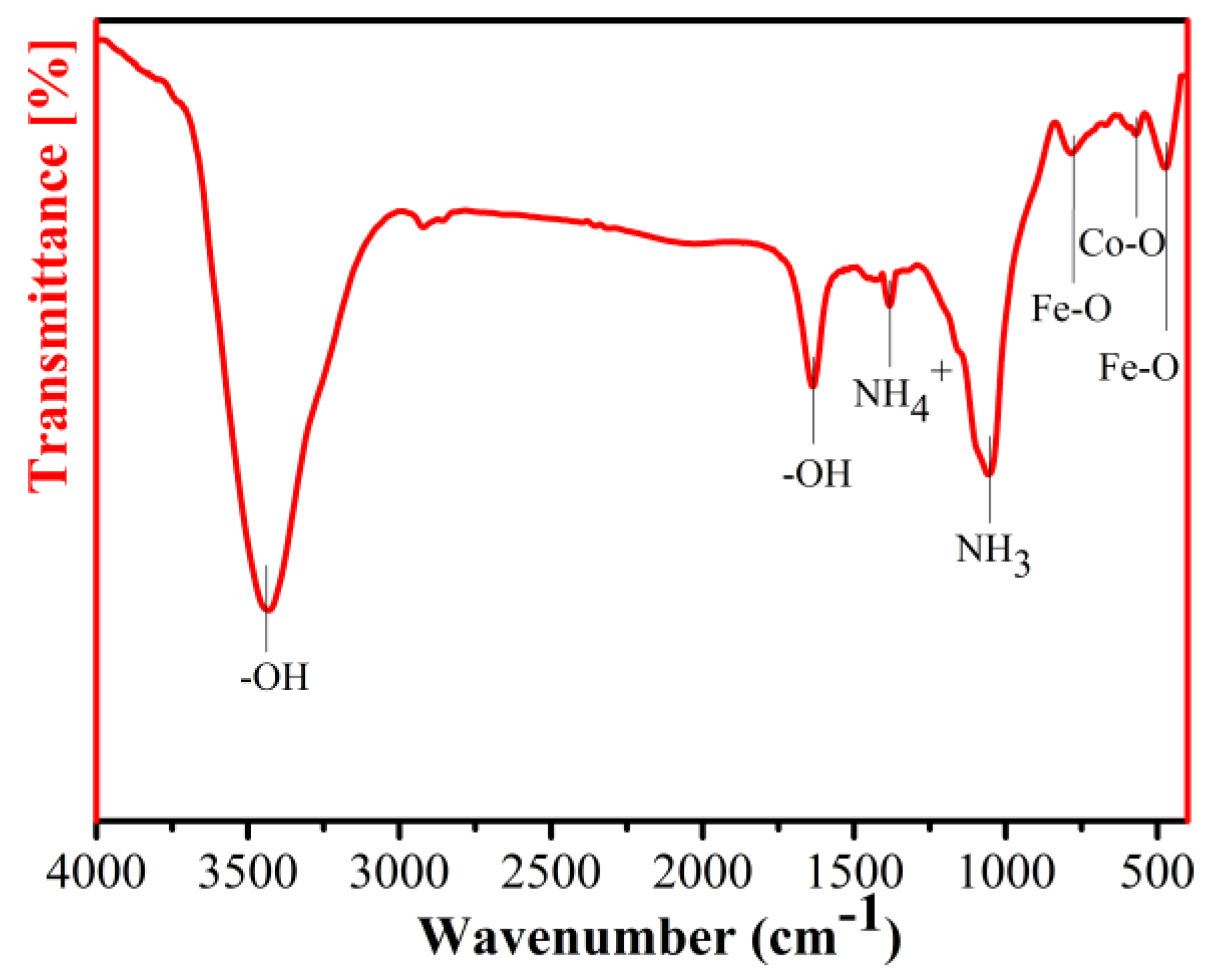
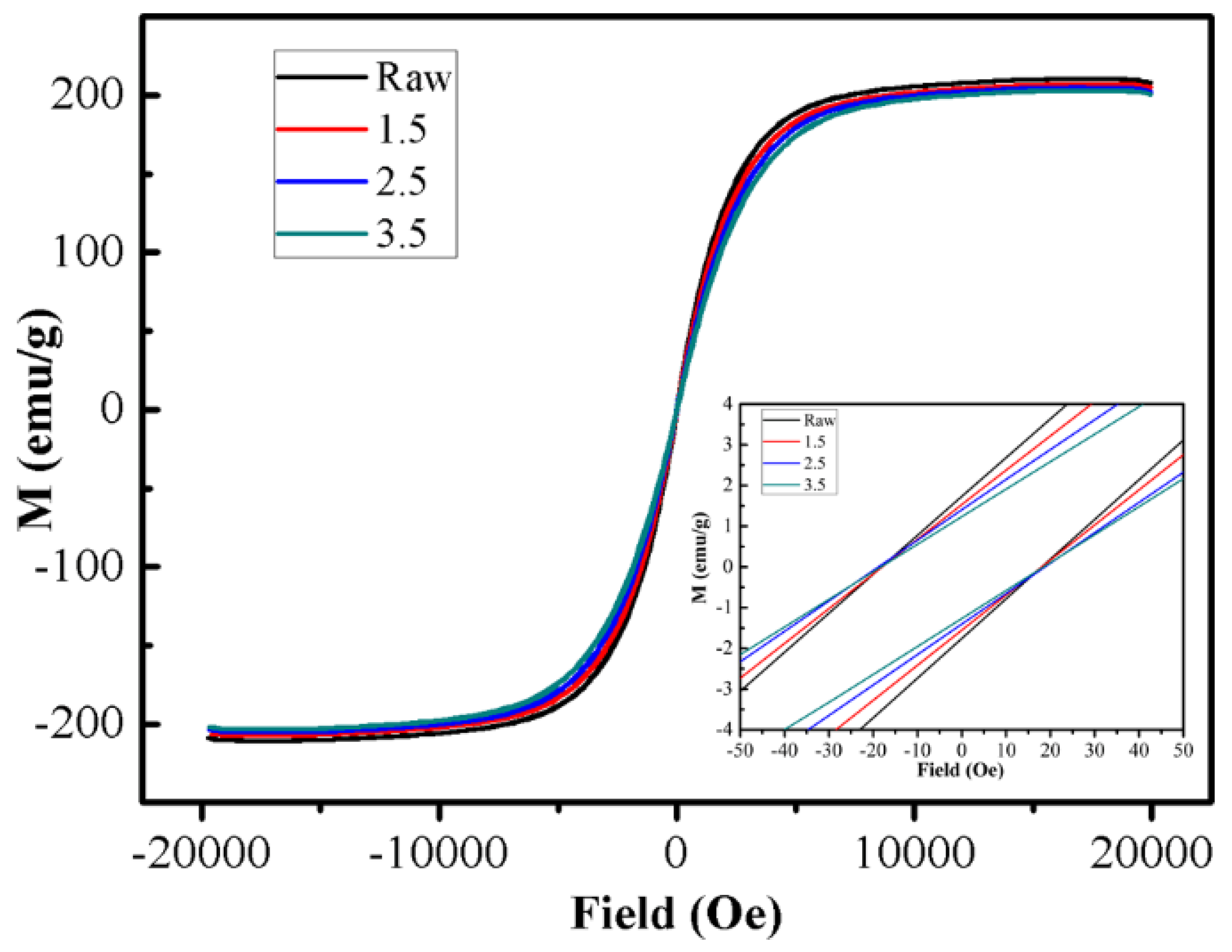
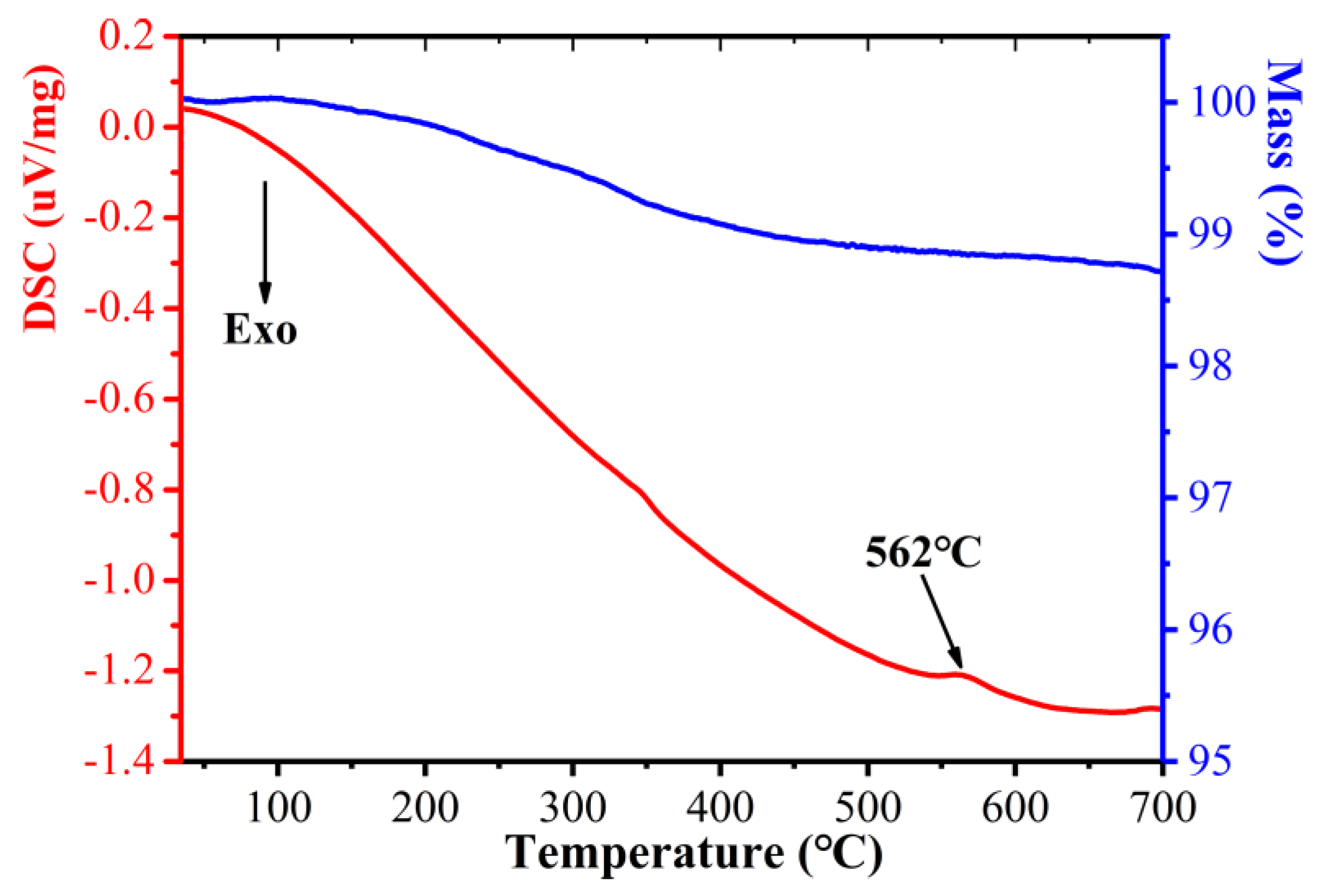
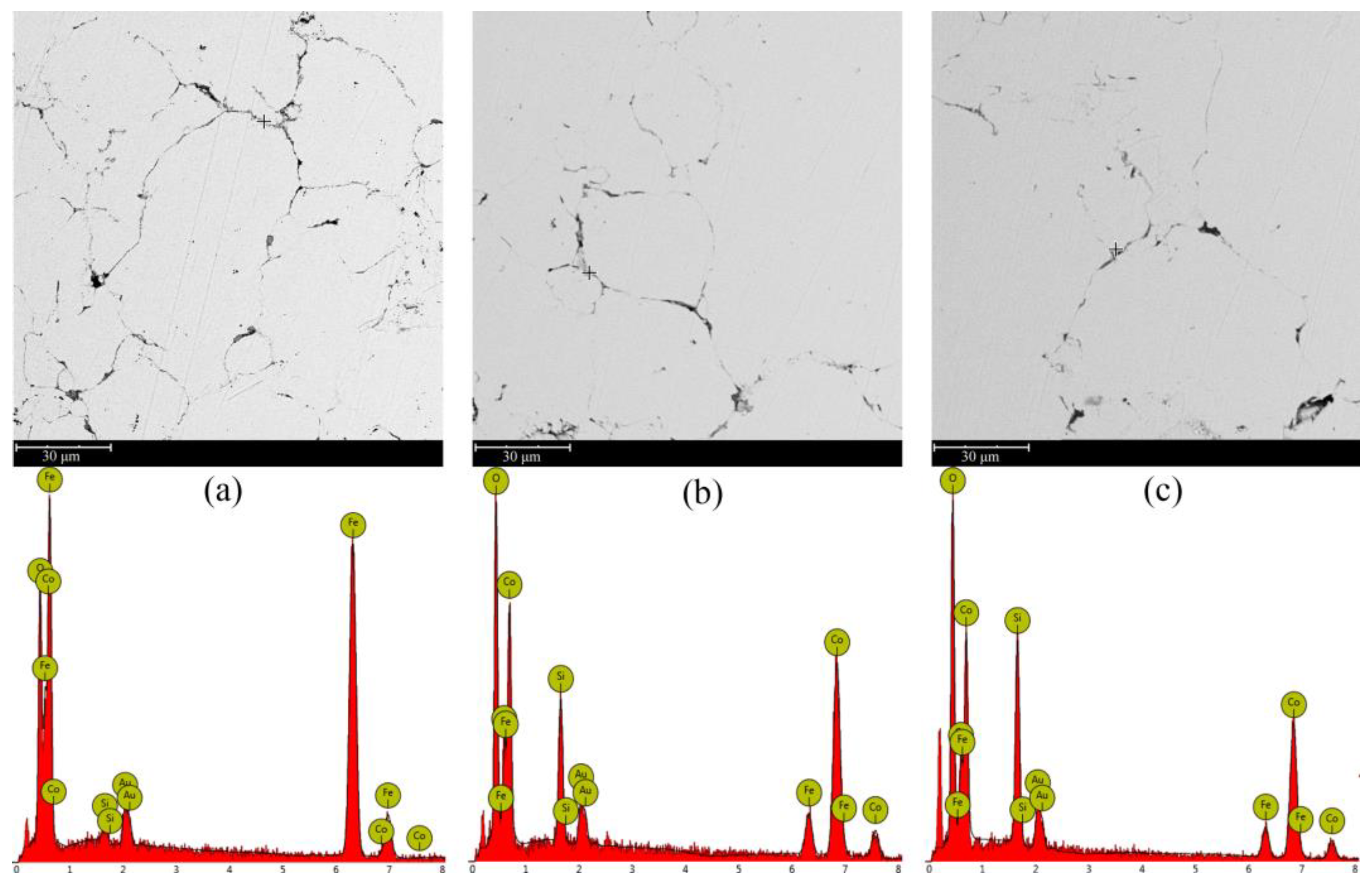
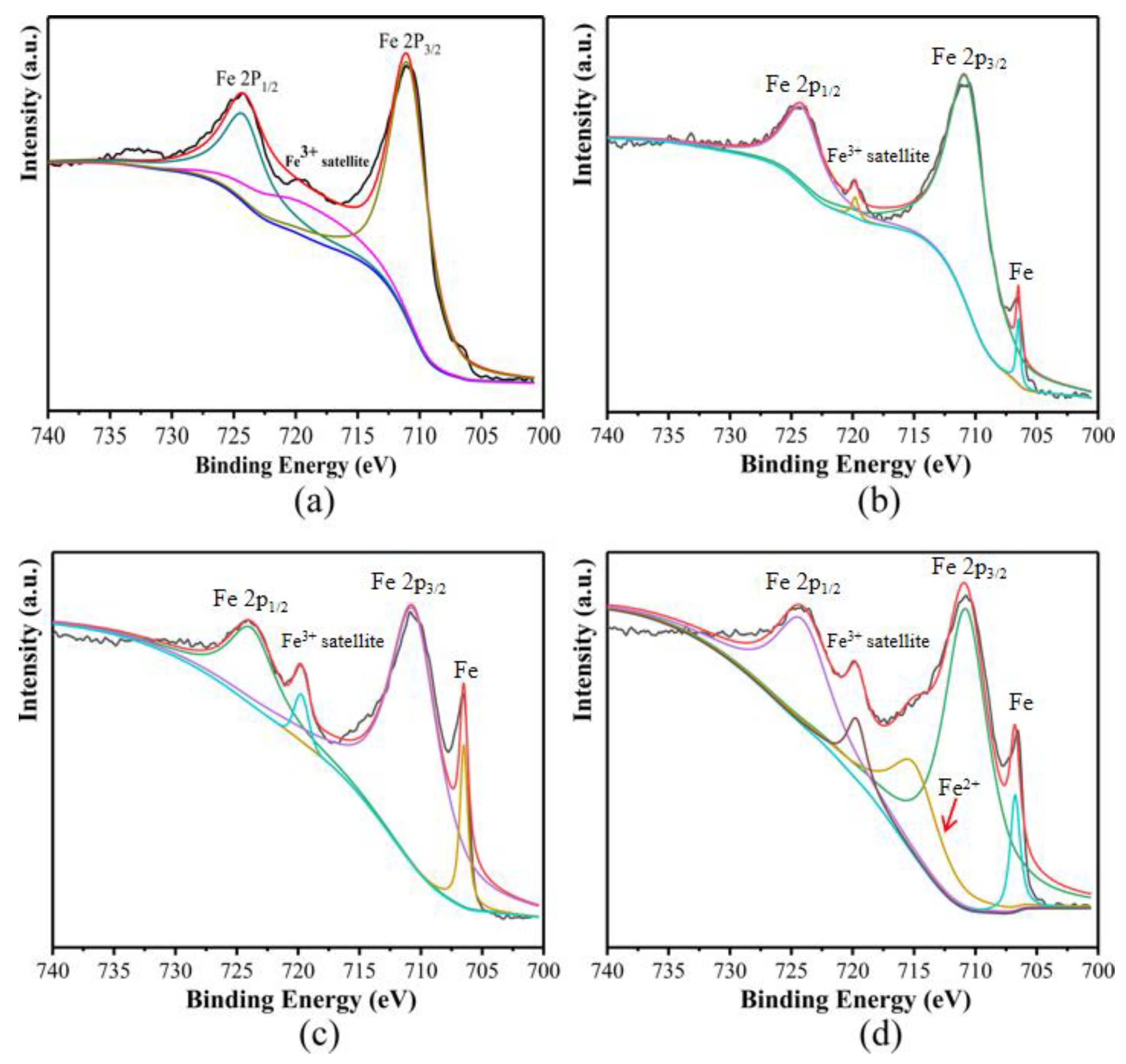
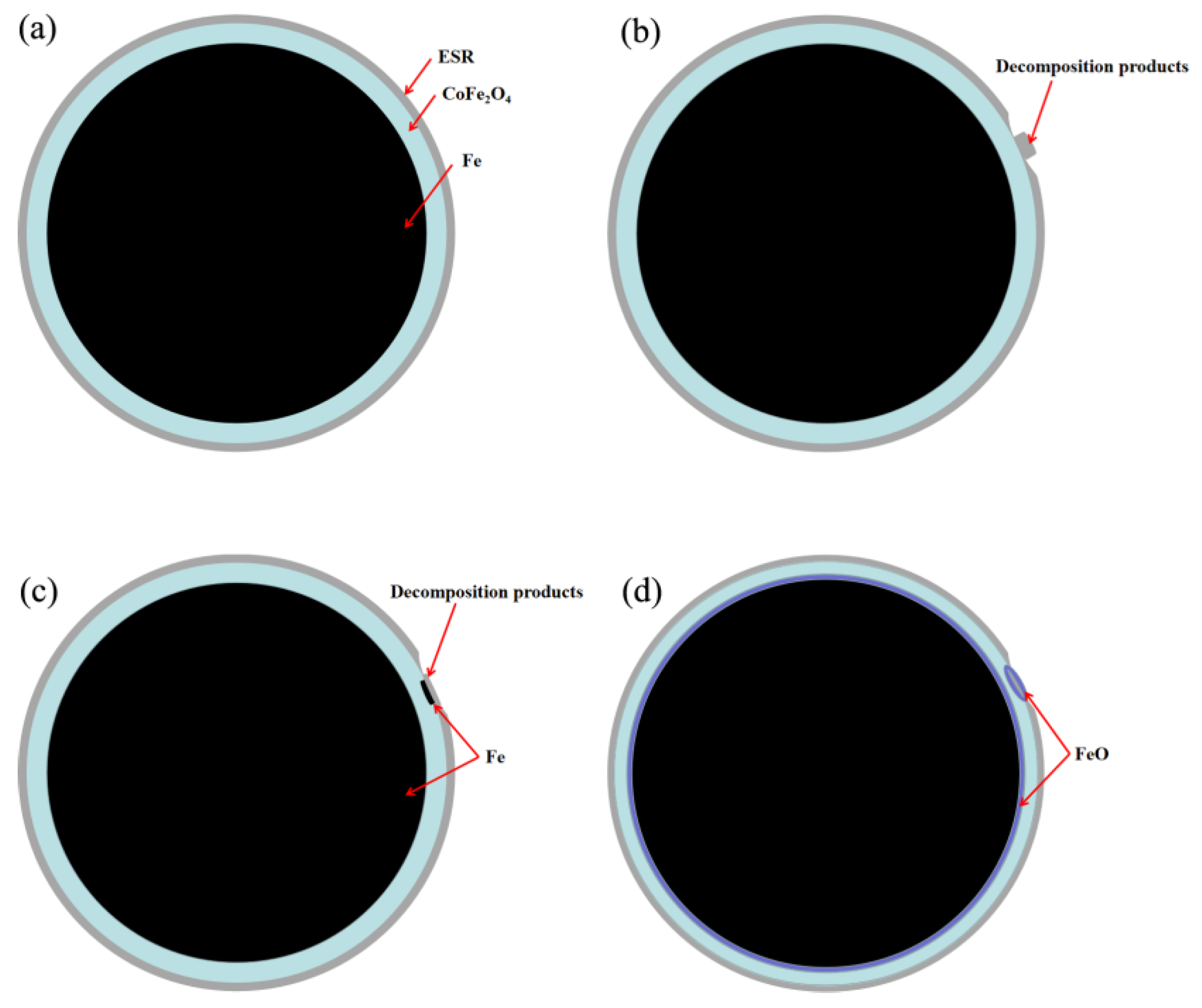
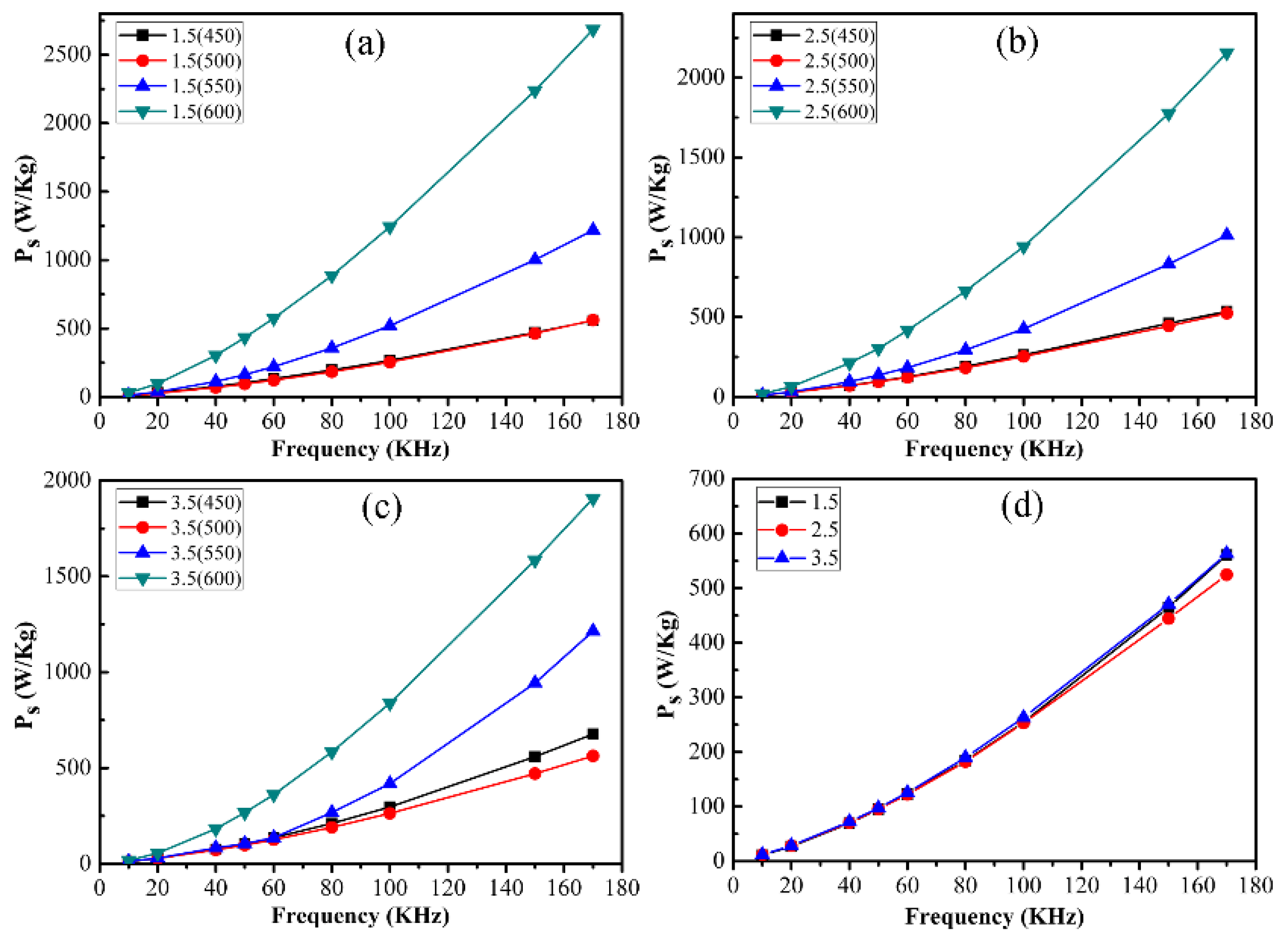
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shokrollahi, H.; Janghorban, K. Soft magnetic composite materials (SMCs). J. Mater. Process. Technol. 2007, 189, 1–12. [Google Scholar] [CrossRef]
- Sun, H.B.; Zhou, G.H.; Guo, Z.L.; Wang, C.; Wang, J.H.; Zong, C.B. Efficient synthesis of TiO2-coated layer for Fe-based soft magnetic composites and their regulation mechanism analysis on magnetic properties. J. Mater. Sci. Mater. Electron. 2022, 33, 13956–13967. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Peng, K. In-situ synthesis and magnetic properties of core-shell structured Fe/Fe3O4 composites. J. Magn. Magn. Mater. 2019, 484, 418–423. [Google Scholar] [CrossRef]
- Li, S.G.; Liu, R.T.; Xiong, X. Preparation and characterization of carbonyl iron soft magnetic composites with magnesioferrite insulating coating layer. Trans. Nonferrous Met. Soc. China 2020, 30, 3067–3077. [Google Scholar] [CrossRef]
