Sonochemical Deposition of Gentamicin Nanoparticles at the PCV Tracheostomy Tube Surface Limiting Bacterial Biofilm Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomaterial Characteristics
2.2. Oxygen Plasma Modification
2.3. Preparation of Gentamicin Nanoparticles and Their Instant Deposition on PVC Surface
Nanoparticle Tracking Analyses (NTA)
2.4. Characterization of Functionalized Biomaterial
2.4.1. Fourier Transform Infrared Microscopy (FTIR)
2.4.2. Analysis of Drug Loading on the Biomaterial Surface
2.4.3. Atomic Force Microscopy (AFM)
2.5. Microbiological Tests
2.5.1. Bacterial Strains
2.5.2. Quantification of Bacterial Adhesion on the Surface of Polyvinyl Chloride
2.5.3. Evaluation of the Cytotoxicity of Gentamicin (GMNPs) on the A549 Cell Line
2.5.4. Evaluation of the Effect of Polyvinyl Chloride Surface on the A549 Cell Line Using the LIVE/DEAD Method
2.6. Statistic
3. Results
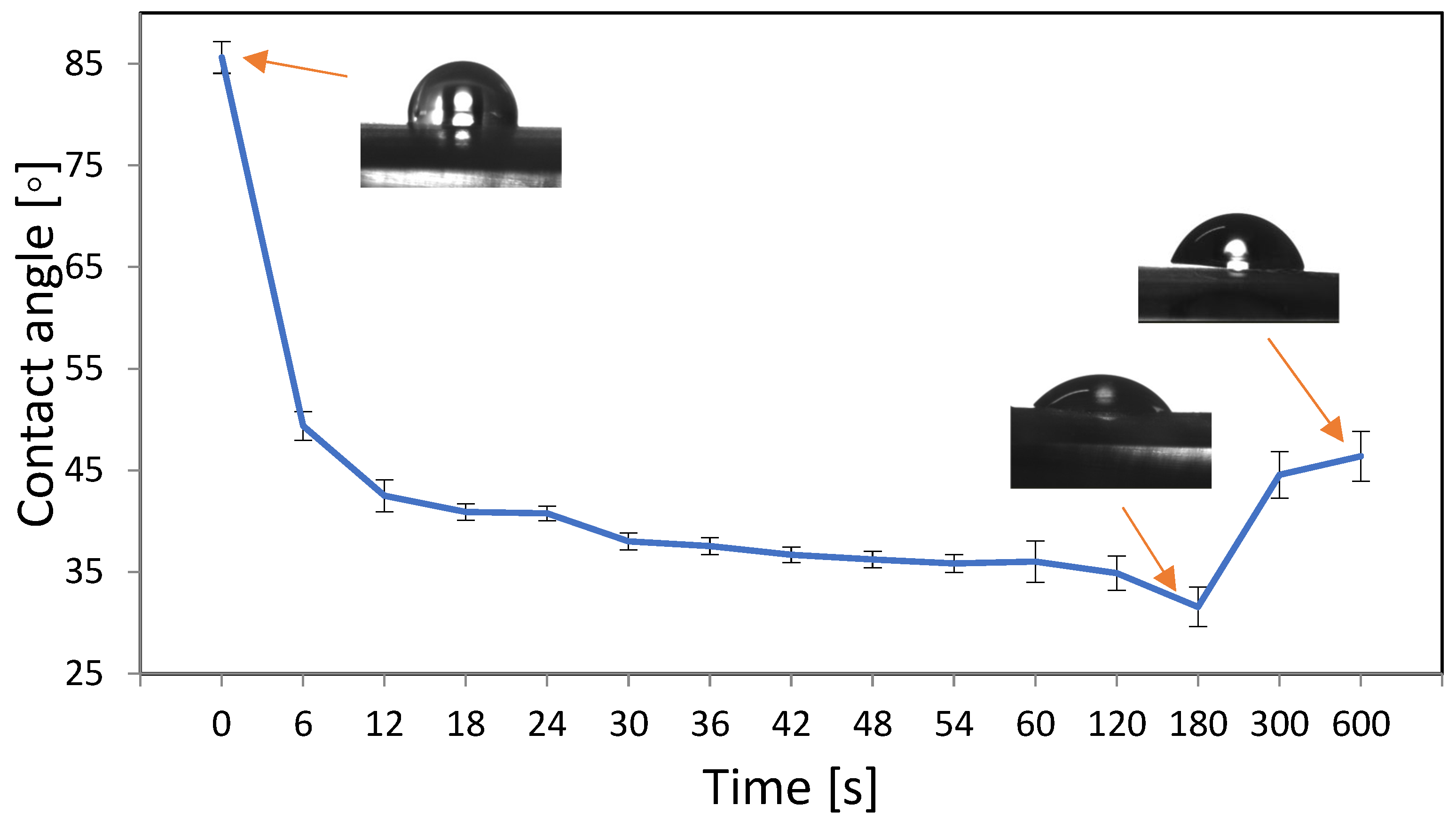
3.1. Optimization of Oxygen Plasma Parameters
3.2. AFM Imaging for Polymer Topography
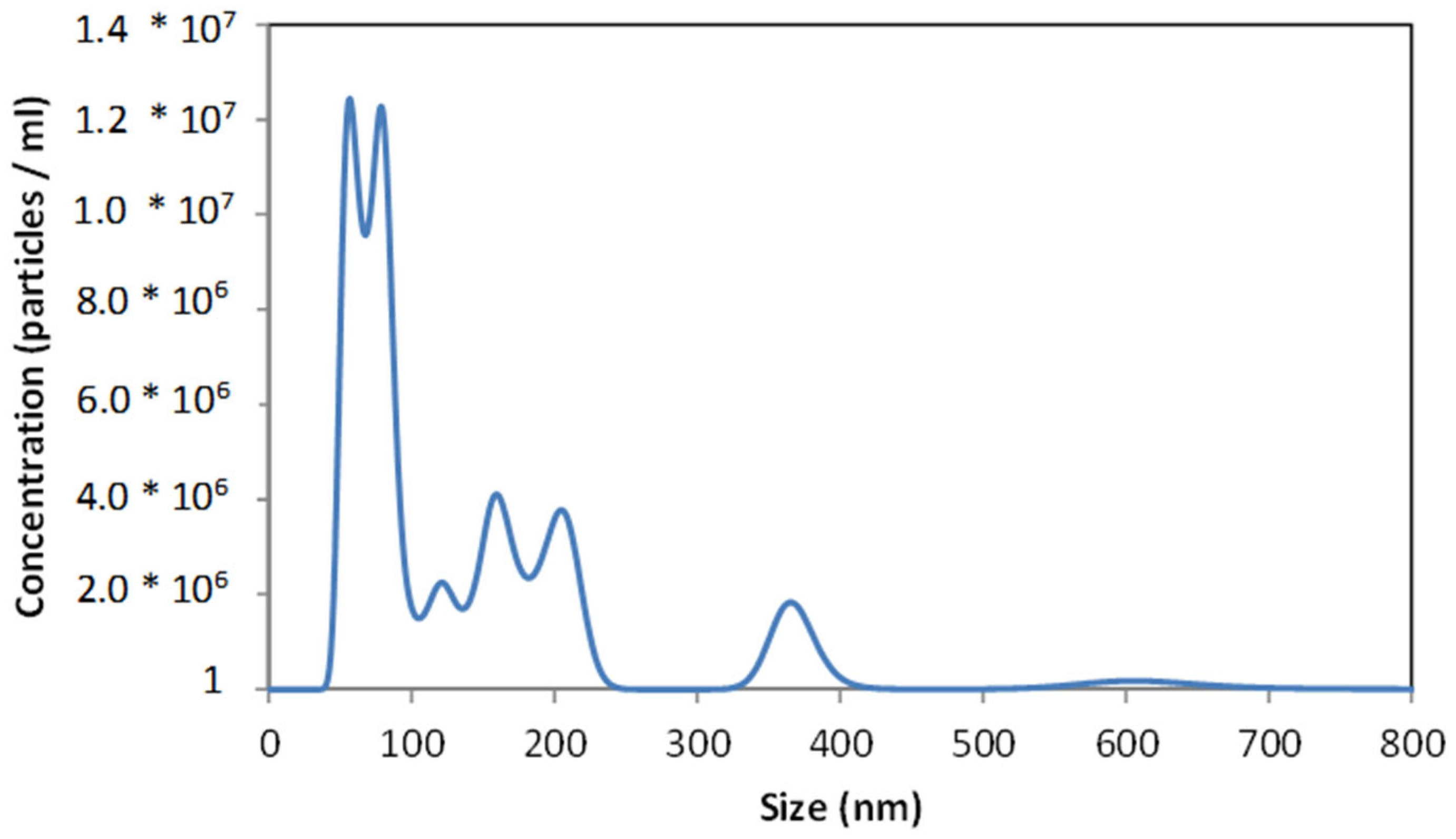
3.3. Analysis of Gentamicin Nanoparticles’ Size
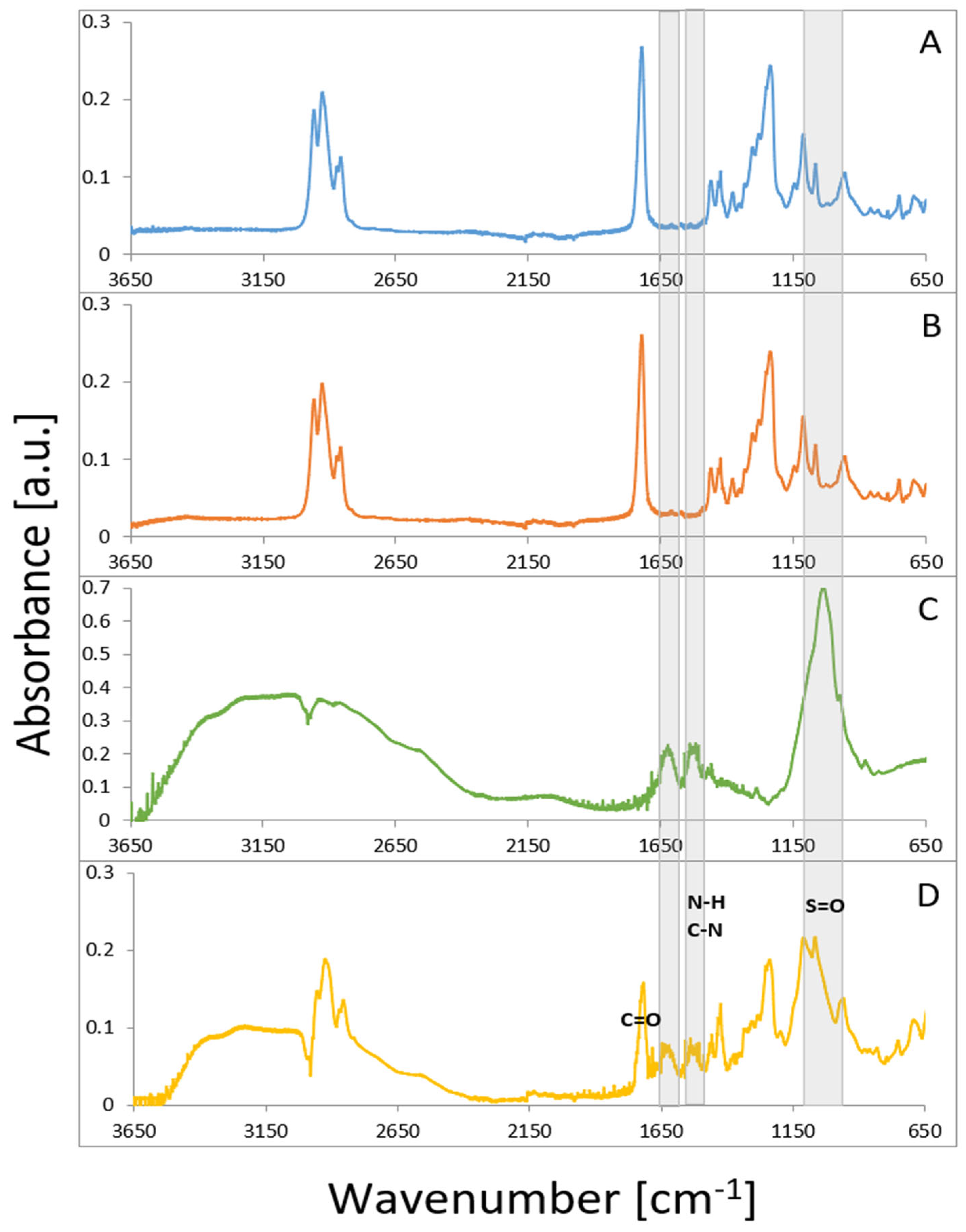
3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
