Effects of Dopants and Processing Parameters on the Properties of ZnO-V2O5-Based Varistors Prepared by Powder Metallurgy: A Review
Abstract
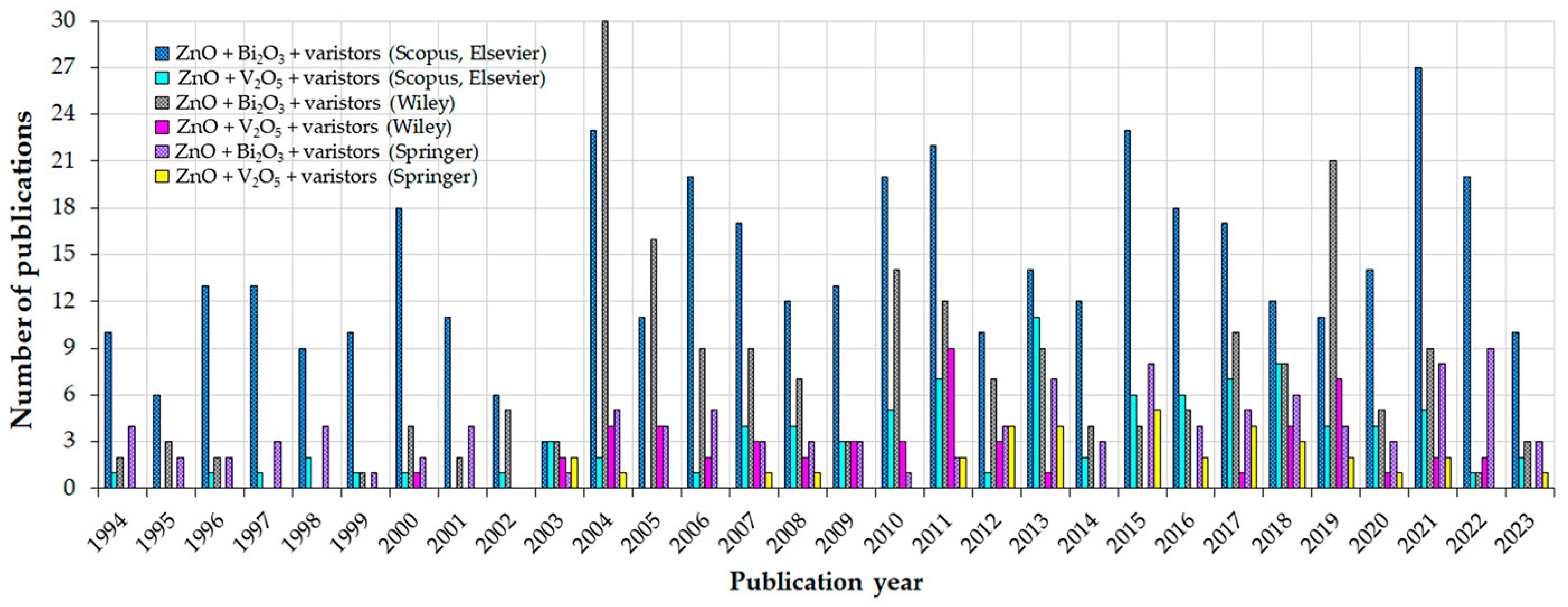
1. Introduction
1.1. Specific Features of ZnO-Based Varistors
1.2. Brief History of ZnO-Based Varistors
2. Overview of the Development of ZnO-Based Varistors
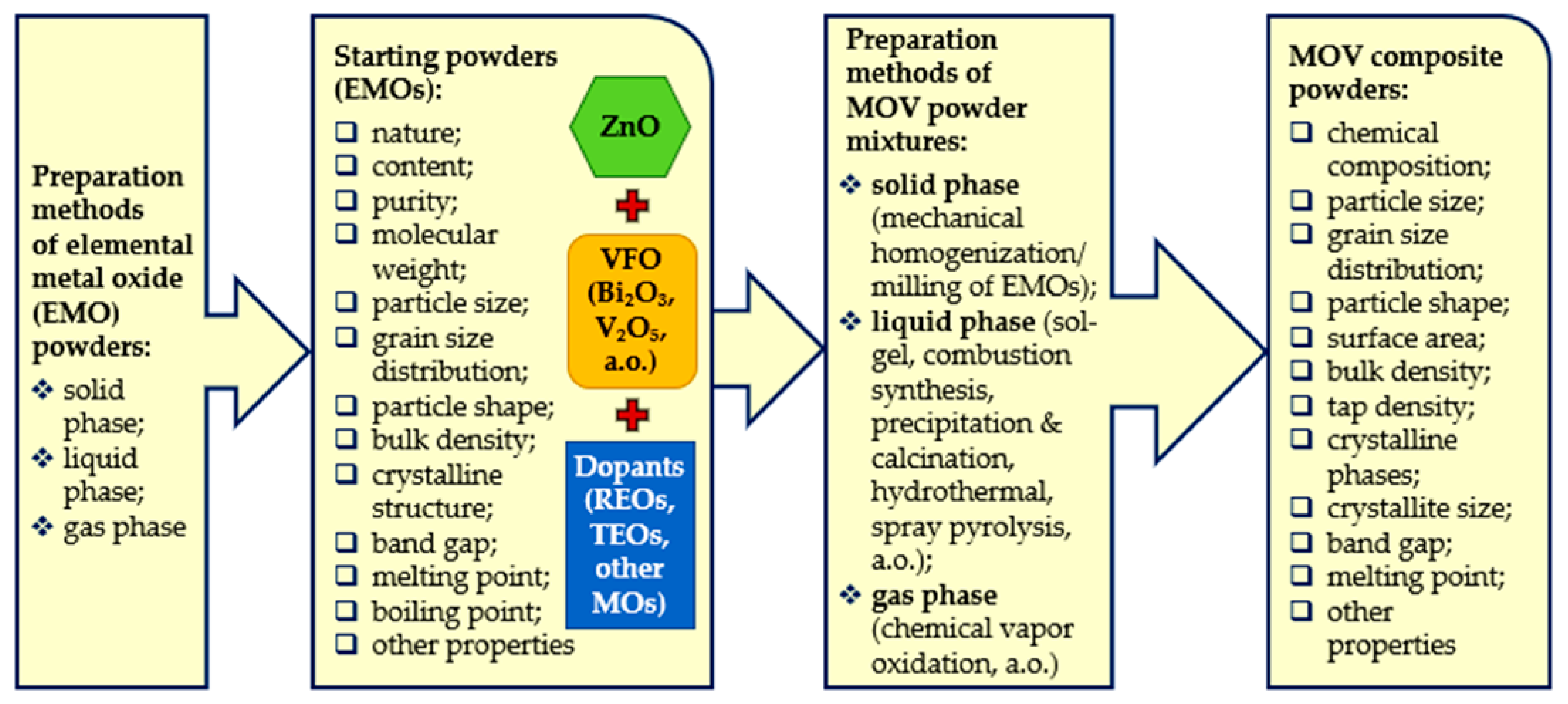
2.1. Preparation Methods of Elemental MOs and Composite Powders for MOVs
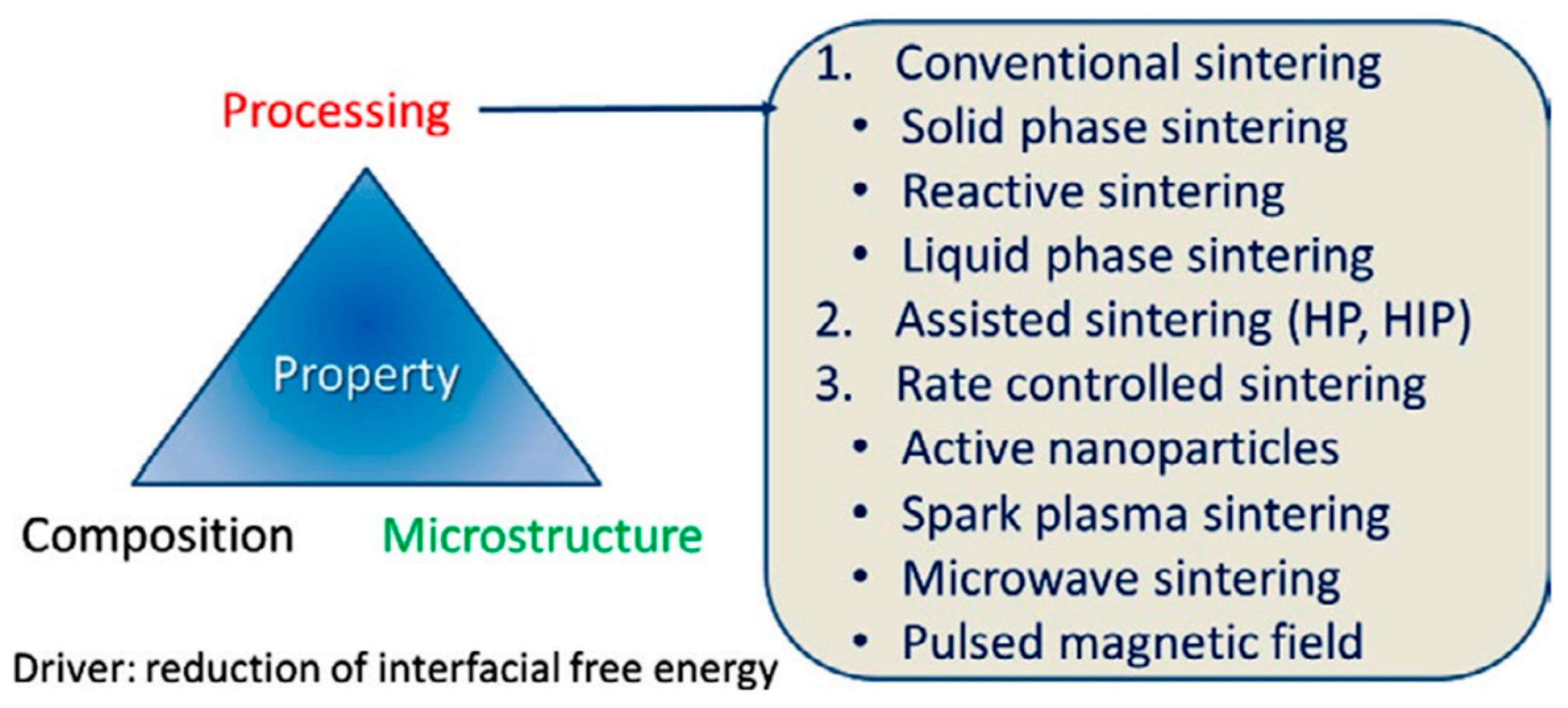
2.2. Preparation Methods and Major Assessment Criteria of ZnO-Based MOVs
- ➢
- conventional sintering (CS), which includes solid-phase sintering (CS-SPS), reactive sintering (CS-RS), and liquid-phase sintering (CS-LPS);
- ➢
- assisted sintering involving hot pressing (HP) or hot isostatic pressing (HIP);
- ➢
- rate-controlled sintering (RCS) by using (i) active nanoparticles (NPs); (ii) spark plasma sintering (SPS), which is an electric-field-assisted process; (iii) microwave sintering (MWS), or (iv) pulsed magnetic field (PMF) processes.
2.3. Liquid-Phase Sintering (LPS) of ZnO-Bi2O3- and ZnO-V2O5-Based Systems
3. Considerations of the Crystalline Structure and Physics of ZnO-Based Varistors
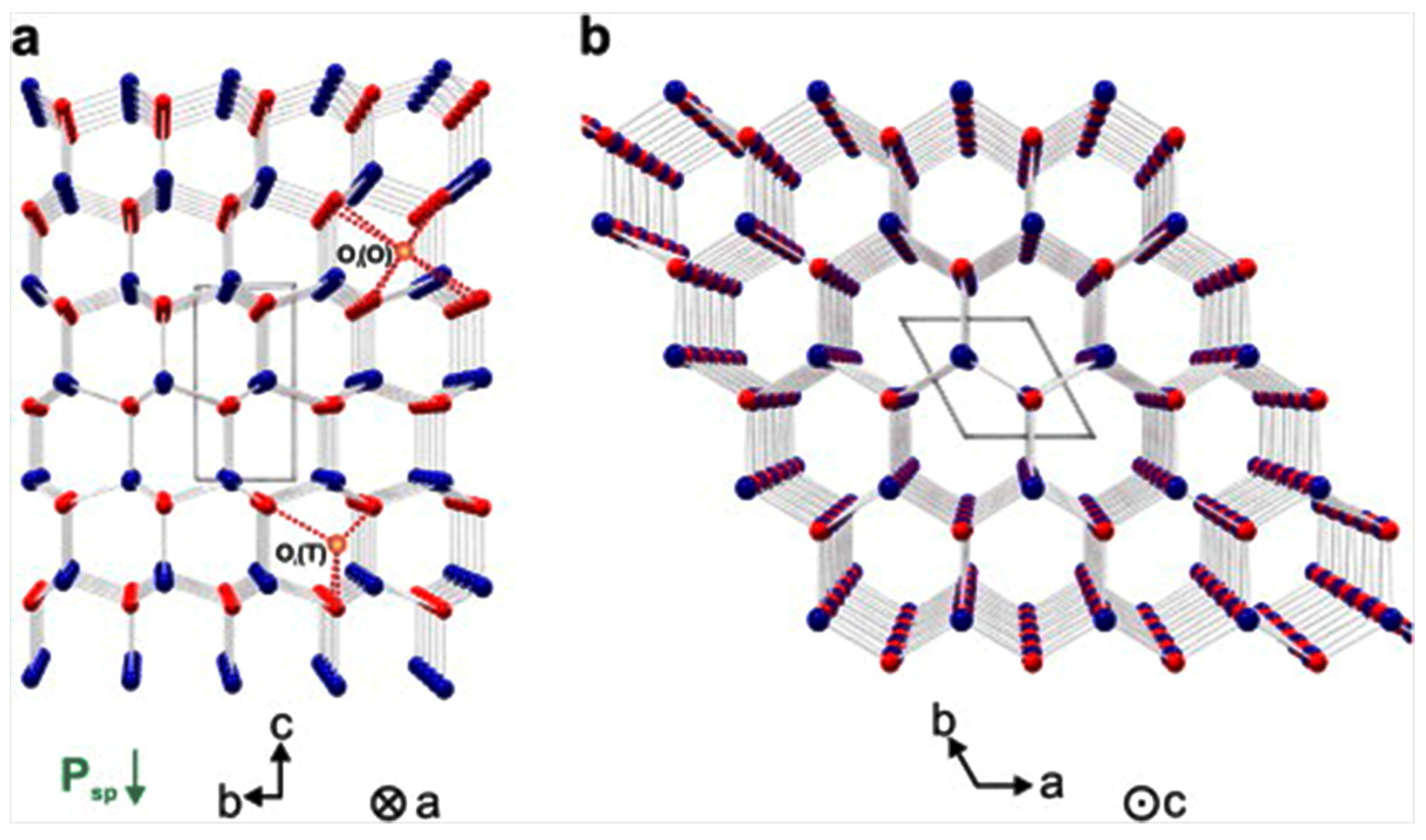
3.1. Crystalline Structure of ZnO
3.2. Polymorphisms of Bi2O3 and V2O5
3.3. Crystallographic Defects and Doping of ZnO
3.4. Inversion Boundary (IB)-Induced Grain Growth in ZnO-Based Systems
3.5. Conduction Mechanisms in ZnO-Based Systems
- ▪
- space-charge-limited current in the Bi2O3-rich intergranular thin layer;
- ▪
- tunneling through a thin layer at the GBs or ZnO homojunctions;
- ▪
- tunneling through DSBs without or with ZnO-Bi2O3 heterojunctions generated by interface states or with the creation of holes (minority carriers) at the GBs;
- ▪
- hole-induced electrical breakdown (EBD) with DSBs created by interface states and bulk traps;
- ▪
- space-charge-induced current or bypass effect at ZnO-Bi2O3 heterojunctions.
4. Preparation of MOV Powders and Disc-Shaped MOVs from ZnO-V2O5 Systems
4.1. Preparation of MOV Powders from ZnO-V2O5-Based Systems
4.2. Preparation of MOV Discs from ZnO-V2O5 Systems by Conventional PM Routes
- ➢
- Granulation of MOV powders containing organic additives (binders, lubricants, plasticizers, and deflocculants), which have been previously milled and spray-dried;
- ➢
- Pressing of the granulated MOV powders into disc-shaped compacts;
- ➢
- Burning out of the organic additives from the MOV discs;
- ➢
- High-temperature sintering of the MOV discs in air;
- ➢
- Glass coating on the cylindrical surfaces of the MOV discs for passivation;
- ➢
- Glass firing of the coatings;
- ➢
- Grinding of the contact surfaces of the MOV discs using a warm ultrasonic process and forced air convection;
- ➢
- Metallization of the plane contact surfaces of the MOV discs with Al metal.
5. Properties of Disc-Shaped MOVs from ZnO-V2O5 Systems
5.1. Structural Properties
- ➢
- ➢
- Zn3(VO4)2, ZnV2O4, and VO2 phases [27,38,50,124,144,162,172] (chemical reactions (3) and (4) [124]) were observed in ZnO-V2O5-Mn3O4 and ZnO-V2O5-MnO2-Nb2O5 systems with 0.5% V2O5, 0.5–2% Mn3O4, or MnO2 and 0.05–0.1% Nb2O5 sintered at 825–900 °C for up to 4 h. The MOVs sintered at 875 °C exhibited a supplementary Mn-rich phase [163]:ZnO + V2O5 → ZnV2O4 + O2V2O5 → 2VO2 + (1/2)O2
- ➢
- Zn3(VO4)2, Zn4V2O9, or ZnV2O4, unreacted V2O5 or VO2, Mn-rich, and ErVO4, DyVO4, GdVO4, YbVO4, BiVO4, or TbVO4 phases (chemical reactions (2)–(7), where M = Er, Dy, Gd, Yb, or Bi), were observed in ZnO-V2O5-MnO2-Nb2O5 systems with 0.5% V2O5, 2% MnO2, and 0.05–0.1% Nb2O5 doped with up to 0.25% Er2O3 [124,163], Dy2O3 [24], Gd2O3 [173], Yb2O3 [27], Bi2O3 [172], or Tb4O7 [162] and sintered at 875–1300 °C for 0.5–8 h:4ZnO + V2O5 → Zn4V2O9V2O5 + M2O3 → 2MVO42V2O5 + Tb4O7 → 4TbVO4 + (1/2)O2
- ➢
- Zn3(VO4)2 and ErVO4 phases [161] were observed in ZnO-V2O5-Mn3O4-Nb2O5-Er2O3 systems with 0.5% V2O5, 0.5% Mn3O4, 0.05% Nb2O5, and 0.05% Er2O3 sintered at 925–950 °C for 3 h.
- ➢
- Zn3(VO4)2, Zn4V2O9, V2O5, Mn-rich, Er-rich, and ErVO4 phases [62,63,169,174,175] were observed in ZnO-V2O5-MnO2-Nb2O5-Er2O3 (ZVMNE) systems with 0.5% V2O5, 2% MnO2, 0.05–0.1% Nb2O5, and 0.5–2% Er2O3 sintered at 900–1300 °C for 0.5–8 h. The MOVs sintered at 950–1100 °C for ≤8 h also yielded the Zn2MnO4 phase [62,175]. Figure 9 depicts the XRD patterns of the ZVMNE systems developed by Roy et al. [62].
