Investigation on Physico Chemical and X-ray Shielding Performance of Zinc Doped Nano-WO3 Epoxy Composite for Light Weight Lead Free Aprons
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Zn-WO3 Nanoparticles
2.2. Fabrication of Zn-WO3 Nanocomposite Apron and Measurement Set-Up
2.3. Characterization
3. Results
3.1. XRD Analysis
3.2. Raman Analysis
3.3. Optical Properties
3.4. HR-TEM and SAED Analysis
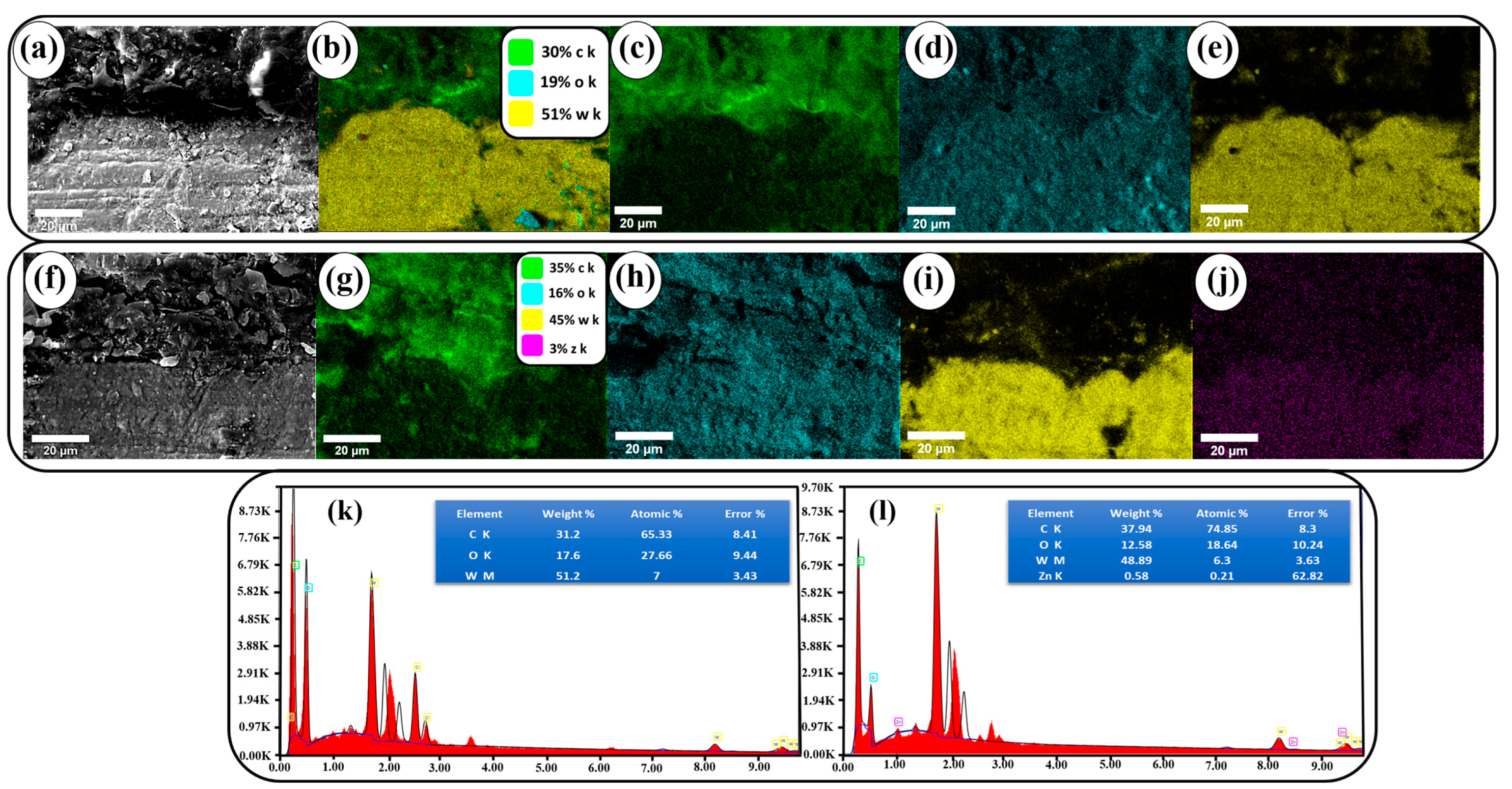
3.5. SEM-EDAX Analysis
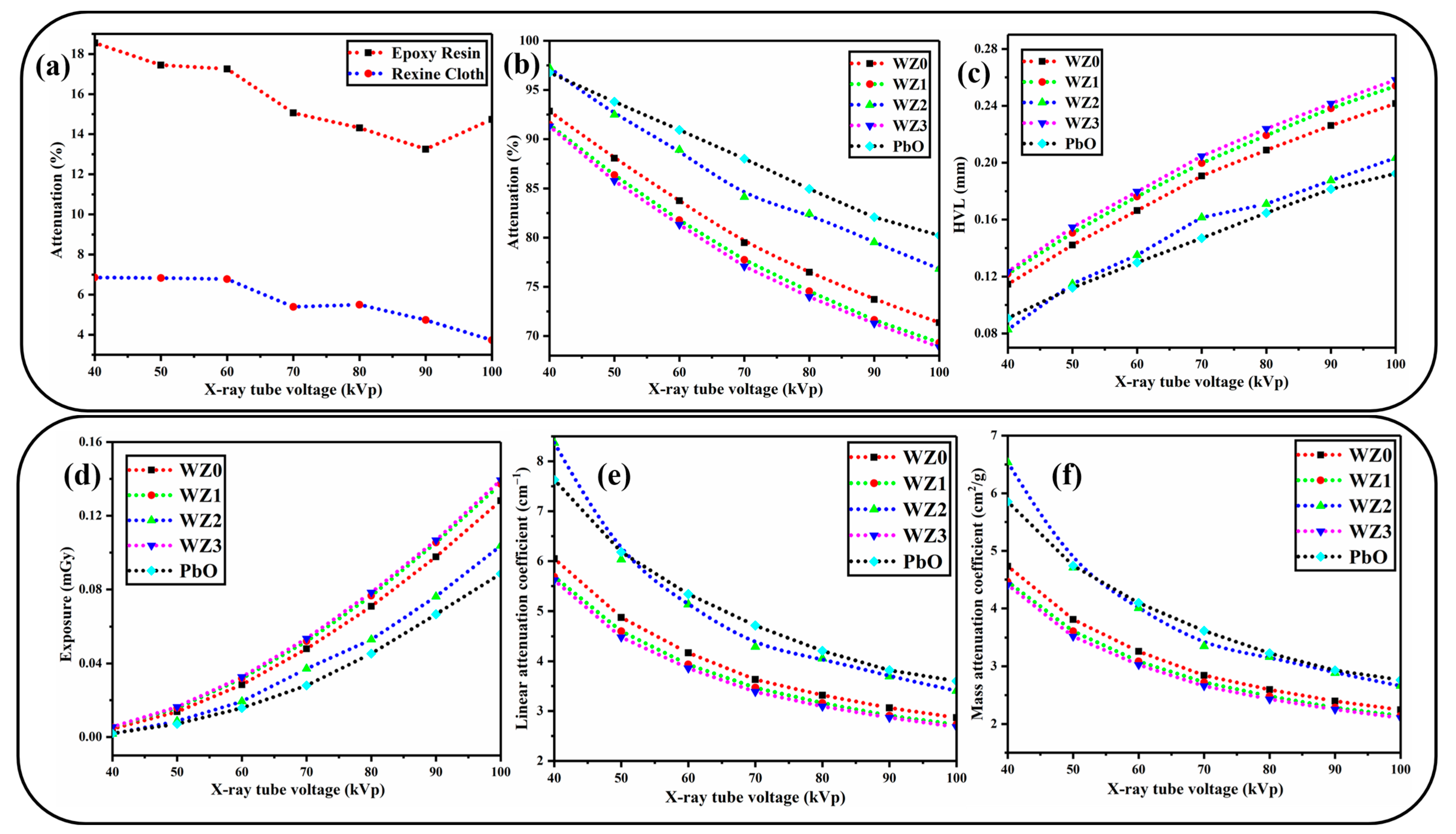
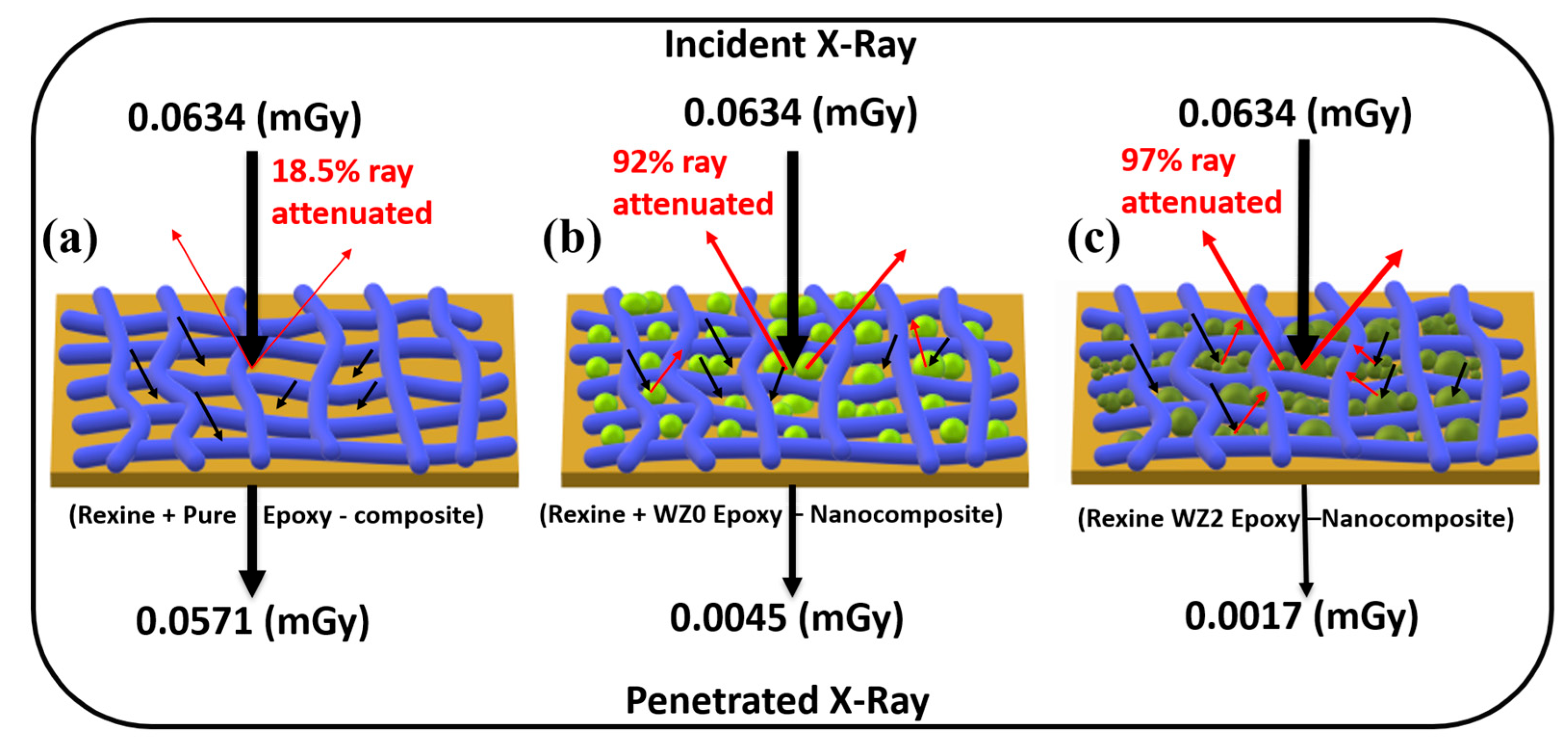
3.6. X-ray Shielding Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maghrabi, H.A.; Vijayan, A.; Deb, P.; Wang, L. Bismuth Oxide-Coated Fabrics for X-ray Shielding. Text. Res. J. 2016, 86, 649–658. [Google Scholar] [CrossRef]
- Le Heron, J.; Padovani, R.; Smith, I.; Czarwinski, R. Radiation Protection of Medical Staff. Eur. J. Radiol. 2010, 76, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, T.; Güngör, A.; Akbay, I.K.; Uzun, H.; Babucçuoglu, Y. Nano Lead Oxide and Epdm Composite for Development of Polymer Based Radiation Shielding Material: Gamma Irradiation and Attenuation Tests. Radiat. Phys. Chem. 2018, 144, 248–255. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Abbas, M.I.; Hammoury, S.I.; Gouda, M.M.; Zard, K.; Elsafi, M. Effect of PbO-Nanoparticles on Dimethyl Polysiloxane for Use in Radiation Shielding Applications. Sci. Rep. 2022, 12, 15722. [Google Scholar] [CrossRef]
- Cinan, Z.M.; Baskan, T.; Erol, B.; Mutlu, S.; Misirlioglu, Y.; Yilmaz, S.S.; Yilmaz, A.H. Gamma Irradiation, Thermal Conductivity, and Phase Change Tests of the Cement-Hyperbranched Poly Amino-Ester-Block-Poly Cabrolactone-Polyurathane Plaster-Lead Oxide and Arsenic Oxide Composite for Development of Radiation Shielding Material. Int. J. Energy Res. 2021, 45, 20729–20762. [Google Scholar] [CrossRef]
- Cinan, Z.M.; Erol, B.; Baskan, T.; Mutlu, S.; Yilmaz, S.S.; Yilmaz, A.H. Gamma Irradiation and the Radiation Shielding Characteristics: For the Lead Oxide Doped the Crosslinked Polystyrene-b-Polyethyleneglycol Block Copolymers and the Polystyrene-b-Polyethyleneglycol-Boron Nitride Nanocomposites. Polymers 2021, 13, 3246. [Google Scholar] [CrossRef]
- Osman, A.F.; El Balaa, H.; El Samad, O.; Awad, R.; Badawi, M.S. Assessment of X-ray Shielding Properties of Polystyrene Incorporated with Different Nano-Sizes of PbO. Radiat. Environ. Biophys. 2023, 62, 235–251. [Google Scholar] [CrossRef]