- Zhang, Z.; Chang, J.S.; Peng, X.L.; Li, J.; Yang, Y.T.; Xu, J.C.; Hong, B.; Jin, D.F.; Jin, H.X.; Wang, X.Q.; et al. Structural and magnetic properties of flaky FeSiB/Al2O3 soft magnetic composites with orientation of a magnetic field. J. Mater. Res. Technol. 2022, 18, 1381–1390. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, Y.B.; Zou, H.H.; Yang, B.; Zhou, B.H.; Yu, R.H. High-strength and corrosion-resistant Fe/Al2SiO5 soft magnetic composites fabricated by a nanoscale solid-reaction coating method. J. Alloys Compd. 2022, 912, 165174. [Google Scholar] [CrossRef]
- Xia, C.; Peng, Y.D.; Yi, Y.; Deng, H.; Zhu, Y.Y.; Hu, G. The magnetic properties and microstructure of phosphated amorphous FeSiCr/silane soft magnetic composite. J. Magn. Magn. Mater. 2019, 474, 424–433. [Google Scholar] [CrossRef]
- Zhao, G.L.; Wu, C.; Yan, M. Evolution of the insulation matrix and influences on the magnetic performance of Fe soft magnetic composites during annealing. J. Alloys Compd. 2016, 685, 231–236. [Google Scholar] [CrossRef]