- ➢
- Zn3(VO4)2, Zn2V2O7, Zn(VO3)2, InVO4, and Zn7I2O10 phases (chemical reactions (6) and (8)–(10), where M = In) were observed in ZnO-V2O5-Nb2O5-In2O3 systems with 0.5% V2O5, 1% Nb2O5, and 0.1% In2O3 sintered at 875–900 °C for 1 h. No secondary phases related to Nb were found, which could be attributed to the possible incorporation of Nb into the ZnO solid solution [44].2ZnO + V2O5 → Zn2V2O7ZnO + V2O5 → Zn(VO3)27ZnO + In2O3 → Zn7I2O10
- ➢
- Zn3(VO4)2, ZnV2O4, and ZnMn2O4 phases were observed in ZnO-V2O5-MnCO3-Nb2O5 systems with 0.5% V2O5, 2% MnCO3, and 0.1% Nb2O5 sintered at 900–930 °C for 3 h. Doping this system with 0.05 mol.% Y2O3, Nd2O3, or Sm2O3 resulted in the formation of an additional phase (YVO4, NdVO4, or SmVO4) [25] (chemical reaction (6), where M = Y, Nd, or Sm).
- ➢
- Zn3(VO4)2, Zn4V2O9, ZnV2O4, V2O5, and Zn2MnO4 phases were observed in ZnO-V2O5-MnO2-Nb2O5 systems with 0.5% V2O5, 2% MnO2, and 0.1% Nb2O5 sintered by SSS at 1100 °C for 0.5–8 h or TSS (T1 = 1050 °C, t1 = 1/6 h, T2 = 750–800 °C, t2 = 2.5–40 h). Additionally, an ErVO4 phase was formed in 0.5% Er2O3-doped systems [62,63].
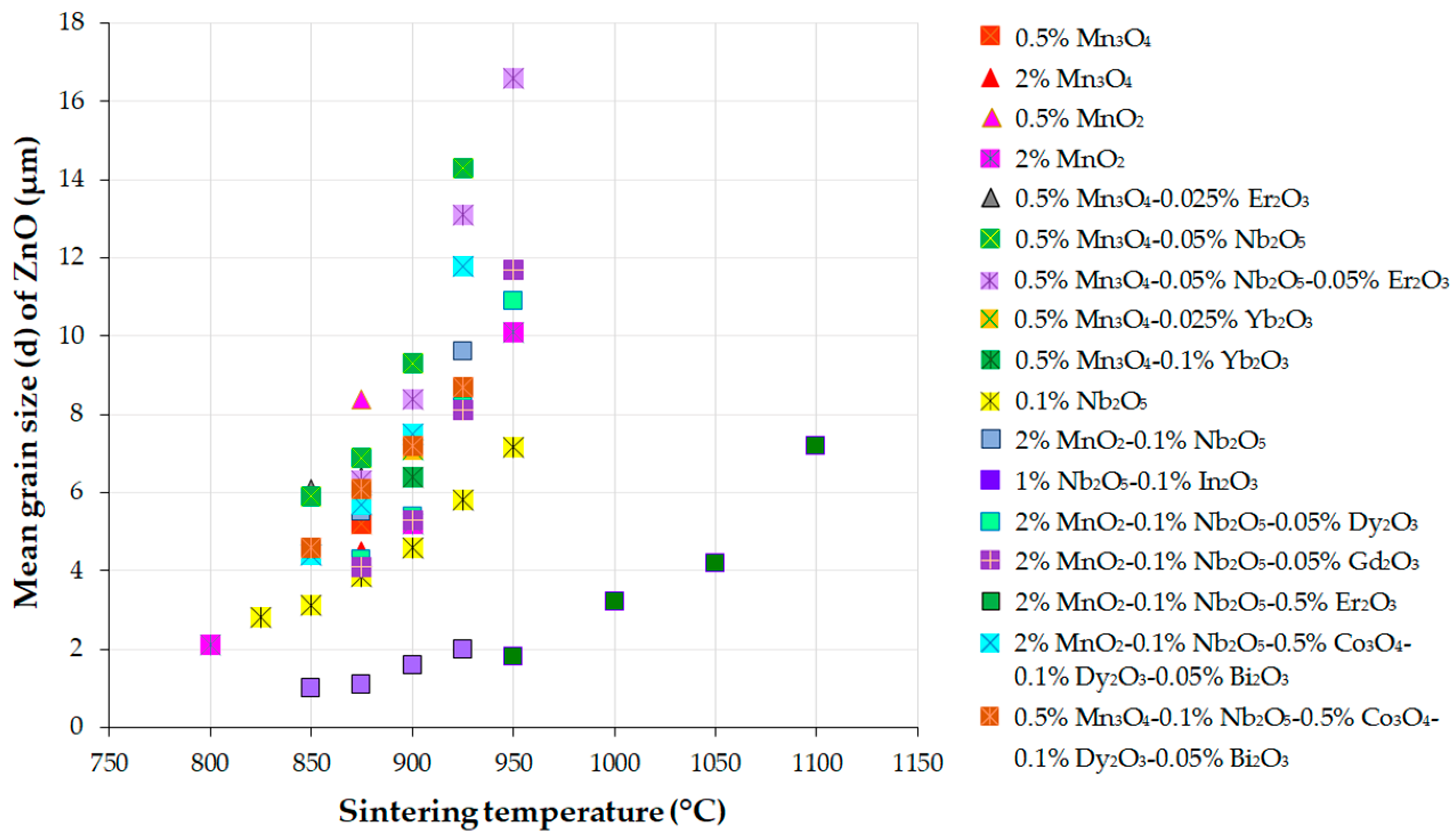
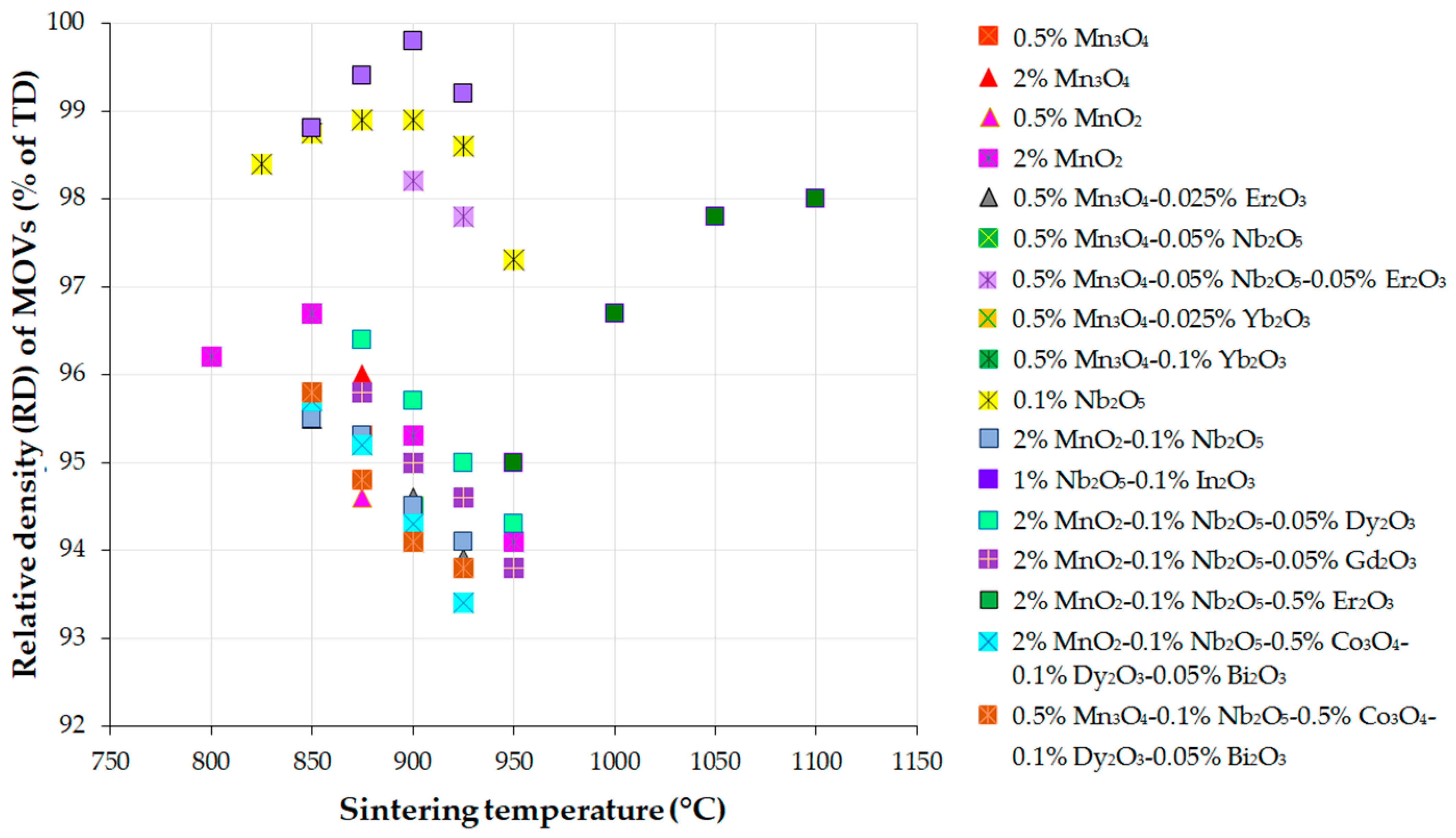
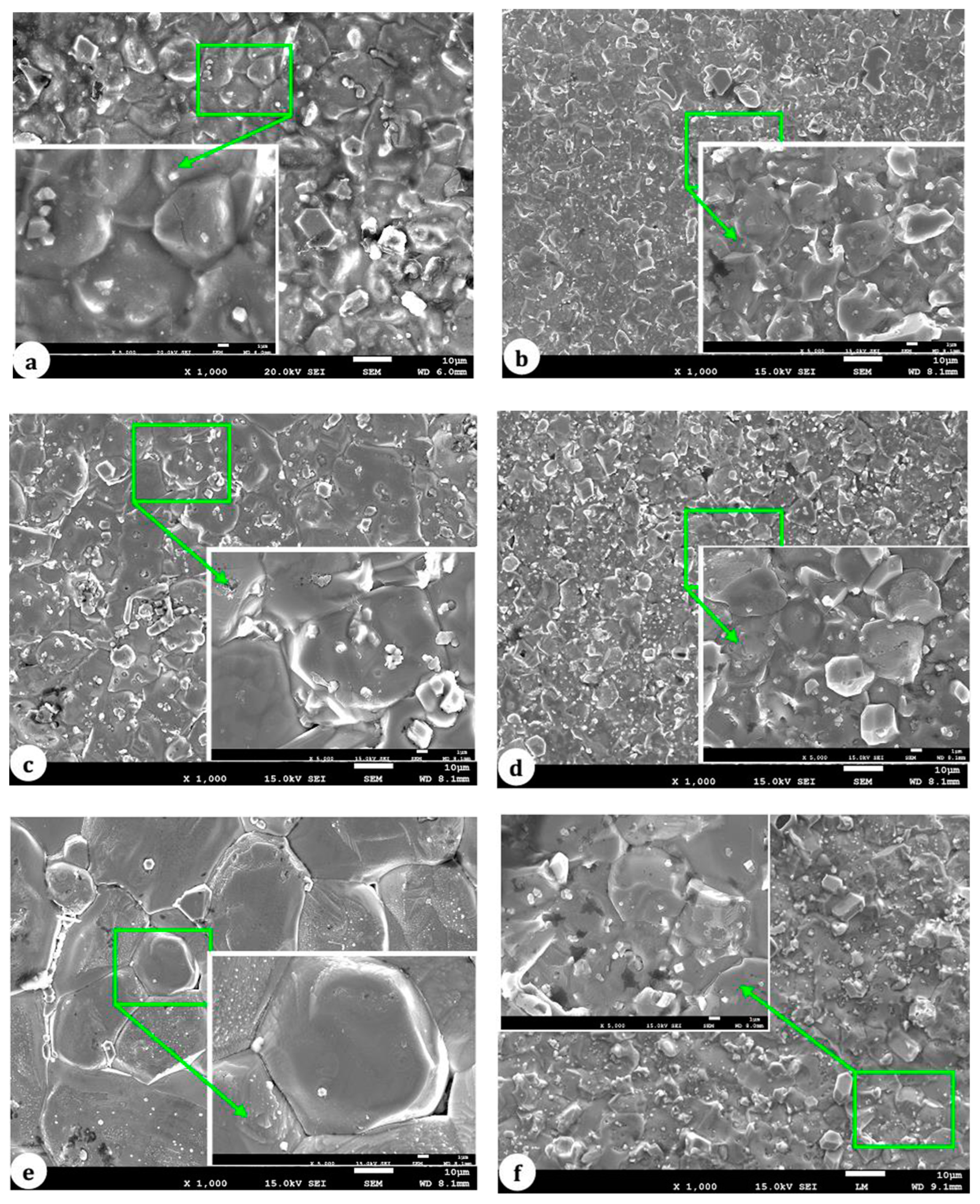
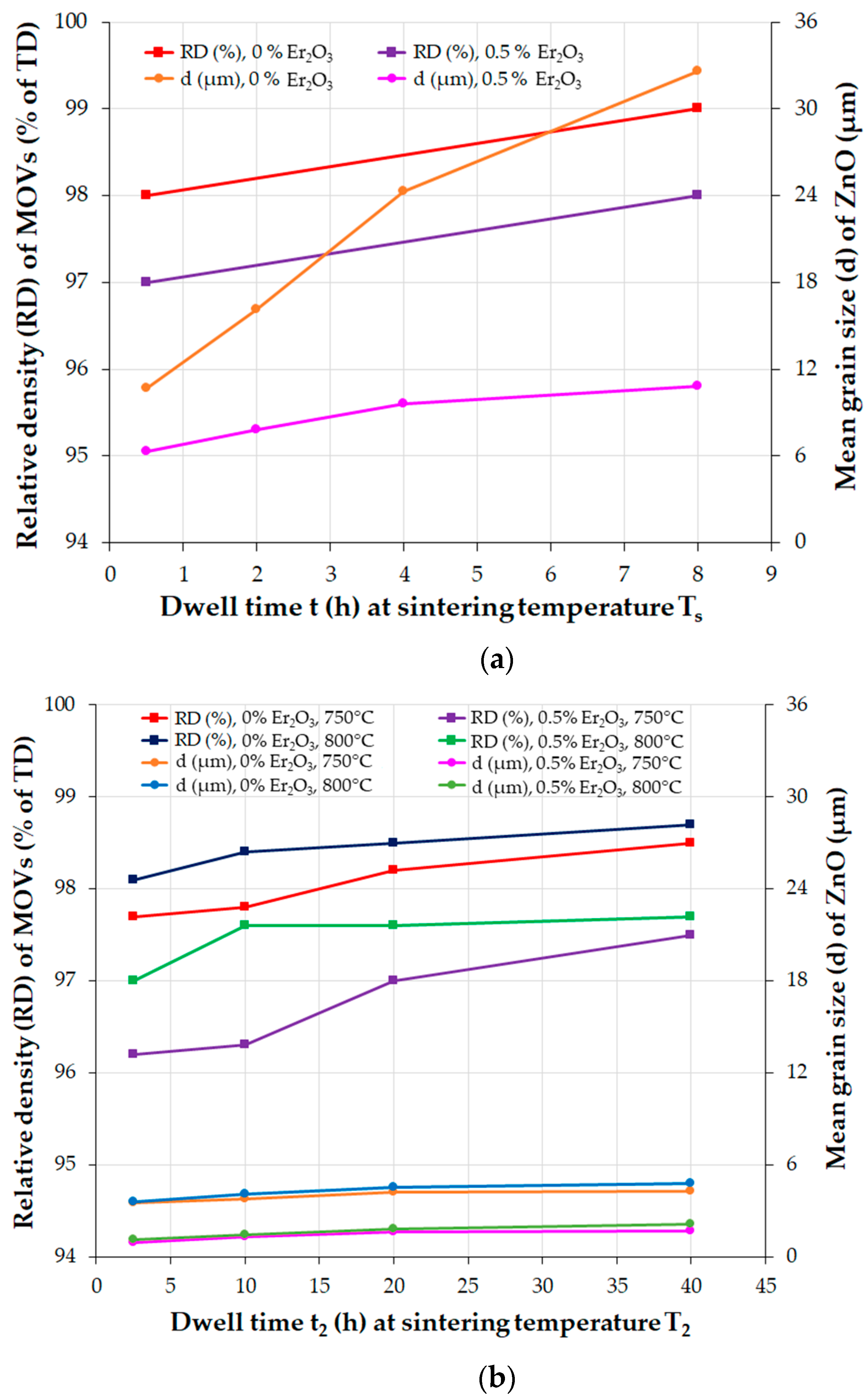
5.2. Grain Growth Behavior, Densification, and Microstructure of ZnO-V2O5-Based Systems
5.3. Electrical and Dielectric Properties of MOV Discs from ZnO-V2O5-Based Systems
5.3.1. Electrical Properties of MOV Discs from ZnO-V2O5-Based Systems
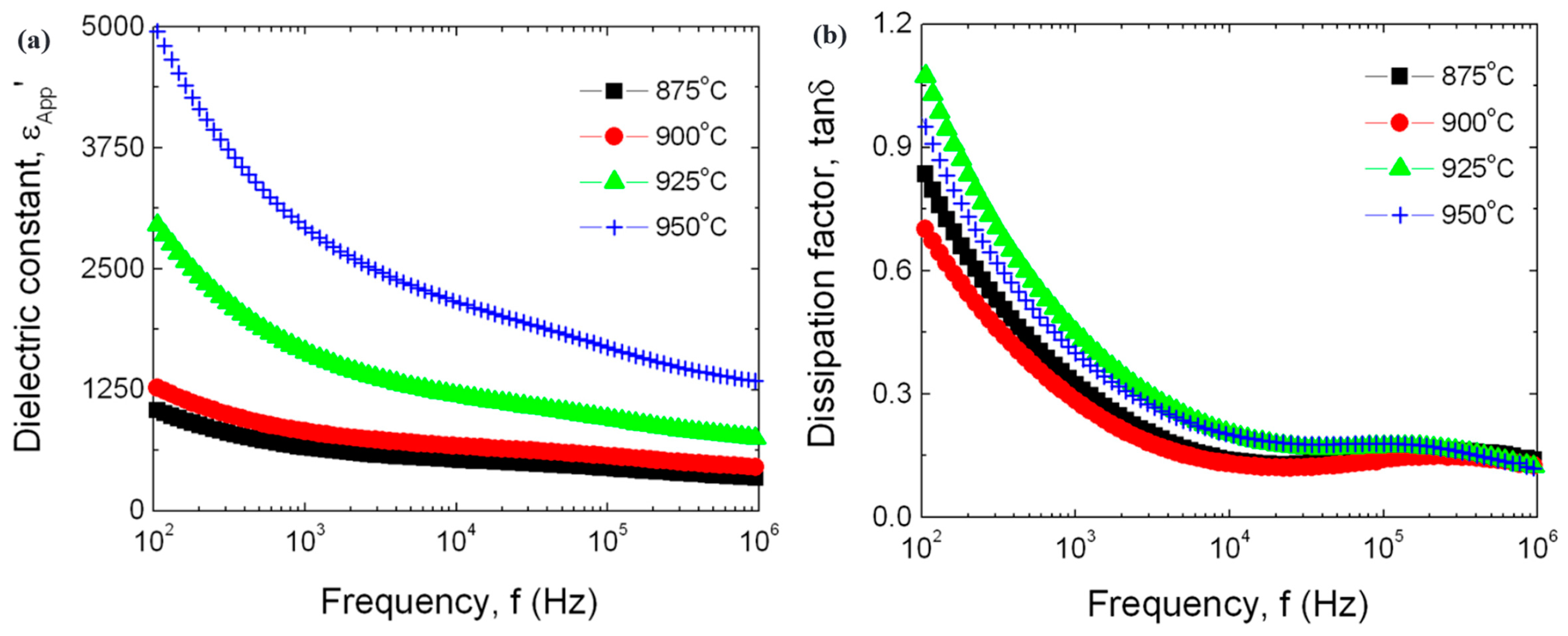
5.3.2. Dielectric Properties of MOVs from ZnO-V2O5 Systems
5.4. Factors Inducing the Failure Modes of ZnO-Based Varistors for Surge Arresters
5.5. Strategies to Improve the Characteristics of MOVs from ZnO-V2O5-Based Systems
6. Conclusions
7. Future Research Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Wang, S.; Cui, Y. Research on protection performance of surge protective devices against electromagnetic pulse. Appl. Bionics Biomech. 2022, 2022, 7194855. [Google Scholar] [CrossRef] [PubMed]
- Drabkin, M.M. Surge protection of low-voltage AC power by MOV-based SPDs. In Proceedings of the 10th International Conference on Harmonics and Quality of Power, Rio de Janeiro, Brazil, 6–9 October 2002; Volume 1, pp. 13–16. [Google Scholar] [CrossRef]
- Danzer, R.; Kaufmann, B.; Supancic, P. Failure of high power varistor ceramic components. J. Eur. Ceram. Soc. 2020, 40, 3766–3770. [Google Scholar] [CrossRef]
- Karim, A.N.M.; Begum, S.; Hashmi, M.S.J. Role of surface to volume ratio of zinc oxide arrester blocks on the energy absorption capability. IOP Conf. Ser. Earth Environ. Sci. 2013, 16, 012008. [Google Scholar] [CrossRef]
- He, H. Metal oxide semiconductors and conductors. In Solution Processed Metal Oxide Thin Films for Electronic Applications; Cui, Z., Korotcenkov, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–30. [Google Scholar] [CrossRef]
- Matsuoka, M. Nonohmic properties of zinc oxide ceramics. Jpn. J. Appl. Phys. 1971, 10, 736–746. [Google Scholar] [CrossRef]
- Chen, B.; Wang, B.; Gao, P.; Zhang, P.; Chen, H. Effects of raw particle size and annealing on microstructure, electrical and mechanical behaviors of ZnO-based varistors. J. Alloys Compd. 2021, 872, 159638. [Google Scholar] [CrossRef]
- Tian, T.; Zheng, L.; Podlogar, M.; Zeng, H.; Bernik, S.; Xu, K.; Ruan, X.; Shi, X.; Li, G. Novel ultrahigh-performance ZnO-based varistor ceramics. ACS Appl. Mater. Interfaces 2021, 13, 35924–35929. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Liu, W.; Lin, J.; He, J.; Cheng, P. Advances in ZnO varistors in China during the past 30 years—Fundamentals, processing, and applications. IEEE Electr. Insul. Mag. 2015, 31, 35–44. [Google Scholar] [CrossRef]
- Pianaro, S.A.; Bueno, P.R.; Longo, E.; Varela, J.A. A new SnO2-based varistor system. J. Mater. Sci. Lett. 1995, 14, 692–694. [Google Scholar] [CrossRef]
- Lungu, M.V.; Pătroi, D.; Marinescu, V.; Caramitu, A.; Marin, M.; Tălpeanu, D.; Lucaci, M.; Godeanu, P. Preparation and study of the optical, electrical and dielectric characteristics of some disc-shaped tin dioxide-based varistors. Rom. J. Phys. 2022, 67, 610. [Google Scholar]
- Liao, X.; Peng, F.; Pu, Y.; Cao, S.; Zhu, D. Effects of Dy2O3 on the electrical properties of a (Nb2O5-Dy2O3-SiO2) Co-doped TiO2 varistor. J. Electron. Mater. 2021, 50, 1963–1979. [Google Scholar] [CrossRef]
- Kang, K.; Zhu, H.; Zhu, G.; Deng, S. Influence of annealing atmosphere on varistor property of TiO2–Nb2O5–SrCO3 ceramics. Mater. Sci. Appl. 2021, 12, 461–474. [Google Scholar] [CrossRef]
- He, J. Metal Oxide Varistors: From Microstructure to Macro-Characteristics; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Chen, X.; Kang, X.; Yuan, C. Sintering temperature dependence of varistor properties and impedance spectroscopy behavior in ZnO based varistor ceramics. J. Mater. Sci. Mater. Electron. 2015, 26, 2389–2396. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Sun, T.; Song, H. Effect of bismuth and vanadium as the varistor forming element in ZnO-based ceramics. J. Mater. Sci. Mater. Electron. 2020, 31, 8206–8211. [Google Scholar] [CrossRef]
- Mirzayi, M.; Hekmatshoar, M.H. Effect of V2O5 on electrical and microstructural properties of ZnO ceramics. Phys. B Condens. Matter. 2013, 414, 50–55. [Google Scholar] [CrossRef]
- Hng, H.-H.; Knowles, K.M.; Midgley, P.A. Zinc vanadates in vanadium oxide-doped zinc oxide varistors. J. Am. Ceram. Soc. 2001, 84, 435–441. [Google Scholar] [CrossRef]
- Cao, W.; Xie, X.; Wang, Y.; Chen, M.; Qiao, Y.; Wang, P.; Zhang, Y.; Liu, J. Effect of Pr6O11 doping on the microstructure and electrical properties of ZnO varistors. Ceram. Int. 2019, 45, 24777–24783. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Gao, J.; Kong, F.; Li, Y.; Li, S. Effects of the Er2O3 doping on the microstructure and electrical properties of ZnO–Bi2O3 based varistor ceramics. Ceram. Int. 2021, 47, 32349–32356. [Google Scholar] [CrossRef]
- Roy, S.; Roy, T.K.; Das, D. Grain growth kinetics of Er2O3 doped ZnO-V2O5 based varistor ceramics. Ceram. Int. 2019, 45, 24835–24850. [Google Scholar] [CrossRef]
- Nahm, C.-W. Er2O3 doping effect on electrical properties of ZnO-V2O5-MnO2-Nb2O5 varistor ceramics. J. Am. Ceram. Soc. 2011, 94, 3227–3229. [Google Scholar] [CrossRef]
- Nahm, C.-W. Major effects on electrical properties of ZnO–V2O5–MnO2–Nb2O5 ceramics with small Gd2O3 doping changes. J. Alloys Compd. 2013, 578, 132–135. [Google Scholar] [CrossRef]
- Nahm, C.-W. Effect of Dy2O3 doping on microstructure, electrical and dielectric properties of ZnO–V2O5-based varistor ceramics. J. Mater. Sci. Mater. Electron. 2015, 26, 10217–10224. [Google Scholar] [CrossRef]
- Zhao, M.; Li, X.; Li, T.; Shi, Y.; Li, B. Effect of Y2O3, Nd2O3 or Sm2O3 on the microstructure and electrical properties of ZnVMnNbO varistor ceramics. J. Mater. Sci. Mater. Electron. 2019, 30, 450–456. [Google Scholar] [CrossRef]
- Nahm, C.-W. Yttrium doping effect on varistor properties of zinc-vanadium-based ceramics. J. Korean Ceram. Soc. 2018, 55, 504–509. [Google Scholar] [CrossRef]
- Nahm, C.-W. Effect of Yb2O3 addition on varistor properties and aging characteristics of ZnO–V2O5–Mn3O4 system. J. Mater. Sci. Mater. Electron 2018, 29, 2958–2965. [Google Scholar] [CrossRef]
- Roy, T.K.; Bhattacharyya, T.K.; Thakur, S.K. Role of sintering temperature on microstructure and nonlinear electrical properties of 0.1 mol.% Nb2O5 added ZnO–V2O5 varistor ceramics. J. Mater. Sci. Mater. Electron. 2019, 30, 5640–5651. [Google Scholar] [CrossRef]
- Guo, M.; Wang, Y.; Wu, K.; Zhang, L.; Zhao, X.; Lin, Y.; Li, J. Revisiting the effects of Co2O3 on multiscale defect structures and relevant electrical properties in ZnO varistors. High Voltage 2020, 5, 241–248. [Google Scholar] [CrossRef]
- Hng, H.-H.; Knowles, K.M. Microstructure and current-voltage characteristics of multicomponent vanadium-doped zinc oxide varistors. J. Am. Ceram. Soc. 2004, 83, 2455–2462. [Google Scholar] [CrossRef]
- Bai, H.; Li, S.; Zhao, Y.; Xu, Z.; Chu, R.; Hao, J.; Chen, C.; Li, H.; Gong, Y.; Li, G. Influence of Cr2O3 on highly nonlinear properties and low leakage current of ZnO–Bi2O3 varistor ceramics. Ceram. Int. 2016, 42, 10547–10550. [Google Scholar] [CrossRef]