- Asari Shik, N.; Gholamzadeh, L. X-ray Shielding Performance of the EPVC Composites with Micro- or Nanoparticles of WO3, PbO or Bi2O3. Appl. Radiat. Isot. 2018, 139, 61–65. [Google Scholar] [CrossRef]
- Jamil, M.; Hazlan, M.H.; Ramli, R.M.; Noor Azman, N.Z. Study of Electrospun PVA-Based Concentrations Nanofibre Filled with Bi2O3 or WO3 as Potential X-ray Shielding Material. Radiat. Phys. Chem. 2019, 156, 272–282. [Google Scholar] [CrossRef]
- Kawady, N.A.; Elkattan, M.; Salah, M.; Galhoum, A.A. Fabrication, Characterization, and Gamma ray Shielding Properties of PVA-Based Polymer Nanocomposite. J. Mater. Sci. 2022, 57, 11046–11061. [Google Scholar] [CrossRef]
- Abulyazied, D.E.; Saudi, H.A.; Zakaly, H.M.H.; Issa, S.A.M.; Henaish, A.M.A. Novel Nanocomposites Based on Polyvinyl Alcohol and Molybdenum Nanoparticles for Gamma Irradiation Shielding. Opt. Laser Technol. 2022, 156, 108560. [Google Scholar] [CrossRef]
- More, C.V.; Alsayed, Z.; Badawi, M.S.; Thabet, A.A.; Pawar, P.P. Polymeric Composite Materials for Radiation Shielding: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 19, ISBN 1031102101189. [Google Scholar]