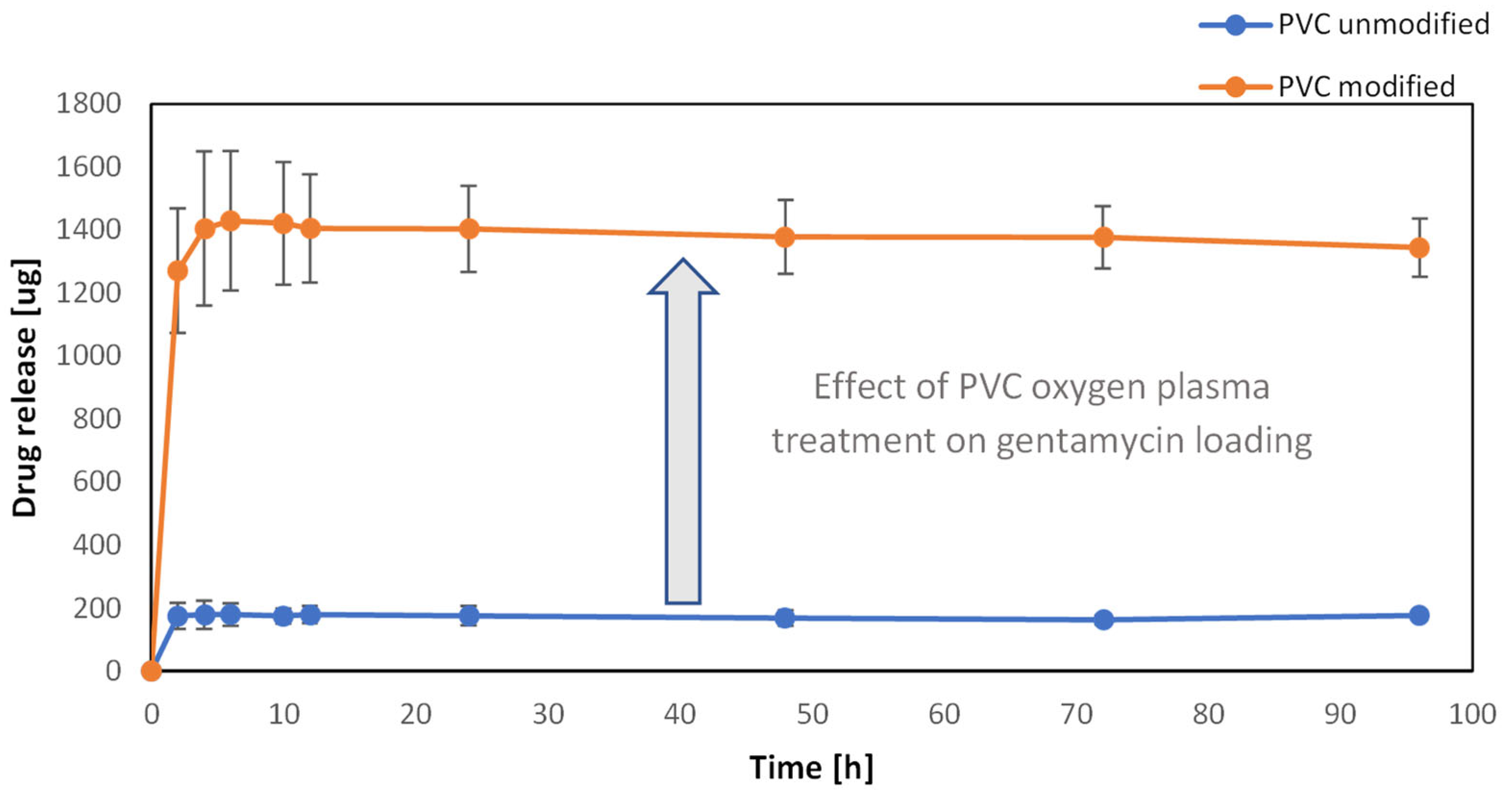
3.5. Drug Loading on the Surface of PVC
3.6. Adhesion of Bacteria on the Surface of the Biomaterial
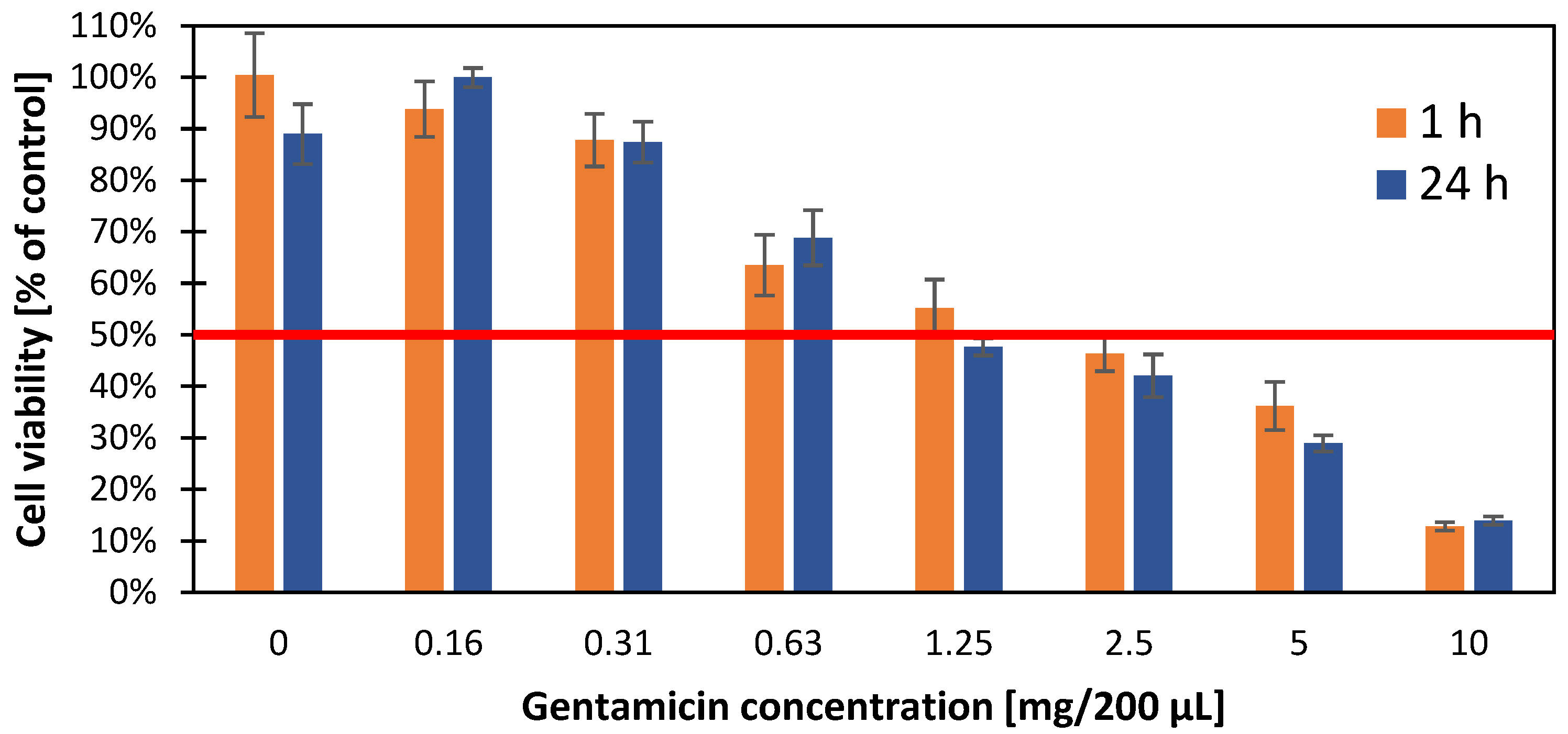
3.7. Cytotoxic Effect of Gentamicin Nanoparticles on A549 Cell Line
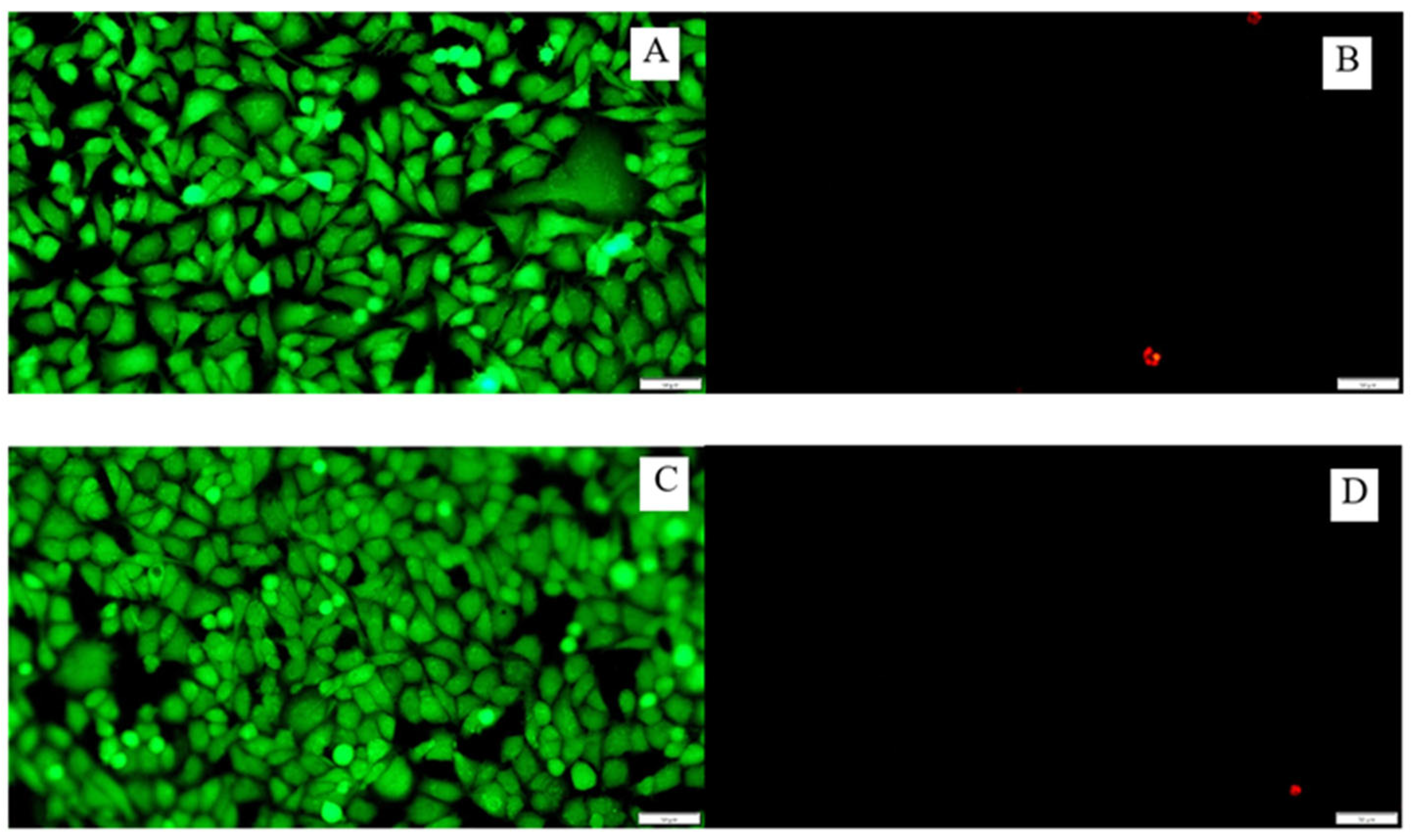
3.8. Cytotoxicity of the Tested Surfaces on the A549 Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abril, M.K.M.; Berkowitz, D.M.; Chen, Y.M.; Waller, L.A.; Martin, G.S.M.; Kempker, J.A.M. The Epidemiology of Adult Tracheostomy in the United States 2002–2017: A Serial Cross-Sectional Study. Crit. Care Explor. 2021, 3, e0523. [Google Scholar] [CrossRef]