- Jiang, H.; Ren, X.; Lao, X.; Kong, A.; Zhong, M.; Sun, Y.; Wu, Y.; Yao, Z.; Shi, L. Effect of NiO doping on grain growth and electrical properties of ZnO-based varistors. J. Eur. Ceram. Soc. 2022, 42, 3898–3904. [Google Scholar] [CrossRef]
- Guo, M.; Zhao, X.; Shi, W.; Zhang, B.; Wu, K.; Li, J. Simultaneously improving the electrical properties and long-term stability of ZnO varistor ceramics by reversely manipulating intrinsic point defects. J. Eur. Ceram. Soc. 2022, 42, 162–168. [Google Scholar] [CrossRef]
- Saadeldin, M.M.; Desouky, O.A.; Ibrahim, M.; Khalil, G.E.; Helali, M.Y. Investigation of structural and electrical properties of ZnO varistor samples doped with different additives. NRIAG J. Astron. Geophys. 2018, 7, 201–207. [Google Scholar] [CrossRef]
- Pandey, S.; Kumar, D.; Parkash, O. Electrical impedance spectroscopy and structural characterization of liquid-phase sintered ZnO–V2O5–Nb2O5 varistor ceramics doped with MnO. Ceram. Int. 2016, 42, 9686–9696. [Google Scholar] [CrossRef]
- Nahm, C.-W. Effect of sintering temperature on varistor properties and aging characteristics of ZnO–V2O5–MnO2 ceramics. Ceram. Int. 2009, 35, 2679–2685. [Google Scholar] [CrossRef]
- Wu, J.; Li, T.; Qi, T.; Qin, Q.; Li, G.; Zhu, B.; Wu, R.; Xie, C. Influence of dopants on electrical properties of ZnO-V2O5 varistors deduced from AC impedance and variable-temperature dielectric spectroscopy. J. Electron. Mater. 2012, 41, 1970–1977. [Google Scholar] [CrossRef]
- El-Rabaie, S.; Khafagy, A.H.; Dawoud, M.T.; Attia, M.T. Mechanical, microstructure and electrical properties of ternary ZnO–V2O5–Mn3O4 varistor with sintering temperature. Bull. Mater. Sci. 2015, 38, 773–781. [Google Scholar] [CrossRef]
- Nahm, C.-W. Impulse aging behavior of ZnO-V2O5-based varistors with Nb2O5 addition. J. Am. Ceram. Soc. 2011, 94, 1305–1308. [Google Scholar] [CrossRef]
- Singh, J.; Virpal Sharma, S.; Singh, R.C. Effect of Fe2O3 doping on structural properties of ZnO-V2O5 based varistor system. AIP Conf. Proc. 2017, 1832, 120021. [Google Scholar] [CrossRef]
- Xie, P.; Hu, J. Influence of sintering temperature and ZrO2 dopants on the microstructure and electrical properties of zinc oxide varistors. IEEE Access 2019, 7, 140126–140133. [Google Scholar] [CrossRef]
- Xiao, X.; Zheng, L.; Cheng, L.; Tian, T.; Ruan, X.; Podlogar, M.; Bernik, S.; Li, G. Influence of WO3-doping on the microstructure and electrical properties of ZnO-Bi2O3 varistor ceramics sintered at 950 °C. J. Am. Ceram. Soc. 2015, 98, 1356–1363. [Google Scholar] [CrossRef]
- Meng, P.; Hu, J.; Zhao, H.; He, J. High voltage gradient and low residual-voltage ZnO varistor ceramics tailored by doping with In2O3 and Al2O3. Ceram. Int. 2016, 42, 19437–19440. [Google Scholar] [CrossRef]
- Roy, T.K. High nonlinearity in 0.1 mol.% In2O3 added ZnO–V2O5 based varistors prepared at different sintering temperatures. Ceram. Int. 2021, 47, 35152–35159. [Google Scholar] [CrossRef]
- Cheng, K.; Zhao, H.; Zhou, Y.; Xie, Q. B2O3- and Y2O3-doped ZnO varistor ceramics: Enhanced voltage gradient and nonlinear properties for UHV. Mater. Sci. Semicond. Process. 2021, 123, 105590. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, M.; Xu, Z.; Chu, R.; Hao, J.; Li, H.; Gong, Y.; Li, G. The effect of SiO2 on electrical properties of low-temperature-sintered ZnO-Bi2O3-TiO2-Co2O3-MnO2-based ceramics. J. Am. Ceram. Soc. 2017, 100, 1057–1064. [Google Scholar] [CrossRef]
- Bernik, S.; Daneu, N.; Rečnik, A. Inversion boundary induced grain growth in TiO2 or Sb2O3 doped ZnO-based varistor ceramics. J. Eur. Ceram. Soc. 2004, 24, 3703–3708. [Google Scholar] [CrossRef]
- Bernik, S.; Daneu, N. Characteristics of SnO2-doped ZnO-based varistor ceramics. J. Eur. Ceram. Soc. 2001, 21, 1879–1882. [Google Scholar] [CrossRef]
- Bernik, S.; Podlogar, M.; Daneu, N.; Rečnik, A. A novel approach to tailoring the microstructure and electrical characteristics of ZnO-based varistor ceramics via inversion-boundary (IB) induced grain growth. Mater. Prot. 2011, 52, 73–79. [Google Scholar]
- Park, J.-H.; Nahm, C.-W. Sintering effect on electrical properties and aging behavior of quaternary ZnO–V2O5–Mn3O4–Nb2O5 ceramics. J. Mater. Sci. Mater. Electron. 2015, 26, 168–175. [Google Scholar] [CrossRef]
- Kularatna, N.; Ross, A.S.; Fernando, J.; James, S. Components used in surge protection circuits. In Design of Transient Protection Systems; Kularatna, N., Ross, A.S., Fernando, J., James, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–42. [Google Scholar] [CrossRef]
- Diwald, O. Zinc oxide nanoparticles for varistors. In Metal Oxide Nanoparticles; Diwald, O., Berger, T., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 783–807. [Google Scholar] [CrossRef]
- Hasse, L.; Konczakowska, A.; Smulko, J. Classification of high-voltage varistors into groups of differentiated quality. Microelectron. Reliab. 2009, 49, 1483–1490. [Google Scholar] [CrossRef]
- Pillai, S.C.; Kelly, J.M.; Ramesh, R.; McCormack, D.E. Advances in the synthesis of ZnO nanomaterials for varistor devices. J. Mater. Chem. C 2013, 1, 3268. [Google Scholar] [CrossRef]
- Rečnik, A.; Bernik, S.; Daneu, N. Microstructural engineering of ZnO-based varistor ceramics. J. Mater. Sci. 2012, 47, 1655–1668. [Google Scholar] [CrossRef]
- Eda, K. Zinc oxide varistors. IEEE Electr. Insul. Mag. 1989, 5, 28–30. [Google Scholar] [CrossRef]
- Simo, A.; Frigura-Iliasa, F.M.; Frigura-Iliasa, M.; Andea, P.; Musuroi, S. Service limits for metal oxide varistors having cylindrical symmetry as function of the ambient temperature. Symmetry 2022, 14, 1351. [Google Scholar] [CrossRef]
- Kubát, M. Properties of a pn junction in a semiconductor; Semiconductor—Metal contact. In Power Semiconductors; Kubát, M., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 54–142. [Google Scholar] [CrossRef]
- Daneu, N.; Bernik, S.; Rečnik, A. Inversion boundary induced grain growth in ZnO ceramics: From atomic-scale investigations to microstructural engineering. J. Phys. Conf. Ser. 2011, 326, 012003. [Google Scholar] [CrossRef]
- Nahm, C.-W. Aging Characteristics of ZnO–V2O5-based varistors for surge protection reliability. Microelectron. Reliab. 2014, 54, 2836–2842. [Google Scholar] [CrossRef]
- Hng, H.H.; Knowles, K.M. Characterisation of Zn3(VO4)2 phases in V2O5-doped ZnO varistors. J. Eur. Ceram. Soc. 1999, 19, 721–726. [Google Scholar] [CrossRef]
- Roy, S.; Roy, T.K.; Das, D. Microstructure and current-voltage characteristics of erbium oxide doped multicomponent zinc oxide varistors. IOP Conf. Ser. Mater. Sci. Eng. 2018, 338, 012046. [Google Scholar] [CrossRef]
- Roy, S.; Das, D.; Roy, T.K. Two stage sintering behaviour of Er2O3 doped high performance ZnO varistors. J. Eur. Ceram. Soc. 2021, 41, 5184–5192. [Google Scholar] [CrossRef]
- Alim, M.A.; Li, S.; Liu, F.; Cheng, P. Electrical barriers in the ZnO varistor grain boundaries. Phys. Status Solidi A 2006, 203, 410–427. [Google Scholar] [CrossRef]
- Gupta, T.K. Application of zinc oxide varistors. J. Am. Ceram. Soc. 1990, 73, 1817–1840. [Google Scholar] [CrossRef]
- Begum, S.; Karim, A.N.M.; Fawzia, S.; Hashmi, M.S.J. Alternative process and design opportunities for ZnO-based surge arrester: An investigation oriented roadmap. In Encyclopedia of Materials: Electronics; Haseeb, A.S.M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 3, pp. 464–483. [Google Scholar] [CrossRef]
- He, J.; Lin, J.; Zhang, L.; Liu, W.; Li, S. Indication of initial degradation of ZnO varistor ceramics. In Proceedings of the 2017 1st International Conference on Electrical Materials and Power Equipment (ICEMPE), Xi’an, China, 14–17 May 2017; pp. 529–532. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, M.; Li, J. Multiscale defect responses in understanding degradation in zinc oxide varistor ceramics. Ceram. Int. 2020, 46, 22134–22139. [Google Scholar] [CrossRef]
- Meng, P.; Zhao, X.; Fu, Z.; Wu, J.; Hu, J.; He, J. Novel zinc-oxide varistor with superior performance in voltage gradient and aging stability for surge arrester. J. Alloys Compd. 2019, 789, 948–952. [Google Scholar] [CrossRef]
