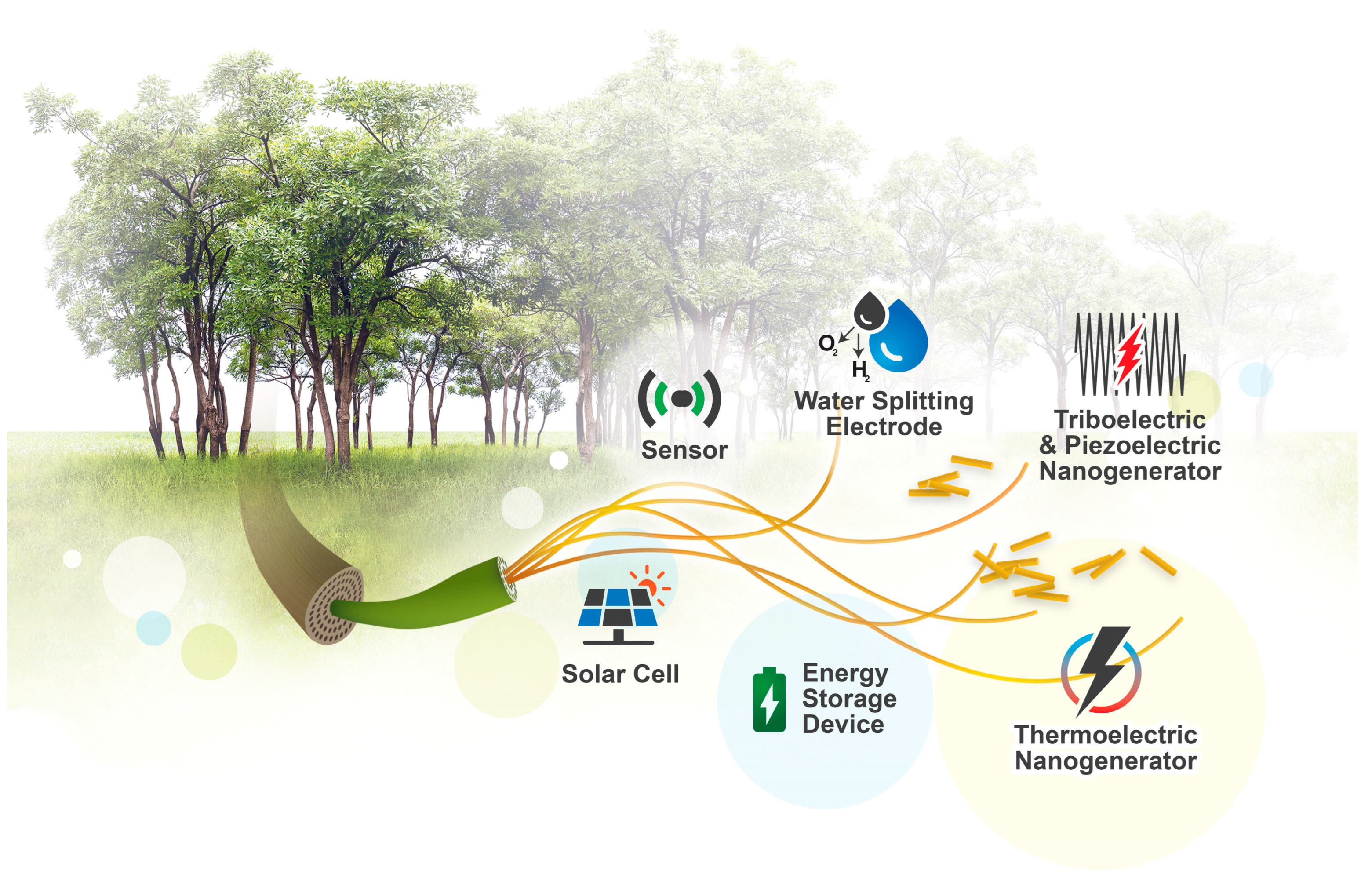
Advances in Cellulose-Based Composites for Energy Applications
Abstract
1. Introduction
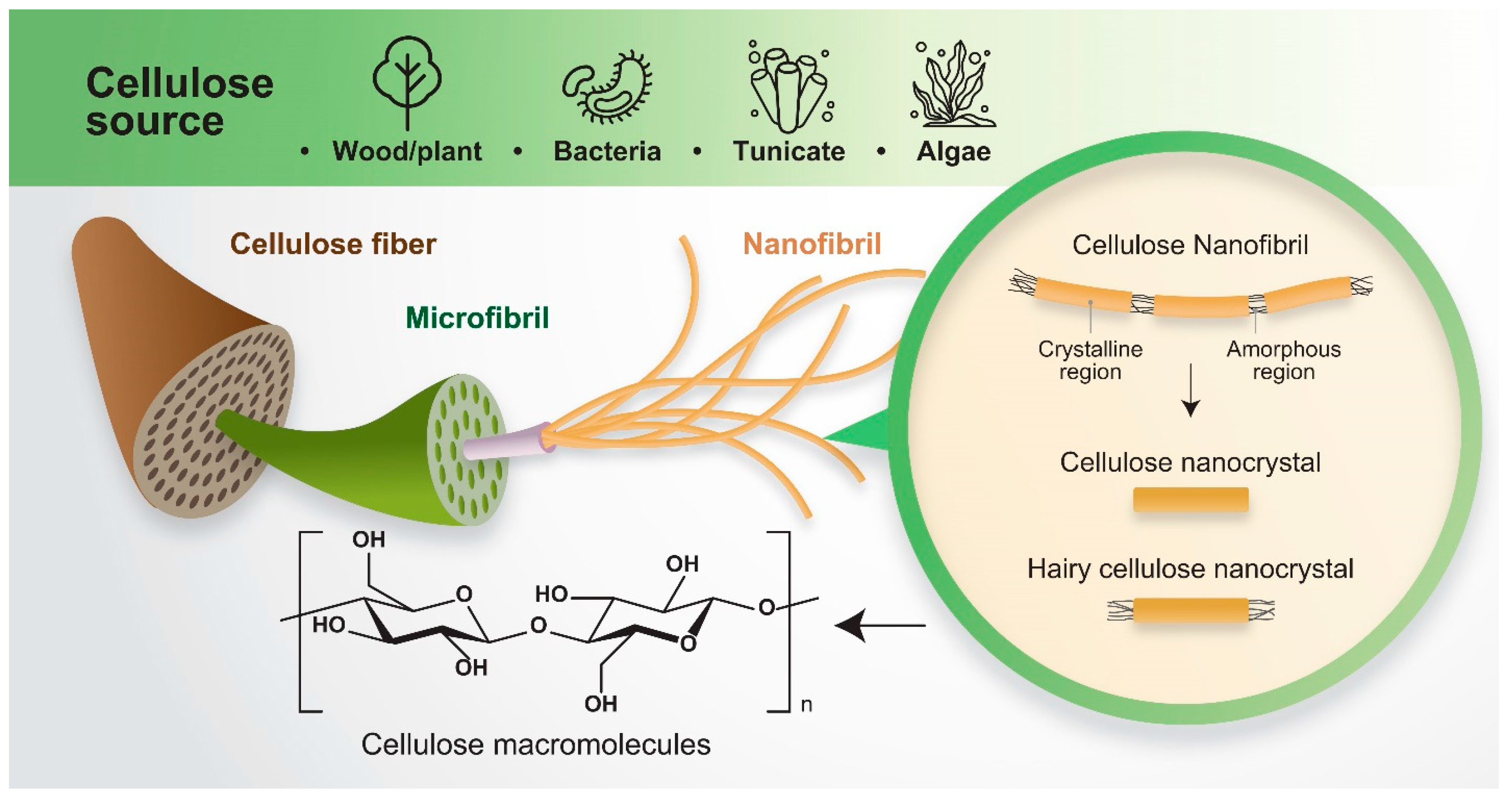
2. Classification of Cellulose and Processing
2.1. Cellulose Nanocrystals
2.2. Cellulose Nanofibrils
2.3. Hairy Cellulose Nanocrystals
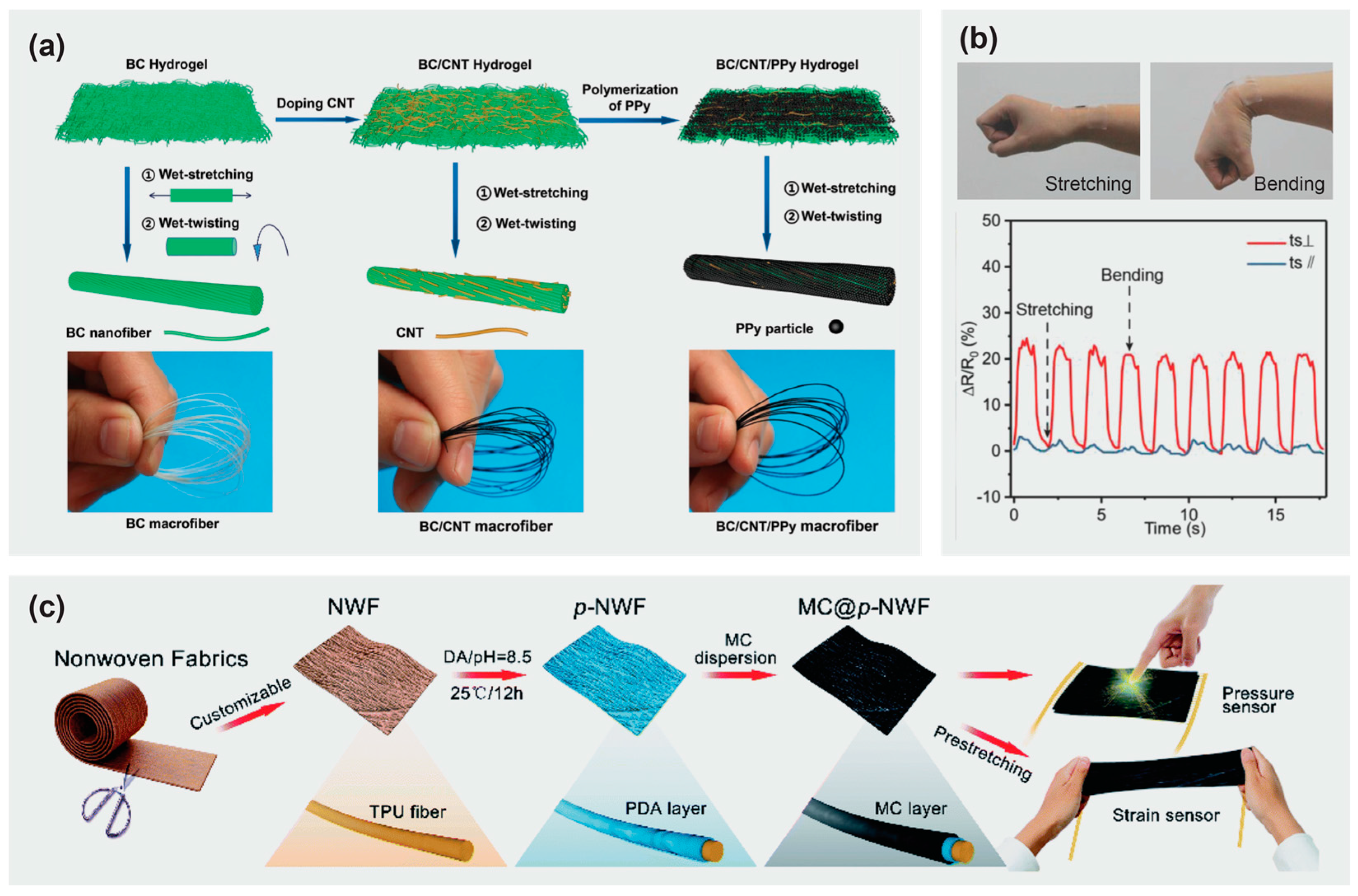
3. Cellulose-Based Composites for Flexible and Wearable Electronics
3.1. Cellulose-Based Materials in Flexible Electronics
3.2. Cellulose-Based Textiles with Integrated Electronics
3.3. Cellulose-Based Materials in Flexible Sensors
4. Cellulose-Based Composites for Energy Conversion
4.1. Mechanical Energy Conversion
4.1.1. Cellulose-Based Triboelectric Generator
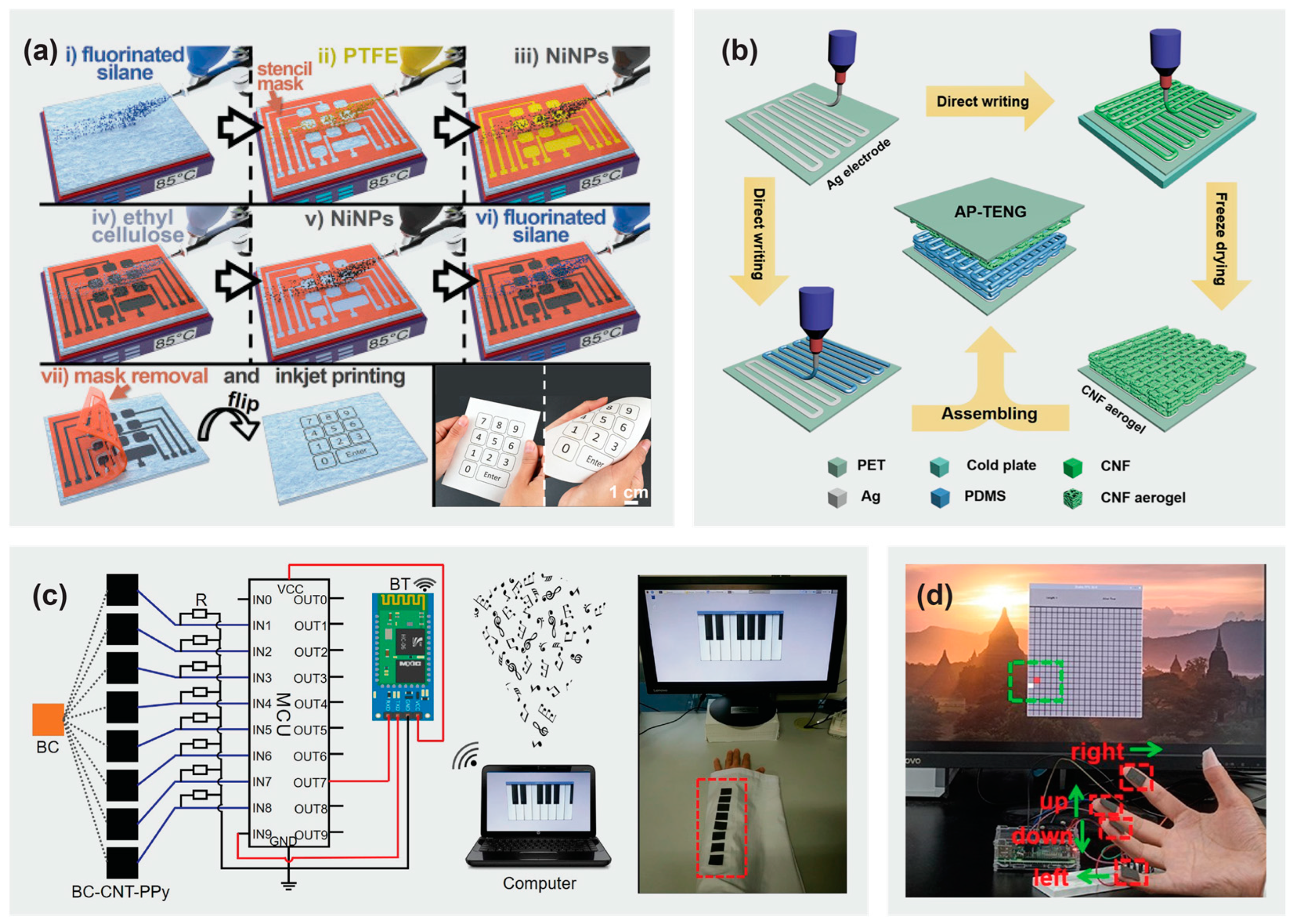
4.1.2. Cellulose-Based Piezoelectric Generators
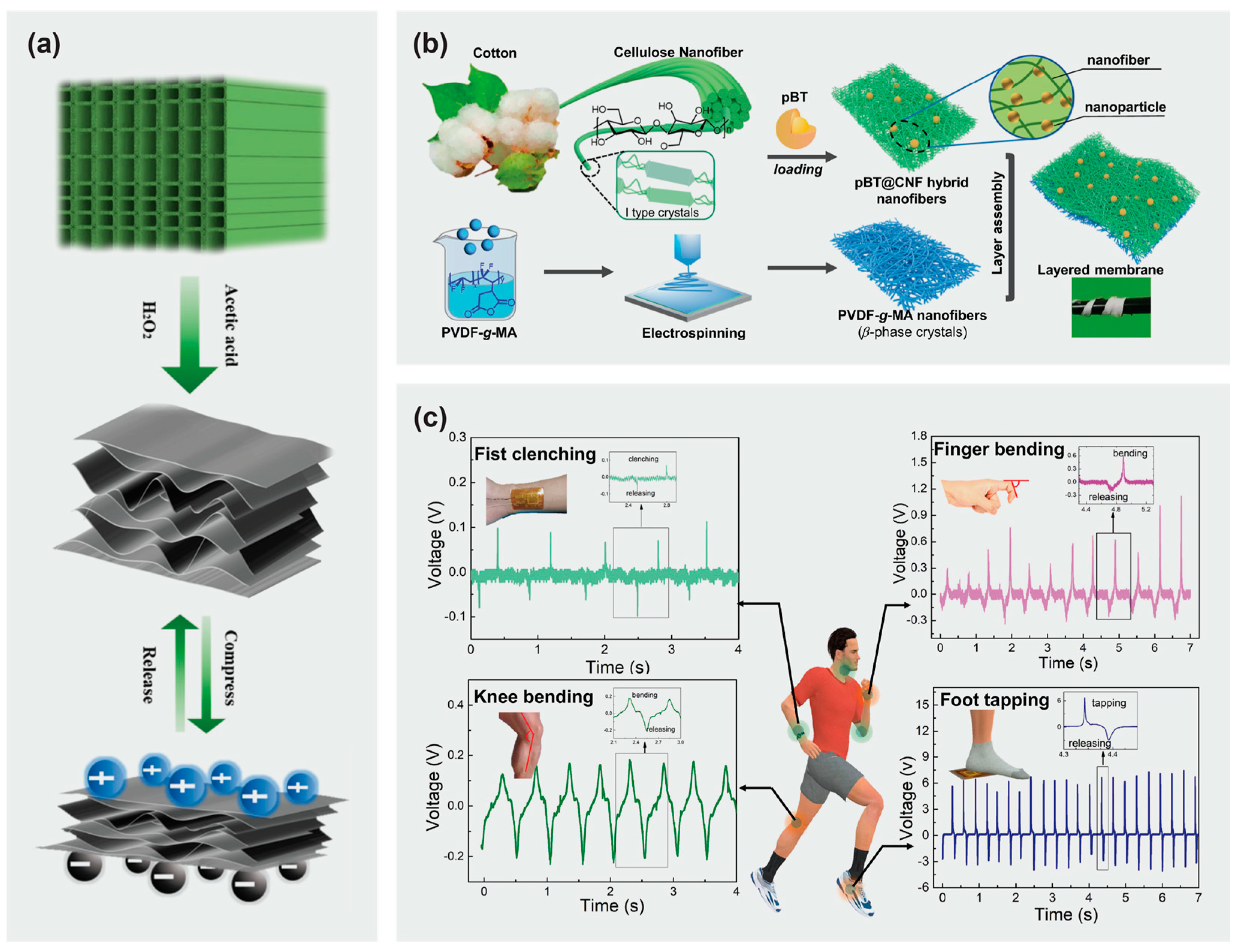
4.2. Thermoelectric Energy Conversion

4.3. Solar Energy Conversion
5. Cellulose-Based Composites for Energy Storage
5.1. Cellulose-Based Electrodes
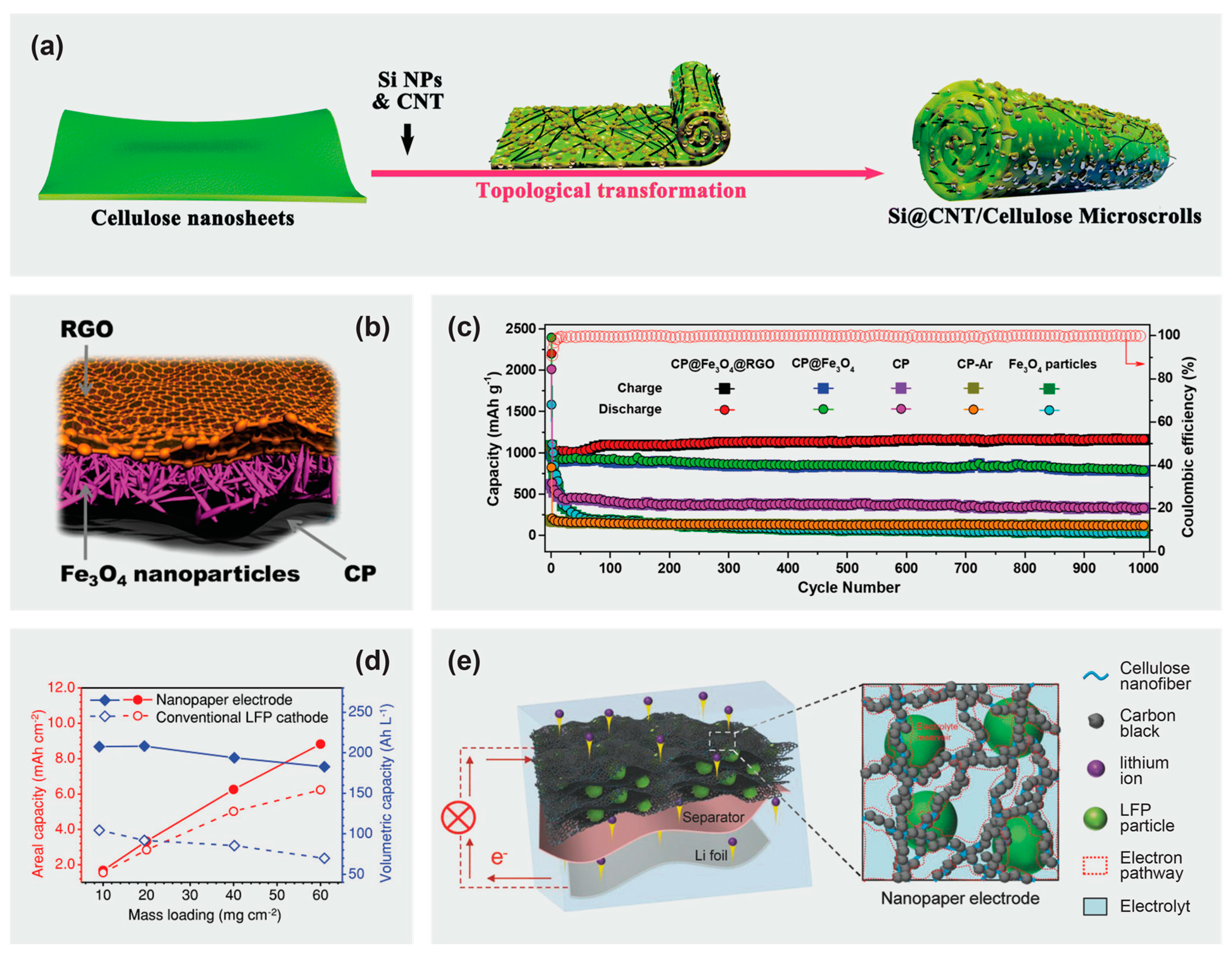
5.2. Cellulose-Based Polymer Separators
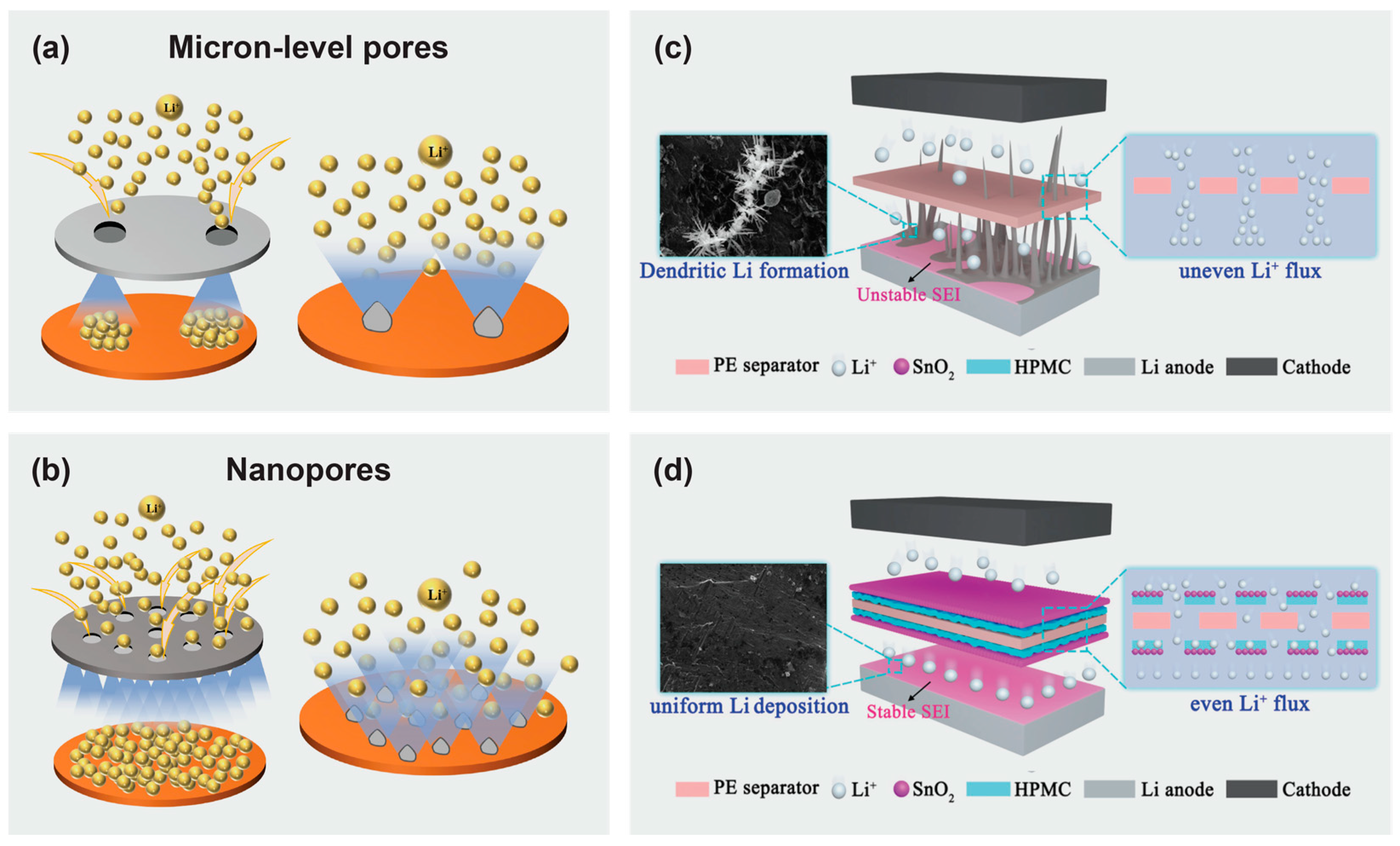
5.3. Cellulose-Based Polymer Electrolytes
5.4. Cellulose-Based Applications in Rechargeable Aqueous Batteries

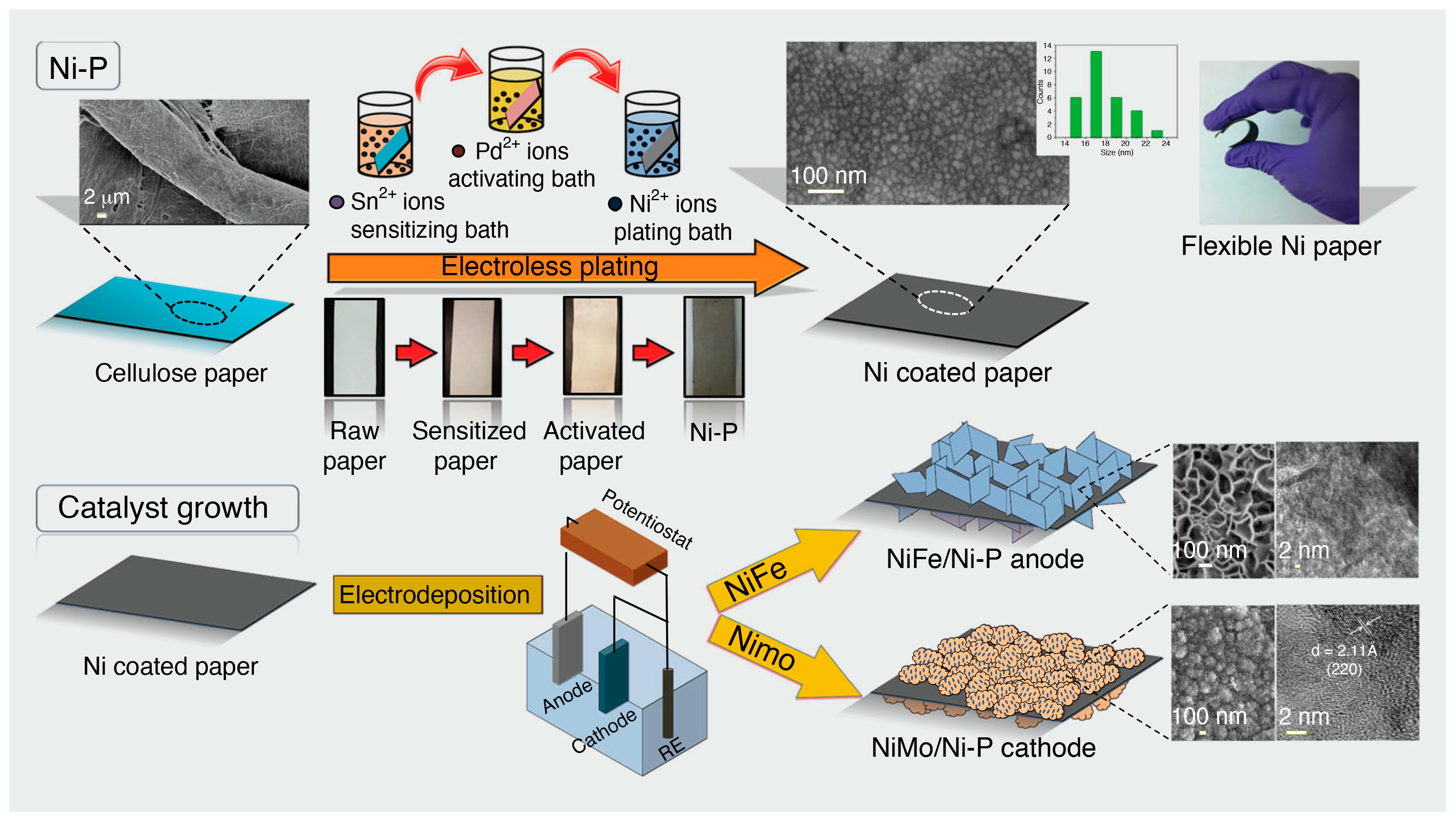
6. Cellulose-Based Composites for Water Splitting Catalysis
6.1. Cellulose-Based Photocatalysts
6.2. Cellulose-Based Electrocatalysts
7. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-Based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2021, 33, e2000619. [Google Scholar] [CrossRef] [PubMed]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Lin, Q.; Tai, Y.A.; Wang, Y. Applications of Cellulose Nanomaterials in Stimuli-Responsive Optics. J. Agric. Food. Chem. 2020, 68, 12940–12955. [Google Scholar] [CrossRef] [PubMed]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and Opportunities in the Characterization of Cellulose—An Important Regulator of Cell Wall Growth and Mechanics. Front. Plant Sci. 2018, 9, 1894. [Google Scholar] [CrossRef]
- Kumar, A.; Jyske, T.; Petrič, M. Delignified Wood from Understanding the Hierarchically Aligned Cellulosic Structures to Creating Novel Functional Materials: A Review. Adv. Sustain. Syst. 2021, 5, 2000251. [Google Scholar] [CrossRef]
- Haigler, C.H.; Betancur, L.; Stiff, M.R.; Tuttle, J.R. Cotton fiber: A powerful single-cell model for cell wall and cellulose research. Front. Plant Sci. 2012, 3, 104. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Mohd Noor, N.H.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.; El-Malkey, S.E.; Abu-Saied, M.A.; Mohammed, A.B.A. Exploration of a novel and efficient source for production of bacterial nanocellulose, bioprocess optimization and characterization. Sci. Rep. 2022, 12, 18533. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Flanagan, B.M.; Dykes, G.A.; Gidley, M.J. Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J. Appl. Microbiol. 2009, 107, 576–583. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites, 1st ed.; Hanieh Kargarzadeh, I.A., Sabu, T., Alain, D., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Volume 1, pp. 1–49. [Google Scholar]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal cellulose, production and potential use in plastics: Challenges and opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Sampath, U.; Ching, Y.C.; Chuah, C.H.; Sabariah, J.J.; Lin, P.C. Fabrication of Porous Materials from Natural/Synthetic Biopolymers and Their Composites. Materials 2016, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.A.; Apperley, D.C.; Evans, B.W.; Huxham, I.M.; Jardine, W.G.; Vietor, R.J.; Reis, D.; Vian, B.; Jarvis, M.C. Fine structure in cellulose microfibrils: NMR evidence from onion and quince. Plant J. 1998, 16, 183–190. [Google Scholar] [CrossRef]
- Mittal, A.; Katahira, R.; Himmel, M.E.; Johnson, D.K. Effects of alkaline or liquid-ammonia treatment on crystalline cellulose: Changes in crystalline structure and effects on enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Takano, K.; Nakamae, K. Elastic modulus of the crystalline regions of cellulose polymorphs. J. Polym. Sci. B Polym. Phys. 1995, 33, 1647–1651. [Google Scholar] [CrossRef]
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836. [Google Scholar] [CrossRef]
- Mittal, N.; Ansari, F.; Gowda, V.K.; Brouzet, C.; Chen, P.; Larsson, P.T.; Roth, S.V.; Lundell, F.; Wagberg, L.; Kotov, N.A.; et al. Multiscale Control of Nanocellulose Assembly: Transferring Remarkable Nanoscale Fibril Mechanics to Macroscale Fibers. ACS Nano 2018, 12, 6378–6388. [Google Scholar] [CrossRef]
- Gui, Z.; Zhu, H.; Gillette, E.; Han, X.; Rubloff, G.W.; Hu, L.; Lee, S.B. Natural cellulose fiber as substrate for supercapacitor. ACS Nano 2013, 7, 6037–6046. [Google Scholar] [CrossRef]
- Wang, X.; Yao, C.; Wang, F.; Li, Z. Cellulose-Based Nanomaterials for Energy Applications. Small 2017, 13, 1702240. [Google Scholar] [CrossRef]
- Dong, S.; Bortner, M.J.; Roman, M. Analysis of the sulfuric acid hydrolysis of wood pulp for cellulose nanocrystal production: A central composite design study. Ind. Crops Prod. 2016, 93, 76–87. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Pawcenis, D.; Lesniak, M.; Szumera, M.; Sitarz, M.; Profic-Paczkowska, J. Effect of hydrolysis time, pH and surfactant type on stability of hydrochloric acid hydrolyzed nanocellulose. Int. J. Biol. Macromol. 2022, 222, 1996–2005. [Google Scholar] [CrossRef]
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Q.; Hirth, K.; Baez, C.; Agarwal, U.P.; Zhu, J.Y. Tailoring the yield and characteristics of wood cellulose nanocrystals (CNC) using concentrated acid hydrolysis. Cellulose 2015, 22, 1753–1762. [Google Scholar] [CrossRef]
- Alonso-Lerma, B.; Barandiaran, L.; Ugarte, L.; Larraza, I.; Reifs, A.; Olmos-Juste, R.; Barruetabeña, N.; Amenabar, I.; Hillenbrand, R.; Eceiza, A.; et al. High performance crystalline nanocellulose using an ancestral endoglucanase. Commun. Mater. 2020, 1, 57. [Google Scholar] [CrossRef]
- Squinca, P.; Bilatto, S.; Badino, A.C.; Farinas, C.S. Nanocellulose Production in Future Biorefineries: An Integrated Approach Using Tailor-Made Enzymes. ACS Sustain. Chem. Eng. 2020, 8, 2277–2286. [Google Scholar] [CrossRef]
- Bondancia, T.J.; Mattoso, L.H.C.; Marconcini, J.M.; Farinas, C.S. A new approach to obtain cellulose nanocrystals and ethanol from eucalyptus cellulose pulp via the biochemical pathway. Biotechnol. Prog. 2017, 33, 1085–1095. [Google Scholar] [CrossRef]
- Camargo, L.A.; Pereira, S.C.; Correa, A.C.; Farinas, C.S.; Marconcini, J.M.; Mattoso, L.H.C. Feasibility of Manufacturing Cellulose Nanocrystals from the Solid Residues of Second-Generation Ethanol Production from Sugarcane Bagasse. BioEnergy Res. 2016, 9, 894–906. [Google Scholar] [CrossRef]
- Pereira, B.; Arantes, V. Production of cellulose nanocrystals integrated into a biochemical sugar platform process via enzymatic hydrolysis at high solid loading. Ind. Crops Prod. 2020, 152, 112377. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Reid, M.S.; Olsen, P.; Berglund, L.A. Eco-Friendly Cellulose Nanofibrils Designed by Nature: Effects from Preserving Native State. ACS Nano 2020, 14, 724–735. [Google Scholar] [CrossRef]
- Muthami, J.; Wamea, P.; Pitcher, M.; Sakib, M.N.; Liu, Z.; Arora, S.; Kennedy, D.; Chang, Y.-J.; Sheikhi, A. Chapter 1 Hairy Cellulose Nanocrystals: From Synthesis to Advanced Applications in the Water–Energy–Health–Food Nexus. In Cellulose Nanoparticles: Volume 2: Synthesis and Manufacturing; The Royal Society of Chemistry: London, UK, 2021; Volume 2, pp. 1–37. [Google Scholar]
- Mahrous, F.; Koshani, R.; Tavakolian, M.; Conley, K.; van de Ven, T.G.M. Formation of hairy cellulose nanocrystals by cryogrinding. Cellulose 2021, 28, 8387–8403. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, M.; Qiu, Q.; Yu, J.; Ding, B. Multilayered fiber-based triboelectric nanogenerator with high performance for biomechanical energy harvesting. Nano Energy 2018, 53, 726–733. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, Y.; Zhou, M. AgNPs@CNTs/Ag hybrid films on thiolated PET substrate for flexible electronics. J. Chem. Eng. 2019, 368, 223–234. [Google Scholar] [CrossRef]