- Lewith, H.; Athanassoglou, V. Update on management of tracheostomy. BJA Educ. 2019, 19, 370–376. [Google Scholar] [CrossRef]
- Ferro, A.; Kotecha, S.; Auzinger, G.; Yeung, E.; Fan, K. Systematic review and meta-analysis of tracheostomy outcomes in COVID-19 patients. Br. J. Oral Maxillofac. Surg. 2021, 59, 1013–1023. [Google Scholar] [CrossRef]
- Klotz, R.; Probst, P.; Deininger, M.; Klaiber, U.; Grummich, K.; Diener, M.K.; Weigand, M.A.; Büchler, M.W.; Knebel, P. Percutaneous versus surgical strategy for tracheostomy: A systematic review and me-ta-analysis of perioperative and postoperative complications. Langenbecks Arch. Surg. 2018, 403, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Ochońska, D.; Ścibik, Ł.; Brzychczy-Włoch, M. Biofilm Formation of Clinical Klebsiella pneumoniae Strains Isolated from Tracheostomy Tubes and Their Association with Antimicrobial Resistance, Virulence and Genetic Diversity. Pathogens 2021, 10, 1345. [Google Scholar] [CrossRef] [PubMed]
- Delaney, A.; Bagshaw, S.M.; Nalos, M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: A systematic review and meta-analysis. Crit. Care 2006, 10, R55. [Google Scholar] [CrossRef]
- Ratner, B.D.; Zhang, G. A History of Biomaterials. Biomater. Sci. 2020, 21–34. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and Its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Chytrosz-Wrobel, P.; Golda-Cepa, M.; Kubisiak, P.; Kulig, W.; Cwiklik, L.; Kotarba, A. Sonochemical Formation of Fluorouracil Nanoparticles: Toward Controlled Drug Delivery from Polymeric Surfaces. ACS Appl. Nano Mater. 2023, 6, 4271–4278. [Google Scholar] [CrossRef]
- Chaves, B.J.; Tadi, P. Gentamicin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557550/ (accessed on 1 May 2022).
