Study on the Possibilities of Developing Cementitious or Geopolymer Composite Materials with Specific Performances by Exploiting the Photocatalytic Properties of TiO2 Nanoparticles
Abstract
:1. Introduction
2. Cementitious Composites with Self-Cleaning Capacity
2.1. Influence on the Physical-Mechanical Characteristics of Fresh Cementitious Composites
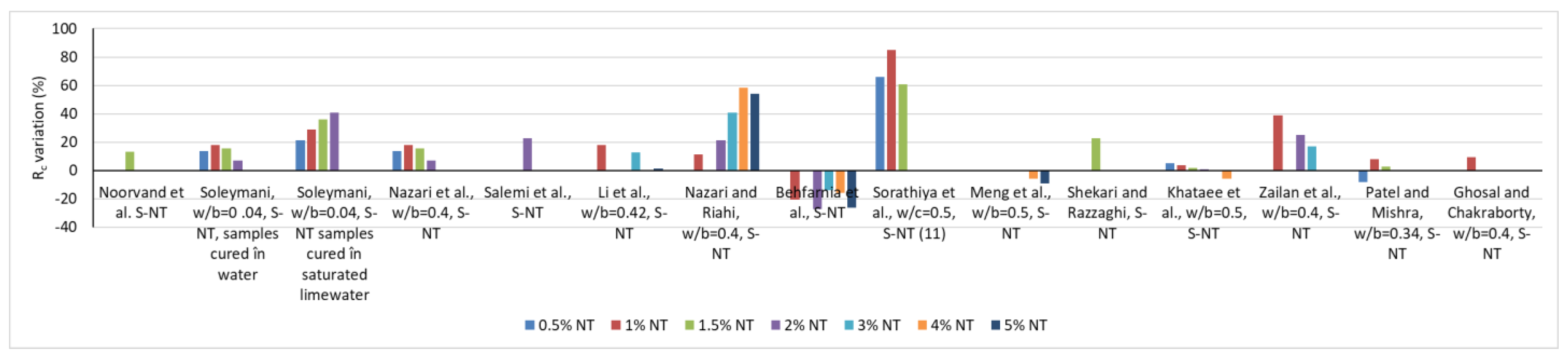
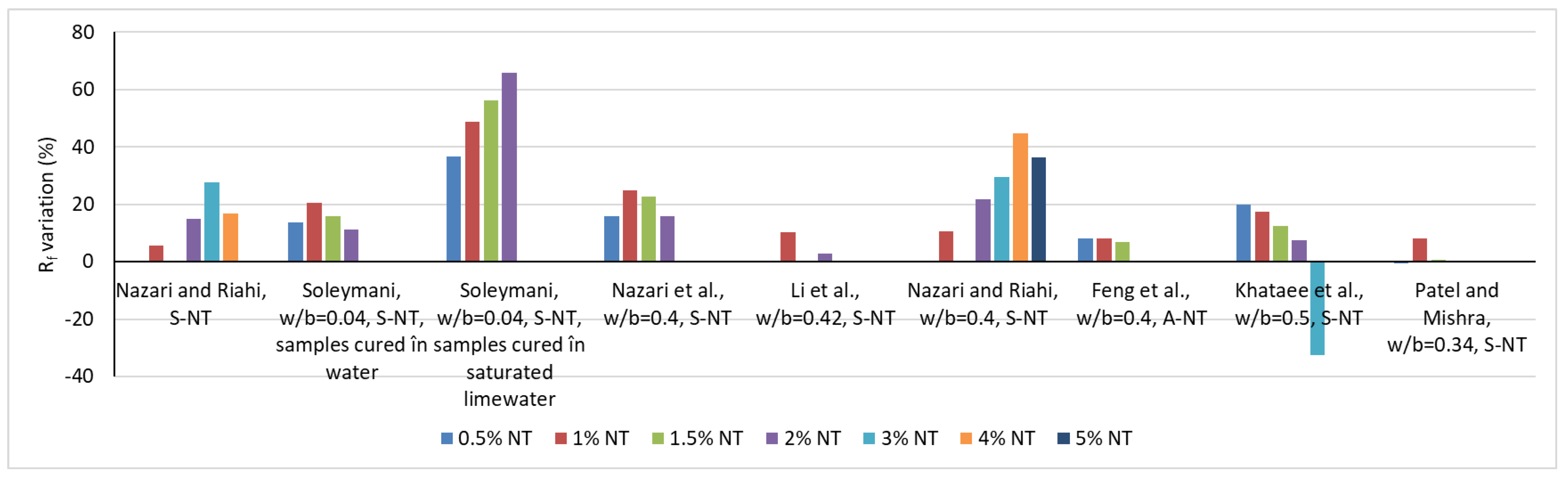
2.2. Influence on the Physical-Mechanical Characteristics of Cementitious Composites in the Hardened State
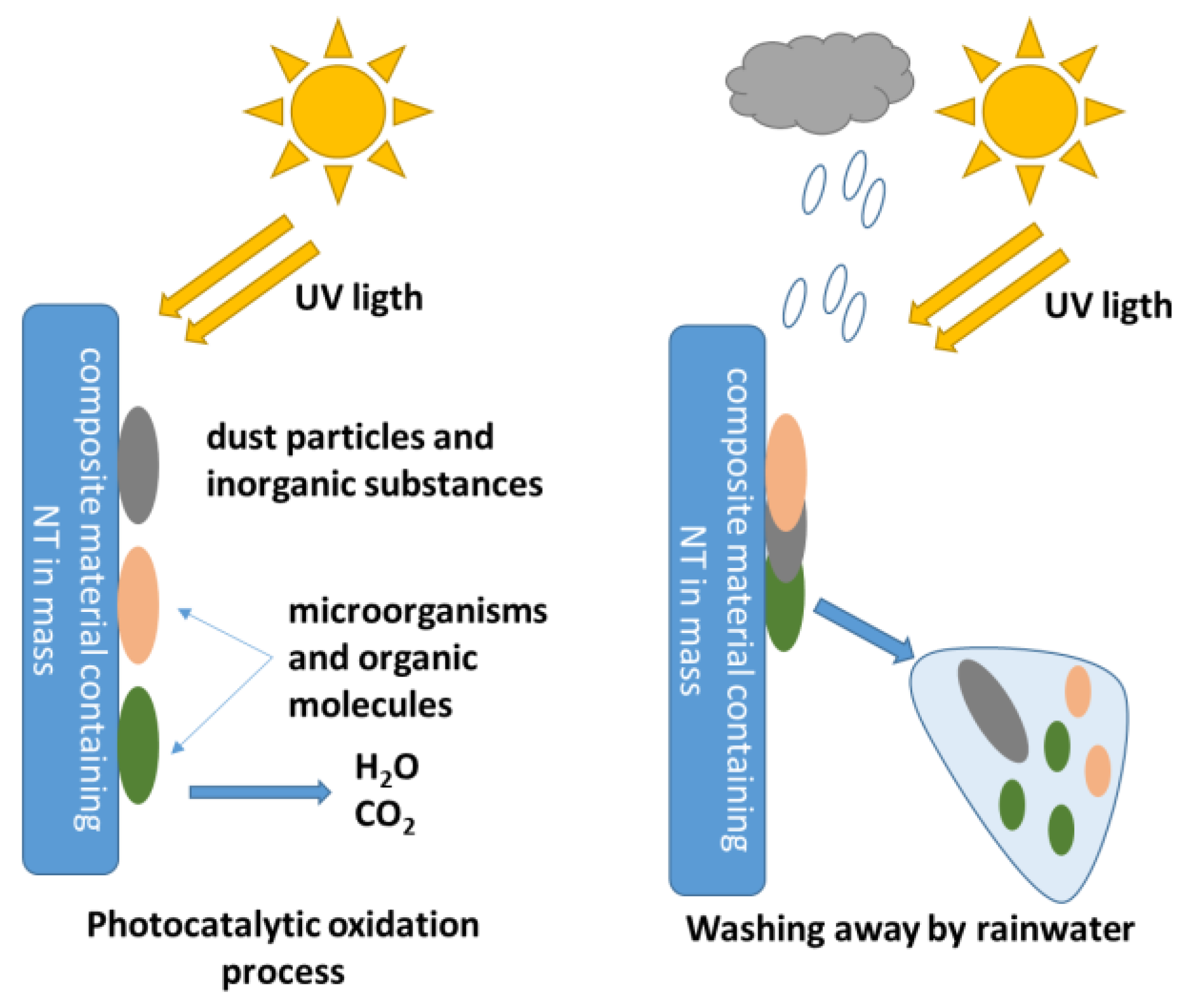
2.3. Influence of the Introduction of TiO2 Nanoparticles into Cementitious Composites on Their Resistance to the Action of Microorganisms—Self-Cleaning Capacity and Biocidal Mechanism
- ASTM E2149—presents a quantitative method for evaluating the behavior of irregular surfaces to bacterial action. The principle of the method is to immerse the material in a suspension with a known concentration of bacteria and to follow the evolution of this concentration over time. The antimicrobial activity of the material is considered positive when the concentration of the bacterial suspension is significantly reduced [121];
- ASTM E2180—presents a quantitative method for evaluating the behavior of hydrophobic surfaces to bacterial action. The principle of the method consists of making a pseudo-film of nutrient medium on the surface of the material, on which bacteria are inoculated in a suspension of known concentration and monitoring the evolution of the concentration compared to a control [122];
- ISO 22196—presents a quantitative method. The principle of this method is also to follow the variation of the concentration of the bacterial suspension inoculated on a nutrient medium [123];
- ASTM E1428—presents a qualitative method featuring the so-called “pink spot test”, in which an inoculation with Streptoverticullium reticulum is used and the appearance of pink spots on the surface of the tested material is observed [124];
- STAS 12718/1989 offers the possibility of semi-quantitative quantification of the microbiological load of the system, providing a quantification grid as follows: 0(−) no growth (sterile); 1(+) 1–10 colonies of microorganisms; 2(++) more than 10 colonies of microorganisms; 3(+++) areas with confluent colonies; 4(++++) growth over the whole surface area [125];
- ISO 27447—presents a method for evaluating the antibacterial activity of semiconducting photocatalytic materials, can be applied for the analysis of some ceramic, photocatalytic materials but not for permeable or rough materials [126].