- Liang, F.-C.; Chang, Y.-W.; Kuo, C.-C.; Cho, C.-J.; Jiang, D.-H.; Jhuang, F.-C.; Rwei, S.-P.; Borsali, R. A mechanically robust silver nanowire–polydimethylsiloxane electrode based on facile transfer printing techniques for wearable displays. Nanoscale 2019, 11, 1520–1530. [Google Scholar] [CrossRef]
- Pei, D.; An, C.; Zhao, B.; Ge, M.; Wang, Z.; Dong, W.; Wang, C.; Deng, Y.; Song, D.; Ma, Z.; et al. Polyurethane-Based Stretchable Semiconductor Nanofilms with High Intrinsic Recovery Similar to Conventional Elastomers. ACS Appl. Mater. Interfaces 2022, 14, 33806–33816. [Google Scholar] [CrossRef]
- Han, Y.K.; Cheon, J.Y.; Kim, T.; Lee, S.B.; Kim, Y.D.; Jung, B.M. A chemically bonded supercapacitor using a highly stretchable and adhesive gel polymer electrolyte based on an ionic liquid and epoxy-triblock diamine network. RSC Adv. 2020, 10, 18945–18952. [Google Scholar] [CrossRef]
- Pang, B.; Jiang, G.; Zhou, J.; Zhu, Y.; Cheng, W.; Zhao, D.; Wang, K.; Xu, G.; Yu, H. Molecular-Scale Design of Cellulose-Based Functional Materials for Flexible Electronic Devices. Adv. Electron. Mater. 2021, 7, 2000944. [Google Scholar] [CrossRef]
- Wang, J.; He, J.; Ma, L.; Zhang, Y.; Shen, L.; Xiong, S.; Li, K.; Qu, M. Multifunctional conductive cellulose fabric with flexibility, superamphiphobicity and flame-retardancy for all-weather wearable smart electronic textiles and high-temperature warning device. J. Chem. Eng. 2020, 390, 124508. [Google Scholar] [CrossRef]
- Fu, Q.; Cui, C.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Emerging cellulose-derived materials: A promising platform for the design of flexible wearable sensors toward health and environment monitoring. Mater. Chem. Front. 2021, 5, 2051–2091. [Google Scholar] [CrossRef]
- Niu, Z.; Cheng, W.; Cao, M.; Wang, D.; Wang, Q.; Han, J.; Long, Y.; Han, G. Recent advances in cellulose-based flexible triboelectric nanogenerators. Nano Energy 2021, 87, 106175. [Google Scholar] [CrossRef]
- Hu, B.; Chen, W.; Zhou, J. High performance flexible sensor based on inorganic nanomaterials. Sens. Actuators B Chem. 2013, 176, 522–533. [Google Scholar] [CrossRef]
- Hu, L.; Kim, H.S.; Lee, J.-Y.; Peumans, P.; Cui, Y. Scalable Coating and Properties of Transparent, Flexible, Silver Nanowire Electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Zhu, Y.; Cheng, W.; Zhao, D.; Xu, G.; Yu, H. Assembly of silver nanowires and PEDOT:PSS with hydrocellulose toward highly flexible, transparent and conductivity-stable conductors. J. Chem. Eng. 2020, 392, 123644. [Google Scholar] [CrossRef]
- Park, G.; Lee, K.; Kwon, G.; Kim, D.; Jeon, Y.; You, J. Green nanoarchitectonics for next generation electronics devices: Patterning of conductive nanowires on regenerated cellulose substrates. Cellulose 2022, 29, 2449–2460. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Cui, K.; Ge, S.; Cheng, X.; Yan, M.; Yu, J.; Liu, H. Flexible Electronics Based on Micro/Nanostructured Paper. Adv. Mater. 2018, 30, 1801588. [Google Scholar] [CrossRef]
- Jung, Y.H.; Chang, T.-H.; Zhang, H.; Yao, C.; Zheng, Q.; Yang, V.W.; Mi, H.; Kim, M.; Cho, S.J.; Park, D.-W.; et al. High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat. Commun. 2015, 6, 7170. [Google Scholar] [CrossRef]
- Xiong, R.; Hu, K.; Grant, A.M.; Ma, R.; Xu, W.; Lu, C.; Zhang, X.; Tsukruk, V.V. Ultrarobust Transparent Cellulose Nanocrystal-Graphene Membranes with High Electrical Conductivity. Adv. Mater. 2016, 28, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Novaković, M.; Popović, D.M.; Mladenović, N.; Poparić, G.B.; Stanković, S.B. Development of comfortable and eco-friendly cellulose based textiles with improved sustainability. J. Clean. Prod. 2020, 267, 122154. [Google Scholar] [CrossRef]
- Liman, M.L.R.; Islam, M.T. Emerging washable textronics for imminent e-waste mitigation: Strategies, reliability, and perspectives. J. Mater. Chem. A 2022, 10, 2697–2735. [Google Scholar] [CrossRef]
- Darabi, S.; Hummel, M.; Rantasalo, S.; Rissanen, M.; Öberg Månsson, I.; Hilke, H.; Hwang, B.; Skrifvars, M.; Hamedi, M.M.; Sixta, H.; et al. Green Conducting Cellulose Yarns for Machine-Sewn Electronic Textiles. ACS Appl. Mater. Interfaces 2020, 12, 56403–56412. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Dai, Y.; Xu, H.; Zhong, Y.; Zhang, L.; Chen, Z.; Sui, X.; Feng, X.; Wang, B.; Mao, Z. Transforming commercial regenerated cellulose yarns into multifunctional wearable electronic textiles. J. Mater. Chem. C 2020, 8, 1309–1318. [Google Scholar] [CrossRef]
- Hu, S.; Han, J.; Shi, Z.; Chen, K.; Xu, N.; Wang, Y.; Zheng, R.; Tao, Y.; Sun, Q.; Wang, Z.L.; et al. Biodegradable, Super-Strong, and Conductive Cellulose Macrofibers for Fabric-Based Triboelectric Nanogenerator. Nanomicro Lett. 2022, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Song, Y.; Ding, D.; Ling, Z.; Xu, F. Flexible and Anisotropic Strain Sensor Based on Carbonized Crepe Paper with Aligned Cellulose Fibers. Adv. Funct. Mater. 2018, 28, 1802547. [Google Scholar] [CrossRef]
- Li, Q.; Yin, R.; Zhang, D.; Liu, H.; Chen, X.; Zheng, Y.; Guo, Z.; Liu, C.; Shen, C. Flexible conductive MXene/cellulose nanocrystal coated nonwoven fabrics for tunable wearable strain/pressure sensors. J. Mater. Chem. A 2020, 8, 21131–21141. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, F.; Cao, P.; Liu, J.; Li, D.; Wu, J.; Wang, N.; Su, Y.; Zhao, Y. Winding-Locked Carbon Nanotubes/Polymer Nanofibers Helical Yarn for Ultrastretchable Conductor and Strain Sensor. ACS Nano 2020, 14, 3442–3450. [Google Scholar] [CrossRef]
- Guan, F.; Xie, Y.; Wu, H.; Meng, Y.; Shi, Y.; Gao, M.; Zhang, Z.; Chen, S.; Chen, Y.; Wang, H.; et al. Silver Nanowire–Bacterial Cellulose Composite Fiber-Based Sensor for Highly Sensitive Detection of Pressure and Proximity. ACS Nano 2020, 14, 15428–15439. [Google Scholar] [CrossRef]
- Jang, S.; Kim, J.; Kim, D.W.; Kim, J.W.; Chun, S.; Lee, H.J.; Yi, G.-R.; Pang, C. Carbon-Based, Ultraelastic, Hierarchically Coated Fiber Strain Sensors with Crack-Controllable Beads. ACS Appl. Mater. Interfaces 2019, 11, 15079–15087. [Google Scholar] [CrossRef] [PubMed]
- Barandun, G.; Soprani, M.; Naficy, S.; Grell, M.; Kasimatis, M.; Chiu, K.L.; Ponzoni, A.; Güder, F. Cellulose Fibers Enable Near-Zero-Cost Electrical Sensing of Water-Soluble Gases. ACS Sens. 2019, 4, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wang, J.; Kang, W.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Highly Stretchable Piezoresistive Graphene–Nanocellulose Nanopaper for Strain Sensors. Adv. Mater. 2014, 26, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Safari, S.; van de Ven, T.G. Effect of Water Vapor Adsorption on Electrical Properties of Carbon Nanotube/Nanocrystalline Cellulose Composites. ACS Appl. Mater. Interfaces 2016, 8, 9483–9489. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gao, S.; Ling, Z.; Lai, C.; Huang, Y.; Wang, J.; Wang, C.; Chu, F.; Xu, F.; Dumont, M.-J.; et al. A multiscale biomimetic strategy to design strong, tough hydrogels by tuning the self-assembly behavior of cellulose. J. Mater. Chem. A 2022, 10, 13685–13696. [Google Scholar] [CrossRef]
- Dandegaonkar, G.; Ahmed, A.; Sun, L.; Adak, B.; Mukhopadhyay, S. Cellulose based flexible and wearable sensors for health monitoring. Mater. Adv. 2022, 3, 3766–3783. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, X.; Wei, X.; Zhang, J.; Wang, D.; Lu, H.; Jia, P. Ultrastretchable, Tough, Antifreezing, and Conductive Cellulose Hydrogel for Wearable Strain Sensor. ACS Appl. Mater. Interfaces 2020, 12, 53247–53256. [Google Scholar] [CrossRef]