- Geng, K.; Xie, Y.; Xu, L.; Yan, B. Fe-Si/ZrO2 composites with core-shell structure and excellent magnetic properties prepared by mechanical milling and spark plasma sintering. J. Alloys Compd. 2017, 718, 53–62. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, J.; Wang, L.; Li, B.; Li, L. Properties of FeSiAl-based soft magnetic composites with AlN/Al2O3 and hybrid phosphate-silane insulation coatings. J. Alloys Compd. 2018, 735, 1603–1610. [Google Scholar] [CrossRef]
- Lei, J.; Zheng, J.W.; Zheng, H.D.; Qiao, L.; Ying, Y.; Cai, W.; Li, W.C.; Yu, J.; Lin, M.; Che, S.L. Effects of heat treatment and lubricant on magnetic properties of iron-based soft magnetic composites with Al2O3 insulating layer by one-pot synthesis method. J. Magn. Magn. Mater. 2019, 472, 7–13. [Google Scholar] [CrossRef]
- Belcher, C.H.; Zheng, B.L.; MacDonald, B.E.; Langlois, E.D.; Lehman, B.; Pearce, C.; Delaney, R.; Apelian, D.; Lavernia, E.J.; Monson, T.C. The role of microstructural evolution during spark plasma sintering on the soft magnetic and electronic properties of a CoFe-Al2O3 soft magnetic composite. J. Mater. Sci. 2022, 57, 5518–5532. [Google Scholar] [CrossRef]