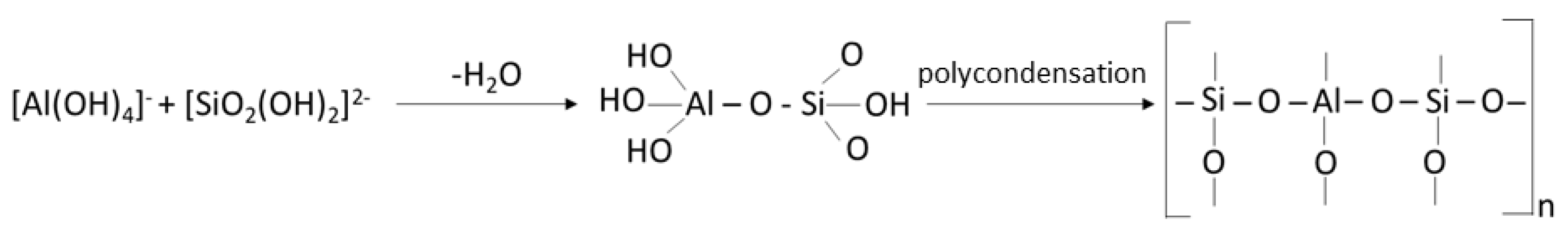
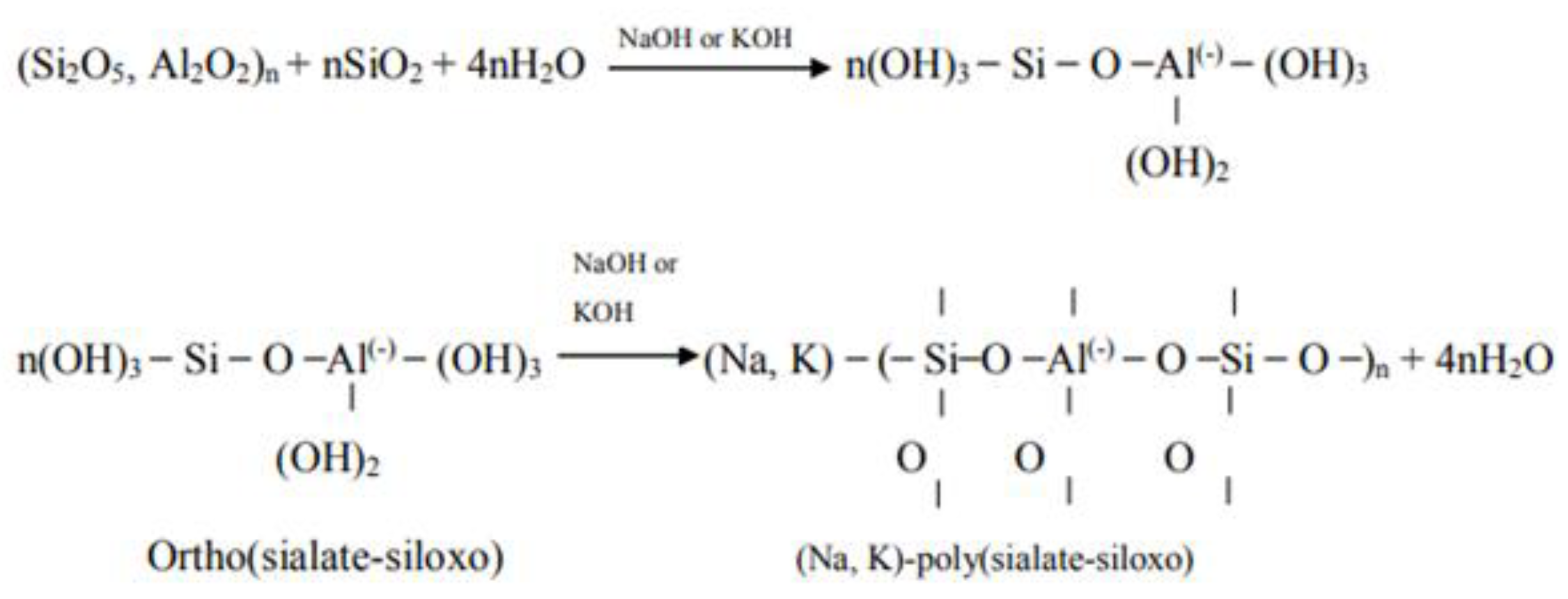
3. Geopolymer Composites with Self-Cleaning Capability
- hydration process for vitreous silica with a pH > 12:
- hydration of Al2O3:
- reaction of CaO and MgO:
- reaction of Na2O and K2O:
- the Fe2O3 reaction:
- hydration of TiO2:
3.1. Physical-Mechanical Characteristics of Geopolymer Composites
3.2. Influence of TiO2 Adding Nanoparticles in Geopolymer Composites
3.2.1. NT Influence on Geopolymer Paste Properties
3.2.2. Influence of NT on the Properties of Geopolymer in Hardened State
4. Future Perspectives
5. Conclusions
- in the current context, where the need to identify sustainable development solutions is imperative, the development of innovative materials contributes to the creation of a favorable framework for increasing the implementation of the principles of the Circular Economy, reducing environmental impact and increasing sustainability in the construction sector;
- innovative directions in this respect, still niche, are the development of cementitious composites or geopolymer composites that include nanoparticles in the matrix, the most used being NT;
- in parallel, the development of geopolymer materials allows for the reuse of waste or industrial by-products which contributes to reducing the environmental impact of other industries.
- In terms of cementitious composites, studies conducted to date have shown that:
- inducing exceptional properties by exploiting specific features of the nanoparticles embedded in the composite matrix has already proven to be a possible way forward;
- to date, although the research carried out is encouraging, there are several controversies and uncertainties, which point to further research;
- the introduction of NT into the cementitious matrix has consequences on the physical-mechanical performance, durability or resistance performance to the action of micro-organisms, improving them;
- the results of the research carried out at microstructural level, corroborated and reflected by the results of the research carried out at macrostructural level, indicate the need for in-depth analysis so as to gain a thorough knowledge of the mechanisms underlying the phenomenon and to be able to determine more easily and more precisely the optimal quantity of nanoparticles and the way in which they are introduced during preparation, so as to achieve good performance in terms of physical-mechanical properties, self-healing capacity and increased surface hygiene.
- In terms of geopolymer materials, studies conducted so far have shown that:
- the field of self-healing geopolymers is an area of interest, but one that has been addressed only in recent years;
- so far, a number of results are reported, but there is still some controversy about the mechanisms of the geopolymerization reaction, the influence of a significant number of factors (e.g., type and oxidative composition of the raw material, characteristics of the alkaline activator, existence or not of nanoparticles or the type of nanoparticles used, working temperature, etc.) on the physical-mechanical performances, which have been studied more intensively, but also on some performances of durability, self-healing capacity, resistance to the action of microorganisms, etc.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zailan, S.N.; Mahmed, N.; Al Bakri Abdullah, M.M.; Sandu, A.V. Self-cleaning geopolymer concrete—A review. IOP Conf. Ser. Mater. Sci. Eng. 2016, 133, 012026. [Google Scholar] [CrossRef]
- Malhotra, V.M. Making Concrete “Greener” With Fly Ash. ACI Conc. Int. 1999, 21, 61–66. [Google Scholar]
- Cement Technology Roadmap: Carbon Emissions Reductions up to 2050. Available online: https://www.iea.org/reports/cement-technology-roadmap-carbon-emissions-reductions-up-to-2050 (accessed on 10 March 2023).
- US Geological Survey. Mineral Commodity Summaries: Cement. 2012. Available online: https://d9-wret.s3.us-west-2.amazonaws.com/assets/palladium/production/mineral-pubs/mcs/mcs2012.pdf (accessed on 28 February 2023).
- Cembureau. Available online: https://www.cembureau.eu/library/reports/2050-carbon-neutrality-roadmap/ (accessed on 21 February 2023).
- Aitcin, P.-C. Cements of yesterday and today; Concrete of tomorrow. Cem. Concr. Res. 2000, 30, 1349–1359. [Google Scholar] [CrossRef]
- Sandu, A.V. Obtaining and Characterization of New Materials. Materials 2021, 14, 6606. [Google Scholar] [CrossRef]
- Jamaludin, L.; Razak, R.A.; Abdullah, M.M.A.B.; Vizureanu, P.; Bras, A.; Imjai, T.; Sandu, A.V.; Abd Rahim, S.Z.; Yong, H.C. The Suitability of Photocatalyst Precursor Materials in Geopolymer Coating Applications: A Review. Coatings 2022, 12, 1348. [Google Scholar] [CrossRef]
- Davidovits, J. Chemistry of Geopolymeric Systems Terminology. In Proceedings of the Geopolymer International Conference, Saint-Quentin, France, 30 June–2 July 1999. [Google Scholar]
- Wazien, A.Z.W.; Mustafa, M.; Abdullah, A.B.; Razak, R.A.; Rozainy, M.M.A.Z.R.; Faheem, M.; Tahir, M.; Faris, M.A.; Hamzah, H.N. Review on Potential of Geopolymer for Concrete Repair and Rehabilitation. MATEC Web Conf. 2016, 78, 01065. [Google Scholar] [CrossRef]
- Lloyd, N.A.; Rangan, B.V. Geopolymer concrete with fly ash. In Proceedings of the Second International Conference on Sustainable Materials and Technologies, Ancona, Italy, 28 June 2010; Zachar, J., Claisse, P., Naik, T.R., Ganjian, E., Eds.; Università Politecnica delle Marche: Ancona, Italy, 2010. [Google Scholar]
- Wang, L.; Zhang, H.; Gao, Y. Effect of TiO2 Nanoparticles on Physical and Mechanical Properties of Cement at Low Temperatures. Adv. Mater. Sci. Eng. 2018, 2018, 8934689. [Google Scholar] [CrossRef]
- Falah, M.; Mackenzie, K.J.D. Photocatalytic Nanocomposite Materials Based on Inorganic Polymers (Geopolymers): A Review. Catalysts 2020, 10, 1158. [Google Scholar] [CrossRef]
- Mohseni, E.; Miyandehi, B.M.; Yang, J.; Yazdi, M.A. Single and combined effects of nano-SiO2, nano-Al2O3 and nano-TiO2 on the mechanical, rheological and durability properties of self-compacting mortar containing fly ash. Constr. Build. Mater. 2015, 84, 331–340. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. The effects of TiO2 nanoparticles on physical, thermal and mechanical properties of concrete using ground granulated blast furnace slag as binder. Mater. Sci. Eng. A 2011, 528, 2085–2092. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. The effects of TiO2 nanoparticles on properties of binary blended concrete. J. Compos. Mater. 2011, 45, 1181–1188. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. The effects of TiO2 nanoparticles on flexural damage of self-compacting concrete. Int. J. Damage Mech. 2011, 20, 1049–1072. [Google Scholar] [CrossRef]
- Zailan, S.N.; Mahmed, N.; Al Bakri Abdullah, M.M.; Sandu, A.V.; Shahedan, N.F. Review on Characterization and Mechanical Performance of Self-cleaning Concrete. MATEC Web Conf. 2017, 97, 01022. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Cordoni, C.; Munafò, P. Self-cleaning and de-polluting stone surfaces: TiO2 nanoparticles for limestone. Constr. Build. Mater. 2012, 37, 51–57. [Google Scholar] [CrossRef]
- Zhang, S.M.-H.; Tanadi, D.; Li, W. Effect of photocatalyst TiO2 on workability, strength, and self-cleaning efficiency of mortars for applications in tropical environment. In Proceedings of the 35th Conference on Our World in Concrete and Structures, Singapore, 25–27 August 2010. [Google Scholar]
- Cassar, L. Nanotechnology and photocatalysis in cementitous materials. In Proceedings of the NICOM 2: 2nd International Symposium on Nanotechnology in Construction, Bilbao, Spain, 13–16 November 2005; de Miguel, Y., Porro, A., Bartos, P.J.M., Eds.; RILEM Publications SARL: Champs-sur-Marne, France, 2006; pp. 277–284. [Google Scholar]
- Hamidi, F.; Aslani, F. TiO2-based Photocatalytic Cementitious Composites: Materials, Properties, Influential Parameters, and Assessment Techniques. Nanomaterials 2019, 9, 1444. [Google Scholar] [CrossRef] [PubMed]
- Reches, Y. Nanoparticles as concrete additives: Review and perspectives. Constr. Build. Mater. 2018, 175, 483–495. [Google Scholar] [CrossRef]
- Li, H.; Xiao, H.-G.; Yuan, J.; Ou, J. Microstructure of cement mortar with nano-particles. Compos. Part B Eng. 2004, 35, 185–189. [Google Scholar] [CrossRef]
- Li, Z.; Han, B.; Yu, X.; Dong, S.; Zhang, L.; Dong, X.; Ou, J. Effect of nano-titanium dioxide on mechanical and electrical properties and microstructure of reactive powder concrete. Mater. Res. Express 2017, 4, 095008. [Google Scholar] [CrossRef]
- Han, B.; Li, Z.; Zhang, L.; Zeng, S.; Yu, X.; Han, B.; Ou, J. Reactive powder concrete reinforced with nano SiO2-coated TiO2. Constr. Build. Mater. 2017, 148, 104–112. [Google Scholar] [CrossRef]
- Yang, L.; Jia, Z.; Zhang, Y.; Dai, J. Effects of nano-TiO2 on strength, shrinkage and microstructure of alkali activated slag pastes. Cem. Concr. Compos. 2015, 57, 1–7. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Plank, J.; Ruiz-Izuriaga, D.; Navarro-Blasco, I.; Fernandez, J.; Alvarez, J.I. Photocatalytically active coatings for cement and air lime mortars: Enhancement of the activity by incorporation of superplasticizers. Constr. Build. Mater. 2018, 162, 628–648. [Google Scholar] [CrossRef]
- Zhao, A.; Yang, J.; Yang, E.-H. Self-cleaning engineered cementitious composites. Cem. Concr. Res. 2015, 64, 74–83. [Google Scholar] [CrossRef]
- Li, C.-Z.; Feng, N.-Q.; Li, Y.-D.; Chen, R.-J. Effects of polyethlene oxide chains on the performance of polycarboxylate-type water reducers. Cem. Concr. Res. 2005, 35, 867–873. [Google Scholar] [CrossRef]