- Han, S.; Alvi, N.U.H.; Granlöf, L.; Granberg, H.; Berggren, M.; Fabiano, S.; Crispin, X. A Multiparameter Pressure–Temperature–Humidity Sensor Based on Mixed Ionic–Electronic Cellulose Aerogels. Adv. Sci. 2019, 6, 1802128. [Google Scholar] [CrossRef]
- Yin, M.; Yu, Y.; Wang, Y.; Wang, Z.; Lu, X.; Cheng, T.; Wang, Z.L. Multi-plate structured triboelectric nanogenerator based on cycloidal displacement for harvesting hydroenergy. Extreme Mech. Lett. 2019, 33, 100576. [Google Scholar] [CrossRef]
- Fouz, D.M.; Carballo, R.; Ramos, V.; Iglesias, G. Hydrokinetic energy exploitation under combined river and tidal flow. Renew. Energ. 2019, 143, 558–568. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Zhang, B.; Yang, O.; Yuan, W.; He, L.; Wei, X.; Wang, J.; Wang, Z.L. Harvesting Wind Energy by a Triboelectric Nanogenerator for an Intelligent High-Speed Train System. ACS Energy Lett. 2021, 6, 1490–1499. [Google Scholar] [CrossRef]
- Hedley, G.J.; Ruseckas, A.; Samuel, I.D.W. Light Harvesting for Organic Photovoltaics. Chem. Rev. 2017, 117, 796–837. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Lim, S.L.; Goh, W.P.; Jiang, C.; Tee, S.Y.; Ye, T.; Li, X.; Nguyen, K.H.; Lee, C.J.J.; Ding, N.; et al. Fabrication of Mesoporous Titania Nanoparticles with Controlled Porosity and Connectivity for Studying the Photovoltaic Properties in Perovskite Solar Cells. ChemNanoMat 2018, 4, 394–400. [Google Scholar] [CrossRef]
- Shin, Y.; Cho, S.; Han, S.; Jung, G.Y. Omni-directional wind-driven triboelectric nanogenerator with cross-shaped dielectric film. Nano Converg. 2021, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, C.; Zhang, B.; Yang, O.; Yuan, W.; Zhou, L.; Zhao, Z.; Wu, Z.; Wang, J.; Wang, Z.L. A Dual-Mode Triboelectric Nanogenerator for Wind Energy Harvesting and Self-Powered Wind Speed Monitoring. ACS Nano 2022, 16, 6244–6254. [Google Scholar] [CrossRef]
- Jiang, Y.; Dong, K.; An, J.; Liang, F.; Yi, J.; Peng, X.; Ning, C.; Ye, C.; Wang, Z.L. UV-Protective, Self-Cleaning, and Antibacterial Nanofiber-Based Triboelectric Nanogenerators for Self-Powered Human Motion Monitoring. ACS Appl. Mater. Interfaces 2021, 13, 11205–11214. [Google Scholar] [CrossRef]
- Proto, A.; Penhaker, M.; Conforto, S.; Schmid, M. Nanogenerators for Human Body Energy Harvesting. Trends Biotechnol. 2017, 35, 610–624. [Google Scholar] [CrossRef]
- Rodrigues, C.; Nunes, D.; Clemente, D.; Mathias, N.; Correia, J.M.; Rosa-Santos, P.; Taveira-Pinto, F.; Morais, T.; Pereira, A.; Ventura, J. Emerging triboelectric nanogenerators for ocean wave energy harvesting: State of the art and future perspectives. Energy Environ. Sci. 2020, 13, 2657–2683. [Google Scholar] [CrossRef]
- Sarkar, D.; Das, N.; Saikh, M.M.; Biswas, P.; Das, S.; Das, S.; Hoque, N.A.; Basu, R. Development of a Sustainable and Biodegradable Sonchus asper Cotton Pappus Based Piezoelectric Nanogenerator for Instrument Vibration and Human Body Motion Sensing with Mechanical Energy Harvesting Applications. ACS Omega 2021, 6, 28710–28717. [Google Scholar] [CrossRef]
- Liu, J.; Cui, N.; Gu, L.; Chen, X.; Bai, S.; Zheng, Y.; Hu, C.; Qin, Y. A three-dimensional integrated nanogenerator for effectively harvesting sound energy from the environment. Nanoscale 2016, 8, 4938–4944. [Google Scholar] [CrossRef]
- Haroun, A.; Tarek, M.; Mosleh, M.; Ismail, F. Recent Progress on Triboelectric Nanogenerators for Vibration Energy Harvesting and Vibration Sensing. Nanomaterials 2022, 12, 2960. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Lin Wang, Z. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Wang, Z.L.; Chen, J.; Lin, L. Progress in triboelectric nanogenerators as a new energy technology and self-powered sensors. Energy Environ. Sci. 2015, 8, 2250–2282. [Google Scholar] [CrossRef]
- Zhang, R.; Olin, H. Material choices for triboelectric nanogenerators: A critical review. EcoMat 2020, 2, e12062. [Google Scholar] [CrossRef]
- Kim, Y.; Wu, X.; Oh, J.H. Fabrication of triboelectric nanogenerators based on electrospun polyimide nanofibers membrane. Sci. Rep. 2020, 10, 2742. [Google Scholar] [CrossRef] [PubMed]
- Dudem, B.; Huynh, N.D.; Kim, W.; Kim, D.H.; Hwang, H.J.; Choi, D.; Yu, J.S. Nanopillar-array architectured PDMS-based triboelectric nanogenerator integrated with a windmill model for effective wind energy harvesting. Nano Energy 2017, 42, 269–281. [Google Scholar] [CrossRef]
- Dudem, B.; Kim, D.H.; Mule, A.R.; Yu, J.S. Enhanced Performance of Microarchitectured PTFE-Based Triboelectric Nanogenerator via Simple Thermal Imprinting Lithography for Self-Powered Electronics. ACS Appl. Mater. Interfaces 2018, 10, 24181–24192. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.X.; Liang, X.; Jiang, T.; Xu, L.; Shao, J.J.; Nie, J.H.; Bai, Y.; Zhong, W.; Wang, Z.L. Spherical Triboelectric Nanogenerators Based on Spring-Assisted Multilayered Structure for Efficient Water Wave Energy Harvesting. Adv. Funct. Mater. 2018, 28, 1802634. [Google Scholar] [CrossRef]
- Hinchet, R.; Yoon, H.-J.; Ryu, H.; Kim, M.-K.; Choi, E.-K.; Kim, D.-S.; Kim, S.-W. Transcutaneous ultrasound energy harvesting using capacitive triboelectric technology. Science 2019, 365, 491–494. [Google Scholar] [CrossRef]
- Lu, L.; Ding, W.; Liu, J.; Yang, B. Flexible PVDF based piezoelectric nanogenerators. Nano Energy 2020, 78, 105251. [Google Scholar] [CrossRef]
- Zheng, Q.; Zou, Y.; Zhang, Y.; Liu, Z.; Shi, B.; Wang, X.; Jin, Y.; Ouyang, H.; Li, Z.; Wang, Z.L. Biodegradable triboelectric nanogenerator as a life-time designed implantable power source. Sci. Adv. 2016, 2, e1501478. [Google Scholar] [CrossRef]
- Strassburg, S.; Zainuddin, S.; Scheibel, T. The Power of Silk Technology for Energy Applications. Adv. Energy Mater. 2021, 11, 2100519. [Google Scholar] [CrossRef]
- Charoonsuk, T.; Pongampai, S.; Pakawanit, P.; Vittayakorn, N. Achieving a highly efficient chitosan-based triboelectric nanogenerator via adding organic proteins: Influence of morphology and molecular structure. Nano Energy 2021, 89, 106430. [Google Scholar] [CrossRef]
- Yang, M.; Tian, X.; Hua, T. Green and recyclable cellulose based TENG for sustainable energy and human-machine interactive system. Chem. Eng. J. 2022, 442, 136150. [Google Scholar] [CrossRef]
- Ccorahua, R.; Huaroto, J.; Luyo, C.; Quintana, M.; Vela, E.A. Enhanced-performance bio-triboelectric nanogenerator based on starch polymer electrolyte obtained by a cleanroom-free processing method. Nano Energy 2019, 59, 610–618. [Google Scholar] [CrossRef]
- Zhang, R.; Dahlström, C.; Zou, H.; Jonzon, J.; Hummelgård, M.; Örtegren, J.; Blomquist, N.; Yang, Y.; Andersson, H.; Olsen, M.; et al. Cellulose-Based Fully Green Triboelectric Nanogenerators with Output Power Density of 300 W m−2. Adv. Mater. 2020, 32, 2002824. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Lu, A. Highly stretchable, transparent cellulose/PVA composite hydrogel for multiple sensing and triboelectric nanogenerators. J. Mater. Chem. A 2020, 8, 13935–13941. [Google Scholar] [CrossRef]
- Sala de Medeiros, M.; Chanci, D.; Martinez, R.V. Moisture-insensitive, self-powered paper-based flexible electronics. Nano Energy 2020, 78, 105301. [Google Scholar] [CrossRef]
- Qian, C.; Li, L.; Gao, M.; Yang, H.; Cai, Z.; Chen, B.; Xiang, Z.; Zhang, Z.; Song, Y. All-printed 3D hierarchically structured cellulose aerogel based triboelectric nanogenerator for multi-functional sensors. Nano Energy 2019, 63, 103885. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, S.; Shi, Z.; Wang, Y.; Lei, Y.; Han, J.; Xiong, Y.; Sun, J.; Zheng, L.; Sun, Q.; et al. Eco-friendly and recyclable all cellulose triboelectric nanogenerator and self-powered interactive interface. Nano Energy 2021, 89, 106354. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric Nanogenerators Based on Zinc Oxide Nanowire Arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. On Maxwell’s displacement current for energy and sensors: The origin of nanogenerators. Mater. Today 2017, 20, 74–82. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Z.; Hu, G.-H.; Xiong, C.; Isogai, A.; Yang, Q. Recent advances in cellulose-based piezoelectric and triboelectric nanogenerators for energy harvesting: A review. J. Mater. Chem. A 2021, 9, 1910–1937. [Google Scholar] [CrossRef]