- Hsiang, H.I.; Fan, L.F.; Ho, K.T. Minor yttrium nitrate addition effect on FeSiCr alloy powder core electromagnetic properties. J. Magn. Magn. Mater. 2017, 444, 1–6. [Google Scholar] [CrossRef]
- Luo, Z.G.; Fan, X.A.; Hu, W.T.; Luo, F.; Li, G.Q.; Li, Y.W.; Liu, X.; Wang, J. Controllable SiO2 insulating layer and magnetic properties for intergranular insulating Fe-6.5wt.%Si/SiO2 composites. Adv. Powder Technol. 2019, 30, 538–543. [Google Scholar] [CrossRef]
- Gao, Z.H.; Jia, J.X.; Zhao, Q.; Kong, H.; Wu, Z.Y.; Li, J.L. Determination of a quantitative relationship between deposition duration and magnetic performance of soft ferromagnetic composites via data-analysis and theoretical models. J. Magn. Magn. Mater. 2022, 549, 168891. [Google Scholar] [CrossRef]
- Zhou, B.; Dong, Y.Q.; Liu, L.; Chang, L.; Bi, F.Q.; Wang, X.M. Enhanced soft magnetic properties of the Fe-based amorphous powder cores with novel TiO2 insulation coating layer. J. Magn. Magn. Mater. 2019, 474, 1–8. [Google Scholar] [CrossRef]
- Chen, Z.H.; Liu, X.S.; Kan, X.C.; Wang, Z.; Zhu, R.W.; Yang, W.; Wu, Q.Y.; Shezad, M. Phosphate coatings evolution study and effects of ultrasonic on soft magnetic properties of FeSiAl by aqueous phosphoric acid solution passivation. J. Alloys Compd. 2019, 783, 434–440. [Google Scholar] [CrossRef]