- Yang, Z.; Kang, S.G.; Zhou, R. Nanomedicine: De novo design of nanodrugs. Nanoscale 2014, 6, 663–677. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Zhang, W.; Radmanesh, M.; Mirmohammadi, S.S.; Maleki, A.; Cathcart, N.; Kitaev, V. Multi-Stimuli Nanocomposite Therapeutic: Docetaxel Targeted Delivery and Synergies in Treatment of Human Breast Cancer Tumor. Small 2022, 16, 2002733. [Google Scholar] [CrossRef]
- Zhang, W.; Taheri-Ledari, R.; Hajizadeh, Z.; Zolfaghari, E.; Ahghari, M.R.; Maleki, A.; Hamblin, M.R.; Tian, Y. Enhanced activity of vancomycin by encapsulation in hybrid magnetic nanoparticles conjugated to a cell-penetrating peptide. Nanoscale 2020, 12, 3855–3870. [Google Scholar] [CrossRef] [PubMed]
- Golda-Cepa, M.; Chytrosz, P.; Chorylek, A.; Kotarba, A. One-step sonochemical fabrication and embedding of gentamicin nanoparticles into parylene C implant coating: Towards controlled drug delivery. Nanomedicine 2018, 14, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, K.; Pudełko, I.; Knap, K.; Reczyńska-Kolman, K.; Krok-Borkowicz, M.; Ochońska, D.; Brzychczy-Włoch, M.; Pamuła, E. Insight in Superiority of the Hydrophobized Gentamycin in Terms of Antibiotics Delivery to Bone Tissue. Int. J. Mol. Sci. 2022, 23, 12077. [Google Scholar] [CrossRef] [PubMed]
- Ścibik, Ł.; Ochońska, D.; Gołda-Cępa, M.; Brzychczy-Włoch, M.; Kotarba, A. Microbiological analysis of tracheostomy tube biofilms and antibiotic resistance profiles of potentially pathogenic microorganisms. Otolaryngol. Pol. 2022, 76, 1–13. [Google Scholar] [CrossRef]
- Śliwka, L.; Wiktorska, K.; Suchocki, P.; Milczarek, M.; Mielczarek, S.; Lubelska, K.; Cierpiał, T.; Łyżwa, P.; Kiełbasiński, P.; Jaromin, A.; et al. The Comparison of MTT and CVS Assays for the Assessment of Anticancer Agent Interactions. PLoS ONE 2016, 11, e0155772. [Google Scholar] [CrossRef]
- ibidi GmbH, Version 2.0 (Online). 2015. Available online: https://ibidi.com/img/cms/support/AN/AN33_Live_Dead_staining_with_FDA_and_PI.pdf (accessed on 2 November 2022).
- Golda-Cepa, M.; Brzychczy-Wloch, M.; Engvall, K.; Aminlashgari, N.; Hakkarainen, M.; Kotarba, A. Microbiological investigations of oxygen plasma treated parylene C surfaces for metal implant coating. Mater. Sci. Eng. C 2015, 52, 273–281. [Google Scholar] [CrossRef]
- Cavalu, S.; Roiu, G.; Pop, O.; Heredea, D.A.P.; Costea, T.O.; Costea, C.F. Nano-Scale Modifications of Amniotic Membrane Induced by UV and Antibiotic Treatment: Histological, AFM and FTIR Spectroscopy Evidence. Materials 2021, 14, 863. [Google Scholar] [CrossRef]
- Maczynska, B.; Secewicz, A.; Smutnicka, D.; Szymczyk-Ziółkowska, P.; Dudek-Wicher, R.; Junka, A.; Bartoszewicz, M. In vitro efficacy of gentamicin released from collagen sponge in eradication of bacterial biofilm preformed on hydroxyapatite surface. PLoS ONE 2019, 14, e0217769. [Google Scholar] [CrossRef]
- Raveendra, N.; Rathnakara, S.H.; Haswani, N.; Subramaniam, V. Bacterial Biofilms on Tracheostomy Tubes. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 4995–4999. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Y.; Wu, P.; Chen, B. Update on new medicinal applications of gentamicin: Evidence-based review. J. Formos. Med. Assoc. 2014, 113, 72–82. [Google Scholar] [CrossRef]
- Akdogan, E.; Sirin, H.T. Plasma surface modification strategies for the preparation of antibacterial biomaterials: A review of the recent literature. Mater. Sci. Eng. C 2021, 131, 112474. [Google Scholar] [CrossRef]
- Rezaei, F.; Shokri, B.; Sharifian, M. Atmospheric-pressure DBD plasma-assisted surface modification of polymethyl methac-rylate: A study on cell growth/proliferation and antibacterial properties. Appl. Surf. Sci. 2016, 360, 641–651. [Google Scholar] [CrossRef]