- Kim, J.; Yun, S.; Ounaies, Z. Discovery of Cellulose as a Smart Material. Macromolecules 2006, 39, 4202–4206. [Google Scholar] [CrossRef]
- Csoka, L.; Hoeger, I.C.; Rojas, O.J.; Peszlen, I.; Pawlak, J.J.; Peralta, P.N. Piezoelectric Effect of Cellulose Nanocrystals Thin Films. ACS Macro Lett. 2012, 1, 867–870. [Google Scholar] [CrossRef]
- Sun, J.; Guo, H.; Ribera, J.; Wu, C.; Tu, K.; Binelli, M.; Panzarasa, G.; Schwarze, F.W.M.R.; Wang, Z.L.; Burgert, I. Sustainable and Biodegradable Wood Sponge Piezoelectric Nanogenerator for Sensing and Energy Harvesting Applications. ACS Nano 2020, 14, 14665–14674. [Google Scholar] [CrossRef]
- Alam, M.M.; Mandal, D. Native Cellulose Microfiber-Based Hybrid Piezoelectric Generator for Mechanical Energy Harvesting Utility. ACS Appl. Mater. Interfaces 2016, 8, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Espinosa, H.D. Giant Piezoelectric Size Effects in Zinc Oxide and Gallium Nitride Nanowires. A First Principles Investigation. Nano Lett. 2011, 11, 786–790. [Google Scholar] [CrossRef]
- Choi, H.Y.; Jeong, Y.G. Microstructures and piezoelectric performance of eco-friendly composite films based on nanocellulose and barium titanate nanoparticle. Compos. B Eng. 2019, 168, 58–65. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, T.; Lian, W.; Zhang, M.; Lu, B.; Dong, B.; Tan, K.; Liu, C.; Shen, C. Flexible layered cotton cellulose-based nanofibrous membranes for piezoelectric energy harvesting and self-powered sensing. Carbohydr. Polym. 2022, 275, 118740. [Google Scholar] [CrossRef]
- Pusty, M.; Shirage, P.M. Gold nanoparticle–cellulose/PDMS nanocomposite: A flexible dielectric material for harvesting mechanical energy. RSC Adv. 2020, 10, 10097–10112. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Du, Y.; Wang, B.; Mao, Z.; Xu, H.; Zhang, L.; Zhong, Y.; Jiang, W.; Wang, L.; Sui, X. Flexible cellulose-based thermoelectric sponge towards wearable pressure sensor and energy harvesting. Chem. Eng. J. 2018, 338, 1–7. [Google Scholar] [CrossRef]
- Tee, S.Y.; Ponsford, D.; Lay, C.L.; Wang, X.; Wang, X.; Neo, D.C.J.; Wu, T.; Thitsartarn, W.; Yeo, J.C.C.; Guan, G.; et al. Thermoelectric Silver-Based Chalcogenides. Adv. Sci. 2022, 9, e2204624. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.Y.; Tan, X.Y.; Wang, X.; Lee, C.J.J.; Win, K.Y.; Ni, X.P.; Teo, S.L.; Seng, D.H.L.; Tanaka, Y.; Han, M.Y. Aqueous Synthesis, Doping, and Processing of n-Type Ag2Se for High Thermoelectric Performance at Near-Room-Temperature. Inorg. Chem. 2022, 61, 6451–6458. [Google Scholar] [CrossRef]
- Tee, S.Y.; Ponsford, D.; Tan, X.Y.; Wang, X.; Lay, C.L.; Lee, C.J.J.; Ni, X.P.; Seng, D.H.L.; Thitsartarn, W.; Guan, G.; et al. Compositionally tuned hybridization of n-type Ag0 : Ag2Se under ambient conditions towards excellent thermoelectric properties at room temperature. Mater. Chem. Front. 2023. [Google Scholar] [CrossRef]
- Lee, B.; Cho, H.; Park, K.T.; Kim, J.-S.; Park, M.; Kim, H.; Hong, Y.; Chung, S. High-performance compliant thermoelectric generators with magnetically self-assembled soft heat conductors for self-powered wearable electronics. Nat. Commun. 2020, 11, 5948. [Google Scholar] [CrossRef]
- Palaporn, D.; Mongkolthanaruk, W.; Faungnawakij, K.; Kurosaki, K.; Pinitsoontorn, S. Flexible Thermoelectric Paper and Its Thermoelectric Generator from Bacterial Cellulose/Ag2Se Nanocomposites. ACS Appl. Energy Mater. 2022, 5, 3489–3501. [Google Scholar] [CrossRef]
- Mo, J.-H.; Kim, J.-Y.; Kang, Y.H.; Cho, S.Y.; Jang, K.-S. Carbon Nanotube/Cellulose Acetate Thermoelectric Papers. ACS Sustain. Chem. Eng. 2018, 6, 15970–15975. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, J.; Kou, T.; Song, Y.; Liu, T.; Li, Y. Paper-Based Electrodes for Flexible Energy Storage Devices. Adv. Sci. 2017, 4, 1700107. [Google Scholar] [CrossRef]
- Sun, C.; Goharpey, A.H.; Rai, A.; Zhang, T.; Ko, D.-K. Paper Thermoelectrics: Merging Nanotechnology with Naturally Abundant Fibrous Material. ACS Appl. Mater. Interfaces 2016, 8, 22182–22189. [Google Scholar] [CrossRef]
- Gao, J.; Miao, L.; Liu, C.; Wang, X.; Peng, Y.; Wei, X.; Zhou, J.; Chen, Y.; Hashimoto, R.; Asaka, T.; et al. A novel glass-fiber-aided cold-press method for fabrication of n-type Ag2Te nanowires thermoelectric film on flexible copy-paper substrate. J. Mater. Chem. A 2017, 5, 24740–24748. [Google Scholar] [CrossRef]
- Jin, Q.; Shi, W.; Zhao, Y.; Qiao, J.; Qiu, J.; Sun, C.; Lei, H.; Tai, K.; Jiang, X. Cellulose Fiber-Based Hierarchical Porous Bismuth Telluride for High-Performance Flexible and Tailorable Thermoelectrics. ACS Appl. Mater. Interfaces 2018, 10, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Mulla, R.; Jones, D.R.; Dunnill, C.W. Thin-films on cellulose paper to construct thermoelectric generator of promising power outputs suitable for low-grade heat recovery. Mater. Today Commun. 2021, 29, 102738. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, H.; Yang, X.; Fan, J.; Bi, H.; Wang, C.; Zhang, Y.; Luo, C.; Chen, X.; Wu, X. Facile fabrication of paper-based flexible thermoelectric generator. Npj Flex. Electron. 2021, 5, 6. [Google Scholar] [CrossRef]
- Li, H.; Zong, Y.; Ding, Q.; Han, W.; Li, X. Paper-based thermoelectric generator based on multi-walled carbon nanotube/carboxylated nanocellulose. J. Power Sources 2021, 500, 229992. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; Dörling, B.; Zapata-Arteaga, O.; Rodríguez-Martínez, X.; Gómez, A.; Reparaz, J.S.; Laromaine, A.; Roig, A.; Campoy-Quiles, M. Farming thermoelectric paper. Energy Environ. Sci. 2019, 12, 716–726. [Google Scholar] [CrossRef]
- Zhao, X.; Han, W.; Jiang, Y.; Zhao, C.; Ji, X.; Kong, F.; Xu, W.; Zhang, X. A honeycomb-like paper-based thermoelectric generator based on a Bi2Te3/bacterial cellulose nanofiber coating. Nanoscale 2019, 11, 17725–17735. [Google Scholar] [CrossRef]
- Yao, X.; Geng, H.; Gu, Y.; Cui, H.; Guan, G.; Han, M. Heterojunction Doping of Poly(triarylamine) with Cesium-Doped Vanadium Oxide via Interfacial Electron Transfer toward High-Performance Perovskite Solar Cells. J. Phys. Chem. C 2021, 125, 23474–23482. [Google Scholar] [CrossRef]
- Pan, T.; Liu, S.; Zhang, L.; Xie, W. Flexible organic optoelectronic devices on paper. iScience 2022, 25, 103782. [Google Scholar] [CrossRef]
- Zhu, H.; Fang, Z.; Preston, C.; Li, Y.; Hu, L. Transparent paper: Fabrications, properties, and device applications. Energy Environ. Sci. 2014, 7, 269–287. [Google Scholar] [CrossRef]
- Jung, M.-H.; Park, N.-M.; Lee, S.-Y. Color tunable nanopaper solar cells using hybrid CH3NH3PbI3−xBrx perovskite. Sol. Energy 2016, 139, 458–466. [Google Scholar] [CrossRef]
- Hu, L.; Zheng, G.; Yao, J.; Liu, N.; Weil, B.; Eskilsson, M.; Karabulut, E.; Ruan, Z.; Fan, S.; Bloking, J.T.; et al. Transparent and conductive paper from nanocellulose fibers. Energy Environ. Sci. 2013, 6, 513–518. [Google Scholar] [CrossRef]
- Wu, J.; Che, X.; Hu, H.-C.; Xu, H.; Li, B.; Liu, Y.; Li, J.; Ni, Y.; Zhang, X.; Ouyang, X. Organic solar cells based on cellulose nanopaper from agroforestry residues with an efficiency of over 16% and effectively wide-angle light capturing. J. Mater. Chem. A 2020, 8, 5442–5448. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Islam, A.; Zheng, Q.; Li, J.; Ji, W.; Chen, L.; Ouyang, X. From Straw to Device Interface: Carboxymethyl-Cellulose-Based Modified Interlayer for Enhanced Power Conversion Efficiency of Organic Solar Cells. Adv. Sci. 2020, 7, 1902269. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Khan, T.M.; Liu, J.C.; Hsu, J.; Shim, J.W.; Dindar, A.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Recyclable organic solar cells on cellulose nanocrystal substrates. Sci. Rep. 2013, 3, 1536. [Google Scholar] [CrossRef]