- Frigura-Iliasa, F.; Musuroi, S.; Sorandaru, C.; Vatau, D. Case study about the energy absorption capacity of metal oxide varistors with thermal coupling. Energies 2019, 12, 536. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, H.; Li, P.; Li, C.; Lv, D.; Yang, S. An experimental study of the failure mode of ZnO varistors under multiple lightning strokes. Electronics 2019, 8, 172. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, J.; Chen, S.; Xie, Q.; He, J. Improving age stability and energy absorption capabilities of ZnO varistors ceramics. Ceram. Int. 2016, 42, 17880–17883. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Kong, F.; Wu, K.; Li, S.; Li, J. Enhanced voltage gradient and energy absorption capability in ZnO varistor ceramics by using nano-sized ZnO powders. J. Alloys Compd. 2020, 828, 154252. [Google Scholar] [CrossRef]
- Wang, J.; Mei, L.; Su, W.; Wang, X.; Zhou, M.; Fan, Y. Impulse current withstanding capability of ZnO varistors with influence of varistor size. IEEJ Trans. Electr. Electron. Eng. 2019, 14, 1016–1022. [Google Scholar] [CrossRef]
- Agrawal, K.C. Surge arresters: Application and selection. In Industrial Power Engineering Handbook; Agrawal, K.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 18–587–18–625. [Google Scholar] [CrossRef]
- Rohini, R.; Pugazhendhi Sugumaran, C. Enhancement of electro-thermal characteristics of micro/nano ZnO based surge arrester. J. Electr. Eng. Technol. 2021, 16, 469–481. [Google Scholar] [CrossRef]
- Huda, D.; El Baradie, M.A.; Hashmi, M.S.J.; Puyane, R. Study of electrical characteristics and microstructure of zinc oxide varistor through rate-controlled sintering. J. Mater. Sci. 1998, 33, 271–278. [Google Scholar] [CrossRef]
- Sakshaug, E.C.; Kresge, J.S.; Miske, S.A. A new concept in station arrester design. IEEE Trans. Power Appar. Syst. 1977, 96, 647–656. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mizuno, M.; Aizawa, T.; Hayashi, M.; Mitani, K. Development of zinc-oxide non-linear resistors and their applications to gapless surge arresters. IEEE Trans. Power Appar. Syst. 1978, PAS-97, 1149–1158. [Google Scholar] [CrossRef]
- Li, J.; Wu, K.; Huang, Y. Breakdown characteristics of varistor ceramics. In Electrical and Electronic Properties of Materials; Kawsar Alam, M.D., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Greuter, F. ZnO varistors: From grain boundaries to power applications. In Oxide Electronics; Ray, A., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 157–234. [Google Scholar] [CrossRef]
- Bokoro, P.; Doorsamy, W. Degradation assessment of metal oxide varistors using a statistical experimental design. IEEJ Trans. Electr. Electron. Eng. 2018, 13, 988–994. [Google Scholar] [CrossRef]
- Xu, M.; Cai, C.; Shi, Y.; Xie, M.; Wu, Y.; Liu, Y.; Peng, J.; Bao, J.; An, S. The grain growth control of ZnO-V2O5 based varistors by PrMnO3 addition. Micromachines 2022, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cheng, C.; Hu, J. Electrical degradation of double-Schottky barrier in ZnO varistors. AIP Adv. 2016, 6, 030701. [Google Scholar] [CrossRef]
- Chu, S.-Y.; Yan, T.-M.; Chen, S.-L. Analysis of ZnO varistors prepared by the sol–gel method. Ceram. Int. 2000, 26, 733–737. [Google Scholar] [CrossRef]
- Yang, R.; Qu, X.; Wang, M. Sol–gel synthesis of Ba-doped ZnO nanoparticles and its use in varistor ceramics. Micro Nano Lett. 2018, 13, 1506–1509. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Ramos-Hernandez, J.J.; Arrieta-Gonzalez, C.D.; Chacon-Nava, J.G.; Gonzalez-Rodriguez, J.G.; Porcayo-Palafox, E.; Sanchez-Carrillo, M.; Flores-De los Rios, J.P.; Martinez-Gomez, L. Synthesis by hydrothermal treatment of ZnO-based varistors doped with rare earth oxides and their characterization by impedance spectroscopy. Crystals 2020, 10, 1134. [Google Scholar] [CrossRef]
- Strachowski, T.; Baran, M.; Małek, M.; Kosturek, R.; Grzanka, E.; Mizeracki, J.; Romanowska, A.; Marynowicz, S. Hydrothermal synthesis of zinc oxide nanoparticles using different chemical reaction stimulation methods and their influence on process kinetics. Materials 2022, 15, 7661. [Google Scholar] [CrossRef]
- Hembram, K.; Rao, T.N.; Srinivasa, R.S.; Kulkarni, A.R. High performance varistors prepared from doped ZnO nanopowders made by pilot-scale flame spray pyrolyzer: Sintering, microstructure and properties. J. Eur. Ceram. Soc. 2015, 35, 3535–3544. [Google Scholar] [CrossRef]
- Polarz, S.; Roy, A.; Merz, M.; Halm, S.; Schröder, D.; Schneider, L.; Bacher, G.; Kruis, F.E.; Driess, M. Chemical vapor synthesis of size-selected zinc oxide nanoparticles. Small 2005, 1, 540–552. [Google Scholar] [CrossRef]
- Sousa, V.C.; Segadães, A.M.; Morelli, M.R.; Kiminami, R.H.G.A. Combustion synthesized ZnO powders for varistor ceramics. Int. J. Inorg. Mater. 1999, 1, 235–241. [Google Scholar] [CrossRef]
- Kelleher, M.C. ZnO varistors—the ideal microstructure and characteristics, and methods investigated and developed to achieve these. In Encyclopedia of Materials: Electronics; Haseeb, A.S.M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 3, pp. 392–418. [Google Scholar] [CrossRef]
- Tan, D.Q. Superior performing nano-enabled metal oxide varistors. Int. J. Ceram. Eng. Sci. 2019, 1, 136–143. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, X.; Sun, J.; Ren, L.; Liao, R.; Yang, L.; Li, W. Enhanced electrical properties of ZnO varistor ceramics by spark plasma sintering: Role of annealing. Ceram. Int. 2020, 46, 15076–15083. [Google Scholar] [CrossRef]
- Perel, T.; Bley, V.; Malec, D.; Bourel, F.; Guillemet-Fritsch, S.; Estournes, C.; Malpiece, F.; Morel, J. Electrical properties of conventional and spark plasma sintering ZnO based varistors. In Proceedings of the 2011 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 16–19 October 2011; pp. 149–152. [Google Scholar] [CrossRef]
- Kharchouche, F.; Belkhiat, S. Effect of spark plasma sintering on microstructure and electrical properties of ZnO-based varistors. J. Mater. Sci. Mater. Electron. 2018, 29, 16238–16247. [Google Scholar] [CrossRef]
- Tokita, M. Progress of spark plasma sintering (SPS) method, systems, ceramics applications and industrialization. Ceramics 2021, 4, 160–198. [Google Scholar] [CrossRef]
- Kuo, C.-T.; Chen, C.-S.; Lin, I.-N. Microstructure and nonlinear properties of microwave-sintered ZnO-V2O5 varistors: II, effect of Mn3O4 doping. J. Am. Ceram. Soc. 2005, 81, 2949–2956. [Google Scholar] [CrossRef]
- Egorov, S.V.; Eremeev, A.G.; Kholoptsev, V.V.; Plotnikov, I.V.; Rybakov, K.I.; Sorokin, A.A.; Balabanov, S.S.; Rostokina, E.Y.e.; Bykov, Y.V. Rapid microwave sintering of zinc oxide-based varistor ceramics. J. Eur. Ceram. Soc. 2021, 41, 6508–6515. [Google Scholar] [CrossRef]
- Gunnewiek, R.F.K.; Kiminami, R.H.G.A. Two-step microwave sintering of nanostructured ZnO-based varistors. Ceram. Int. 2017, 43, 847–853. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide–from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: Material, physics and applications. ChemPhysChem 2007, 8, 782–803. [Google Scholar] [CrossRef]
- Amin, S.A. Structural and electrical properties of ZnO varistor with different particle size for initial oxides materials. Nanoscale Rep. 2019, 2, 20–31. [Google Scholar] [CrossRef]
- Boumezoued, A.; Guergouri, K.; Barille, R.; Rechem, D.; Zaabat, M.; Rasheed, M. ZnO nanopowders doped with bismuth oxide, from synthesis to electrical application. J. Alloys Compd. 2019, 791, 550–558. [Google Scholar] [CrossRef]
- Hamdelou, S.; Guergouri, K.; Arab, L. The effect of the starting powders particle size on the electrical properties of sintered Co doped ZnO varistors. Appl. Nanosci. 2015, 5, 817–825. [Google Scholar] [CrossRef]
- Yan, Y.; Ren, X.; Cheng, Q.; Ruan, X.; Wang, M.; Yu, W.; Yao, Z. Effects of sizes of additive particles on suspensions, microstructures, and electrical properties of ZnO varistors. J. Am. Ceram. Soc. 2020, 103, 3265–3272. [Google Scholar] [CrossRef]