- Navarro-Blasco, I.; Pérez-Nicolás, M.; Fernández, J.M.; Duran, A.; Sirera, R.; Alvarez, J.I. Assessment of the interaction of polycarboxylate superplasticizers in hydrated lime pastes modified with nanosilica or metakaolin as pozzolanic reactives. Constr. Build. Mater. 2014, 73, 1–12. [Google Scholar] [CrossRef]
- Zapata, L.E.; Portela, G.; Suarez, O.M.; Carrasquillo, O. Rheological performance and compressive strength of superplasticized cementitious mixtures with micro/nano-SiO2 additions. Constr. Build. Mater. 2013, 41, 708–716. [Google Scholar] [CrossRef]
- Ranjit, K.; Odedra; Parmar, K.A.; Arora, N.K. Photocatalytic Self cleaning Concrete. IJSRD Int. J. Sci. Res. Dev. 2014, 1, 2521–2523. [Google Scholar]
- Lazăr, M.; Fiat, D.; Hubcă, G. The influence of TiO2 nanometric photocatalytic pigment on the proprieties of the film forming products based on organic binders in aqueous dispersion. Rom. J. Mater. 2015, 40, 178–187. [Google Scholar]
- Aslanidou, D.; Karapanagiotis, I.; Lampakis, D. Waterborne Superhydrophobic Coatings for the Protection of Marble and Sandstone. Materials 2018, 1, 585. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Riahi, S.; Riahi, S.; Shamekhi, S.F.; Khademno, A. Improvement the mechanical properties of the cementitious composite by using TiO2 nanoparticles. Am. J. Sci. 2010, 6, 98–101. [Google Scholar]
- Midtdal, K.; Jelle, B.P. Self-cleaning glazing products: A state-of-the-art review and future research pathways. Sol. Energy Mater. Sol. Cells 2013, 109, 126–141. [Google Scholar] [CrossRef]
- Lee, B.Y.; Jayapalan, A.R.; Kurtis, K.E. Effects of nanoTiO2 on properties of cement-based materials. Mag. Concr. Res. 2013, 65, 1293–1302. [Google Scholar] [CrossRef]
- Pimenta Teixeira, K.; Perdigão Rocha, I.; De S’a Carneiro, L.; Flores, J.; Dauer, E.A.; Ghahremaninezhad, A. The effect of curing temperature on the properties of cement pastes modified with TiO2 nanoparticles. Materials 2016, 9, 952. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.; Fathi, M.; Farzad, M. Effects of fly ash and TiO2 nanoparticles on rheological, mechanical, microstructural and thermal properties of high strength self compacting concrete. Mech. Mater. 2013, 61, 11–27. [Google Scholar] [CrossRef]
- Salemi, N.; Behfamia, K.; Zaree, S.A. Effect of nanoparticles on frost durability of concrete. Asian J. Civ. Eng. (BHRC) 2014, 15, 411–420. [Google Scholar]
- Nazari, A.; Riahi, S.; Shamekhi, S.F.; Khademno, A. Assessment of the Effects of the Cement Paste Composite in Presence TiO2 Nanoparticles. Am. J. Sci. 2010, 6, 43–46. [Google Scholar]
- Li, H.; Zhang, M.H.; Ou, J.P. Flexural fatigue performance of concrete containing nanoparticles for pavement. Int. J. Fatigue 2007, 29, 1292–1301. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Ou, J. Abrasion resistance of concrete containing nano-particles for pavement. Wear 2006, 260, 1262–1266. [Google Scholar] [CrossRef]
- Smits, M.; Chan, C.K.; Tytgat, T.; Craeye, B.; Costarramone, N.; Lacombe, S.; Lenaerts, S. Photocatalytic degradation of soot deposition: Self-cleaning effect on titanium dioxide coated cementitious materials. Chem. Eng. J. 2013, 222, 411–418. [Google Scholar] [CrossRef]
- Jalal, M.; Ramezanianpour, A.A.; Pool, M.K. Effects of titanium dioxide nanopowder on rheological properties of self compacting concrete. Am. J. Sci. 2012, 8, 285–288. [Google Scholar]
- Meng, T.; Yu, Y.; Qian, X.; Zhan, S.; Qian, K. Effect of nano-TiO2 on the mechanical properties of cement mortar. Constr. Build. Mater. 2012, 29, 241–245. [Google Scholar] [CrossRef]
- Noorvand, H.; Ali, A.A.A.; Demirboga, R.; Farzadnia, N.; Noorvand, H. Incorporation of nano TiO2 in black rice husk ash mortars. Constr. Build. Mater. 2013, 47, 1350–1361. [Google Scholar] [CrossRef]
- Lee, B.Y. Effect of Titanium Dioxide Nanoparticles on Early Age and Long Term Properties of Cementitious Materials. Ph.D. Thesis, School of Civil & Environmental Engineering, Georgia Institute of Technology, Atlanta, GA, USA, August 2012. [Google Scholar]
- Chen, J.; Kou, S.; Poon, C. Hydration and properties of nano-TiO2 blended cement composites. Cem. Concr. Comp. 2012, 34, 642–649. [Google Scholar] [CrossRef]
- Essaway, A.A.; El Aleem, S.A. Physico-mechanical properties, potent adsorptive and photocatalytic efficacies of sulfate resisting cement blends containing micro silica and nano-TiO2. Constr. Build. Mater. 2014, 52, 1–8. [Google Scholar] [CrossRef]
- Rashad, A.M. A synopsis about the effect of nano-titanium dioxide on some properties of cementitious materials—A short guide for civil engineer. Rev. Adv. Mater. Sci. 2015, 40, 72–88. [Google Scholar]
- Lee, B.Y.; Kurtis, K.E. Influence of TiO2 Nanoparticles on Early C3S Hydration. J. Am. Ceram. Soc. 2010, 93, 3399–3405. [Google Scholar] [CrossRef]
- Sneff, L.; Hotza, D.; Lucas, S.; Ferreira, V.M.; Labrinca, J.A. Effect of nano-SiO2 and nano-TiO2 addition on the rheological behavior and the hardened properties of cement mortars. Mater. Sci. Eng. A 2012, 532, 354–361. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. The effect of TiO2 nanoparticles on water permeability and thermal and mechanical properties of high strength self-compacting concrete. Mater. Sci. Eng. A 2010, 582, 756–763. [Google Scholar] [CrossRef]
- Jalal, M.; Mortazavi Ali, A.; Nemat, H. Thermal properties of TiO2 nanoparticles binary blended cementitious composites. Am. J. Sci. 2012, 8, 391–394. [Google Scholar]
- Jayapalan, A.R.; Lee, B.Y.; Kurtis, K.E. Effect of Nano-sized Titanium Dioxide on Early Age Hydration of Portland Cement. In Nanotechnology in Construction 3; Bittnar, Z., Bartos, P.J.M., Němeček, J., Šmilauer, V., Zeman, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Jayapalan, A.R.; Lee, B.Y.; Kurtis, K.E. Can nanotechnology be ‘green’? Comparing efficacy of nano and microparticles in cementitious materials. Cem. Concr. Comp. 2013, 36, 16–24. [Google Scholar] [CrossRef]
- Kurihara, R.; Maruyama, I. Influences of Nano-TiO2 particles on alteration of microstructure of cement. JCI 2016, 38, 219–224. [Google Scholar]
- Baoguo, M.A.; Hainan, L.I.; Junpeng, M.E.I.; Pei, O. Effect of Nano-TiO2 Addition on the Hydration and Hardening Process of Sulphoaluminate Cement. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 768–773. [Google Scholar]
- Sakthivel, R.; Arun, K.T.; Dhanabal, M.; Aravindan, V.; Aravindh, S. Experimental study of photocatalytic concrete using titanium dioxide. Int. J. Innov. Res. Sci. Technol. 2018, 4, 117–123. [Google Scholar]
- Khataee, R.; Heydari, V.; Moradkhannejhad, L.; Safarpour, M.; Joo, S.W. Self-cleaning and mechanical properties of modified white cement with nanostructured TiO2. J. Nanosci. Nanotechnol. 2013, 13, 5109–5114. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H. Pore structure and chloride permeability of concrete containing nano-particles for pavement. Constr. Build. Mater. 2011, 25, 608–616. [Google Scholar] [CrossRef]
- Behfarnia, K.; Azarkeivan, A.; Keivan, A. The effects of TiO2 and ZnO nanoparticles on physical and mechanical properties of normal concrete. Asian J. Civ. Eng. (Bhrc) 2013, 14, 517–531. [Google Scholar]
- Zailan, S.N.; Mahmed, N.; Abdullah, M.M.A.B.; Rahim, S.Z.A.; Halin, D.S.C.; Sandu, A.V.; Vizureanu, P.; Yahya, Z. Potential Applications of Geopolymer Cement-Based Composite as Self-Cleaning Coating: A Review. Coatings 2022, 12, 133. [Google Scholar] [CrossRef]
- Soleymani, F. Assessments of the effects of limewater on water permeability of TiO2 nanoparticles binary blended limestone aggregate-based concrete. J. Am. Sci. 2011, 7, 7–12. [Google Scholar]
- Soleymani, F. The filler effects TiO2 nanoparticles on increasing compressive strength of palm oil clinker aggregate-based concrete. J. Am. Sci. 2012, 8, 21–24. [Google Scholar]
- Sorathiya, J.; Shah, S.; Kacha, S. Effect on Addition of Nano “Titanium Dioxide”(TiO2) on Compressive Strength of Cementitious Concrete. Kalpa Publ. Civil Eng. 2017, 1, 219–225. [Google Scholar]
- Shekari, A.H.; Razzaghi, M.S. Influence of Nano Particles on Durability and Mechanical Properties of High Performance Concrete. Proc. Eng. 2011, 14, 3036–3041. [Google Scholar] [CrossRef]
- Patel, N.; Mishra, C.B. Laboratory Investigation of nano titanium dioxide (TiO2) in concrete for pavement. Int. Res. J. Eng. Technol. (IRJET) 2018, 5, 1634–1638. [Google Scholar]
- Ghosal, M.; Chakraborty, A.K. A comparative assessment of nano-SiO2 and nano-TiO2 insertion in concrete. Eur. J. Adv. Eng. Technol. 2015, 2, 44–48. [Google Scholar]
- Nazari, A. The effects of curing medium on flexural strength and water permeability of concrete incorporating TiO2 nanoparticles. Mater. Struct. 2011, 44, 773–786. [Google Scholar] [CrossRef]
- He, X.; Shi, X. Chloride Permeability and Microstructure of Portland Cement Mortars Incorporating Nanomaterials. Transp. Res. Rec. 2008, 2070, 13–21. [Google Scholar] [CrossRef]
- Li, H.; Xiao, H.; Guan, X.; Wang, Z.; Yu, L. Chloride diffusion in concrete containing nano-TiO2 under coupled effect of scouring. Compos. B. Eng. 2014, 56, 698–704. [Google Scholar] [CrossRef]
- Hassan, M.M.; Dylla, H.; Mohammad, L.N.; Rupnow, T. Methods for the application of titanium dioxide coatings to concrete pavement. Int. J. Pavement Res. Technol. 2012, 5, 12–20. [Google Scholar]
- Farzadnia, N.; Ali, A.A.A.; Demirboga, R.; Parvez, M. Characterization of high strength mortars with nano Titania at elevated temperatures. Contr. Build. Mater. 2013, 43, 469–479. [Google Scholar] [CrossRef]
- Feng, D.; Xie, N.; Gong, C. Portland cement paste modified by TiO2 nanoparticles: A microstructure perspective. Ind. Eng. Chem. Res. 2013, 52, 11575–11582. [Google Scholar] [CrossRef]