- Zhou, Y.; Khan, T.M.; Liu, J.-C.; Fuentes-Hernandez, C.; Shim, J.W.; Najafabadi, E.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Efficient recyclable organic solar cells on cellulose nanocrystal substrates with a conducting polymer top electrode deposited by film-transfer lamination. Org. Electron. 2014, 15, 661–666. [Google Scholar] [CrossRef]
- Cheng, Q.; Ye, D.; Yang, W.; Zhang, S.; Chen, H.; Chang, C.; Zhang, L. Construction of Transparent Cellulose-Based Nanocomposite Papers and Potential Application in Flexible Solar Cells. ACS Sustain. Chem. Eng. 2018, 6, 8040–8047. [Google Scholar] [CrossRef]
- Gao, L.; Chao, L.; Hou, M.; Liang, J.; Chen, Y.; Yu, H.-D.; Huang, W. Flexible, transparent nanocellulose paper-based perovskite solar cells. Npj Flex. Electron. 2019, 3, 4. [Google Scholar] [CrossRef]
- Sun, G.; Liu, B.; Niu, H.; Hao, F.; Chen, N.; Zhang, M.; Tian, G.; Qi, S.; Wu, D. In situ welding: Superb strength, good wettability and fire resistance tri-layer separator with shutdown function for high-safety lithium ion battery. J. Membr. Sci. 2020, 595, 117509. [Google Scholar] [CrossRef]
- Tu, S.; Lu, Z.; Zheng, M.; Chen, Z.; Wang, X.; Cai, Z.; Chen, C.; Wang, L.; Li, C.; Seh, Z.W.; et al. Single-Layer-Particle Electrode Design for Practical Fast-Charging Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2202892. [Google Scholar] [CrossRef]
- Chen, C.; Hu, L. Nanocellulose toward Advanced Energy Storage Devices: Structure and Electrochemistry. Acc. Chem. Res. 2018, 51, 3154–3165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shen, F.; Luo, W.; Zhu, S.; Zhao, M.; Natarajan, B.; Dai, J.; Zhou, L.; Ji, X.; Yassar, R.S.; et al. Low temperature carbonization of cellulose nanocrystals for high performance carbon anode of sodium-ion batteries. Nano Energy 2017, 33, 37–44. [Google Scholar] [CrossRef]
- Jeong, C.-U.; Umirov, N.; Jung, D.-H.; Seo, D.-H.; Lee, B.-M.; Choi, B.-S.; Kim, S.-S.; Choi, J.-H. Li-incorporated porous carbon monoliths derived from carboxymethyl cellulose as anode material for high power lithium-ion batteries. J. Power Sources 2021, 506, 230050. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, L.; Yan, X.; Ding, X. Recent Advances of Cellulose-Based Materials and Their Promising Application in Sodium-Ion Batteries and Capacitors. Small 2018, 14, 1802444. [Google Scholar] [CrossRef]
- Liu, H.; Du, H.; Zheng, T.; Liu, K.; Ji, X.; Xu, T.; Zhang, X.; Si, C. Cellulose based composite foams and aerogels for advanced energy storage devices. Chem. Eng. J. 2021, 426, 130817. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Luo, B.; Yang, J.; Wang, X.; Song, Q.; Chen, S.; Zhi, L. Pyrolyzed Bacterial Cellulose: A Versatile Support for Lithium Ion Battery Anode Materials. Small 2013, 9, 2399–2404. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Shi, J.; Liu, H.; Wang, D.; Zhai, W.; Meng, Z. A nanotubular TiO2/SiOx/Si composite derived from cellulosic cotton as an anode material for lithium-ion batteries with enhanced electrochemical performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126870. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.; Zhang, J.; Jiang, Q.; Yin, H.; Wang, Z.; Zuo, S. Silicon-Based Nanorod Anodes by Employing Bacterial Cellulose Derived Carbon Skeleton Towards Lithium-Ion Batteries. Batter. Supercaps 2022, 5, e202100260. [Google Scholar] [CrossRef]
- Shen, D.; Huang, C.; Gan, L.; Liu, J.; Gong, Z.; Long, M. Rational Design of Si@SiO2/C Composites Using Sustainable Cellulose as a Carbon Resource for Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 7946–7954. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, W.; Wang, W.; Wang, H.; Wang, Y.; Shi, J.; Chen, J.; Liu, S.; Huang, M.; Mitlin, D. Sulfur and nitrogen codoped cyanoethyl cellulose-derived carbon with superior gravimetric and volumetric capacity for potassium ion storage. Carbon Energy 2022, 4, 986–1001. [Google Scholar] [CrossRef]
- Xu, D.; Chen, C.; Xie, J.; Zhang, B.; Miao, L.; Cai, J.; Huang, Y.; Zhang, L. A Hierarchical N/S-Codoped Carbon Anode Fabricated Facilely from Cellulose/Polyaniline Microspheres for High-Performance Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1501929. [Google Scholar] [CrossRef]
- Pang, Q.; Tang, J.; Huang, H.; Liang, X.; Hart, C.; Tam, K.C.; Nazar, L.F. A Nitrogen and Sulfur Dual-Doped Carbon Derived from Polyrhodanine@Cellulose for Advanced Lithium–Sulfur Batteries. Adv. Mater. 2015, 27, 6021–6028. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Wang, P.; Niu, X.; Li, Y.; Li, J. Cellulosic filter paper derived MoO3/TiO2 composites with variable micromorphologies as anode materials for lithium storage. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129731. [Google Scholar] [CrossRef]
- Oh, S.-I.; Kim, J.-C.; Kim, D.-W. Cellulose-derived tin-oxide-nanoparticle-embedded carbon fibers as binder-free flexible Li-ion battery anodes. Cellulose 2019, 26, 2557–2571. [Google Scholar] [CrossRef]
- Liu, M.; Li, N.; Wang, S.; Li, Y.; Liang, C.; Yu, K. 3D nanoflower-like MoS2 grown on wheat straw cellulose carbon for lithium-ion battery anode material. J. Alloys Compd. 2023, 933, 167689. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Tu, J.; Chen, F.; Xie, J.; Zhu, T.; Zhao, X. Cross-linked binder enables reversible volume changes of Si-based anodes from sustainable photovoltaic waste silicon. Mater. Today Sustain. 2022, 19, 100178. [Google Scholar] [CrossRef]
- Wang, H.; Fu, J.; Wang, C.; Wang, J.; Yang, A.; Li, C.; Sun, Q.; Cui, Y.; Li, H. A binder-free high silicon content flexible anode for Li-ion batteries. Energy Environ. Sci. 2020, 13, 848–858. [Google Scholar] [CrossRef]
- Illa, M.P.; Pathak, A.D.; Sharma, C.S.; Khandelwal, M. Bacterial Cellulose–Polyaniline Composite Derived Hierarchical Nitrogen-Doped Porous Carbon Nanofibers as Anode for High-Rate Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 8676–8687. [Google Scholar] [CrossRef]
- Gao, T.; Xu, C.; Li, R.; Zhang, R.; Wang, B.; Jiang, X.; Hu, M.; Bando, Y.; Kong, D.; Dai, P.; et al. Biomass-Derived Carbon Paper to Sandwich Magnetite Anode for Long-Life Li-Ion Battery. ACS Nano 2019, 13, 11901–11911. [Google Scholar] [CrossRef]
- Dong, G.-H.; Mao, Y.-Q.; Li, Y.-Q.; Huang, P.; Fu, S.-Y. MXene-carbon nanotubes-Cellulose-LiFePO4 based self-supporting cathode with ultrahigh-area-capacity for lithium-ion batteries. Electrochim. Acta 2022, 420, 140464. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Pastel, G.; Li, Y.; Song, J.; Mi, R.; Kong, W.; Liu, B.; Jiang, Y.; Yang, K.; et al. Conductive Cellulose Nanofiber Enabled Thick Electrode for Compact and Flexible Energy Storage Devices. Adv. Energy Mater. 2018, 8, 1802398. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Z.; Xiang, Y.; Liu, D.; Xie, Z.; Qu, D.; Sun, M.; Tang, H.; Li, J. Cellulose-based material in lithium-sulfur batteries: A review. Carbohydr. Polym. 2021, 255, 117469. [Google Scholar] [CrossRef]
- Mao, H.; Liu, L.; Shi, L.; Wu, H.; Lang, J.; Wang, K.; Zhu, T.; Gao, Y.; Sun, Z.; Zhao, J.; et al. High loading cotton cellulose-based aerogel self-standing electrode for Li-S batteries. Sci. Bull. 2020, 65, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Y.; Bai, Q.; Wu, R. Discarded cigarette filter-derived hierarchically porous carbon@graphene composites for lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 3558–3562. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, J.; Zhang, F.; Du, M.; Hui, H.; Zhao, F.; He, X.; Sun, Z. A porous, mechanically strong and thermally stable zeolitic imidazolate framework-8@bacterial cellulose/aramid nanofibers composite separator for advanced lithium-ion batteries. J. Membr. Sci. 2022, 652, 120461. [Google Scholar] [CrossRef]
- Pavlin, N.; Hribernik, S.; Kapun, G.; Talian, S.D.; Njel, C.; Dedryvère, R.; Dominko, R. The Role of Cellulose Based Separator in Lithium Sulfur Batteries. J. Electrochem. Soc. 2019, 166, A5237. [Google Scholar] [CrossRef]