- Boumezoued, A.; Guergouri, K.; Barille, R.; Rechem, D.; Mourad, Z. Synthesis and characterization of ZnO-based nano-powders: Study of the effect of sintering temperature on the performance of ZnO–Bi2O3 varistors. J. Mater. Sci. Mater. Electron. 2021, 32, 3125–3139. [Google Scholar] [CrossRef]
- Cheng, L.; Zheng, L.; Zeng, J.; Zhao, K.; Shi, X.; Ruan, X.; Li, G.; Yang, J.; Liu, Y. Further analysis of high-voltage ZnO varistor prepared from novel chemically aided method. J. Am. Ceram. Soc. 2017, 100, 1261–1264. [Google Scholar] [CrossRef]
- Wang, X.; Ren, X.; Li, Z.; You, W.; Jiang, H.; Yu, W.; Jin, L.; Yao, Z.; Shi, L. A unique tuning effect of Mg on grain boundaries and grains of ZnO varistor ceramics. J. Eur. Ceram. Soc. 2021, 41, 2633–2640. [Google Scholar] [CrossRef]
- Szwagierczak, D.; Kulawik, J.; Skwarek, A. Influence of processing on microstructure and electrical characteristics of multilayer varistors. J. Adv. Ceram. 2019, 8, 408–417. [Google Scholar] [CrossRef]
- Dugar, J.; Ikram, A.; Pušavec, F. Evaluation of chip formation mechanisms in the turning of sintered ZnO electro-ceramics. Processes 2021, 9, 1422. [Google Scholar] [CrossRef]
- Hassazadeh, M.; Puyane, R.; Malpiece, F. Ecological design up-grade of a zinc oxide varistor suitable for distribution polymeric surge arresters. In Proceedings of the 18th International Conference and Exhibition on Electricity Distribution (CIRED 2005), Turin, Italy, 6–9 June 2005; Volume 2005, p. v1-50. [Google Scholar] [CrossRef]
- Bernik, S. Low-temperature sintering of ZnO-Bi2O3-based varistor ceramics for enhanced microstructure development and current-voltage characteristics. Ceram. Silik. 2017, 62, 8–14. [Google Scholar] [CrossRef]
- Arefin, M.L.; Raether, F.; Dolejš, D.; Klimera, A. Phase formation during liquid phase sintering of ZnO ceramics. Ceram. Int. 2009, 35, 3313–3320. [Google Scholar] [CrossRef]
- Kaufmann, B.; Billovits, T.; Supancic, P. Observation of an electrical breakdown at ZnO Schottky contacts in varistors. J. Eur. Ceram. Soc. 2021, 41, 1969–1974. [Google Scholar] [CrossRef]
- Sendi, R.K. Structural, nonlinear electrical characteristics, and stability against DC-accelerated aging stress behavior of Er-doped 20 nm ZnO–Bi2O3–Mn2O3-based varistors. Microsc. Res. Tech. 2021, 84, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Wang, Z.; Wu, K. Evolution of intrinsic and extrinsic electron traps at grain boundary during sintering ZnO based varistor ceramics. Materials 2022, 15, 1098. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kimura, T.; Yamaguchi, T. Effect of bismuth of oxide content on the sintering of zinc oxide. J Am. Ceram. Soc. 1989, 72, 1541–1544. [Google Scholar] [CrossRef]
- Xu, D.; Cheng, X.; Yan, X.; Xu, H.; Shi, L. Sintering process as relevant parameter for Bi2O3 vaporization from ZnO-Bi2O3-based varistor ceramics. Trans. Nonferrous Met. Soc. China 2009, 19, 1526–1532. [Google Scholar] [CrossRef]
- Izoulet, A.; Guillemet-Fritsch, S.; Estournès, C.; Morel, J. Microstructure control to reduce leakage current of medium and high voltage ceramic varistors based on doped ZnO. J. Eur. Ceram. Soc. 2014, 34, 3707–3714. [Google Scholar] [CrossRef]
- Choi, S.-K.; Jung, H.-H.; Kang, S.-M. The correlation between surge energy capability and Bi2O3 volatilization in ZnO varistors. J. Ceram. Soc. Japan 2018, 126, 236–240. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, C.; Li, J.; Zheng, D.; Pang, C.; Fei, Z. Effect of Zn7Sb2O12 pre-synthesis on microstructure and properties of ZnO varistor ceramics. AIP Adv. 2022, 12, 065214. [Google Scholar] [CrossRef]
- Chen, T.; Zu, H.; Gao, C.; Ke, C.; Zhou, D.; Hu, Y.; Zheng, Z.; Fu, Q. Preparation and electrical properties of ZnO-Bi2O3-based multilayer varistors with base metal nickel inner electrodes. J. Am. Ceram. Soc. 2017, 100, 3024–3032. [Google Scholar] [CrossRef]
- Nahm, C.-W. Effect of Bi2O3 doping on microstructure and electrical properties of ZnO–V2O5–Mn3O4 semiconducting ceramics. J. Mater. Sci. Mater. Electr. 2017, 28, 903–908. [Google Scholar] [CrossRef]
- Pandey, S.; Kumar, D.; Parkash, O. Investigation of the electrical properties of liquid-phase sintered ZnO–V2O5 based varistor ceramics using impedance and dielectric spectroscopy. J. Mater. Sci. Mater. Electron. 2016, 27, 3748–3758. [Google Scholar] [CrossRef]
- Kurzawa, M.; Rychlowska-Himmel, I.; Bosacka, M.; Blonska-Tabero, A. Reinvestigation of phase equilibria in the V2O5 system. J. Therm. Anal. Calorim. 2001, 64, 1113–1119. [Google Scholar] [CrossRef]
- Wu, J.; Qi, T.; Li, T.T.; Qin, Q.W.; Li, G.Q.; Zhu, B.L.; Xiang, Z.D.; Xie, C.S. The AC impedance and variable temperature dielectric spectroscopic analysis of MnO2 doped and un-doped ZnO–V2O5 ceramics. J. Mater. Sci. Mater. Electron. 2012, 23, 1143–1150. [Google Scholar] [CrossRef]
- Qu, X.; Sun, W.-J.; Wang, M.-H.; Chen, Y.; Zhang, H.-P. Microstructures and electrical properties of ZnO–V2O5–MnO2 varistors with low-temperature sintering. J. Mater. Sci. Mater. Elect. 2017, 28, 1909–1913. [Google Scholar] [CrossRef]
- von Wenckstern, H.; Schmidt, H.; Brandt, M.; Lajn, A.; Pickenhain, R.; Lorenz, M.; Grundmann, M.; Hofmann, D.M.; Polity, A.; Meyer, B.K.; et al. Anionic and cationic substitution in ZnO. Prog. Solid State Chem. 2009, 37, 153–172. [Google Scholar] [CrossRef]
- Ayoub, I.; Kumar, V.; Abolhassani, R.; Sehgal, R.; Sharma, V.; Sehgal, R.; Swart, H.C.; Mishra, Y.K. Advances in ZnO: Manipulation of defects for enhancing their technological potentials. Nanotechnol. Rev. 2022, 11, 575–619. [Google Scholar] [CrossRef]
- Klinkova, L.A.; Nikolaichik, V.I.; Barkovskii, N.V.; Fedotov, V.K. Thermal stability of Bi2O3. Russ. J. Inorg. Chem. 2007, 52, 1822–1829. [Google Scholar] [CrossRef]
- Mielcarek, W.; Gubański, A.; Paściak, G.; Prociów, K.; Warycha, J.; Wrobel, J.M. The effect of bismuth oxide polymorph forms on degradation processes in ZnO varistors. Ceram. Int. 2013, 39, 8219–8226. [Google Scholar] [CrossRef]
- Cao, W.; Guo, Y.; Su, J.; Liu, J. Effect of sintering temperature on the microstructural evolution of ZnO varistors. J. Electron. Mater. 2023, 52, 1266–1273. [Google Scholar] [CrossRef]
- Parija, A.; Waetzig, G.R.; Andrews, J.L.; Banerjee, S. Traversing energy landscapes away from equilibrium: Strategies for accessing and utilizing metastable phase space. J. Phys. Chem. C 2018, 122, 25709–25728. [Google Scholar] [CrossRef]
- Tadeu Cestarolli, D.; Maria Guerra, E. Vanadium pentoxide (V2O5): Their obtaining methods and wide applications. In Transition Metal Compounds—Synthesis, Properties, and Application; Haider, S., Haider, A., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Shvets, P.; Dikaya, O.; Maksimova, K.; Goikhman, A. A review of Raman spectroscopy of vanadium oxides. J. Raman Spectrosc. 2019, 50, 1226–1244. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Gurylev, V.; Perng, T.P. Defect engineering of ZnO: Review on oxygen and zinc vacancies. J. Eur. Ceram. Soc. 2021, 41, 4977–4996. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- McCluskey, M.D.; Jokela, S.J. Defects in ZnO. J. Appl. Phys. 2009, 106, 071101. [Google Scholar] [CrossRef]
- Md, T. Doped zinc oxide nanostructures for photovoltaic solar cells application. In Zinc Oxide Based Nano Materials and Devices; Nahhas, A.M., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wei, S.-H.; Zunger, A. Intrinsic n-type versus p-type doping asymmetry and the defect physics of ZnO. Phys. Rev. B 2001, 63, 075205. [Google Scholar] [CrossRef]
- Yüksel, P.; Hardal, G.; Kınacı, B. Influence of V2O5 and B2O3 addition on the sintering behaviour and physical properties of ZnO ceramics. Process. Appl. Ceram. 2022, 16, 48–54. [Google Scholar] [CrossRef]
- Nahm, C.-W. Effect of Mn doping on electrical properties and accelerated ageing behaviours of ternary ZVM varistors. Bull. Mater. Sci. 2011, 34, 1385–1391. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Beynet, Y.; Izoulet, A.; Guillemet-Fritsch, S.; Chevallier, G.; Bley, V.; Pérel, T.; Malpiece, F.; Morel, J.; Estournès, C. ZnO-based varistors prepared by spark plasma sintering. J. Eur. Ceram. Soc. 2015, 35, 1199–1208. [Google Scholar] [CrossRef]
- Ahmed, Z.W.; Khadim, A.I.; ALsarraf, A.H.R. The effect of doping with some rare earth oxides on electrical features of ZnO varistor. Energy Procedia 2019, 157, 909–917. [Google Scholar] [CrossRef]