- Lackhoff, M.; Prieto, X.; Nestle, N.; Dehn, F.; Niessner, R. Photocatalytic activity of semiconductor-modified cement—Influence of semiconductor type and cement ageing. Appl. Catal. B Environ. 2003, 43, 205–216. [Google Scholar] [CrossRef]
- Kwon, J.M.; Kim, Y.H.; Song, B.K.; Yeom, S.H.; Kim, B.S.; Im, J.B. Novel immobilization of titanium dioxide (TiO2) on the fluidizing carrier and its application to the degradation of azo-dye. J. Hazard. Mater. 2006, 134, 230–236. [Google Scholar] [CrossRef]
- De Ceukelaire, L.; Van Nieuwenburg, D. Accelerated carbonation of a blast-furnace cement concrete. Cem. Concr. Res. 1993, 23, 442–452. [Google Scholar] [CrossRef]
- Castellote, M.; Fernandez, L.; Andrade, C.; Alonso, C. Chemical changes and phase analysis of OPC pastes carbonated at different CO2 concentrations. Mater. Struct. 2009, 42, 515–525. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Nakamura, N.; Komine, T. Continuous-sterilization system that uses photosemiconductor powders. Appl. Environ. Microbiol. 1988, 54, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Folli, A.; Jakobsen, U.H.; Guerrini, G.L.; Macphee, D.E. Rhodamine B discolouration on TiO2 in the cement environment: A look at fundamental aspects of the self-cleaning effect in concretes. J. Adv. Oxid. Technol. 2009, 12, 126–133. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Castellote, M. Quantification of hydroxyl radicals on cementitious materials by fluorescence spectrophotometry as a method to assess the photocatalytic activity. Cem. Concr. Res. 2015, 74, 108–115. [Google Scholar] [CrossRef]
- Dharma, H.N.C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Othman, M.H.D.; Rahman, M.A.; Jafri, N.N.M.; Suhaimin, N.S.; Nasir, A.M.; et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium Dioxide (TiO2)-Based Photocatalyst Materials Activity Enhancement for Contaminants of Emerging Concern (CECs) Degradation: In the Light of Modification Strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Sharifi, T.; Crmaric, D.; Kovacic, M.; Popovic, M.; Rokovic, M.K.; Kusic, H.; Jozić, D.; Ambrožić, G.; Kralj, D.; Kontrec, J.; et al. Tailored BiVO4 for Enhanced Visible-Light Photocatalytic Performance. J. Environ. Chem. Eng. 2021, 9, 106025. [Google Scholar] [CrossRef]
- Baxter, D.M.; Perkins, J.L.; McGhee, C.R.; Seltzer, J.M. A Regional Comparison of Mold Spore Concentrations Outdoors and Inside “Clean” and “Mold Contaminated” Southern California Buildings. J. Occup. Environ. Hyg. 2005, 2, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Hui Chen, P.E.; Song Deng, P.E.; Homer Bruner, C.E.M., Jr.; Garcia, J. Roots of Mold Problems and Humidity Control Measures in Institutional Buildings with Pre-Existing Mold Condition. In Proceedings of the Fourteenth Symposium on Improving Building Systems in Hot and Humid Climates, Richardson, TX, USA, 17–20 May 2004. [Google Scholar]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Kühn, K.P.; Chaberny, I.F.; Massholder, K.; Stickler, M.; Benz, V.W.; Sonntag, H.G.; Erdinger, L. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere 2003, 53, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Norimoto, K.; Kimura, T. Antibacterial Activity of Photocatalytic Titanium Dioxide Thin Films with Photodeposited Silver on the Surface of Sanitary Ware. J. Am. Ceram. Soc. 2005, 88, 95–100. [Google Scholar] [CrossRef]
- Watts, R.J.; Kong, S.; Orr, M.P.; Miller, G.C.; Henry, B.E. Photocatalytic inactivation of coliform bacteria and viruses in secondary wastewater effluent. Water Res. 1995, 29, 95–100. [Google Scholar] [CrossRef]
- Vohra, A.; Goswami, D.Y.; Deshpande, D.A.; Block, S.S. Enhanced photocatalytic inactivation of bacterial spores on surfaces in air. J. Ind. Microbiol. Biotechnol. 2005, 32, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Haleem Khan, A.A.; Mohan Karuppayil, S. Fungal pollution of indoor environments and its management. Saudi J. Biol. Sci. 2012, 19, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Bactericidal Activity of Copper-Deposited TiO2 Thin Film under Weak UV Light Illumination. Environ. Sci. Technol. 2003, 37, 4785–4789. [Google Scholar] [CrossRef] [PubMed]
- Oguma, K.; Katayama, H.; Ohgaki, S. Photoreactivation of Escherichia coli after Low- or Medium-Pressure UV Disinfection Determined by an Endonuclease Sensitive Site Assay. Appl. Environ. Microbiol. 2002, 68, 6029–6035. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of photocatalytic bactericidal action of powdered semiconductor TiO2 on mutans streptococci. J. Photochem. Photobiol. B. 1992, 14, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Linkous, C.A.; Carter, G.J.; Locuson, D.B.; Ouellette, A.J.; Slattery, D.K.; Smitha, L.A. Photocatalytic inhibition of algae growth using TiO2, WO3, and cocatalyst modifications. Environ. Sci. Technol. 2000, 34, 4754–4758. [Google Scholar] [CrossRef]
- Gogniat, G.; Thyssen, M.; Denis, M.; Pulgarin, C.; Dukan, S. The bactericidal effect of TiO2 photocatalysis involves adsorption onto catalyst and the loss of membrane integrity. FEMS Microbiol. Lett. 2006, 258, 18–24. [Google Scholar] [CrossRef]
- Mazurkova, N.A.; Spitsyna, Y.E.; Shikina, N.V.; Ismagilov, Z.R.; Zagrebel’Nyi, S.N.; Ryabchikova, E.I. Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnol. Russ. 2010, 5, 417–420. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; McIntosh, A.; Alvarez, P.J.J. Comparative toxicity of nano-scale TiO2, SiO2 and ZnO water suspensions. Water Sci. Technol. 2006, 54, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Armelao, L.; Barreca, D.; Bottaro, G.; Gasparotto, A.; Maccato, C.; Maragno, C.; Tondello, E.; Stangar, U.L.; Bergant, M.; Mahne, D. Photocatalytic and antibacterial activity of TiO2 and Au/TiO2 nanosystems. Nanotechnology 2007, 18, 375709. [Google Scholar] [CrossRef]
- Dědková, K.; Matějová, K.; Lang, J.; Peikertová, P.; Kutláková, K.M.; Neuwirthová, L.; Frydrýšek, K.; Kukutschová, J. Antibacterial activity of kaolinite/nanoTiO2 composites in relation to irradiation time. J. Photochem. Photobiol. B. 2014, 135, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Gurr, J.R.; Wang, A.S.S.; Chen, C.H. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, I.G.; El-Sayad, H.; El-Ghaly, A.E.; Moussa, S. Effect of micro TiO2 on cement mortar. EJME 2020, 5, 58–68. [Google Scholar] [CrossRef]
- Davidson, H.; Poon, M.; Saunders, R.; Shapiro, I.M.; Hickok, N.J.; Adams, C.S. Tetracycline tethered to titanium inhibits colonization by Gram-negative bacteria. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1381–1389. [Google Scholar] [CrossRef]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The Influence of Surface Modification on Bacterial Adhesion to Titanium-Based Substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ni, J.; Zheng, K.; Shen, Y.; Wang, X.; He, G.; Jin, S.; Tang, T. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int. J. Nanomed. 2013, 8, 3093–3105. [Google Scholar]
- Daly, M.J.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Zhai, M.; Leapman, R.D.; Lai, B.; Ravel, B.; Li, S.-M.W.; Kemner, K.M.; et al. Protein Oxidation Implicated as the Primary Determinant of Bacterial Radioresistance. PLoS Biol. 2007, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Carre, G.; Estner, M.; Gies, J.-P.; Andre, P.; Hamon, E.; Ennahar, S.; Keller, V.; Keller, N.; Lett, M.-C.; Horvatovich, P. TiO2 Photocatalysis Damages Lipids and Proteins in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.P.; Fernández-garcía, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Yu, K.-P.; Yang, K.-R.; Yang, S.-C.; Chen, Y.-L. Evaluation the antifungal effects of nano-metals loaded titanium dioxide on fungal spore. In Proceedings of the 10th International Conference on Healthy Buildings 2012, Brisbane, Australia, 8–12 July 2012. [Google Scholar]
- Yadav, H.M.; Kim, J.S.; Pawar, S.H. Developments in photocatalytic antibacterial activity of nano TiO2: A review. Korean J. Chem. Eng. 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.-S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Dubosc, A.; Escadeillas, G.; Blanc, P. Characterization of biological stains on external concrete walls and influence of concrete as underlying material. Cem. Concr. Res. 2001, 31, 1613–1617. [Google Scholar] [CrossRef]
- Kurth, J.C.; Giannantonio, D.J.; Allain, F.; Sobecky, P.A.; Kurtis, K.E. Mitigating biofilm growth through the modification of concrete design and practice. In Proceedings of the International RILEM Symposium on Photocatalysis, Environment and Construction Materials, Florence, Italy, 8–9 October 2007; pp. 8–9. [Google Scholar]
- Maury, A.; De Belie, N. State of the art of TiO2 containing cementitious materials: Self-cleaning properties. Mater. Constr. 2010, 60, 33–50. [Google Scholar] [CrossRef]
- Mejía, J.M.; Mendoza, J.D.; Yucuma, J.; Mejía de Gutiérrez, R.; Mejía, D.E.; Astudillo, M. Mechanical, in-vitro biological and antimicrobial characterization as an evaluation protocol of a ceramic material based on alkaline activated metakaolin. Appl. Clay Sci. 2019, 178, 105141. [Google Scholar] [CrossRef]
- Damian, L.; Patachia, S. Method for testing the antimicrobial character of the materials and their fitting to the scope. Bull. Transilv. Univ. Bras. 2014, 7, 37–44. [Google Scholar]
- ASTM E2149; Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents under Dynamic Contact Conditions. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM E2180; Test for Hydrophobic Antimicrobial Surfaces. ASTM International: West Conshohocken, PA, USA, 2018.