- Vogler, E.A. Water and the Acute Biological Response to Surfaces. J. Biomater. Sci. Polym. Ed. 1999, 10, 1015–1045. [Google Scholar] [CrossRef] [PubMed]
- Shafei, S.; Foroughi, J.; Chen, Z.; Wong, C.S.; Naebe, M. Short Oxygen Plasma Treatment Leading to Long-Term Hydrophilicity of Conductive PCL-PPy Nanofiber Scaffolds. Polymers 2017, 9, 614. [Google Scholar] [CrossRef]
- Balazs, D.; Favez, D.; Chevolot, Y.; Xanthopoulos, N.; Granges, C.; Aronsson, B.-O.; Sidouni, F.; Descouts, P.; Mathieu, H.J. Surface modification of PVC endotracheal tubes: Oxygen plasma treatment and aging effects. Eur. Cells Mater. 2001, 1, 18–24. [Google Scholar]
- Kumar, R.M.; Gupta, P.; Sharma, S.K.; Mittal, A.; Shekhar, M.; Kumar, V.; Kumar, B.M.; Roy, P.; Lahiri, D. Sustained drug release from surface modified UHMWPE for acetabular cup lining in total hip implant. Mater. Sci. Eng. C 2017, 77, 649–661. [Google Scholar] [CrossRef]
- Peng, B.-Y.; Chen, Z.; Chen, J.; Yu, H.; Zhou, X.; Criddle, C.S.; Wu, W.-M.; Zhang, Y. Biodegradation of Polyvinyl Chloride (PVC) in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae. Environ. Int. 2020, 145, 106106. [Google Scholar] [CrossRef]
- Kamal, R.; Kahdhum, Q.A.; Mohammed, A.I.; Essa, A.J.; Elhamid, M.A.A.; Mohamad, E.A. Evaluation the Biological Activity of Nano-Gentamicin Prepared and Char-acterization of Nanoparticles. Iraqi J. Ind. Res. 2021, 8, 84–90. [Google Scholar] [CrossRef]
- Barar, J. Bioimpacts of nanoparticle size: Why it matters? Bioimpacts 2015, 5, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Beraud, G.; Moal, G.; Elsendoorn, A.; Tattevin, P.; Godet, C.; Alfandari, S.; Couet, W.; Roblot, P.; Roblot, F. A survey on the use of gentamicin in infective endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.E.; Reynolds, H.Y. Concentrations of gentamicin and carbenicillin in bronchial secretions. J. Infect. Dis. 1973, 128, 63–68. [Google Scholar] [CrossRef]
- Kovacik, A.; Tvrda, E.; Fulopova, D.; Cupka, P.; Kovacikova, E.; Zbynovska, K.; Massanyi, P. In Vitro Assessment of Gentamicin Cytotoxicity on the Selected Mammalian Cell Line (Vero cells). Adv. Res. Life Sci. 2017, 1, 111–116. [Google Scholar] [CrossRef]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. 2011, 29, 1070–1074. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ścibik, Ł.; Ochońska, D.; Gołda-Cępa, M.; Kwiecień, K.; Pamuła, E.; Kotarba, A.; Brzychczy-Włoch, M. Sonochemical Deposition of Gentamicin Nanoparticles at the PCV Tracheostomy Tube Surface Limiting Bacterial Biofilm Formation. Materials 2023, 16, 3765. https://doi.org/10.3390/ma16103765
Ścibik Ł, Ochońska D, Gołda-Cępa M, Kwiecień K, Pamuła E, Kotarba A, Brzychczy-Włoch M. Sonochemical Deposition of Gentamicin Nanoparticles at the PCV Tracheostomy Tube Surface Limiting Bacterial Biofilm Formation. Materials. 2023; 16(10):3765. https://doi.org/10.3390/ma16103765
Chicago/Turabian StyleŚcibik, Łukasz, Dorota Ochońska, Monika Gołda-Cępa, Konrad Kwiecień, Elżbieta Pamuła, Andrzej Kotarba, and Monika Brzychczy-Włoch. 2023. "Sonochemical Deposition of Gentamicin Nanoparticles at the PCV Tracheostomy Tube Surface Limiting Bacterial Biofilm Formation" Materials 16, no. 10: 3765. https://doi.org/10.3390/ma16103765
APA StyleŚcibik, Ł., Ochońska, D., Gołda-Cępa, M., Kwiecień, K., Pamuła, E., Kotarba, A., & Brzychczy-Włoch, M. (2023). Sonochemical Deposition of Gentamicin Nanoparticles at the PCV Tracheostomy Tube Surface Limiting Bacterial Biofilm Formation. Materials, 16(10), 3765. https://doi.org/10.3390/ma16103765