- Rečnik, A.; Daneu, N.; Bernik, S. Nucleation and growth of basal-plane inversion boundaries in ZnO. J. Eur. Ceram. Soc. 2007, 27, 1999–2008. [Google Scholar] [CrossRef]
- Vahidi, H.; Syed, K.; Guo, H.; Wang, X.; Wardini, J.L.; Martinez, J.; Bowman, W.J. A review of grain boundary and heterointerface characterization in polycrystalline oxides by (scanning) transmission electron microscopy. Crystals 2021, 11, 878. [Google Scholar] [CrossRef]
- Rečnik, A.; Daneu, N.; Walther, T.; Mader, W. Structure and chemistry of basal-plane inversion boundaries in antimony oxide-doped zinc oxide. J. Am. Ceram. Soc. 2001, 84, 2657–2668. [Google Scholar] [CrossRef]
- Scheiber, D.; Popov, M.N.; Supancic, P.; Spitaler, J. Understanding and controlling inversion boundaries in ZnO. Acta Mater. 2022, 229, 117804. [Google Scholar] [CrossRef]
- Ribić, V.; Rečnik, A.; Komelj, M.; Kokalj, A.; Branković, Z.; Zlatović, M.; Branković, G. New inversion boundary structure in Sb-doped ZnO predicted by DFT calculations and confirmed by experimental HRTEM. Acta Mater. 2020, 199, 633–648. [Google Scholar] [CrossRef]
- Ribic, V.; Recnik, A.; Drazic, G.; Podlogar, M.; Brankovic, Z.; Brankovic, G. TEM and DFT study of basal-plane inversion boundaries in SnO2-doped ZnO. Sci. Sintering 2021, 53, 237–252. [Google Scholar] [CrossRef]
- Lee, J.-S.; Maier, J. High barrier effects of (0001−)|(0001−) zinc oxide bicrystals: Implication for varistor ceramics with inversion boundaries. J. Mater. Res. 2005, 20, 2101–2109. [Google Scholar] [CrossRef]
- Guo, R.; Fang, L.; Zhou, H.; Chen, X.; Chu, D.; Chan, B.; Qin, Y. SbVO4 doped ZnO–V2O5-based varistor ceramics: Microstructure, electrical properties and conductive mechanism. J. Mater. Sci. Mater. Electron. 2013, 24, 2721–2726. [Google Scholar] [CrossRef]
- Li, W.-B.; Easterling, K.E. The influence of particle shape on Zener drag. Acta Metall. Mater. 1990, 38, 1045–1052. [Google Scholar] [CrossRef]
- Levinson, L.M.; Philipp, H.R. The physics of metal oxide varistors. J. Appl. Phys. 1975, 46, 1332–1341. [Google Scholar] [CrossRef]
- Gupta, T.K.; Carlson, W.G. A grain-boundary defect model for instability/stability of a ZnO varistor. J. Mater. Sci. 1985, 20, 3487–3500. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, Y.; Zhang, X.; Xu, Y.; Liu, P.; Zeng, Y.; Wang, Q.; Zhang, G. Effect of Zn doping on stability of ZnO varistors under high pulse-current stress. Ceram. Int. 2015, 41, 11611–11617. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.-H.; Li, X.; Song, H.-H.; Sun, T.-T. Sintering mechanism and properties of ZnVMnNbO varistor ceramic and their evolutions by Ce–La doping. Ceram. Int. 2020, 46, 20923–20931. [Google Scholar] [CrossRef]
- Nahm, C.-W. Effect of sintering temperature on nonlinearity and surge degradation characteristics of Mn3O4/Nb2O5/Er2O3-doped ZnO–V2O5-based varistors. J. Korean Ceram. Soc. 2020, 57, 65–72. [Google Scholar] [CrossRef]
- Nahm, C.-W. Varistor characteristics of ZnO/V2O5/MnO2/Nb2O5 semiconducting ceramics with Tb4O7 addition. J. Mater. Sci. Mater. Electron. 2015, 26, 4144–4151. [Google Scholar] [CrossRef]
- Nahm, C.-W. Electrical and dielectric characteristics of erbium-added ZnO–V2O5-based varistor ceramics. Ceram. Int. 2012, 38, 6651–6658. [Google Scholar] [CrossRef]
- Begum, S.; Karim, A.N.M.; Hashmi, M.S.J. Characterization and performance evaluation of zinc oxide varistor blocks processed by latex binders. J. Am. Ceram. Soc. 2008, 91, 3216–3221. [Google Scholar] [CrossRef]
- Begum, S.; Karim, A.N.M.; Fawzia, S.; Hashmi, M.S.J. Industrial fabrication of ZnO varistor: Leveraging the powder processing parameters. In Encyclopedia of Materials: Electronics; Haseeb, A.S.M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 3, pp. 580–594. [Google Scholar] [CrossRef]
- Lao, X.; Ren, X.; Yan, Y.; Jiang, H.; Zhong, M.; Kong, A.; Yao, Z.; Shi, L. Effects of dispersant on electrical properties of zinc-oxide varistors and methods to improve protection performance. J. Electron. Mater. 2022, 51, 5169–5180. [Google Scholar] [CrossRef]
- Kelleher, M.C.; Hashmi, M.S.J. The effect of vibratory milling on the powder properties of zinc oxide varistors. J. Mater. Process. Technol. 2008, 201, 645–650. [Google Scholar] [CrossRef]
- Kelleher, M.C.; Hashmi, M.S.J. Effect on the processing characteristics of ZnO varistors produced using vibratory milling. AIP Conf. Proc. 2011, 1315, 253–258. [Google Scholar] [CrossRef]
- Roy, S.; Das, D.; Roy, T.K. Influence of sintering temperature on microstructure and electrical properties of Er2O3 added ZnO-V2O5-MnO2-Nb2O5 varistor ceramics. J. Alloys Compd. 2018, 749, 687–696. [Google Scholar] [CrossRef]
- Tsai, J.-K.; Wu, T.-B. Microstructure and nonohmic properties of binary ZnO-V2O5 ceramics sintered at 900 °C. Mater. Lett. 1996, 26, 199–203. [Google Scholar] [CrossRef]
- Huda, M.D.; Puyane, R.; El Baradie, M.A.; Hashmi, M.S.J. Failure modelling for ZnO varistors. J. Mater. Process. Technol. 1998, 77, 273–277. [Google Scholar] [CrossRef]
- Nahm, C.-W. Influence of Bi2O3 doping on microstructure and electrical properties of ZnO-V2O5-MnO2-Nb2O5 varistor ceramics. J. Am. Ceram. Soc. 2012, 95, 2093–2095. [Google Scholar] [CrossRef]
- Nahm, C.W. Effect of small changes in sintering temperature on varistor properties and degradation behavior of V–Mn–Nb–Gd co-doped zinc oxide ceramics. Trans. Nonferrous Met. Soc. China 2015, 25, 1176–1184. [Google Scholar] [CrossRef]
- Roy, S.; Das, D.; Roy, T.K. Nonlinear electrical properties of ZnO-V2O5 based rare earth (Er2O3) added varistors. J. Electron. Mater. 2019, 48, 5650–5661. [Google Scholar] [CrossRef]
- Roy, S.; Kundu Roy, T.; Das, D. Sintering of nanocrystalline multicomponent zinc oxide varistor powders prepared by ball milling. Mater. Today: Proc. 2018, 5, 9899–9909. [Google Scholar] [CrossRef]
- Phapale, S.; Dawar, R.; Pathak, P.; Achary, S.N.; Mishra, R. Thermodynamic investigations on compounds of ZnO-V2O5 system. Mater. Today Commun. 2021, 29, 102763. [Google Scholar] [CrossRef]
- Hng, H.H.; Tse, K.Y. Grain growth of ZnO in binary ZnO-V2O5 ceramics. J. Mater. Sci. 2003, 38, 2367–2372. [Google Scholar] [CrossRef]
- Ming, Z.; Yu, S.; Sheng, T.C. Grain growth of ZnO–V2O5 based varistor ceramics with different antimony dopants. J. Eur. Ceram. Soc. 2011, 31, 2331–2337. [Google Scholar] [CrossRef]
- Nahm, C.-W. Sintering effect on electrical properties and pulse aging behavior of (V2O5-Mn3O4-Er2O3)-doped zinc oxide varistor ceramics. J. Rare Earths 2014, 32, 29–36. [Google Scholar] [CrossRef]
- Nahm, C.-W. Effect of sintering process on electrical properties and ageing behavior of ZnO–V2O5–MnO2–Nb2O5 varistor ceramics. J. Mater. Sci. Mater. Electron. 2012, 23, 457–463. [Google Scholar] [CrossRef]
- Nahm, C.-W. Microstructure and varistor properties of ZVMND ceramics with sintering temperature. Trans. Electr. Electron. Mater. 2015, 16, 221–225. [Google Scholar] [CrossRef]
- Nahm, C.-W. Effects of low-temperature sintering on varistor properties and stability of VMCDNB-doped zinc oxide ceramics. J. Korean Ceram. Soc. 2019, 56, 84–90. [Google Scholar] [CrossRef]
- Nahm, C.-W. Effect of low-temperature sintering on electrical properties and aging behavior of ZVMNBCD varistor ceramics. Korean. J. Mater. Res. 2020, 30, 502–508. [Google Scholar] [CrossRef]
- Nahm, C.-W. Microstructure, electrical and dielectric properties, and impulse clamping characteristics of ZnO–V2O5–Mn3O4 semiconducting ceramics modified with Er2O3. J. Mater. Sci. Mater. Electron. 2013, 24, 4129–4136. [Google Scholar] [CrossRef]
- Rizwan, Z.; Zakaria, A.; Ghazali, M.S.M. Photopyroelectric spectroscopic studies of ZnO-MnO2-Co3O4-V2O5 ceramics. Int. J. Mol. Sci. 2011, 12, 1625–1632. [Google Scholar] [CrossRef]
- Gaucher, P.; Perrier, R.L.; Ganne, J.P. Prebreakdown in low voltage varistors and its relation with deep levels. Rev. Phys. Appl. 1990, 25, 823–830. [Google Scholar] [CrossRef]
- Meng, P.; Wu, J.; Yang, X.; Hu, J.; He, J. Electrical properties of ZnO varistor ceramics modified by rare earth-yttrium and gallium dopants. Mater. Lett. 2018, 233, 20–23. [Google Scholar] [CrossRef]