- ISO 22196:2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. International Organization for Standardization: Geneva, Switzerland, 2020.
- ASTM E1428; Antimicrobial Pink Stain Test. ASTM International: West Conshohocken, PA, USA, 2015.
- STAS 12718/1989; Lacquers and Paints. Determination of the Sterility or Degree of Contamination with Micro-Organisms of Film-Forming Products. Romanian Institute for Standardization: Bucharest, Romania, 1989. (In Romanian)
- ISO 27447; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Antibacterial Activity of Semiconducting Photocatalytic Materials. International Organization for Standardization: Geneva, Switzerland, 2019.
- Renz, C. Lichtreaktionen der Oxyde des Titans, Cers und der Erdsauren. Helv. Chim. Acta 1921, 4, 961–968. [Google Scholar] [CrossRef]
- Matusunga, T. Sterilization with particulate photosemiconductor. J. Antibact. Antifung. Agents 1985, 13, 211–220. [Google Scholar]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Aissa, A.H.; Puzenat, E.; Plassais, A.; Herrmann, J.-M.; Haehnel, C.; Guillard, C. Characterization and photocatalytic performance in air of cementitious materials containing TiO2. Case study of formaldehyde removal. Appl. Catal. B Environ. 2011, 107, 1–8. [Google Scholar] [CrossRef]
- Giannantonio, D.J.; Kurth, J.C.; Kurtis, K.E.; Sobecky, P.A. Effects of concrete properties and nutrients on fungal colonization and fouling. Int. Biodeterior. Biodegrad. 2009, 63, 252–259. [Google Scholar] [CrossRef]
- Sökmen, M.; Candan, F.; Sümer, Z. Disinfection of E. coli by the Ag–TiO2/UV system: Lipidperoxidation. J. Photochem. Photobiol. A 2001, 143, 241–244. [Google Scholar] [CrossRef]
- Sökmen, M.; Degerli, S.; Aslan, A. Photocatalytic disinfection of Giardia intestinalis and Acanthamoeba castellani cysts in water. Exp. Parasitol. 2008, 119, 44–48. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Sunada, K.; Iyoda, T.; Hashimoto, K.; Fujishima, A. Photocatalytic bactericidal effect of TiO2 thin films: Dynamic view of the active oxygen species responsible for the effect. J. Photochem. Photobiol. A 1997, 106, 51–56. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Ditta, I.B.; Steele, A.; Liptrot, C.; Tobin, J.; Tyler, H.; Yates, H.M.; Sheel, D.W.; Foster, H.A. Photocatalytic antimicrobial activity of thin surface films of TiO2, CuO and TiO2/CuO dual layers on Escherichia coli and bacteriophage T4. Appl. Microbiol. Biotechnol. 2008, 79, 127–133. [Google Scholar] [CrossRef]
- Brook, L.A.; Evans, P.; Foster, H.A.; Pemble, M.E.; Steele, A.; Sheel, D.W.; Yates, H.M. Highly bioactive silver and silver/titania composite films grown by chemical vapour deposition. J. Photochem. Photobiol. 2007, 187, 53–63. [Google Scholar] [CrossRef]
- Yates, H.M.; Brook, L.A.; Ditta, I.B.; Evans, P.; Foster, H.A.; Sheel, D.W.; Steele, A. Photo-induced self-cleaning and biocidal behaviour of titania and copper oxide multilayers. J. Photochem. Photobiol. A 2008, 195, 197–205. [Google Scholar] [CrossRef]
- Yates, H.M.; Brook, L.A.; Sheel, D.W.; Ditta, I.B.; Steele, A.; Foster, H.A. The growth of copper oxides on glass by flame assisted chemical vapour deposition. Thin Solid Films 2008, 517, 517–521. [Google Scholar] [CrossRef]
- Sahana, R. Setting Time, Compressive Strength and Microstructure of Geopolymer Paste. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 311–316. [Google Scholar]
- Yahya, Z.; Abdullah, M.M.A.B.; Ramli, N.M.; Burduhos-Nergis, D.D.; Abd Razak, R. Influence of Kaolin in Fly Ash Based Geopolymer Concrete: Destructive and Non-Destructive Testing. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012068. [Google Scholar] [CrossRef]
- Sassolini, A.; Malizia, A.; D’Amico, F.; Carestia, M.; Di Giovanni, D.; Cenciarelli, O.; Bellecci, C.; Gaudio, P. Evaluation of the effectiveness of titanium dioxide (TiO2) self-cleaning coating for increased protection against cbrn incidents in critical infrastructures. Def. S&T Tech. Bull. 2014, 7, 9–17. [Google Scholar]
- Al Bakri Abdullah, A.M.; Kamarudin, H.; Binhussain, M.; Nizar, K.; Mastura, W.I.W. Mechanism and Chemical Reaction of Fly Ash Geopolymer Cement—A Review. Asian J. Sci. Res. 2011, 1, 247–253. [Google Scholar]
- Adewuyi, Y.G. Recent Advances in Fly-Ash-Based Geopolymers: Potential on the Utilization for Sustainable Environmental Remediation. ACS Omega 2021, 6, 15532–15542. [Google Scholar] [CrossRef]
- Abbas, R.; Khereby, M.A.; Ghorab, H.Y.; Elkhoshkhany, N. Preparation of Geopolymer Concrete Using Egyptian Kaolin Clay and the Study of Its Environmental Effects and Economic Cost. Clean Technol. Environ. Policy 2020, 22, 669–687. [Google Scholar] [CrossRef]
- Albidah, A.; Alghannam, M.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Characteristics of Metakaolin-Based Geopolymer Concrete for Different Mix Design Parameters. J. Mater. Res. Technol. 2021, 10, 84–98. [Google Scholar] [CrossRef]
- Ionescu, B.A.; Lăzărescu, A.-V.; Hegyi, A. The Possibility of Using Slag for the Production of Geopolymer Materials and Its Influence on Mechanical Performances—A Review. Proceedings 2020, 63, 30. [Google Scholar]
- Davidovits, J. Synthesis of new high-temperature Geopolymers for reinforced plastics and composites. In Proceedings of the PACTEC‘79 Society of Plastics Engineers, Costa Mesa, CA, USA, 1 January–2 February 1979; pp. 151–154. [Google Scholar]
- Assi, L.; Ghahari, S.; Deaver, E.; Leaphart, D.; Ziehl, P. Improvement of the early and final compressive strength of fly ash-based geopolymer concrete at ambient conditions. Constr. Build. Mater. 2016, 123, 806–813. [Google Scholar] [CrossRef]
- De Silva, P.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerisation: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-Activated Fly Ashes. A Cement for the Future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; Van Deventer, J.S.J. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers of the first generation: SILIFACE-Process. In Proceedings of the Geopolymer’88, First Europrean Conference on Soft Mineralogy, Compiegne, France, 1–3 June 1988; pp. 49–67. [Google Scholar]
- Wallah, S. Drying shrinkage of heat-cured fly ash-based geopolymer concrete. Mod. Appl. Sci. 2009, 3, 14–21. [Google Scholar] [CrossRef]
- Al Bakri Abdullah, A.M.; Hussin, K.; Bnuhussain, M.; Ismail, K.N.; Ahmad, M.I. Chemical Reactions in the Geopolymerisation Process Using Fly Ash-Based Geopolymer: A review. AJBAS 2011, 5, 1199–1203. [Google Scholar]
- Skvara, F. Alkali Activated Material—Geopolymer; Department of Glass and Ceramics, Faculty of Chemical Technology, ICT Prague: Prague, Czech Republic, 2007; pp. 661–676. [Google Scholar]
- Amran, M.; Fediuk, R.; Murali, G.; Avudaiappan, S.; Ozbakkaloglu, T.; Vatin, N.; Karelina, M.; Klyuev, S.; Gholampour, A. Fly Ash-Based Eco-Efficient Concretes: A Comprehensive Review of the Short-Term Properties. Materials 2021, 14, 4264. [Google Scholar] [CrossRef]
- Buchwald, A. What are geopolymers? Current State of Research and Technology, The Opportunities They Offer, and Their Significance for The Precast Industry, Concrete Precasting Plant and Technology. Betonw. Fert.-Tech. 2006, 72, 42. [Google Scholar]
- Xu, H.; van Deventer, J. The effect of alkali metals on the formation of geopolymeric gels from alkali-feldspats. Colloids Surf. A Physicochem. Eng. Asp. 2013, 216, 27–44. [Google Scholar] [CrossRef]
- Weng, L.; Sagoe-Crentsil, K. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part I—Low Si/Al ratio systems. J. Mater. Sci. 2007, 42, 2997–3006. [Google Scholar] [CrossRef]