- Lv, D.; Chai, J.; Wang, P.; Zhu, L.; Liu, C.; Nie, S.; Li, B.; Cui, G. Pure cellulose lithium-ion battery separator with tunable pore size and improved working stability by cellulose nanofibrils. Carbohydr. Polym. 2021, 251, 116975. [Google Scholar] [CrossRef]
- Boriboon, D.; Vongsetskul, T.; Limthongkul, P.; Kobsiriphat, W.; Tammawat, P. Cellulose ultrafine fibers embedded with titania particles as a high performance and eco-friendly separator for lithium-ion batteries. Carbohydr. Polym. 2018, 189, 145–151. [Google Scholar] [CrossRef]
- Deng, J.; Cao, D.; Yang, X.; Zhang, G. Cross-linked cellulose/carboxylated polyimide nanofiber separator for lithium-ion battery application. J. Chem. Eng. 2022, 433, 133934. [Google Scholar] [CrossRef]
- Hänsel, C.; Lizundia, E.; Kundu, D. A Single Li-Ion Conductor Based on Cellulose. ACS Appl. Mater. Interfaces 2019, 2, 5686–5691. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Huang, C.; Luo, L.; Deng, Z.; Guo, W.; Xu, J.; Meng, Z. A novel cellulose membrane from cattail fibers as separator for Li-ion batteries. Cellulose 2021, 28, 9309–9321. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, Y.-H.; Cho, S.-J.; Gwon, J.-G.; Cho, H.-J.; Jang, M.; Lee, S.-Y.; Lee, S.-Y. Nanomat Li–S batteries based on all-fibrous cathode/separator assemblies and reinforced Li metal anodes: Towards ultrahigh energy density and flexibility. Energy Environ. Sci. 2019, 12, 177–186. [Google Scholar] [CrossRef]
- Pan, R.; Xu, X.; Sun, R.; Wang, Z.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Nanocellulose Modified Polyethylene Separators for Lithium Metal Batteries. Small 2018, 14, 1704371. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Yang, S.; Zhang, M.; Huang, K.; Long, J.; Xiao, J. Eco-friendly xonotlite nanowires/wood pulp fibers ceramic hybrid separators through a simple papermaking process for lithium ion battery. J. Membr. Sci. 2020, 597, 117725. [Google Scholar] [CrossRef]
- Jiang, F.; Yin, L.; Yu, Q.; Zhong, C.; Zhang, J. Bacterial cellulose nanofibrous membrane as thermal stable separator for lithium-ion batteries. J. Power Sources 2015, 279, 21–27. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Guan, M.; Shang, Z.; Sun, Y.; Lu, Z.; Li, H.; An, X.; Liu, H. Nanofibrillated Cellulose (NFC) as a Pore Size Mediator in the Preparation of Thermally Resistant Separators for Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 4838–4844. [Google Scholar] [CrossRef]
- Gonçalves, R.; Lizundia, E.; Silva, M.M.; Costa, C.M.; Lanceros-Méndez, S. Mesoporous Cellulose Nanocrystal Membranes as Battery Separators for Environmentally Safer Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 2, 3749–3761. [Google Scholar] [CrossRef]
- Xie, W.; Liu, W.; Dang, Y.; Tang, A.; Deng, T.; Qiu, W. Investigation on electrolyte-immersed properties of lithium-ion battery cellulose separator through multi-scale method. J. Power Sources 2019, 417, 150–158. [Google Scholar] [CrossRef]
- Liu, J.; Yang, K.; Mo, Y.; Wang, S.; Han, D.; Xiao, M.; Meng, Y. Highly safe lithium-ion batteries: High strength separator from polyformaldehyde/cellulose nanofibers blend. J. Power Sources 2018, 400, 502–510. [Google Scholar] [CrossRef]
- Seo, J.-Y.; Lee, Y.-H.; Kim, J.-H.; Hong, Y.-K.; Chen, W.; Lee, Y.-G.; Lee, S.-Y. Electrode-customized separator membranes based on self-assembled chiral nematic liquid crystalline cellulose nanocrystals as a natural material strategy for sustainable Li-metal batteries. Energy Storage Mater. 2022, 50, 783–791. [Google Scholar] [CrossRef]
- Love, C.T. Thermomechanical analysis and durability of commercial micro-porous polymer Li-ion battery separators. J. Power Sources 2011, 196, 2905–2912. [Google Scholar] [CrossRef]
- Deng, L.; Wang, Y.; Cai, C.; Wei, Z.; Fu, Y. 3D-cellulose acetate-derived hierarchical network with controllable nanopores for superior Li+ transference number, mechanical strength and dendrites hindrance. Carbohydr. Polym. 2021, 274, 118620. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Ojanguren, A.; Cavasin, N.; Lizundia, E.; Niederberger, M. Transient Rechargeable Battery with a High Lithium Transport Number Cellulosic Separator. Adv. Funct. Mater. 2021, 31, 2101827. [Google Scholar] [CrossRef]
- Su, Z.; He, Y.; Liu, S.; Li, J.; Xiao, X.; Nan, J.; Zuo, X. Uniform Lithium Deposition Achieved by SnO2/Hydroxypropyl Methyl Cellulose Composite Separator toward Ultrastable Lithium Metal Batteries. ACS Appl. Energy Mater. 2022, 5, 10264–10275. [Google Scholar] [CrossRef]
- Sheng, J.; Tong, S.; He, Z.; Yang, R. Recent developments of cellulose materials for lithium-ion battery separators. Cellulose 2017, 24, 4103–4122. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, K.; Yang, T.; Wei, J.; Wang, C.; Chen, M. Sandwich composite PEO@(Er0.5Nb0.5)0.05Ti0.95O2@cellulose electrolyte with high cycling stability for all-solid-state lithium metal batteries. J. Alloys Compd. 2021, 877, 160307. [Google Scholar] [CrossRef]
- Tan, M.Y.; Goh, L.; Safanama, D.; Loh, W.W.; Ding, N.; Chien, S.W.; Goh, S.S.; Thitsartarn, W.; Lim, J.Y.C.; Fam, D.W.H. Upcycling waste poly(ethylene terephthalate) into polymer electrolytes. J. Mater. Chem. A 2022, 10, 24468–24474. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Zeng, Q.; Liu, X.; Lai, W.-Y.; Zhang, L. Cellulose Microcrystals with Brush-Like Architectures as Flexible All-Solid-State Polymer Electrolyte for Lithium-Ion Battery. ACS Sustain. Chem. Eng. 2020, 8, 3200–3207. [Google Scholar] [CrossRef]
- Youcef, H.B.; Orayech, B.; Del Amo, J.M.L.; Bonilla, F.; Shanmukaraj, D.; Armand, M. Functionalized cellulose as quasi single-ion conductors in polymer electrolyte for all-solid–state Li/Na and LiS batteries. Solid State Ion. 2020, 345, 115168. [Google Scholar] [CrossRef]
- Xie, H.; Yang, C.; Fu, K.; Yao, Y.; Jiang, F.; Hitz, E.; Liu, B.; Wang, S.; Hu, L. Flexible, Scalable, and Highly Conductive Garnet-Polymer Solid Electrolyte Templated by Bacterial Cellulose. Adv. Energy Mater. 2018, 8, 1703474. [Google Scholar] [CrossRef]
- Nirmale, T.C.; Karbhal, I.; Kalubarme, R.S.; Shelke, M.V.; Varma, A.J.; Kale, B.B. Facile Synthesis of Unique Cellulose Triacetate Based Flexible and High Performance Gel Polymer Electrolyte for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 34773–34782. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Qu, W.; Su, Q.; Chen, S.; Xing, Y.; Huang, Y.; Chen, N.; Li, Y.; Li, L.; Wu, F.; et al. Biodegradable Bacterial Cellulose-Supported Quasi-Solid Electrolyte for Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 13950–13958. [Google Scholar] [CrossRef] [PubMed]
- Flouda, P.; Bukharina, D.; Pierce, K.J.; Stryutsky, A.V.; Shevchenko, V.V.; Tsukruk, V.V. Flexible Sustained Ionogels with Ionic Hyperbranched Polymers for Enhanced Ion-Conduction and Energy Storage. ACS Appl. Mater. Interfaces 2022, 14, 27028–27039. [Google Scholar] [CrossRef] [PubMed]
- Hyun, W.J.; Thomas, C.M.; Luu, N.S.; Hersam, M.C. Layered Heterostructure Ionogel Electrolytes for High-Performance Solid-State Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2007864. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.B.; Nirmale, T.C.; Khupse, N.D.; Kale, B.B.; Kulkarni, M.V.; Pavitran, S.; Gosavi, S.W. Cellulose-Derived Flame-Retardant Solid Polymer Electrolyte for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 1559–1567. [Google Scholar] [CrossRef]
- Tan, M.Y.; Safanama, D.; Goh, S.S.; Lim, J.Y.C.; Lee, C.-H.; Yeo, J.C.C.; Thitsartarn, W.; Srinivasan, M.; Fam, D.W.H. Concepts and Emerging Trends for Structural Battery Electrolytes. Chem. Asian J. 2022, 17, e202200784. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.; Li, G.; Wang, C.; Li, B.; Li, M.; Wang, Y.; Ming, H.; Wen, Y.; Qiu, J.; et al. A cross-linked gel polymer electrolyte employing cellulose acetate matrix and layered boron nitride filler prepared via in situ thermal polymerization. J. Power Sources 2021, 484, 229235. [Google Scholar] [CrossRef]