- Khafagy, A.-M.H.; El-Rabaie, S.M.; Dawoud, M.T.; Attia, M.T. Microhardness, microstructure and electrical properties of ZVM ceramics. J. Adv. Ceram. 2014, 3, 287–296. [Google Scholar] [CrossRef]
- Mon, T.L.; Ismail, N.Q.A.; Sa’at, N.K.; Azis, R.S.; Zaid, M.H.M. Influence of Co doping on structural and band gap energy properties of ZnO based varistor ceramic. J. Penelit. Pendidik. IPA 2022, 8, 1644–1650. [Google Scholar] [CrossRef]
- Ganesh, K.S. A Review of zinc oxide varistors for surge arrester. In Proceedings of the 2018 4th International Conference on Electrical Energy Systems (ICEES), Chennai, India, 7–9 February 2018; pp. 470–474. [Google Scholar] [CrossRef]
- Christodoulou, C.A.; Avgerinos, M.V.; Ekonomou, L.; Gonos, I.F.; Stathopulos, I.A. Measurement of the resistive leakage current in surge arresters under artificial rain test and impulse voltage subjection. IET Sci. Meas. Technol. 2009, 3, 256–262. [Google Scholar] [CrossRef]
- Yang, P.; Gomez, C.A.; Andrews, S.; Sorenson, J.D.; Chen, K.S. High-voltage surge protection by a varistor-filled air gap. J. Am. Ceram. Soc. 2021, 104, 3247–3259. [Google Scholar] [CrossRef]
- Ranjbar, B.; Darvishi, A.; Dashti, R.; Shaker, H.R. A survey of diagnostic and condition monitoring of metal oxide surge arrester in the power distribution network. Energies 2022, 15, 8091. [Google Scholar] [CrossRef]
- Lengauer, M.; Rubeša, D.; Danzer, R. Finite element modelling of the electrical impulse induced fracture of a high voltage varistor. J. Eur. Ceram. Soc. 2000, 20, 1017–1021. [Google Scholar] [CrossRef]
- Channe, A.; Mohale, V. Surge protective device failure modes, impact and corrective actions. Int. J. Eng. Res. Technol. 2020, 9, IJERTV9IS060963. [Google Scholar] [CrossRef]
- Rojo Ceballos, C.; Chejne, F.; Pérez, E.; Osorio, A.; Correa, A. Study of the behavior of low voltage ZnO varistors against very fast transient overvoltages (VFTO). Electr. Power Syst. Res. 2023, 214, 108937. [Google Scholar] [CrossRef]
- Lončar, B.; Vujisić, M.; Stanković, K.; Osmokrović, P. Stability of metal-oxide varistor characteristics in exploitation conditions. Acta Phys. Pol. A 2009, 116, 1081–1084. [Google Scholar] [CrossRef]
- Živanović, E.; Živković, M.; Veljković, S. Study of breakdown voltage stability of gas-filled surge arresters in the presence of gamma radiation. Electronics 2022, 11, 2447. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Cheng, P.; Alim, M.A. Advances in ZnO–Bi2O3 based varistors. J. Mater. Sci. Mater. Electron. 2015, 26, 4782–4809. [Google Scholar] [CrossRef]
- Banerjee, A.; Ramamohan, T.R.; Patni, M.J. Smart technique for fabrication of zinc oxide varistor. Mater. Res. Bull. 2001, 36, 1259–1267. [Google Scholar] [CrossRef]
- Lin, J.; Li, S.; He, J.; Zhang, L.; Liu, W.; Li, J. Zinc interstitial as a universal microscopic origin for the electrical degradation of ZnO-based varistors under the combined DC and temperature condition. J. Eur. Ceram. Soc. 2017, 37, 3535–3540. [Google Scholar] [CrossRef]
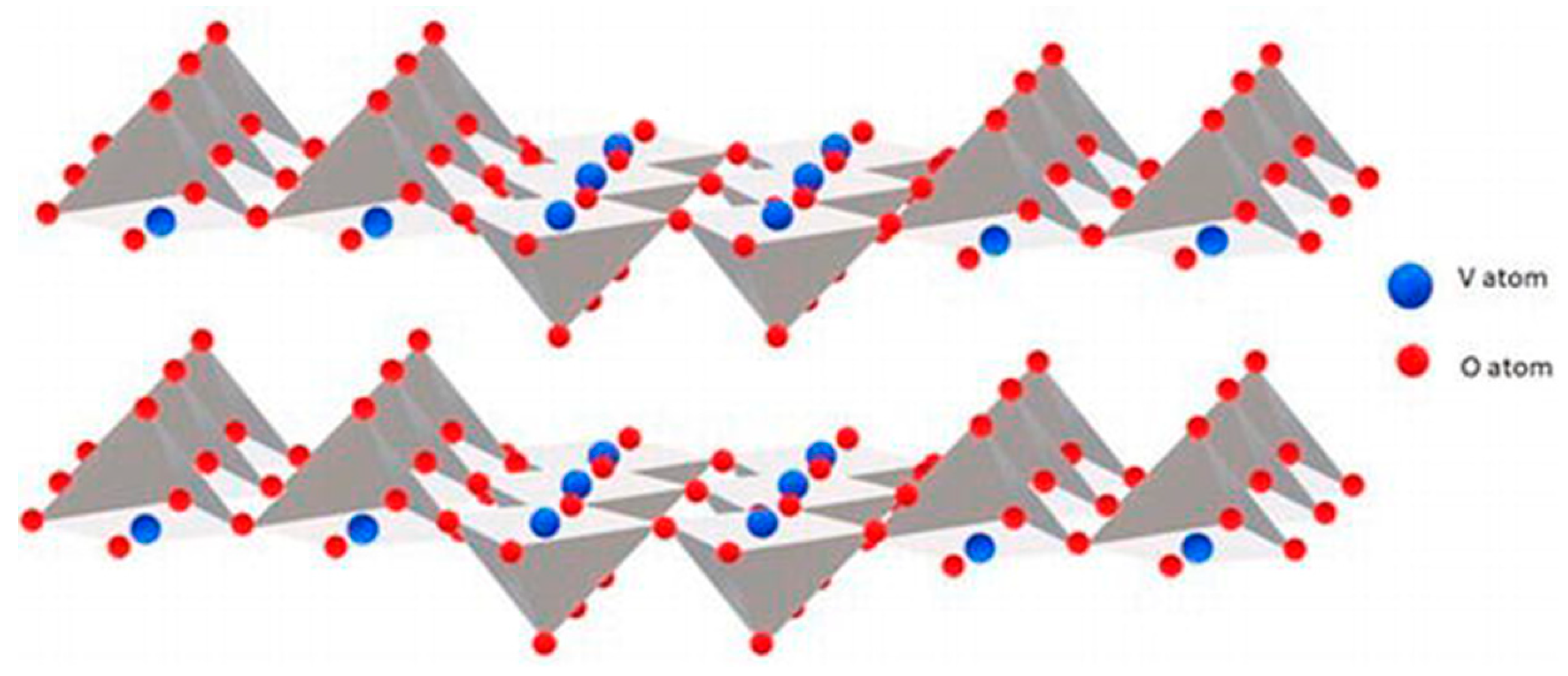
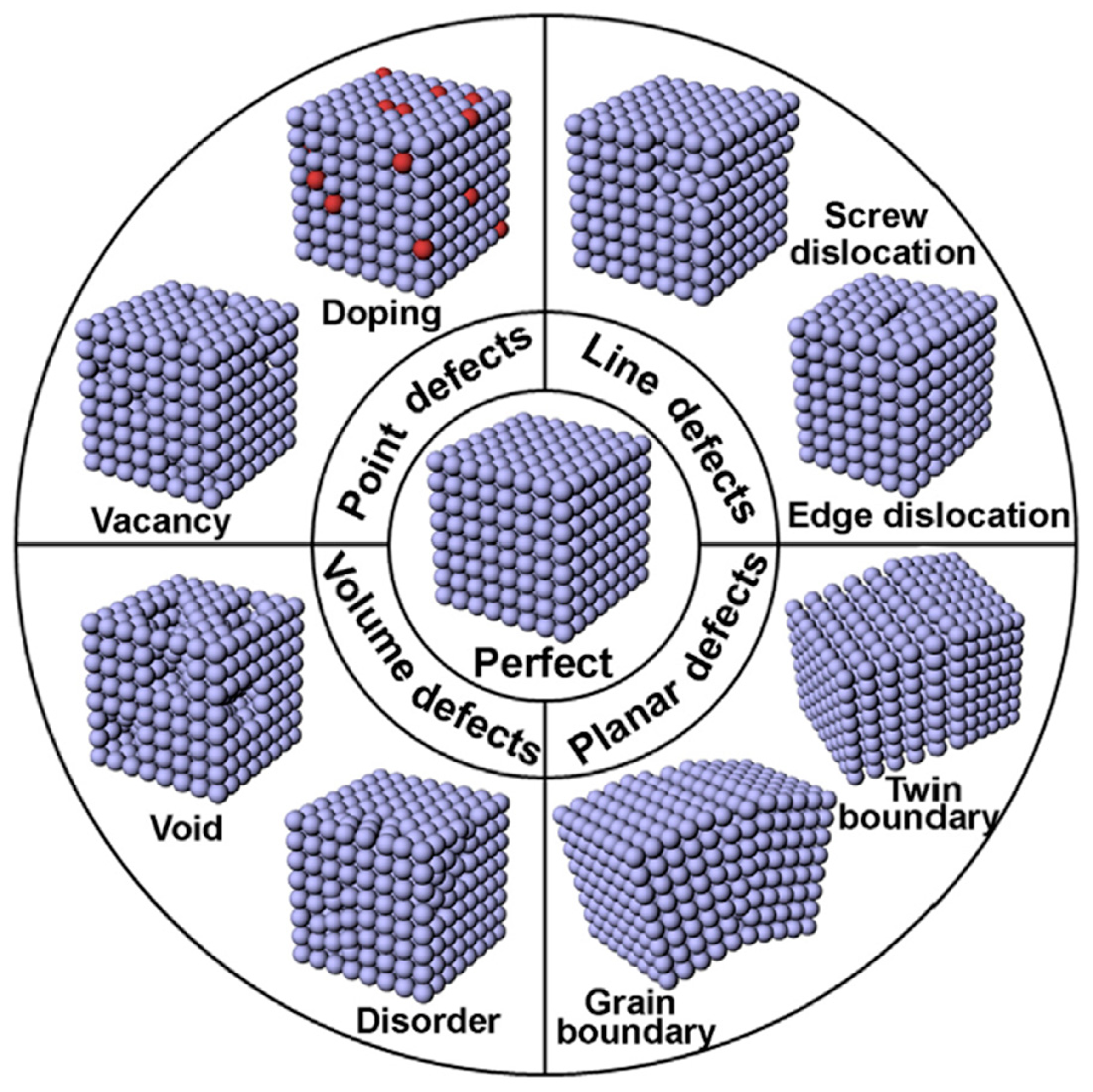

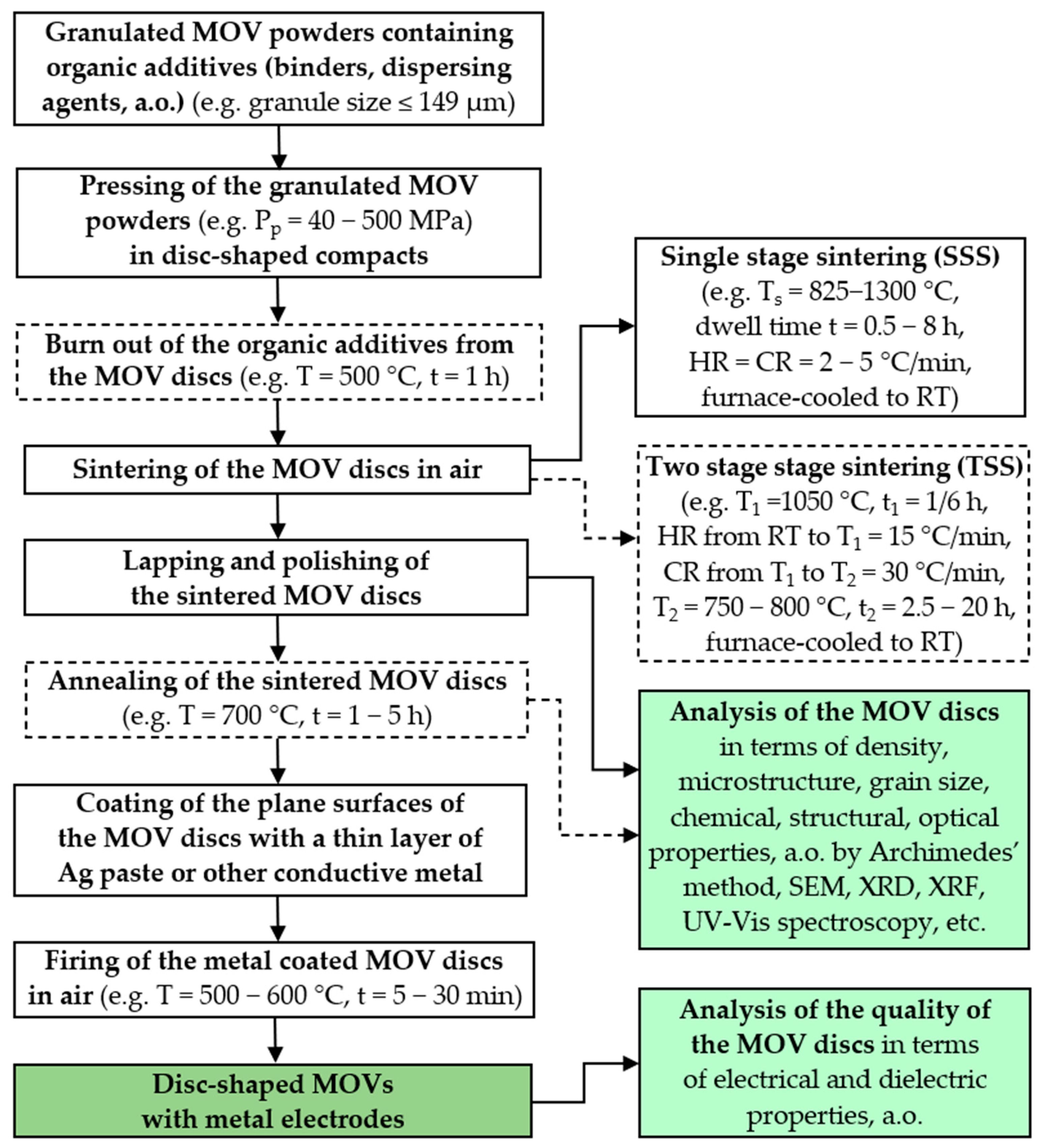
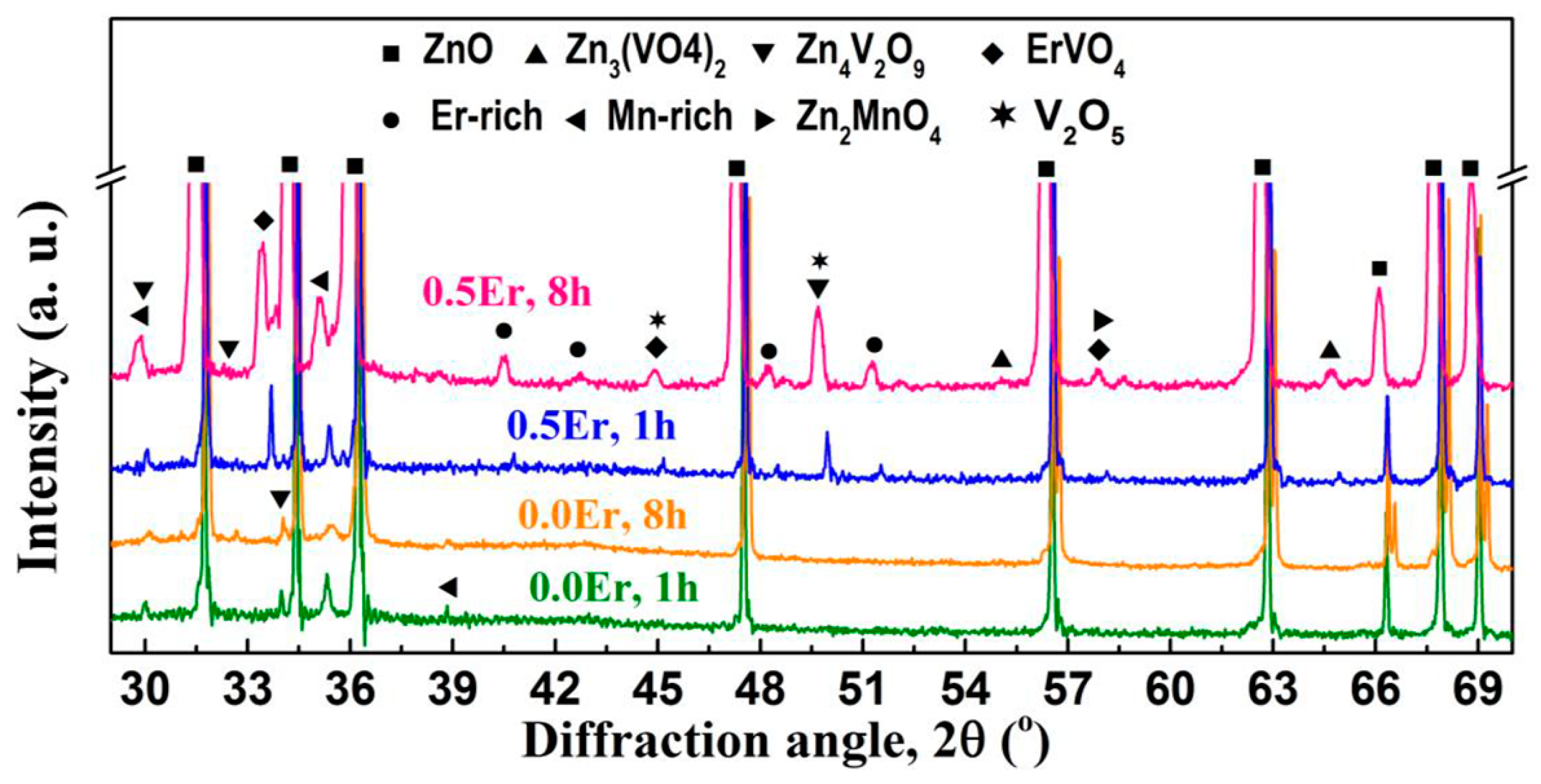

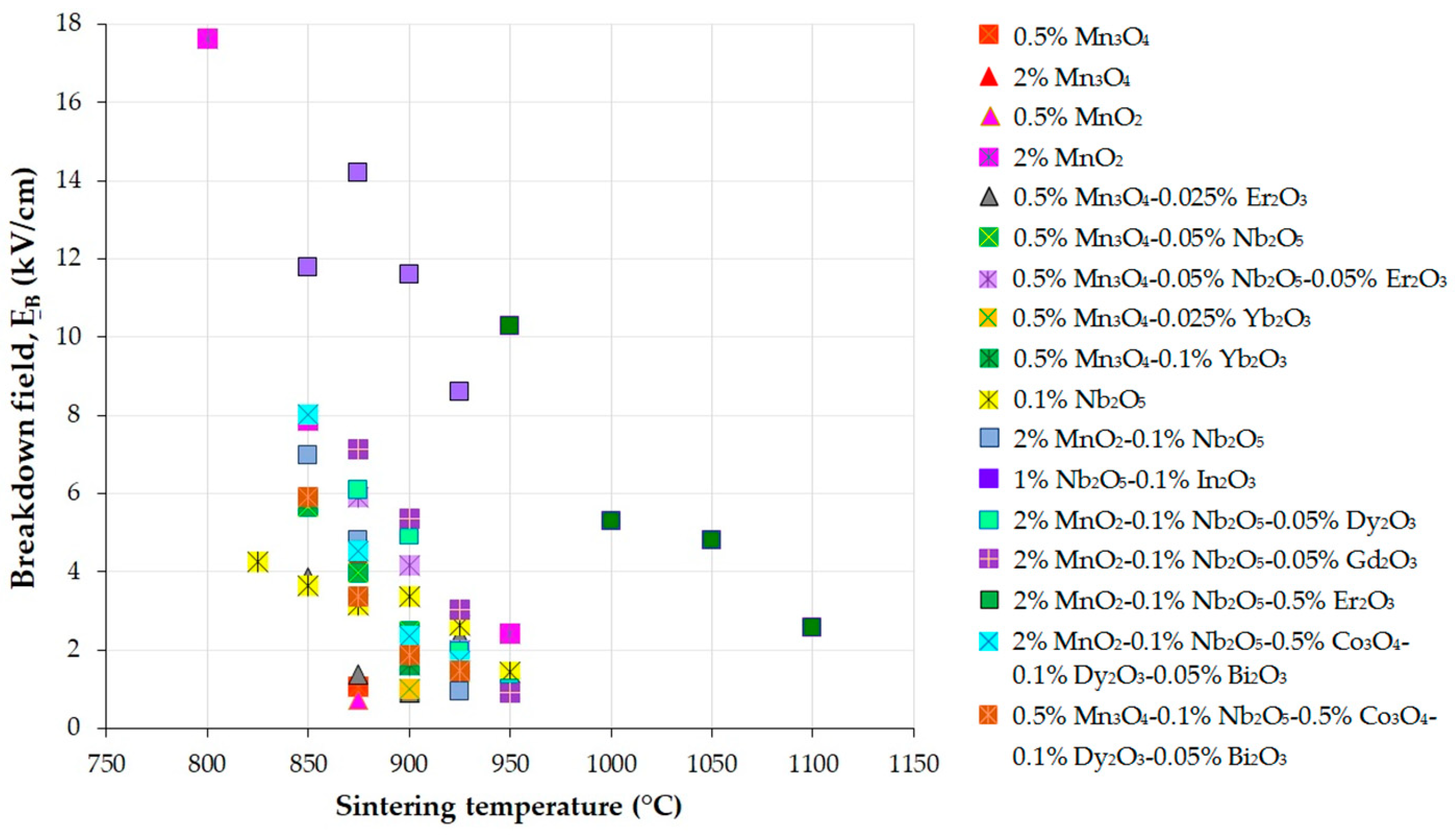
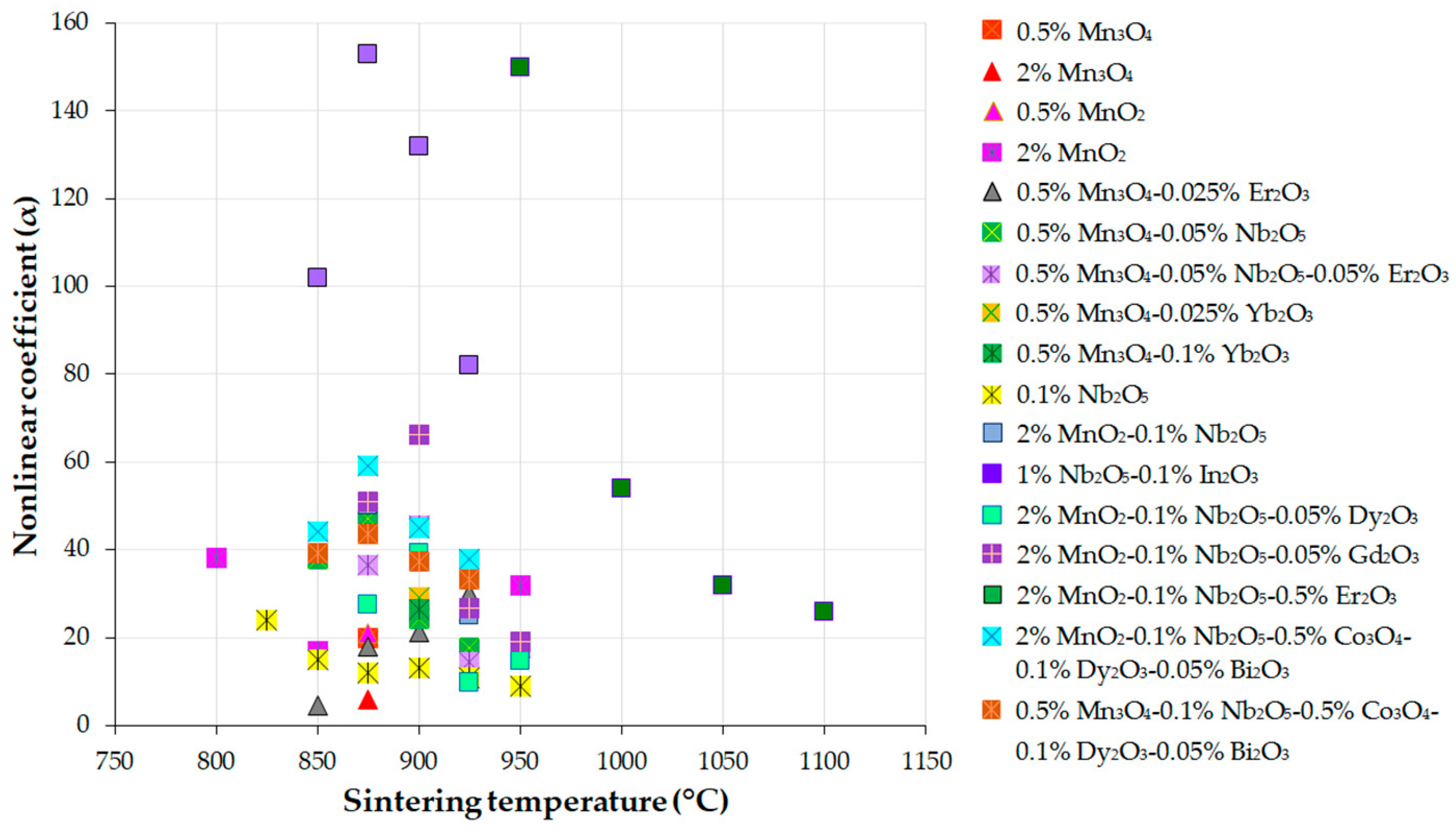
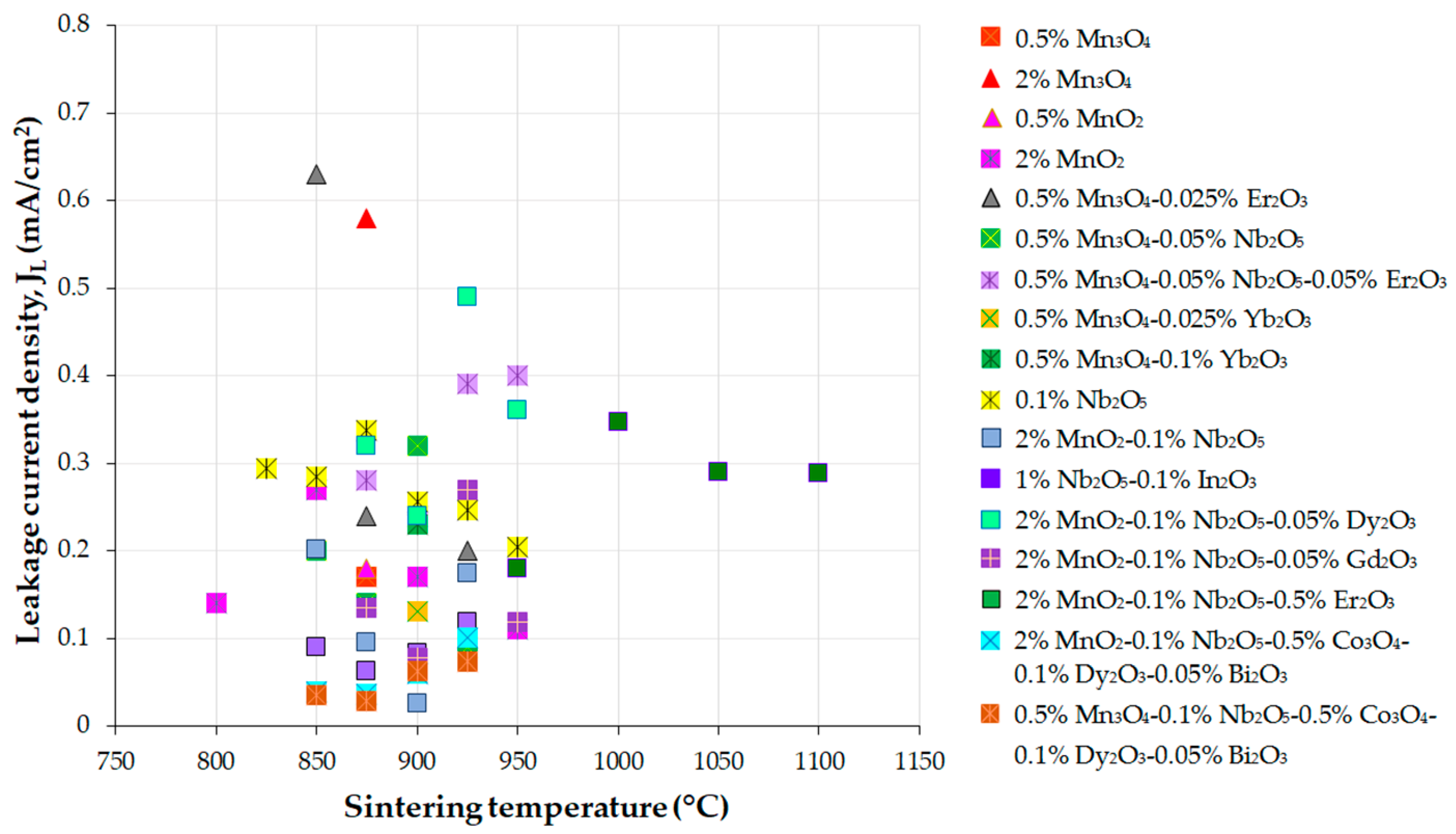
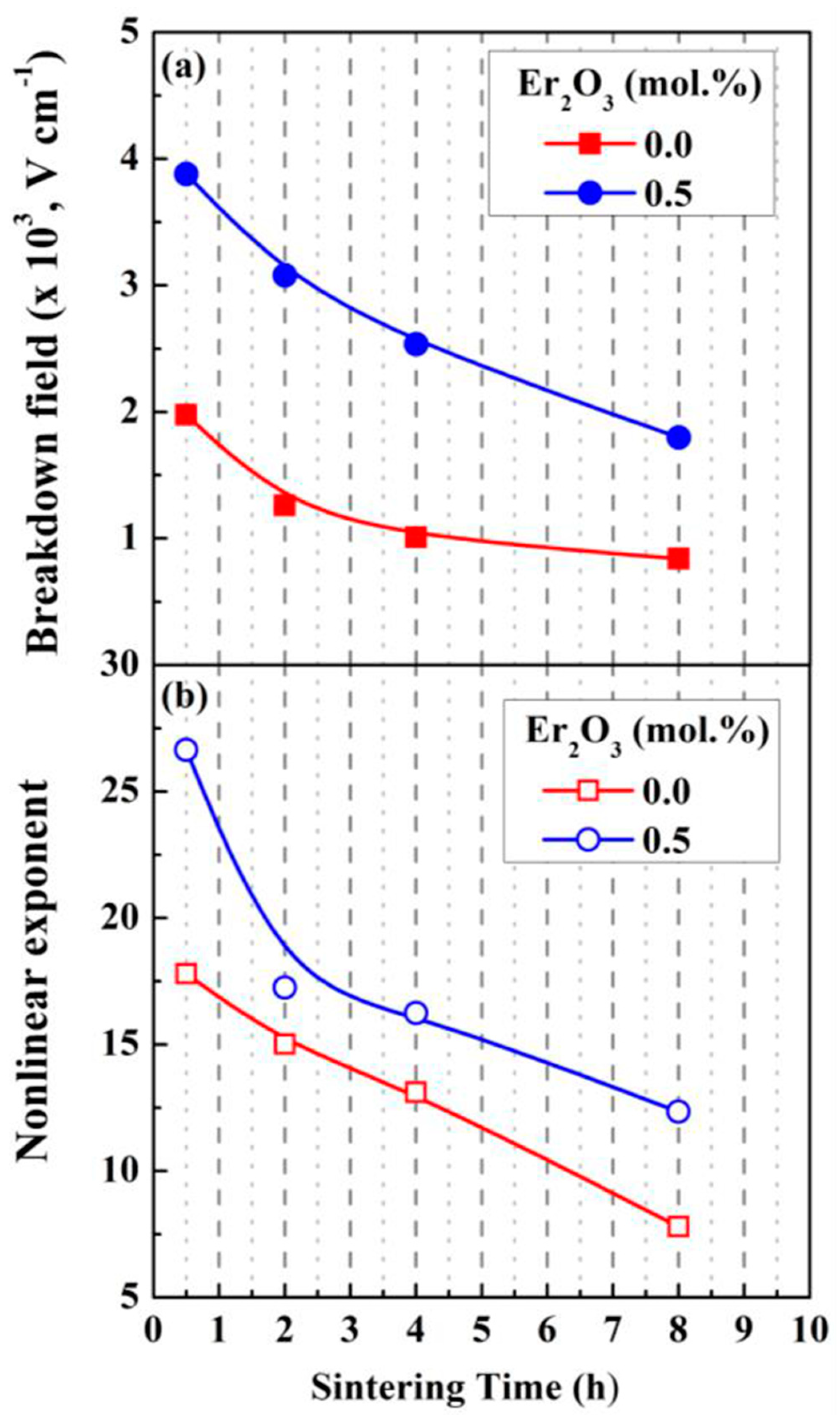
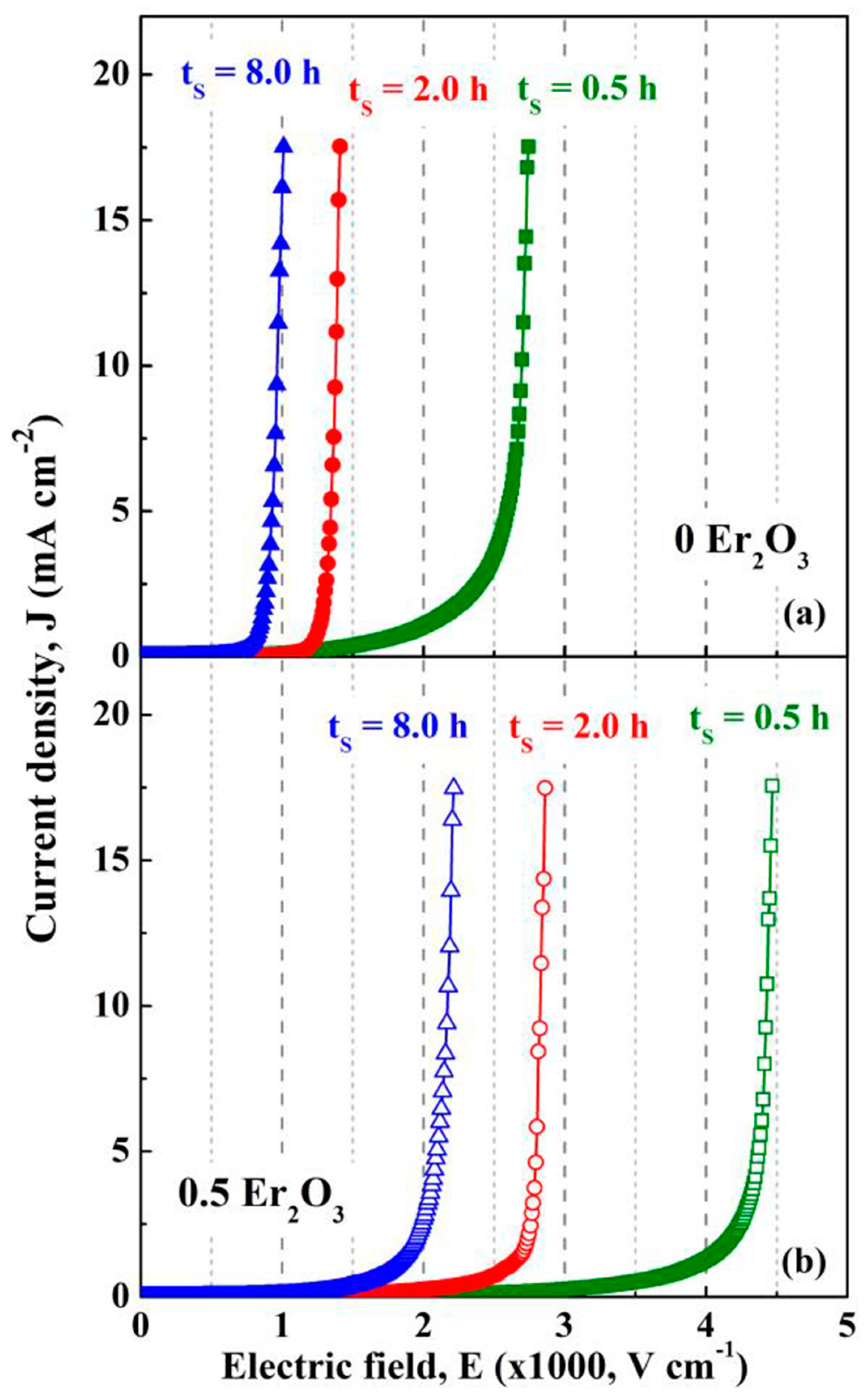
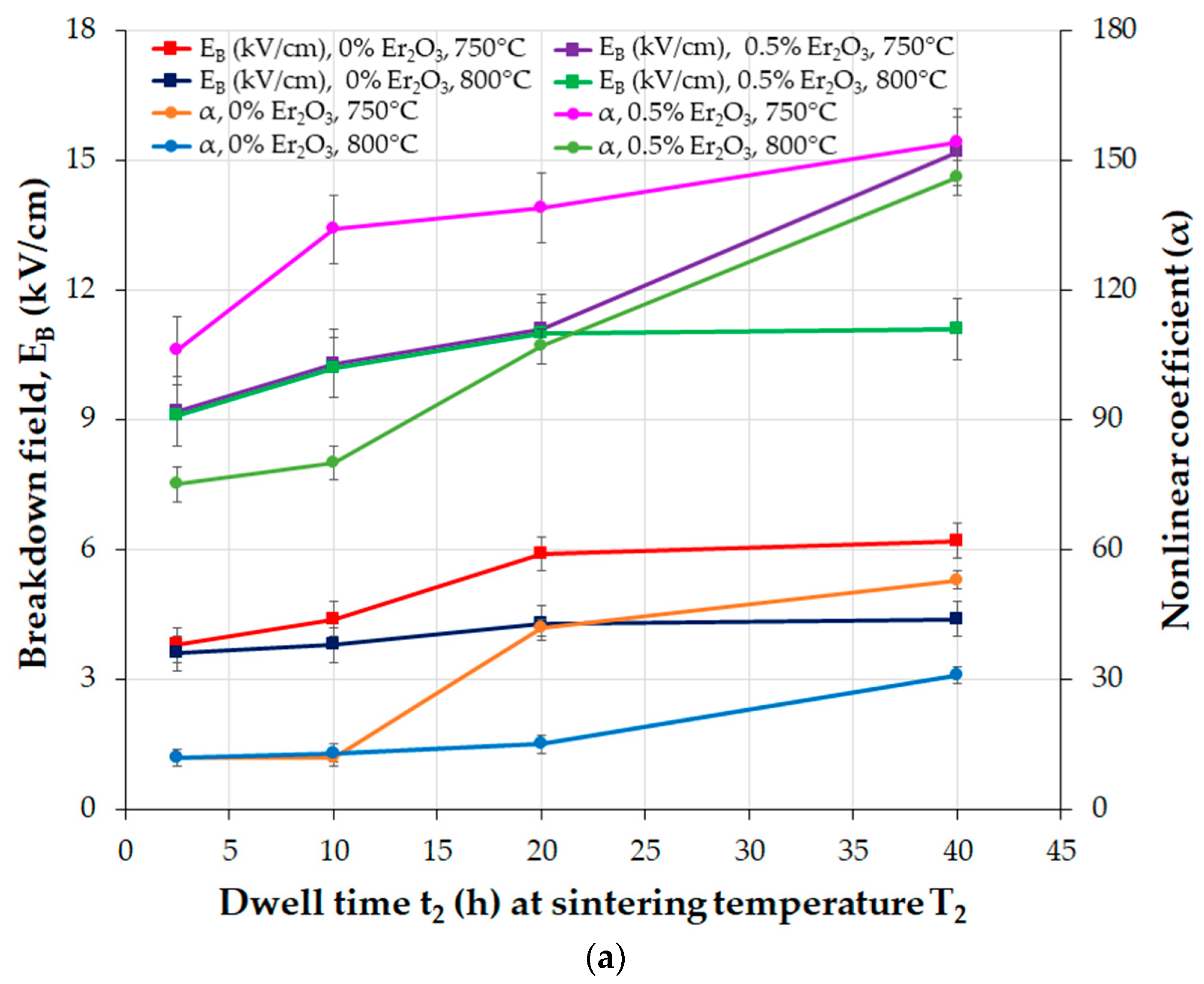
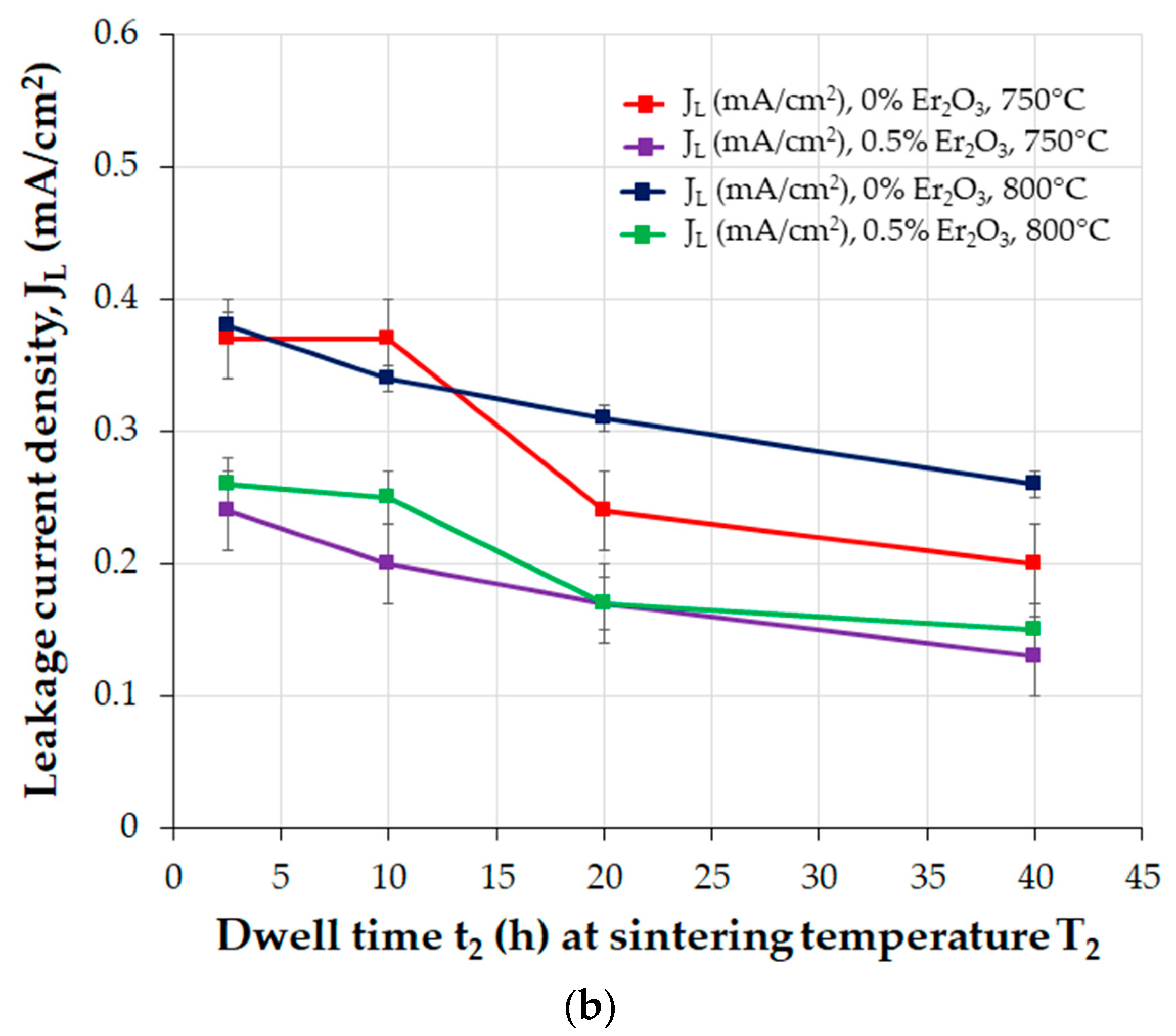
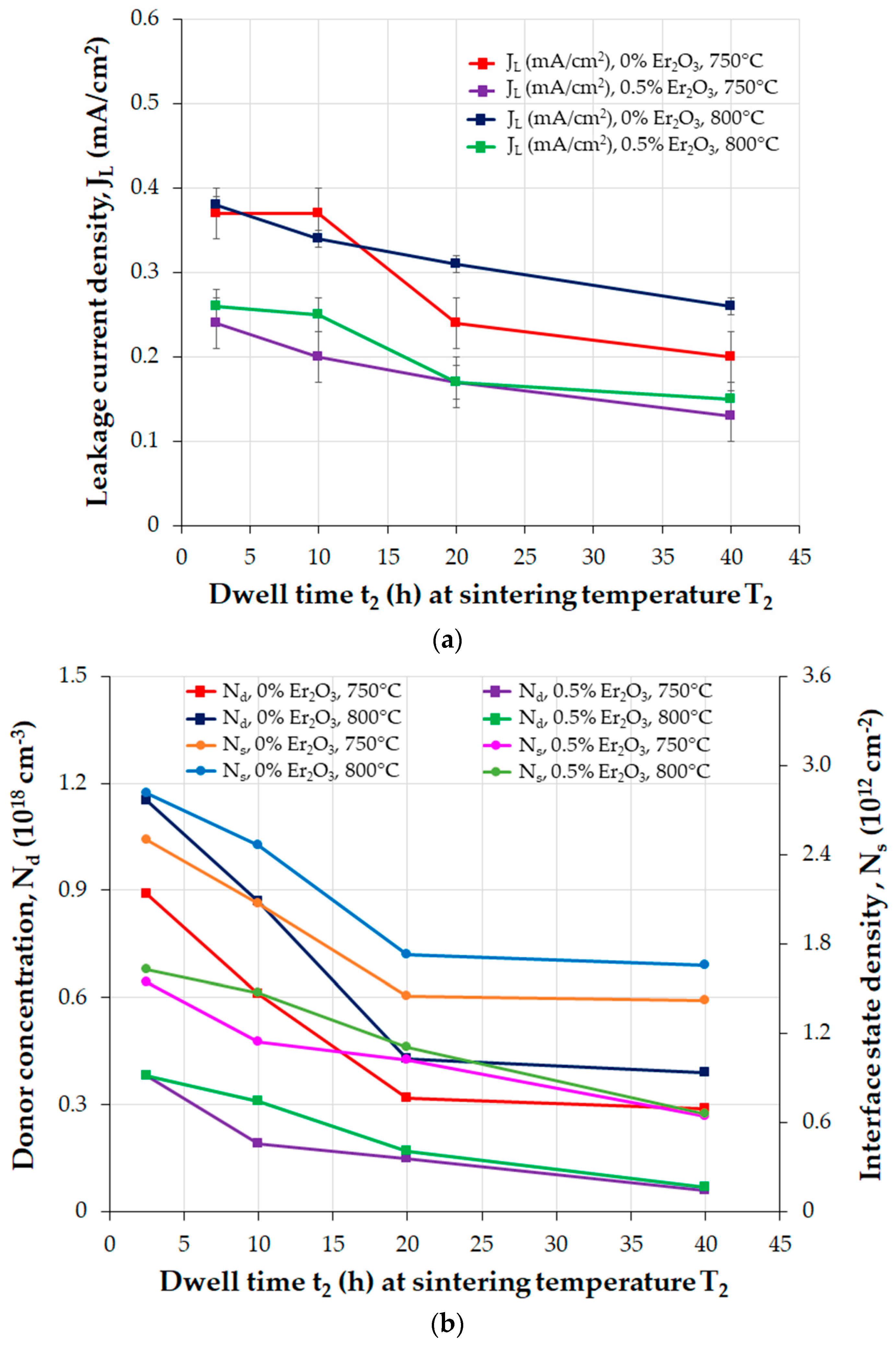
| Parameter | Function | Comments | Reference |
|---|---|---|---|
| Nonlinear coefficient (α) | Protective level | Typical α values in the low-current region are 20–70. The increase in temperature and pressure to which the MOV device is subjected in service causes a decrease in α values. | [57,65] |
| Nonlinear voltage (V) | Voltage rating | It is the threshold or breakdown voltage (VB) at a current of 1 mA. Typical EB values are in the range of 2–5 kV/cm. | [8,65] |
| Leakage current (IL) | Watt loss/operating voltage | DC IL values ≤ 100 μA for small-sized MOV discs, and ≤200 μA for large-sized MOV discs equipping electric power and telecommunication SPDs; AC IL = IR + IC. | [54,65,66] |
| Lifetime | Stability | Generated power (PG) < dissipated power (PD). | [65] |
| Energy absorption capability (E) | Survival of the electrical components | E depends on the size of MOV discs; a high surface-to-volume ratio of the MOV discs leads to a high E. | [65,66] |
| Preparation Methods of EMO and MOV Powders | Advantages | Disadvantages |
|---|---|---|
|
|
|
|
|
|
| Content of MO Additives of ZnO-0.5% V2O5 Systems (mol.%) (MOV System Type) | Sintering Temperature (t = 3 h) | Breakdown Field, EB (kV/cm) | Nonlinear Exponent (α) in the Low-Current Region | Leakage Current Density, JL (mA/cm2) | Degradation Rate Coefficient KT (µA h−1/2) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Stressed | ΔEB/EB (%) | Initial | Stressed | Δα/α (%) | Initial | Stressed | ΔJL/JL (%) | ||||
| 0.5% Mn3O4 (ZVM*) | 900 °C | 1.072 | 0.960 | −10.4 | 20 | 11 | −45.0 | 0.17 | 0.48 | 182.4 | 12.0 | [144] |
| 2% Mn3O4 (ZVM*) | 4.444 | 2.556 | −42.5 | 6 | 3 | −50.0 | 0.58 | 0.74 | 27.6 | thermal runaway | ||
| 0.5% MnO2 (ZVM) | 0.722 | 0.502 | −30.5 | 21 | 5 | −76.2 | 0.18 | 0.62 | 244.4 | 35.3 | ||
| 2% MnO2 (ZVM) | 0.999 | 0.978 | −2.1 | 27 | 20 | −25.9 | 0.042 | 0.21 | 400.0 | 3.8 | ||
| 2% MnO2 (ZVM) | 800 °C 850 °C 900 °C 950 °C | 17.640 7.881 0.992 2.430 | ~5.3 ~6.1 0.998 ~2.2 | −70.0 −22.6 0.6 −9.5 | 38.1 17.0 27.2 32.0 | 2.8 ~8.5 20.1 ~22 | −92.7 −50.0 −26.1 −31.3 | 0.14 0.27 0.17 0.11 | − − − − | − − − − | − − 3.8 9.0 | [36] |
| 0.5% Mn3O4 (ZVM*) | 900 °C | 0.922 | 0.880 | −4.6 | 20.7 | 14.3 | −30.9 | 0.31 | − | − | 9.4 | [27] |
| 0.5% Mn3O4 + 0.025% Yb2O3 | 1.025 | 0.943 | −8.0 | 29.2 | 14.9 | −49.0 | 0.13 | − | − | 16.0 | ||
| 0.5% Mn3O4 + 0.1% Yb2O3 | 1.637 | 1.648 | 0.7 | 26.3 | 25.2 | −4.2 | 0.23 | − | − | 5.5 | ||
| 0.5% Mn3O4+ 0.25% Yb2O3 (ZVM*Y) | 3.774 | 2.833 | −24.9 | 5.7 | 3.8 | −33.3 | 0.60 | − | − | − 19.4 | ||
| 0.5% Mn3O4 +0.05% Nb2O5 (ZVM*N) | 875 °C 900 °C 925 °C 950 °C | 5.671 3.967 2.489 1.443 | 0.675 3.498 0.703 1.449 | −88.1 −11.8 −71.8 0.4 | 37.9 47.0 24.2 17.8 | 1.2 16.0 1.8 17.1 | −96.8 −66.0 −92.6 −0.6 | 0.20 0.14 0.32 0.09 | 0.81 1.00 0.77 0.11 | 305.0 614.3 140.6 22.2 | − 19.6 − 3.5 | [50] |
| 2% MnO2 + 0.1% Nb2O5 (ZVMN) | 875 °C 900 °C 925 °C 950 °C | 6.991 4.800 2.241 0.943 | 4.305 4.389 2.274 0.603 | −38.4 −8.6 1.5 −36.1 | 44 50 38 25 | 4 20 43 5 | −90.9 −60.0 13.2 −80.0 | 0.2013 0.0949 0.0258 0.1737 | 0.7208 0.2824 0.0548 0.5095 | 258.1 197.6 112.4 193.3 | failure 27.6 0.38 20.8 | [180] |
| 2% MnO2 + 0.1% Nb2O5 + 0.5% Co3O4 + 0.1% Dy2O3 +0.05% Bi2O3 (ZVMNCDB) | 850 °C 875 °C 900 °C 925 °C | 8.016 4.522 2.351 1.715 | 7.533 4.265 2.370 1.343 | −6.0 −5.7 0.8 −21.7 | 44 59 45 38 | 21 24 45 8 | −52.3 −59.3 0 −78.9 | 0.0390 0.0362 0.0600 0.1006 | 0.1869 0.2390 0.1661 0.5457 | 379.2 560.2 176.8 442.4 | − − − − | [182] |
| 0.5% Mn3O4 + 0.1% Nb2O5 + 0.5% Co3O4 + 0.1% Dy2O3 + 0.05% Bi2O3 (ZVM*NCDB) | 850 °C 875 °C 900 °C 925 °C | 5.919 3.370 1.871 1.465 | 5.798 3.249 1.640 1.204 | −2.0 −3.6 −12.3 −17.8 | 39.1 43.6 37.2 33.3 | 30.0 27.1 13.2 9.6 | −23.3 −37.8 −64.5 −71.2 | 0.0357 0.0282 0.0630 0.0741 | 0.1229 0.1592 0.4019 0.4507 | 244.3 464.5 537.9 508.2 | 1.38 2.17 5.03 12.0 | [183] |
| Content of MO Additives of ZnO-0.5% V2O5 Systems (mol.%) (MOV System Type) | Sintering Temperature (t = 3 h) | Aging Stress State | Breakdown Field, EB (kV/cm) | Nonlinear Exponent (α) in the Low-Current Region | Leakage Current Density, JL (mA/cm2) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Stressed | ΔEB/EB (%) | Initial | Stressed | Δα/α (%) | Initial | Stressed | ΔJL/JL (%) | ||||
| 0.5% Mn3O4 + 0.025% Er2O3 (ZVM*E) | 850 °C 875 °C 900 °C 925 °C | Is = 10 A (5 times) | 3.856 1.385 0.922 2.352 | 3.560 1.161 0.743 2.275 | −7.7 −16.2 −19.4 −3.3 | 4.6 17.9 21.3 30.0 | 4.4 7.9 7.3 19.6 | −4.3 −55.9 −65.7 −34.7 | 0.63 0.24 0.13 0.20 | 0.63 0.47 0.47 0.30 | 0 95.8 261.5 50.0 | [179] |