- Andini, S.; Cioffi, R.; Colangelo, F.; Grieco, T.; Montagnaro, F.; Santoro, L. Coal fly ash as raw material for the manufacture of geopolymer-based product. J. Waste Manag. 2008, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Duxon, P.; Fernande-Jimenez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Andrés, J.M.; Stanton, K.; Towler, M.; Jurcovicova, M.; Jones, R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1563. [Google Scholar] [CrossRef]
- Chen-Tan, N.W.; Van Riessen, A.; Ly, C.V.; Southam, D. Determining the reactivity of a fly ash for production of geopolymer. J. Am. Ceram. Soc. 2009, 92, 881–887. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Sarker, P.K.; Rangan, V.B. Early Age Properties of Low-calcium Fly Ash Geopolymer Concrete Suitable for Ambient Curing. Procedia Eng. 2015, 125, 601–607. [Google Scholar] [CrossRef]
- Swanepoel, J.C.; Strydom, C.A. Utilisation of fly ash in a geopolymeric material. Appl. Geochem. 2002, 17, 114–148. [Google Scholar] [CrossRef]
- Goretta, K.C.; Gutierrez-Mora, F.; Singh, D.; Routbort, J.L.; Lukey, G.C.; van Deventer, J.S.J. Erosion of geopolymers made from industrial waste. J. Mater. Sci. 2007, 42, 3066–3072. [Google Scholar] [CrossRef]
- Puertas, F.; Martinez-Ramirez, S.; Alonso, S.; Vazquez, T. Alkali activated fly ash/slag cements: Strength behavior and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Farhana, Z.; Kamarudin, H.; Rahmat, A.; Al Bakri, A.M. The Relationship between Water Absorption and Porosity for Geopolymer Paste. Mater. Sci. Forum 2014, 803, 166–172. [Google Scholar] [CrossRef]
- Aly, M.; Hashmi, M.S.; Olabi, A.G.; Messeiry, M. Effect of colloidal nano-silica on the mechanical and physical behavior of waste-glass cement mortar. Mater. Des. 2012, 33, 127135. [Google Scholar] [CrossRef]
- Khater, M.H. Effect of nano-silica on microstructure formation of low-cost geopolymer binder. Nanocomposites 2016, 2, 84–97. [Google Scholar] [CrossRef]
- Khater, M.H. Physicomechanical properties of nano-silica effect on geopolymer composites. J. Build. Mater. Struct. 2016, 3, 1–14. [Google Scholar] [CrossRef]
- Assaedi, H.; Shaikh, F.U.; Low, I.M. Effect of nanoclay on durability and mechanical properties of flax fabric reinforced geopolymer composites. J. Asian Ceram. Soc. 2017, 5, 62–70. [Google Scholar] [CrossRef]
- Adak, D.; Sarkar, M.; Mandal, S. Effect of nano-silica on strength and durability of fly ash based geopolymer mortar. Construct. Build. Mater. 2014, 70, 453–459. [Google Scholar] [CrossRef]
- Shaikh, F.U.; Supit, S.W.; Sarker, P.K. A study on the effect of nano silica on compressive strength of high volume fly ash mortars and concretes. Mater. Des. 2014, 60, 433–442. [Google Scholar] [CrossRef]
- Zhaoheng, L.; Wei, Z.; Ruilan, W.; Fangzhu, C.; Xichun, J.; Peitong, G. Effects of Reactive MgO on the Reaction Process of Geopolymer. Materials 2019, 12, 526. [Google Scholar]
- Hu, M.; Zhu, X.; Long, F. Alkali-activated fly ash-based geopolymers with zeolite or bentonite as additives. Cem. Concr. Compos. 2009, 31, 762–768. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Atis, C.D.; Görür, E.B.; Karahan, O.; Bilim, C.; Ilkentapar, S.; Luga, E. Very high strength (120 MPa) Class F fly ash geopolymer mortar activated at different NaOH amount, heat curing temperature and heat curing duration. Constr Build. Mater. 2015, 96, 673–678. [Google Scholar] [CrossRef]
- Al Bakri, M.M.; Mohammed, H.; Kamarudin, H.; Niza, K.; Zarina, Y. Review of Fly Ash-Based Geopolymer Concrete Without Portland Cement. J. Eng. Technol. 2011, 3, 1–4. [Google Scholar]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Technical Report GC1; Civil Engineering Faculty, Technical University: Perth, Australia, 2005. [Google Scholar]
- Al Bakri Mustafa, A.M.; Kamarudin, H.; Binhussain, M.; Niza, I.K. The effect of curing temperature on physical and chemical properties of geopolymers. Phys. Procedia 2011, 22, 286–291. [Google Scholar]
- Al Bakri Mustafa, A.M.; Kamarudin, H.; Bnhussain, M.; Nizar, I.K.; Rafiza, A.R.; Zarina, Y. The processing, characterization, and properties of fly ash based geopolymer concrete. Rev. Adv. Mater. Sci. 2012, 30, 90–97. [Google Scholar]
- Chindaprasirt, P.; Chareerat, T.; Sirivivatnano, V. Workability and strength of coarse high calcium fly ash geopolymer. Cem. Conc. Comp. 2007, 29, 224–229. [Google Scholar] [CrossRef]
- Morsy, M.S.; Alsaye, S.H.; Al-Salloum, Y.; Almusallam, T. Effect of sodium silicate to sodium hydroxide ratios on strength and microstructure of fly ash geopolymer binder. Arab. J. Sci. Eng. 2014, 39, 4333–4339. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vasquez, K.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Technical Report GC2; Civil Engineering Faculty, Technical University: Perth, Australia, 2005. [Google Scholar]
- Provis, J.L.; Yong, C.Z.; Duxson, P.; van Deventer, J. Correlating mechanical and thermal properties of sodium silicate-fly ash geopolymers. Colloids Surf. A Physicochem. Eng. 2009, 336, 57–63. [Google Scholar] [CrossRef]
- Sumajouw, D.; Hardjito, D.; Wallah, S.; Rangan, B. Fly ash-based geopolymer concrete: Study of slender reinforced columns. J. Mater. Sci. 2007, 42, 3124–3130. [Google Scholar] [CrossRef]
- Vora, P.; Dave, U. Parametric Studies on Compressive Strength of Geopolymer Concrete. Procedia Eng. 2013, 51, 210–219. [Google Scholar] [CrossRef]
- Sindhunata; van Deventer, J.S.J.; Lukey, G.C.; Xu, H. Effect of Curing Temperature and Silicate Concentration on Fly-Ash-Based Geopolymerization. Ind. Eng. Chem. Res. 2006, 45, 3559–3569. [Google Scholar] [CrossRef]
- Raijiwala, D.; Patil, H. Geopolymer concrete: A green concrete. In Proceedings of the 2nd International Conference on Chemical, Biological and Environmental Engineering (ICBEE 2010), Cairo, Egypt, 2–4 November 2010. [Google Scholar]
- Mishra, A.; Choudhary, D.; Jain, N.; Kumar, M.; Sharda, N.; Dutta, D. Effect of concentration of alkaline liquid and curing time on strength and water absorption of geopolymer concrete. J. Eng. Appl. Sci. 2008, 3, 14–18. [Google Scholar]
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- Memon, F.; Nuruddin, M.F.; Khan, S.H.; Shafiq, N.R. Effect of sodium hydroxide concentration on fresh properties and compressive strength of self-compacting geopolymer concrete. J. Eng. Sci. Technol. 2013, 8, 44–56. [Google Scholar]
- Barbosa, V.; Mackenzie, K.; Thaumaturgo, C. Synthesis and characterisation of sodium polysialate inorganic polymer based on alumina and silica. In Proceedings of the Geopolymer’99 International Conference, Saint-Quentin, Geopolymer Institute, Saint-Quentin, France, 30 June–2 July 1999. [Google Scholar]
- Luhar, S.; Dave, U. Investigations on mechanical properties of fly ash and slag based geopolymer concrete. Ind. Concr. J. 2016, 34–41. [Google Scholar]
- Ma, Y.; Hu, J.; Ye, G. The effect of activating solution on the mechanical strength, reaction rate, mineralogy, and microstructure of alkali-activated fly ash. J. Mater. Sci. 2012, 47, 4568–4578. [Google Scholar] [CrossRef]
- Xie, J.; Yin, J.; Chen, J.; Xu, J. Study on the geopolymer based on fly ash and slag. Energy Environ. 2009, 3, 578–581. [Google Scholar]
- van Jaarsveld, J.; van Deventer, J.; Lukey, G. The effect of composition and temperature on the properties of fly ash-and kaolinite-based geopolymers. J. Chem. Eng. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Hatanaka, S.; Cao, T. High-strength geopolymer using fine high-calcium fly ash. Mater. Civ. Eng. 2010, 23, 264–270. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Garcia-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Sinsiri, T. Workability and compressive strength of geopolymer mortar from fly ash containing diatomite. Eng. J. 2011, 38, 11–26. [Google Scholar]