- Cai, D.; Zhang, S.; Su, M.; Ma, Z.; Zhu, J.; Zhong, Y.; Luo, X.; Wang, X.; Xia, X.; Gu, C.; et al. Cellulose mesh supported ultrathin ceramic-based composite electrolyte for high-performance Li metal batteries. J. Membr. Sci. 2022, 661, 120907. [Google Scholar] [CrossRef]
- Cai, D.; Qi, X.; Xiang, J.; Wu, X.; Li, Z.; Luo, X.; Wang, X.; Xia, X.; Gu, C.; Tu, J. A cleverly designed asymmetrical composite electrolyte via in-situ polymerization for high-performance, dendrite-free solid state lithium metal battery. J. Chem. Eng. 2022, 435, 135030. [Google Scholar] [CrossRef]
- Foran, G.; Mankovsky, D.; Verdier, N.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. The Impact of Absorbed Solvent on the Performance of Solid Polymer Electrolytes for Use in Solid-State Lithium Batteries. iScience 2020, 23, 101597. [Google Scholar] [CrossRef]
- Mankovsky, D.; Lepage, D.; Lachal, M.; Caradant, L.; Aymé-Perrot, D.; Dollé, M. Water content in solid polymer electrolytes: The lost knowledge. Chem. Commun. 2020, 56, 10167–10170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xie, Y.; Gao, X.; Chen, Z.; Jiang, H.; Tong, Y.; Fan, X.; Lai, Y.; Zhang, Z. The influence of water in electrodes on the solid electrolyte interphase film of micro lithium-ion batteries for the wireless headphone. J. Colloid Interface Sci. 2022, 606, 1729–1736. [Google Scholar] [CrossRef]
- Bin, D.; Wang, F.; Tamirat, A.G.; Suo, L.; Wang, Y.; Wang, C.; Xia, Y. Progress in Aqueous Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703008. [Google Scholar] [CrossRef]
- Chen, D.; Lu, M.; Cai, D.; Yang, H.; Han, W. Recent advances in energy storage mechanism of aqueous zinc-ion batteries. J. Energy Chem. 2021, 54, 712–726. [Google Scholar] [CrossRef]
- Han, X.; Chen, L.; Yanilmaz, M.; Lu, X.; Yang, K.; Hu, K.; Liu, Y.; Zhang, X. From nature, requite to nature: Bio-based cellulose and its derivatives for construction of green zinc batteries. J. Chem. Eng. 2023, 454, 140311. [Google Scholar] [CrossRef]
- von Wald Cresce, A.; Xu, K. Aqueous lithium-ion batteries. Carbon Energy 2021, 3, 721–751. [Google Scholar] [CrossRef]
- Gheytani, S.; Liang, Y.; Wu, F.; Jing, Y.; Dong, H.; Rao, K.K.; Chi, X.; Fang, F.; Yao, Y. An Aqueous Ca-Ion Battery. Adv. Sci. 2017, 4, 1700465. [Google Scholar] [CrossRef]
- Lim, G.J.H.; Chua, R.; Koh, J.J.; Chan, K.K.; Tang, E.J.J.; Teh, V.; Srinivasan, M. Structural and conformable designs for aqueous multifunctional batteries. Mater. Today Energy 2023, 33, 101255. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, Y.; Zhao, C.; Liu, L.; Zhang, J.; Zhang, Q.; Shen, X.; Zhao, J.; Yu, X.; Li, H.; et al. Building aqueous K-ion batteries for energy storage. Nat. Energy 2019, 4, 495–503. [Google Scholar] [CrossRef]
- Yang, C.; Chen, J.; Qing, T.; Fan, X.; Sun, W.; von Cresce, A.; Ding, M.S.; Borodin, O.; Vatamanu, J.; Schroeder, M.A.; et al. 4.0 V Aqueous Li-Ion Batteries. Joule 2017, 1, 122–132. [Google Scholar] [CrossRef]
- Jin, T.; Ji, X.; Wang, P.-F.; Zhu, K.; Zhang, J.; Cao, L.; Chen, L.; Cui, C.; Deng, T.; Liu, S.; et al. High-Energy Aqueous Sodium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2021, 60, 11943–11948. [Google Scholar] [CrossRef] [PubMed]
- Leonard, D.P.; Wei, Z.; Chen, G.; Du, F.; Ji, X. Water-in-Salt Electrolyte for Potassium-Ion Batteries. ACS Energy Lett. 2018, 3, 373–374. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.; Chi, X.; Wen, B.; Wang, W.; Liu, Y. Water/Sulfolane Hybrid Electrolyte Achieves Ultralow-Temperature Operation for High-Voltage Aqueous Lithium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2106811. [Google Scholar] [CrossRef]
- Hu, J.; Guo, H.; Li, Y.; Wang, H.; Wang, Z.; Huang, W.; Yang, L.; Chen, H.; Lin, Y.; Pan, F. Understanding Li-ion thermodynamic and kinetic behaviors in concentrated electrolyte for the development of aqueous lithium-ion batteries. Nano Energy 2021, 89, 106413. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, J.; Wang, L.; Long, X.; Liu, X.; Li, F.; Chen, J. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 405. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Z.-Z.; Lv, W.; Lv, R.; Shen, W.; Kang, F.; Yang, Q.-H.; Weng, Y.; Huang, Z.-H. Wasp nest-imitated assembly of elastic rGO/p-Ti3C2Tx MXene-cellulose nanofibers for high-performance sodium-ion batteries. Carbon 2019, 153, 625–633. [Google Scholar] [CrossRef]
- Jiang, L.; Li, L.; Luo, S.; Xu, H.; Xia, L.; Wang, H.; Liu, X.; Wu, Y.; Qing, Y. Configuring hierarchical Ni/NiO 3D-network assisted with bamboo cellulose nanofibers for high-performance Ni–Zn aqueous batteries. Nanoscale 2020, 12, 14651–14660. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, L.; Qing, Y.; Zeng, Y.; Zhang, Z.; Xiao, L.; Lu, X.; Wu, Y. Manipulating nickel oxides in naturally derived cellulose nanofiber networks as robust cathodes for high-performance Ni–Zn batteries. J. Mater. Chem. A 2020, 8, 565–572. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, W.; Huang, A.; Chen, M.; Chen, J.; Tian, Q.; Xu, J. Modifying the Zn anode with carbon black coating and nanofibrillated cellulose binder: A strategy to realize dendrite-free Zn-MnO2 batteries. J. Colloid Interface Sci. 2020, 577, 256–264. [Google Scholar] [CrossRef]
- Profili, J.; Rousselot, S.; Tomassi, E.; Briqueleur, E.; Aymé-Perrot, D.; Stafford, L.; Dollé, M. Toward More Sustainable Rechargeable Aqueous Batteries Using Plasma-Treated Cellulose-Based Li-Ion Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 4728–4733. [Google Scholar] [CrossRef]
- Liu, X.; Han, Q.; Ma, Q.; Wang, Y.; Liu, C. Cellulose-Acetate Coating by Integrating Ester Group with Zinc Salt for Dendrite-Free Zn Metal Anodes. Small 2022, 18, 2203327. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Z.; Zhang, X.; Xu, W.; Chen, W.; Zhao, K.; Wang, Y.; Hong, S.; Wu, Q.; Li, M.-C.; et al. Synergic Effect of Dendrite-Free and Zinc Gating in Lignin-Containing Cellulose Nanofibers-MXene Layer Enabling Long-Cycle-Life Zinc Metal Batteries. Adv. Sci. 2022, 9, 2202380. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, T.; Chen, M.; Tian, Q.; Han, X.; Xu, X.; Chen, J. Wood-based electrodes enabling stable, anti-freezing, and flexible aqueous zinc-ion batteries. Energy Storage Mater. 2022, 51, 286–293. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, J.-M.; Ahn, D.B.; Lee, S.-Y. Cellulose Nanofiber/Carbon Nanotube-Based Bicontinuous Ion/Electron Conduction Networks for High-Performance Aqueous Zn-Ion Batteries. Small 2020, 16, 2002837. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhou, W.; Chen, M.; Huang, A.; Tian, Q.; Xu, X.; Chen, J. Integrated design of aqueous zinc-ion batteries based on dendrite-free zinc microspheres/carbon nanotubes/nanocellulose composite film anode. J. Colloid Interface Sci. 2021, 594, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, H.; Xiao, P.; Zeng, C.; Sun, Q.; Li, H. A high strength, anti-corrosion and sustainable separator for aqueous zinc-based battery by natural bamboo cellulose. Energy Storage Mater. 2022, 48, 191–197. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, R.; Xu, R.; Li, Y.; Tian, W.; Gao, M.; Wang, M.; Li, D.; Liang, X.; Xie, L.; et al. Super-Assembled Hierarchical Cellulose Aerogel-Gelatin Solid Electrolyte for Implantable and Biodegradable Zinc Ion Battery. Adv. Funct. Mater. 2022, 32, 2111406. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, M.; Tian, Q.; Chen, J.; Xu, X.; Wong, C.-P. Cotton-derived cellulose film as a dendrite-inhibiting separator to stabilize the zinc metal anode of aqueous zinc ion batteries. Energy Storage Mater. 2022, 44, 57–65. [Google Scholar] [CrossRef]