- Zhou, M.M.; Han, Y.; Guan, W.W.; Han, S.J.; Meng, Q.S.; Xu, T.T.; Su, H.L.; Guo, X.; Zou, Z.Q.; Yang, F.Y.; et al. Magnetic properties and loss mechanism of Fe-6.5wt%Si powder core insulated with magnetic Mn-Zn ferrite nanoparticles. J. Magn. Magn. Mater. 2019, 482, 148–154. [Google Scholar] [CrossRef]
- Li, N.W.; Zheng, M.B.; Chang, X.F.; Ji, G.B.; Lu, H.L.; Xue, L.P.; Pan, L.J.; Cao, J.M. Preparation of magnetic CoFe2O4-functionalized graphene sheets via a facile hydrothermal method and their adsorption properties. J. Solid State Chem. 2011, 184, 953–958. [Google Scholar] [CrossRef]
- Gao, L.L.; Deng, J.Q.; Li, T.; Qi, K.; Zhang, J.D.; Yi, Q. A facial strategy to efficiently improve catalytic performance of CoFe2O4 to peroxymonosulfate. J. Environ. Sci. 2022, 116, 1–13. [Google Scholar] [CrossRef]
- Bi, J.H.; Lin, C.C.; Lu, D.W.; Chen, A.; Meng, X.F. Exchange coupled CoFe2O4/CoFe composites for enhanced microwave absorption properties by in-situ hydrothermal reduction. J. Phys. Chem. Solids. 2022, 164, 110624. [Google Scholar] [CrossRef]