- Kong, D.; Sanjayan, J. Effect of elevated temperatures on geopolymer paste, mortar and concrete. Cem. Concr. Res. 2010, 40, 334–339. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H. Self-solidification/stabilization of heavy metal wastes of class C fly ash-based geopolymers. J. Mater. Civ. Eng. 2012, 25, 491–496. [Google Scholar] [CrossRef]
- Rashad, A.; Zeedan, S. The effect of activator concentration on the residual strength of alkali-activated fly ash pastes subjected to thermal load. Constr. Build. Mater. 2011, 25, 3098–3107. [Google Scholar] [CrossRef]
- Sukmak, P.; Horpibulsuk, S.; Shen, S. Strength development in clay-fly ash geopolymer. Contr. Build. Mater. 2013, 40, 566–574. [Google Scholar] [CrossRef]
- Taebuanhuad, S.; Rattanasak, U.; Jenjirapanya, S. Strength behavior of fly ash geopolymer with microwave pre-radiation curing. J. Ind. Technol. 2012, 8, 1–8. [Google Scholar]
- Lăzărescu, A.V.; Szilagyi, H.; Baeră, C.; Ioani, A. Parameters Affecting the Mechanical Properties of Fly Ash-Based Geopolymer Binders–Experimental Results. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012035. [Google Scholar] [CrossRef]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Technical Report GC3; Civil Engineering Faculty, Technical University: Perth, Australia, 2006. [Google Scholar]
- Fernandez-Jimenez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Omar, O.M.; Heniegal, A.M.; Abd Elhameed, G.D.; Mohamadien, H.A. Effect of Local Steel Slag as a Coarse Aggregate on Properties of Fly Ash Based-Geopolymer Concrete. Int. J. Civ. Environ. 2015, 3, 1452–1460. [Google Scholar]
- Perera, D.S.; Uchida, O.; Vance, E.R.; Finnie, K.S. Influence of curing schedule on the integrity of geopolymers. J. Mater. Sci. 2007, 42, 3099–3106. [Google Scholar] [CrossRef]
- Davidovits, J. Properties of geopolymer cements. In Proceedings of the First International Conference on Alkaline Cements and Concretes, Kiev, Ukraine, 11–14 October 1994; SRIBM, Kiev State Technical University: Kiev, Ukraine, 1994; pp. 131–149. [Google Scholar]
- Sikora, S.; Gapys, E.; Michalowski, B.; Horbanowicz, T.; Hynowski, M. Geopolymer coating as protection of concrete against chemical attack and corrosion. E3S Web Conf. 2018, 49, 00101. [Google Scholar] [CrossRef]
- Cassar, L.; Pepe, C.; Tognon, G.; Guerrini, G.L.; Amadelli, R. White Cement for Architectural Concrete Possessing Photocatalytic Properties. In Proceedings of the 11th International Congress on the Chemistry of Cement, Durban, South Africa, 11–16 May 2003. [Google Scholar]
- Andaloro, A.; Mazzucchelli, E.S.; Lucchini, A.; Pedeferri, M.P. Photocatalytic self-cleaning coating for building façade maintainance. Performance analysis through a case-study application. J. Façade Des. Eng. 2016, 4, 115–129. [Google Scholar]
- Guerrero, L.E.; Gómez-Zamorano, L.; Jiménez-Relinque, E. Effect of the addition of TiO2 nanoparticles in alkali-activated materials. Constr. Build. Mater. 2020, 245, 118370. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Yan, C.; Peng, R.; Wang, H. Geopolymer-TiO2 Nanocomposites for Photocsatalysis: Synthesis by One-Step Adding Treatment Versus Two-Step Acidification Calcination. Minerals 2019, 9, 658. [Google Scholar] [CrossRef]
- Singh, B.; Ishwaraya, G.; Gupta, M.; Bhattacharyya, S.K. Review: Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structure, Processing, Properties and Industrial Applications; Woodhead Publishing Limited: Cambridge, UK, 2009. [Google Scholar]
- Ma, C.K.; Awang, A.Z.; Omar, W. Structural and material perfromance of geopolymer concrete: A review. Constr. Build Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Khalil, K.A. Application of thermal treatment on cement kiln dust and feldspar to create one-part geopolymer cement. Constr. Build. Mater. 2018, 187, 231–237. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; Palomo, A. Characterization of fly ashes. Potential reactivity as alkaline cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Malek, R.I.A.; Roy, D.M. Structure and properties of alkaline activated cementitious materials. In Proceedings of the 97th Annual Meeting and the 1995 Fall Meetings of the Materials & Equipment and Whitewares Divisions, Cincinnati, OH, USA, 30 April–3 May 1995. [Google Scholar]
- Provis, J.L.; Brice, D.G.; Buchwald, A.; Duxson, P.; Kavalerova, E.; Krivenko, P.V.; Shi, C.; van Deventer, J.S.J.; Wiercx, J.A. Demonstration Projects in Building and Civil Infrastructure; Alkali-Activated Materials: State-of-the-Art Report; RILEM TC 224-AAM; Springer: Dordrecht, The Netherlands, 2014; pp. 309–338. [Google Scholar]
- Hoy, M.; Horpibulsuk, S.; Arulrajah, A. Strength development of recycled asphalt pavement—Fly ash geopolymer as a road construction material. Constr. Build. Mater. 2016, 117, 209–219. [Google Scholar] [CrossRef]
- Murgod, G.; Shetty, K.; Raja, A. Self-consolidating paving grade geopolymer concrete. IOP Conf. Ser. Mater. Sci. Eng. 2018, 431, 92006. [Google Scholar]
- Moutinho, S.; Costa, C.; Cerqueira, A.; Rocha, F.; Velosa, A. Geopolymers and polymers in the conservatiopn of tile facades. Constr. Build. Mater. 2019, 197, 175. [Google Scholar] [CrossRef]
- Salwa, M.; Al-Bakri Mustafa, M.M.; Kamarudin, H.; Ruzaidi, C.; Binhussain, M.; Syed Zuber, S.Z. Review on current geopolymer as a coating material. Aust. J. Basic Appl. Sci. 2013, 7, 246–257. [Google Scholar]
- Aguirre-Guerreo, A.M.; Robayo-Salazar, R.A.; de Gutierrez, R.M. A novel geopolymer application: Coatings to protect reinforced concrete against corrosion. Appl Clay Sci. 2017, 135, 437–446. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, B.; Wang, L.; Xing, M.; Lei, J. Mechanism of Photocatalysis. In Photocatalysis. Lecture Notes in Chemistry; Springer: Singapore, 2018; Volume 100. [Google Scholar]
- Rabajczyk, A.; Zielecka, M.; Klapsa, W.; Dziechciarz, A. Self-Cleaning Coatings and Surfaces of Modern Building Materials for the Removal of Some Air Pollutants. Materials 2021, 14, 2161. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.C.; Gururani, P.; Gairola, S.P. Metal Oxide Nanoparticles and Their Nanocomposite-Based Materials as Photocatalysts in the Degradation of Dyes. Biointerface Res. Appl. Chem. 2022, 12, 6557–6579. [Google Scholar]
- Burduhos Nergis, D.D.; Vizureanu, P.; Ardelean, I.; Sandu, A.V.; Corbu, O.C.; Matei, E. Revealing the Influence of Microparticles on Geopolymers’ Synthesis and Porosity. Materials 2020, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Meor Ahmad Tajudin, M.A.F.; Abdullah, M.M.A.B.; Sandu, A.V.; Nizar, K.; Moga, L.; Neculai, O.; Muniandy, R. Assessment of Alkali Activated Geopolymer Binders as an Alternative of Portland Cement. Mater. Plastice. 2017, 54, 145–154. [Google Scholar]
- Vizureanu, P.; Samoila, C.; Cotfas, D. Materials Processing using Solar Energy. Environ. Eng. Manag. J. 2009, 8, 301–306. [Google Scholar] [CrossRef]
- Azimi, E.A.; Abdullah, M.M.A.B.; Vizureanu, P.; Salleh, M.A.A.M.; Sandu, A.V.; Chaiprapa, J.; Yoriya, S.; Hussin, K.; Aziz, I.H. Strength Development and Elemental Distribution of Dolomite/Fly Ash Geopolymer Composite under Elevated Temperature. Materials 2020, 13, 1015. [Google Scholar] [CrossRef] [PubMed]
- Burduhos Nergis, D.D.; Vizureanu, P.; Corbu, O. Synthesis and Characteristics of Local Fly Ash Based Geopolymers Mixed with Natural Aggregates. Rev. De Chim. 2019, 70, 1262–1267. [Google Scholar] [CrossRef]
- Krishnan, U.; Sanalkumar, A.; Yahg, E.-H. Self-cleaning performance of nano-TiO2 modified metakaolin-based geopolymers. Cem. Concr. Res. 2021, 115, 103847. [Google Scholar]
- ASTM C1437–07; Standard Test Metod for Flow of Hydrauloc Cement Mortar. ASTM International: West Conshohocken, PA, USA, 2007.