| 0.5% Mn3O4 + 0.025% Er2O3 (ZVM*E) | 850 °C 875 °C 900 °C 925 °C | Is = 25 A (5 times) | 3.856 1.385 0.922 2.352 | 3.225 1.066 0.601 2.150 | −16.4 −23.0 −34.8 −8.6 | 4.6 17.9 21.3 30.0 | 4.1 6.1 4.5 13.6 | −10.9 −65.9 −78.9 −54.7 | 0.63 0.24 0.13 0.20 | 0.63 0.50 0.56 0.36 | 0 108.3 330.8 80.0 | [179] |
| 0.5% Mn3O4 + 0.05% Nb2O5 + 0.05% Er2O3 (ZVM*NE) | 875 °C 900 °C 925 °C 950 °C | Is = 25 A (3 times) | 5.909 4.175 1.995 1.028 | 5.403 3.702 1.965 0.839 | −8.6 −11.3 −1.5 −18.4 | 36.4 45.6 14.5 17.8 | 16.3 13.7 13.2 7.7 | −55.2 −69.9 −9.0 −56.7 | 0.28 0.24 0.39 0.40 | 0.28 0.31 0.39 0.39 | 0 29.2 0 −2.5 | [161] |
| 0.5% Mn3O4 + 0.05% Nb2O5 + 0.05% Er2O3 (ZVM*NE) | 875 °C 900 °C 925 °C 950 °C | Is = 200 A (3 times) | 5.909 4.175 1.995 1.028 | − − 1.784 − | − − −10.6 − | 36.4 45.6 14.5 17.8 | − − 9.1 − | − − −37.2 − | 0.28 0.24 0.39 0.40 | − − ~0.43 − | − − 10.2 − | [161] |
| 2% MnO2 + 0.1% Nb2O5 (ZVMN) | 875 °C 900 °C 925 °C 950 °C | Is = 10 A (3 times) | 6.830 4.791 2.292 0.968 | 6.643 4.689 2.250 0.887 | −2.7 −2.1 −1.8 −8.4 | 35.4 49.5 39.5 25.3 | 24.0 27.1 28.8 15.7 | −32.2 −45.3 −27.1 −37.9 | 0.19 0.11 0.03 0.08 | 0.21 0.17 0.11 0.21 | 10.5 54.5 266.7 162.5 | [60] |
| 2% MnO2 + 0.1% Nb2O5 (ZVMN) | 875 °C 900 °C 925 °C 950 °C | Is = 25 A (3 times) | 6.830 4.791 2.292 0.968 | 6.400 4.524 2.068 0.827 | −6.3 −5.6 −9.8 −14.6 | 35.4 49.5 39.5 25.3 | 18.7 20.2 14.7 11.2 | −47.2 −59.2 −62.8 −55.7 | 0.19 0.11 0.03 0.08 | 0.24 0.19 0.18 0.21 | 26.3 72.7 500.0 162.5 | [60] |
| 2% MnO2 + 0.1% Nb2O5 (ZVMN) | 875 °C 900 °C 925 °C 950 °C | Is = 50 A (3 times) | 6.830 4.791 2.292 0.968 | 6.205 4.292 1.999 0.778 | −9.2 −10.4 −12.8 −19.6 | 35.4 49.5 39.5 25.3 | 16.0 15.4 12.4 9.0 | −54.8 −68.9 −68.6 −64.4 | 0.19 0.11 0.03 0.08 | 0.26 0.22 0.21 0.33 | 36.8 100.0 600.0 312.5 | [60] |
| 2% MnO2 + 0.1% Nb2O5 (ZVMN) | 875 °C 900 °C 925 °C 950 °C | Is = 100 A (3 times) | 6.830 4.791 2.292 0.968 | 5.861 4.089 1.798 0.697 | −14.2 −14.7 −21.6 −28.0 | 35.4 49.5 39.5 25.3 | 13.0 13.0 8.8 6.9 | −63.3 −73.7 −77.7 −72.7 | 0.19 0.11 0.03 0.08 | 0.30 0.26 0.29 0.39 | 57.9 136.4 866.7 387.5 | [60] |
| MOV System Type (mol.%) | State | Pp, Ts/DT (PM Process) | EB (kV/cm) | α | IL (μA) | JL (mA/cm2) | MOV Producers | Reference |
|---|---|---|---|---|---|---|---|---|
| ZnO-V2O5-based systems | ||||||||
| ZnO-0.5% V2O5-0.5% Mn3O4 | Initial | 80 MPa, 900 °C/ 3 h (SSS) | 1.07 | 20 | − | 0.170 | Semiconductor Ceramics Laboratory, Department of Electrical Engineering, Dongeui University, Republic of Korea | [144] |
| Stressed | 0.96 | 11 | − | 0.480 | ||||
| ZnO-0.5% V2O5-0.5% Mn3O4 | Initial | 80 MPa, 900 °C/ 3 h (SSS) | 0.72 | 27 | − | 0.042 | [144] | |
| Stressed | 0.99 | 20 | − | 0.210 | ||||
| ZnO-0.5% V2O5-0.5% Mn3O4-0.1% Yb2O3 | Initial | 100 MPa, 900 °C/ 3 h (SSS) | 1.64 | 26.3 | − | 0.230 | [27] | |
| Stressed | 1.65 | 25.2 | − | − | ||||
| ZnO-0.5% V2O5-1% Nb2O5-0.1% In2O3 | Initial | 500 MPa, 875 °C/1 h (SSS) | 14.2 ± 0.1 | 153 ± 7 | − | 0.062 | Variable Energy Cyclotron Centre and Homi Bhabha National Institute, India | [44] |
| Initial | 500 MPa, 900 °C/1 h (SSS) | 11.6 ± 0.2 | 132 ± 8 | − | 0.083 | |||
| ZnO-0.5% V2O5-0.5% Mn3O4-0.05% Bi2O3 | Initial | 100 MPa, 825 °C/3 h (SSS) | 6.027 | 31 | − | 0.043 | Semiconductor Ceramics Laboratory, Department of Electrical Engineering, Dongeui University, Republic of Korea | [124] |
| ZnO-0.5% V2O5-0.5% Mn3O4-0.1% Bi2O3 | Initial | 3.357 | 24.9 | − | 0.052 | |||
| ZnO-Bi2O3-based systems | ||||||||
| ZnO-1% Bi2O3-1% Sb2O3-0.75% MnO2-1% Co2O3-0.5% Cr2O3-1% Sb2O3, 1.2% SiO2-0.2% Al2O3-0.005-0.02% In2O3 | Initial | ~39 MPa 1200 °C/2 h (SSS) | 3.56–4.34 | 52–60 | − | 0.002–0.004 | State Key Laboratory of Control and Simulation of Power System and Generation Equipment, Department of Electrical Engineering, Tsinghua University, China | [43] |
| ZnO-0.5% Bi2O3-1% Sb2O3-0.5% CoO-0.5% MnO2-0.5% Cr2O3 | Initial | ~33 MPa 1350 °C (SSS) | 1.35 | 50 | − | − | Matsushita Electric Industrial Co., Japan (actual Panasonic) | [6] |
| Initial | 1100 °C/1 min and 850 °C/1 h (MW-TSS) | 10.7 | 40 | 58 | − | PPG-CEM, Federal University of São Carlos, Brazil | [100] | |
| Commercial ZNR 10K 270 (ZnO-Bi2O3-MO dopants) | Initial | NA | 0.22 | ≤11 | − | 0.070 | Matsushita Electric Industrial Co., Japan (actual Panasonic) | [186] |
| Commercial ZNR 10K 470 (ZnO-Bi2O3-MO dopants) | Initial | NA | 0.42 | ≤25 | − | 0.013 | ||
| Commercial GEMOV 27Z 1 (ZnO-Bi2O3-MO dopants) | Initial | NA | 0.27 | ≤16 | − | 0.035 | General Electric Co., USA | |
| Commercial (ZnO-Bi2O3 + MO dopants) | Initial | 1095 °C/2.5 h (SSS) | 1.85 | 27 | 17 | − | Unknown producer | [120] |
| ZnO-Bi2O3-Sb2O3-Co3O4-Mn2O3-NiO-B2O3-Al2O3 | Initial | 95 MPa, 1115 °C/5 h (SSS) | 1.79 | 56 | 3.4 | − | Tridelta Parafoudres S.A. and UMR CNRS-UPSINP, France | [120] |
| Content of MO Additives of ZnO-0.5% V2O5 Systems (mol.%) | MOV System Type | Sintering Temperature (t = 3 h) | Apparent Dielectric at 1 kHz | Dissipation Factor (tanδ) at 1 kHz | Reference |
|---|---|---|---|---|---|
| 0.5% Mn3O4 | ZVM* | 825 °C | 1324.5 | 0.506 | [124] |
| 0.5% Mn3O4 + 0.025% Bi2O3 | ZVM*B | 528.3 | 0.225 | ||
| 0.5% Mn3O4 + 0.05% Bi2O3 | 544.7 | 0.176 | |||
| 0.5% Mn3O4 + 0.1% Bi2O3 | 734.4 | 0.212 | |||
| 0.5% Mn3O4 | ZVM* | 900 °C | 2438.3 | 0.424 | [27] |
| 0.5% Mn3O4 + 0.025% Yb2O3 | ZVM*Y | 2126.8 | 0.309 | ||
| 0.5% Mn3O4 + 0.1% Yb2O3 | 1635.1 | 0.404 | |||
| 0.5% Mn3O4 + 0.25% Yb2O3 | 1061.7 | 0.482 | |||
| 0.5% Mn3O4 + 0.025% Er2O3 | ZVM*E | 850 °C 875 °C 900 °C 925 °C | 1223.8 1626.9 2239.8 1489.3 | 0.543 0.403 0.332 0.273 | [179] |
| 0.5% Mn3O4 | ZVM* | 900 °C | 2104.2 | 0.354 | [184] |
| 0.5% Mn3O4 + 0.05% Er2O3 | ZVM*E | 1845.3 | 0.297 | ||
| 0.5% Mn3O4 + 0.1% Er2O3 | 1445.0 | 0.395 | |||
| 0.5% Mn3O4 + 0.25% Er2O3 | 1314.1 | 0.468 | |||
| 0.5% Mn3O4 + 0.05% Nb2O5 | ZVM*N | 875 °C 900 °C 925 °C 950 °C | 779.2 1054.9 1631.8 1470.2 | 0.205 0.145 0.235 0.279 | [50] |
| 2% MnO2 + 0.1% Nb2O5 | ZVMN | 875 °C 900 °C 925 °C 950 °C | 443.2 604.2 961.8 2062.6 | 0.281 0.228 0.134 0.287 | [180] |
| 2% MnO2 + 0.1% Nb2O5 | ZVMN | 900 °C | 592.7 | 0.209 | [26] |
| 2% MnO2 + 0.1% Nb2O5 + 0.05% Yb2O3 | ZVMNY | 559.8 | 0.233 | ||
| 2% MnO2 + 0.1% Nb2O5 + 0.1% Yb2O3 | 549.1 | 0.248 | |||
| 2% MnO2 + 0.1% Nb2O5 + 0.25% Yb2O3 | 568.6 | 0.313 | |||
| 2% MnO2 + 0.1% Nb2O5 + 0.05% Dy2O3 | ZVMND | 875 °C 900 °C 925 °C 950 °C | 658.6 823.2 1644.3 2928.8 | 0.324 0.284 0.450 0.400 | [181] |
| 2% MnO2 + 0.1% Nb2O5 | ZVMN | 900 °C | 822.0 | 0.261 | [24] |
| 2% MnO2 + 0.1% Nb2O5 + 0.05% Dy2O3 | ZVMND | 770.6 | 0.241 | ||
| 2% MnO2 + 0.1% Nb2O5 + 0.1% Dy2O3 | 713.1 | 0.205 | |||
| 2% MnO2 + 0.1% Nb2O5 + 0.25% Dy2O3 | 917.6 | 0.355 | |||
| 2% MnO2 + 0.1% Nb2O5 + 0.05% Bi2O3 + 0.5% Co3O4 + 0.1% Dy2O3 | ZVMNBCD | 800 °C 875 °C 900 °C 925 °C | 330.6 512.7 985.8 1391.4 | 0.211 0.203 0.206 0.238 | [183] |
| Sintering Temperature (t = 3 h) | Apparent Dielectric Constant at 1 kHz | Dissipation Factor (tanδ) at 1 kHz | ||||
|---|---|---|---|---|---|---|
| Initial | Stressed | (%) | Initial | Stressed | Δtanδ/tanδ (%) | |
| 800 °C 850 °C 900 °C 950 °C | 135.2 299.8 1163.5 800.9 | ~170 ~350 1160 ~880 | 25.7 16.7 −0.3 9.9 | 0.0781 0.1838 0.3160 0.2090 | ~1.125 ~0.610 0.385 ~0.338 | 1340.5 231.9 21.8 61.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, M.V. Effects of Dopants and Processing Parameters on the Properties of ZnO-V2O5-Based Varistors Prepared by Powder Metallurgy: A Review. Materials 2023, 16, 3725. https://doi.org/10.3390/ma16103725
Lungu MV. Effects of Dopants and Processing Parameters on the Properties of ZnO-V2O5-Based Varistors Prepared by Powder Metallurgy: A Review. Materials. 2023; 16(10):3725. https://doi.org/10.3390/ma16103725
Chicago/Turabian StyleLungu, Magdalena Valentina. 2023. "Effects of Dopants and Processing Parameters on the Properties of ZnO-V2O5-Based Varistors Prepared by Powder Metallurgy: A Review" Materials 16, no. 10: 3725. https://doi.org/10.3390/ma16103725
APA StyleLungu, M. V. (2023). Effects of Dopants and Processing Parameters on the Properties of ZnO-V2O5-Based Varistors Prepared by Powder Metallurgy: A Review. Materials, 16(10), 3725. https://doi.org/10.3390/ma16103725