- Wang, W.; Ding, Z.; Zhao, X.R.; Wu, S.Z.; Li, F.; Yue, M.; Liu, J.P. Microstructure and magnetic properties of MFe2O4 (M = Co, Ni, and Mn) ferrite nanocrystals prepared using colloid mill and hydrothermal method. J. Appl. Phys. 2015, 117, 17A328. [Google Scholar] [CrossRef]
- Yu, S.H.; Yoshimura, M. Direct fabrication of ferrite MFe2O4 (M = Zn, Mg)/Fe composite thin films by soft solution processing. Chem. Mater. 2000, 12, 3805–3810. [Google Scholar] [CrossRef]
- Bennet, J.; Tholkappiyan, R.; Vishista, K.; Jaya, N.V.; Hamed, F. Attestation in selfpropagating combustion approach of spinel AFe2O4(A = Co, Mg and Mn) complexes bearing mixed oxidation states: Magnetostructural properties. Appl. Surf. Sci. 2016, 383, 113–125. [Google Scholar] [CrossRef]
- Naik, C.C.; Salker, A.V. Investigation of the effect of fractional In3+ ion substitution on the structural, magnetic, and dielectric properties of Co-Cu ferrite. J. Phys. Chem. Solids 2019, 133, 151–162. [Google Scholar] [CrossRef]
- Wu, Z.B.; Jiang, B.Q.; Liu, Y.; Wang, H.Q.; Jin, R.B. DRIFT study of manganese/titania-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Environ. Sci. Technol. 2007, 41, 5812–5817. [Google Scholar] [CrossRef]