- Duan, P.; Yan, C.; Luo, W.; Zhou, W. Effects of adding nano-TiO2 on compressive strength, drying shrinkage, carbonation and microstructure of fluidized bed fly ash based geopolymer paste. Constr. Build. Mater. 2016, 106, 115–125. [Google Scholar] [CrossRef]
- Zulkifly, K.; Heah, C.Y.; Liew, Y.M.; Abdullah, M.M.A.B.; Abdullah, S.F.A. The Synergetic Compressive Strength and Microstructure of Fly Ash and Metakaolin Blend Geopolymer Pastes. AIP Conf. Proc. 2018, 2045, 020100. [Google Scholar]
- Syamsidar, D. The properties of nano TiO2-geopolymer composite as a material for functional surface application. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2017; Volume 97, p. 01013. [Google Scholar]
- Guzmán-Aponte, L.A.; de Gutiérrez, R.M.; Maury-Ramírez, A. Metakaolin-Based Geopolymer with Added TiO2 Particles: Physicomechanical Characteristics. Coatings 2017, 7, 233. [Google Scholar] [CrossRef]
- Sastry, K.G.K.; Sahitya, P.; Ravitheja, A. Influence of nano TiO2 on strength and durability properties of geopolymer concrete. Mater. Today Proc. 2020, 45, 1017–1025. [Google Scholar] [CrossRef]
- Subaer; Haris, A.; Noor Afifah, K.; Akifah, N.; Zulwiyati, R. Thermo-Mechanical Properties of Geopolymer/Carbon Fiber/TiO2 Nanoparticles (NPs) Composite. Mater. Sci. Forum 2019, 967, 267–273. [Google Scholar] [CrossRef]
- Bonilla, A.; Villaquirán-Caicedo, M.A.; Mejía de Gutiérrez, R. Novel Alkali-Activated Materials with Photocatalytic and Bactericidal Properties Based on Ceramic Tile Waste. Coatings 2022, 12, 35. [Google Scholar] [CrossRef]
- Sakai, N.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Enhancement of the photoinduced hydrofilic conversion rate of TiO2 film electrode surfaces by anodic polarization. J. Phys. Chem. B. 2001, 105, 3023–3026. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sakamoto, K.; Martra, G.; Coluccia, S.; Anpo, M. Mechanism of photoinduced superhydrophilicity on the TiO2 photocatalyst surface. J. Phys. Chem. B. 2005, 109, 15422–15428. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.; Gaylarde, C.C.; Shirakawa, M.A. Photocatalytic Activity of ZnO and TiO2 ‘Nanoparticles’ for Use in Cement Mixes. Constr. Build. Mater. 2018, 167, 853–859. [Google Scholar] [CrossRef]
- Zailan, S.N.; Bouaissi, A.; Mahmed, N. Influence of ZnO Nanoparticles on Mechanical Properties and Photocatalytic Activity of Self-cleaning ZnO-Based Geopolymer Paste. J. Inorg. Organomet. Polym. 2020, 30, 2007–2016. [Google Scholar] [CrossRef]
- Min Li, C.; He, Y.; Tang, Q.; Tuo Wang, K.; Min Cui, X.; Min Li, C.; He, Y.; Tang, Q.; Tuo Wang, K.; Min Cui, X. Study of the preparation of CdS on the surface of geopolymer spheres and photocatalyst performance. Mater. Chem. Phys. 2016, 178, 204–210. [Google Scholar]
- Gasca-Tirado, J.R.; Manzano-Ramırez, A.; Villasenor-Mora, C.; Muniz-Villarreal, M.S.; Zaldivar-Cadena, A.A.; Rubio-Avalos, J.C.; Borras, V.A.; Mendoza, R.N. Incorporation of photoactive TiO2 in an aluminosilicate inorganic polymer by ion exchange. Microporous Mesoporous Mater. 2012, 153, 282–287. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L. Fly ash-based geopolymer as a novel photocatalyst for degradation of dye from wastewater. Particuology 2013, 11, 353–358. [Google Scholar] [CrossRef]
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Razak, R.A.; Vizureanu, P.; Sandu, A.V.; Matasaru, P.-D. A State-of-the-Art Review on Innovative Geopolymer Composites Designed for Water and Wastewater Treatment. Materials 2021, 14, 7456. [Google Scholar] [CrossRef] [PubMed]
- Kaya-Özkiper, K.; Uzun, A.; Soyer-Uzun, S. Red Mud- and Metakaolin-Based Geopolymers for Adsorption and Photocatalytic Degradation of Methylene Blue: Towards Self-Cleaning Construction Materials. J. Clean. Prod. 2021, 288, 125120. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, K.; Liu, Y. Geopolymer-supported photocatalytic TiO2 film: Preparation and characterization. Constr. Build. Mater. 2017, 151, 63–70. [Google Scholar] [CrossRef]
- Saufi, H.; el Alouani, M.; Alehyen, S.; el Achouri, M.; Aride, J.; Taibi, M. Photocatalytic Degradation of Methylene Blue from Aqueous Medium onto Perlite-Based Geopolymer. Int. J. Chem. Eng. 2020, 2020, 9498349. [Google Scholar] [CrossRef]
- Jdm, K.; Yusoff, M.M.; Aqilah, N.S. Degradation of Methylene Blue via Geopolymer Composite Photocatalyst. Solid State Sci. Technol. 2013, 21, 23–30. [Google Scholar]
- Yang, L.; Wang, F.; Du, D.; Liu, P.; Zhang, W.; Hu, S. Enhanced photocatalytic efficiency and long-term performance of TiO2 in cementitious materials by activated zeolite fly ash bead carrier. Constr. Build. Mater. 2016, 126, 886–893. [Google Scholar] [CrossRef]
- El Alouani, M.; Alehyen, S.; El Achouri, M.; Taibi, M. Preparation, Characterization, and Application of Metakaolin-Based Geopolymer for Removal of Methylene Blue from Aqueous Solution. J. Chem. 2019, 2019, 4212901. [Google Scholar] [CrossRef]
- Strini, A.; Roviello, G.; Ricciotti, L.; Ferone, C.; Messina, F.; Schiavi, L.; Corsaro, D.; Cioffi, R. TiO2-Based Photocatalytic Geopolymers for Nitric Oxide Degradation. Materials 2016, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Geddes, D.A.; Walkley, B.; Provis, J.L.; Mechtcherine, V.; Tsang, D.C.W. The Role of Zinc in Metakaolin-Based Geopolymers. Cem. Concr. Res. 2020, 136, 106194. [Google Scholar] [CrossRef]
- Qin, Y.; Fang, Z.; Chai, X.; Cui, X. A Superhydrophobic Alkali Activated Materials Coating by Facile Preparation. Coatings 2022, 12, 864. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jitsangiam, P.; Pachana, P.K. Self-cleaning superhydrophobic fly ash geopolymer. Sci. Rep. 2023, 13, 44. [Google Scholar] [CrossRef]
- Permatasari, A.D.; Fahira, N.; Husna Muslimin, N.; Subaer. Development of Photoactive Nano TiO2 Thin Film-Geopolymer Based on Laterite Soils Deposit Gowa Regency as Self-Cleaning Materia. Mater. Sci. Forum 2019, 967, 274–280. [Google Scholar] [CrossRef]
- Vancea, D.P.C.; Kamer-Ainur, A.; Simion, L.; Vanghele, D. Export expansion policies. An analysis of Romanian exports between 2005–2020 using Principal Component Analysis method and short recommendations for increasing this activity. Transform. Bus. Econ. 2021, 20, 614–634. [Google Scholar]
- Kamer-Ainur, A.; Munteanu, I.F.; Stan, M.-I.; Chiriac, A. A Multivariate Analysis on the Links Between Transport Noncompliance and Financial Uncertainty in Times of COVID-19 Pandemics and War. Sustainability 2022, 14, 10040. [Google Scholar]
- Batrancea, L.M.; Pop, M.C.; Rathnaswamy, M.M.; Batrancea, I.; Rus, M.-I. An Empirical Investigationon the Transition Process toward a Green Economy. Sustainability 2021, 13, 13151. [Google Scholar] [CrossRef]
- Batrancea, L.; Rathnaswamy, M.M.; Rus, M.I.; Tulai., H. Determinants of Economic Growth for the Last Half Century: A Panel Data Analysis on 50 Countries. J. Knowl. Econ. 2022, 1–25. [Google Scholar] [CrossRef]
- Yao, Q.; Jahanshahi, H.; Batrancea, L.M.; Alotaibi, N.D.; Rus, M.-I. Fixed-Time Output-Constrained Synchronization of Unknown Chaotic Financial Systems Using Neural Learning. Mathematics 2022, 10, 3682. [Google Scholar] [CrossRef]
- Cheng, G.; Xu, F.; Xiong, J.; Tian, F.; Ding, J.; Stadler, F.J.; Chen, R. Enhanced adsorption and photocatalysis capability of generally synthesized TiO2-carbon materials hybrids. Adv. Powder Technol. 2016, 27, 1949–1962. [Google Scholar] [CrossRef]
- Tian, H.; Shen, K.; Hu, X.; Qiao, L.; Zheng, W. N, S co-doped graphene quantum dots-graphene-TiO2 nanotubes composite with enhanced photocatalytic activity. J. Alloys Compd. 2017, 691, 369–377. [Google Scholar] [CrossRef]
- Razzaq, A.; Grimes, C.A.; In, S. Il Facile fabrication of a noble metal-free photocatalyst: TiO2 nanotube arrays covered with reduced graphene oxide. Carbon 2016, 98, 537–544. [Google Scholar] [CrossRef]
- Lettieri, S.; Gargiulo, V.; Pallotti, D.K.; Vitiello, G.; Maddalena, P.; Alfè, M.; Marotta, R. Evidencing opposite charge-transfer processes at TiO2/graphene-related materials interface through combined EPR, photoluminescence and photocatalysis assessment. Catal. Today 2018, 315, 19–30. [Google Scholar] [CrossRef]
- Andreozzi, M.; Álvarez, M.G.; Contreras, S.; Medina, F.; Clarizia, L.; Vitiello, G.; Llorca, J.; Marotta, R. Treatment of saline produced water through photocatalysis using rGO-TiO2 nanocomposites. Catal. Today 2018, 315, 194–204. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Hanus, M.J.; Harris, A.T. Nanotechnology innovations for the construction industry. Prog. Mater. Sci. 2013, 58, 1056–1102. [Google Scholar] [CrossRef]
- Tsai, S.-J.; Cheng, S. Effect of TiO2 crystalline structure in photocatalytic degradation of phenolic contaminants. Catal. Today 1997, 33, 227–237. [Google Scholar] [CrossRef]
- Folli, A.; Macphee, D. Photocatalytic Concretes–The interface between photocatalysis and cement chemistry. In Proceedings of the 33rd Cement and Concrete Science Conference, Portsmouth, UK, 2–3 September 2013. [Google Scholar]
- Cassar, L. Photocatalysis of cementitious materials: Clean buildings and clean air. Mrs Bull. 2004, 29, 328–331. [Google Scholar] [CrossRef]
- Bellardita, M.; Di Paola, A.; Megna, B.; Palmisano, L. Determination of the crystallinity of TiO2 photocatalysts. J. Photochem. Photobiol. A Chem. 2018, 367, 312–320. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Rodriguez-Garcia, J.R.; Castillo, A.; Castellote, M. Characteristics and efficiency of photocatalytic cementitious materials: Type of binder, roughness and microstructure. Cem. Concr. Res. 2015, 71, 124–131. [Google Scholar] [CrossRef]
- Addamo, M.; Augugliaro, V.; Bellardita, M.; Di Paola, A.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Environmentally friendly photocatalytic oxidation of aromatic alcohol to aldehyde in aqueous suspension of brookite TiO2. Catal. Lett. 2008, 126, 58–62. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, S.-K.; Kim, S.-J. Effect of particle size and phase composition of titanium dioxide nanoparticles on the photocatalytic properties. J. Nanopart. Res. 2001, 3, 141–147. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegyi, A.; Lăzărescu, A.-V.; Ciobanu, A.A.; Ionescu, B.A.; Grebenişan, E.; Chira, M.; Florean, C.; Vermeşan, H.; Stoian, V. Study on the Possibilities of Developing Cementitious or Geopolymer Composite Materials with Specific Performances by Exploiting the Photocatalytic Properties of TiO2 Nanoparticles. Materials 2023, 16, 3741. https://doi.org/10.3390/ma16103741
Hegyi A, Lăzărescu A-V, Ciobanu AA, Ionescu BA, Grebenişan E, Chira M, Florean C, Vermeşan H, Stoian V. Study on the Possibilities of Developing Cementitious or Geopolymer Composite Materials with Specific Performances by Exploiting the Photocatalytic Properties of TiO2 Nanoparticles. Materials. 2023; 16(10):3741. https://doi.org/10.3390/ma16103741
Chicago/Turabian StyleHegyi, Andreea, Adrian-Victor Lăzărescu, Adrian Alexandru Ciobanu, Brăduţ Alexandru Ionescu, Elvira Grebenişan, Mihail Chira, Carmen Florean, Horaţiu Vermeşan, and Vlad Stoian. 2023. "Study on the Possibilities of Developing Cementitious or Geopolymer Composite Materials with Specific Performances by Exploiting the Photocatalytic Properties of TiO2 Nanoparticles" Materials 16, no. 10: 3741. https://doi.org/10.3390/ma16103741
APA StyleHegyi, A., Lăzărescu, A.-V., Ciobanu, A. A., Ionescu, B. A., Grebenişan, E., Chira, M., Florean, C., Vermeşan, H., & Stoian, V. (2023). Study on the Possibilities of Developing Cementitious or Geopolymer Composite Materials with Specific Performances by Exploiting the Photocatalytic Properties of TiO2 Nanoparticles. Materials, 16(10), 3741. https://doi.org/10.3390/ma16103741