- Yang, Z.; Li, W.; Zhang, Q.; Xie, C.; Ji, H.; Tang, Y.; Li, Y.; Wang, H. A piece of common cellulose paper but with outstanding functions for advanced aqueous zinc-ion batteries. Mater. Today Energy 2022, 28, 101076. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Gu, C.; Zhang, X.; Okhawilai, M.; Wang, S.; Han, J.; Qin, J.; Huang, Y. Modulating Zn deposition via ceramic-cellulose separator with interfacial polarization effect for durable zinc anode. Nano Energy 2021, 89, 106322. [Google Scholar] [CrossRef]
- Li, L.; Peng, J.; Jia, X.; Zhu, X.; Meng, B.; Yang, K.; Chu, D.; Yang, N.; Yu, J. PBC@cellulose-filter paper separator design with efficient ion transport properties toward stabilized zinc-ion battery. Electrochim. Acta 2022, 430, 141129. [Google Scholar] [CrossRef]
- Mo, F.; Chen, Z.; Liang, G.; Wang, D.; Zhao, Y.; Li, H.; Dong, B.; Zhi, C. Zwitterionic Sulfobetaine Hydrogel Electrolyte Building Separated Positive/Negative Ion Migration Channels for Aqueous Zn-MnO2 Batteries with Superior Rate Capabilities. Adv. Energy Mater. 2020, 10, 2000035. [Google Scholar] [CrossRef]
- Xu, W.; Liu, C.; Ren, S.; Lee, D.; Gwon, J.; Flake, J.C.; Lei, T.; Baisakh, N.; Wu, Q. A cellulose nanofiber–polyacrylamide hydrogel based on a co-electrolyte system for solid-state zinc ion batteries to operate at extremely cold temperatures. J. Mater. Chem. A 2021, 9, 25651–25662. [Google Scholar] [CrossRef]
- Quan, Y.; Zhou, W.; Wu, T.; Chen, M.; Han, X.; Tian, Q.; Xu, J.; Chen, J. Sorbitol-modified cellulose hydrogel electrolyte derived from wheat straws towards high-performance environmentally adaptive flexible zinc-ion batteries. Chem. Eng. J. 2022, 446, 137056. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nano-Micro Lett. 2020, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.Y.; Lee, C.J.; Dinachali, S.S.; Lai, S.C.; Williams, E.L.; Luo, H.K.; Chi, D.; Andy Hor, T.S.; Han, M.Y. Amorphous ruthenium nanoparticles for enhanced electrochemical water splitting. Nanotechnology 2015, 26, 415401. [Google Scholar] [CrossRef]
- Zeng, D.; Ong, W.-J.; Chen, Y.; Tee, S.Y.; Chua, C.S.; Peng, D.-L.; Han, M.-Y. Photocatalysis: Co2P Nanorods as an Efficient Cocatalyst Decorated Porous g-C3N4 Nanosheets for Photocatalytic Hydrogen Production under Visible Light Irradiation. Part. Part. Syst. Charact. 2018, 35, 1700251. [Google Scholar] [CrossRef]
- Li, N.; Jayaraman, S.; Tee, S.Y.; Kumar, P.S.; Jun Lee, C.J.; Liew, S.L.; Chi, D.; Hor, T.S.A.; Ramakrishna, S.; Luo, H.-K. Effect of La-Doping on optical bandgap and photoelectrochemical performance of hematite nanostructures. J. Mater. Chem. A 2014, 2, 19290–19297. [Google Scholar] [CrossRef]
- Li, R.; Weng, Y.; Zhou, X.; Wang, X.; Mi, Y.; Chong, R.; Han, H.; Li, C. Achieving overall water splitting using titanium dioxide-based photocatalysts of different phases. Energy Environ. Sci. 2015, 8, 2377–2382. [Google Scholar] [CrossRef]
- Schwarze, M.; Thiel, T.A.; Tasbihi, M.; Schroeter, M.; Menezes, P.W.; Walter, C.; Driess, M.; Schomäcker, R. Use of Cellulose for the Production of Photocatalytic Films for Hydrogen Evolution Along the Lines of Paper Production. Energy Technol. 2021, 10, 2100525. [Google Scholar] [CrossRef]
- Li, Z.; Yao, C.; Yu, Y.; Cai, Z.; Wang, X. Highly efficient capillary photoelectrochemical water splitting using cellulose nanofiber-templated TiO2 photoanodes. Adv. Mater. 2014, 26, 2262–2267. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yao, C.; Wang, F.; Cai, Z.; Wang, X. Cellulose nanofiber-templated three-dimension TiO2 hierarchical nanowire network for photoelectrochemical photoanode. Nanotechnology 2014, 25, 504005. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Qin, J.; Volokh, M.; Shalom, M. Freestanding Hierarchical Carbon Nitride/Carbon-Paper Electrode as a Photoelectrocatalyst for Water Splitting and Dye Degradation. ACS Appl. Mater. Interfaces 2019, 11, 29139–29146. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.D.; Liu, S.; Teng, C.P.; Han, M.Y. Recent Progress in Energy-Driven Water Splitting. Adv. Sci. 2017, 4, 1600337. [Google Scholar] [CrossRef]
- Tetik, H.; Wang, Y.; Sun, X.; Cao, D.; Shah, N.; Zhu, H.; Qian, F.; Lin, D. Additive Manufacturing of 3D Aerogels and Porous Scaffolds: A Review. Adv. Funct. Mater. 2021, 31, 2103410. [Google Scholar] [CrossRef]
- Shin, D.W.; Barnes, M.D.; Walsh, K.; Dimov, D.; Tian, P.; Neves, A.I.S.; Wright, C.D.; Yu, S.M.; Yoo, J.B.; Russo, S.; et al. A New Facile Route to Flexible and Semi-Transparent Electrodes Based on Water Exfoliated Graphene and their Single-Electrode Triboelectric Nanogenerator. Adv. Mater. 2018, 30, e1802953. [Google Scholar] [CrossRef]
- Dong, H.; Yu, S.; Song, L.; Wang, X.; Wu, C. Fabrication of high-quality flexible transparent conductive thin films with a Nb2O5/AgNWs/Nb2O5 sandwich structure. Ceram. Int. 2022, 48, 15348–15354. [Google Scholar] [CrossRef]
- Hao, W.; Wu, R.; Huang, H.; Ou, X.; Wang, L.; Sun, D.; Ma, X.; Guo, Y. Fabrication of practical catalytic electrodes using insulating and eco-friendly substrates for overall water splitting. Energy Environ. Sci. 2020, 13, 102–110. [Google Scholar] [CrossRef]
- Sahasrabudhe, A.; Dixit, H.; Majee, R.; Bhattacharyya, S. Value added transformation of ubiquitous substrates into highly efficient and flexible electrodes for water splitting. Nat. Commun. 2018, 9, 2014. [Google Scholar] [CrossRef]
- Rajan, H.; Christy, M.; Jothi, V.R.; Anantharaj, S.; Yi, S.C. A bifunctional hexa-filamentous microfibril multimetallic foam: An unconventional high-performance electrode for total water splitting under industrial operation conditions. J. Mater. Chem. A 2021, 9, 4971–4983. [Google Scholar] [CrossRef]
- Li, G.; Yu, J.; Zhou, Z.; Li, R.; Xiang, Z.; Cao, Q.; Zhao, L.; Wang, X.; Peng, X.; Liu, H.; et al. N-Doped Mo2C Nanobelts/Graphene Nanosheets Bonded with Hydroxy Nanocellulose as Flexible and Editable Electrode for Hydrogen Evolution Reaction. iScience 2019, 19, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Amiinu, I.S.; Pu, Z.; He, D.; Monestel, H.G.R.; Mu, S. Scalable cellulose-sponsored functionalized carbon nanorods induced by cobalt for efficient overall water splitting. Carbon 2018, 137, 274–281. [Google Scholar] [CrossRef]
- Jiang, S.; Li, J.; Fang, J.; Wang, X. Fibrous-Structured Freestanding Electrodes for Oxygen Electrocatalysis. Small 2021, 17, e1903760. [Google Scholar] [CrossRef]
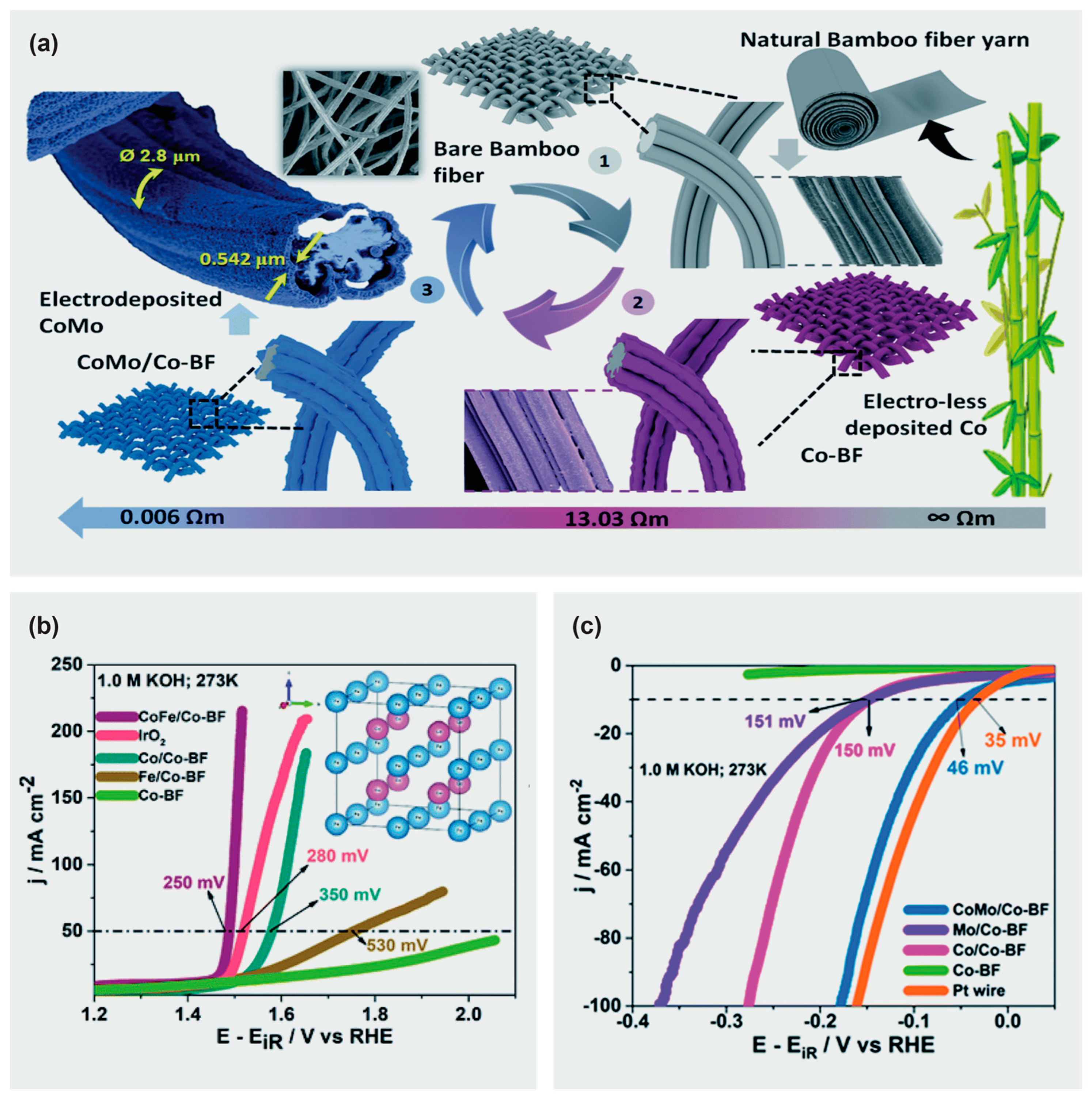
- Wu, Z.-Y.; Hu, B.-C.; Wu, P.; Liang, H.-W.; Yu, Z.-L.; Lin, Y.; Zheng, Y.-R.; Li, Z.; Yu, S.-H. Mo2C nanoparticles embedded within bacterial cellulose-derived 3D N-doped carbon nanofiber networks for efficient hydrogen evolution. NPG Asia Mater. 2016, 8, e288. [Google Scholar] [CrossRef]
| Energy Devices | Advantages | Disadvantages |
|---|---|---|
| Flexible and Wearable Electronics |
|
|
| Piezoelectric nanogenerators (PENGs) |
|
|
| Triboelectric nanogenerators (TENGs) |
|
|
| Thermoelectric generators |
|
|
| Solar cells |
|
|
| Batteries |
|
|
| Water splitting catalysts |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, C.P.; Tan, M.Y.; Toh, J.P.W.; Lim, Q.F.; Wang, X.; Ponsford, D.; Lin, E.M.J.; Thitsartarn, W.; Tee, S.Y. Advances in Cellulose-Based Composites for Energy Applications. Materials 2023, 16, 3856. https://doi.org/10.3390/ma16103856
Teng CP, Tan MY, Toh JPW, Lim QF, Wang X, Ponsford D, Lin EMJ, Thitsartarn W, Tee SY. Advances in Cellulose-Based Composites for Energy Applications. Materials. 2023; 16(10):3856. https://doi.org/10.3390/ma16103856
Chicago/Turabian StyleTeng, Choon Peng, Ming Yan Tan, Jessica Pei Wen Toh, Qi Feng Lim, Xiaobai Wang, Daniel Ponsford, Esther Marie JieRong Lin, Warintorn Thitsartarn, and Si Yin Tee. 2023. "Advances in Cellulose-Based Composites for Energy Applications" Materials 16, no. 10: 3856. https://doi.org/10.3390/ma16103856
APA StyleTeng, C. P., Tan, M. Y., Toh, J. P. W., Lim, Q. F., Wang, X., Ponsford, D., Lin, E. M. J., Thitsartarn, W., & Tee, S. Y. (2023). Advances in Cellulose-Based Composites for Energy Applications. Materials, 16(10), 3856. https://doi.org/10.3390/ma16103856