- Liu, F.D.; He, H.; Ding, Y.; Zhang, C.B. Effect of manganese substitution on the structure and activity of iron titanate catalyst for the selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 2009, 93, 3760–3769. [Google Scholar] [CrossRef]
- Zhou, G.Z.; Wang, Y.; Zhou, R.S.; Wang, C.Z.; Jin, Y.Q.; Qiu, J.; Hua, C.Y.; Cao, Y.Y. Synthesis of amino-functionalized bentonite/CoFe2O4@MnO2 magnetic recoverable nanoparticles for aqueous Cd2+ Removal. Sci. Total Environ. 2019, 682, 505–513. [Google Scholar] [CrossRef]
- Venturini, J.; Tonelli, A.M.; Wermuth, T.B.; Zampiva, R.Y.S.; Arcaro, S.; Viegas, A.D.C.; Bergmanna, C.P. Excess of cations in the sol-gel synthesis of cobalt ferrite (CoFe2O4): A pathway to switching the inversion degree of spinels. J. Magn. Magn. Mater. 2019, 482, 1–8. [Google Scholar] [CrossRef]
- Peng, Y.D.; Yi, Y.; Li, L.Y.; Ai, H.Y.; Wang, X.X.; Chen, L.L. Fe-based soft magnetic composites coated with NiZn ferrite prepared by a co-precipitation method. J. Magn. Magn. Mater. 2017, 428, 148–153. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, Y.; Ding, C.; Yang, L.; Yu, L. Annealing effects on magnetic properties and strength of organic-silicon epoxy resin-coated soft magnetic composites. J. Mech. Eng. Sci. 2014, 228, 2049–2058. [Google Scholar] [CrossRef]
- Li, S.G.; Liu, R.T.; Xiong, X. Fe-based soft magnetic composites with high permeability and low core loss by in situ coating ZnFe2O4 layer. J. Mater. Sci. 2020, 55, 274–282. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, X.; Ouyang, F.; Liu, R.; Xiong, X. Novel Functional Soft Magnetic CoFe2O4/Fe Composites: Preparation, Characterization, and Low Core Loss. Materials 2023, 16, 3665. https://doi.org/10.3390/ma16103665
Li S, Wang X, Ouyang F, Liu R, Xiong X. Novel Functional Soft Magnetic CoFe2O4/Fe Composites: Preparation, Characterization, and Low Core Loss. Materials. 2023; 16(10):3665. https://doi.org/10.3390/ma16103665
Chicago/Turabian StyleLi, Shigeng, Xianzhong Wang, Fangping Ouyang, Rutie Liu, and Xiang Xiong. 2023. "Novel Functional Soft Magnetic CoFe2O4/Fe Composites: Preparation, Characterization, and Low Core Loss" Materials 16, no. 10: 3665. https://doi.org/10.3390/ma16103665