- Wang, B.; Qiu, T.; Yuan, L.; Fang, Q.; Wang, X.; Guo, X.; Zhang, D.; Lai, C.; Wang, Q.; Liu, Y. A Comparative Study between Pure Bismuth/Tungsten and the Bismuth Tungsten Oxide for Flexible Shielding of Gamma/X rays. Radiat. Phys. Chem. 2023, 208, 110906. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Li, S.; Dong, K.; Wang, S.; Cai, P.; Hou, L.; Dou, H.; Liang, D.; Algadi, H.; et al. Lead-Free and Wearing Comfort 3D Composite Fiber-Needled Fabric for Highly Efficient X-ray Shielding. Adv. Compos. Hybrid Mater. 2023, 6, 76. [Google Scholar] [CrossRef]
- Alsaab, A.H.; Zeghib, S. Study of Prepared Lead-Free Polymer Nanocomposites for X- and Gamma-ray Shielding in Healthcare Applications. Polymers 2023, 15, 2142. [Google Scholar] [CrossRef]
- Mahmoud, K.G.; Tashlykov, O.L.; Praveenkumar, S.; Sayyed, M.I.; Hashim, S. Synthesis of a New Epoxy Resin Reinforced by ZnO Nanoparticles for γ-ray Shielding Purposes: Experimental and Monte Carlo Simulation Assesments. Radiat. Phys. Chem. 2023, 208, 110938. [Google Scholar] [CrossRef]
- La, L.B.T.; Leatherday, C.; Leong, Y.K.; Watts, H.P.; Zhang, L.C. Green Lightweight Lead-Free Gd2O3/Epoxy Nanocomposites with Outstanding X-ray Attenuation Performance. Compos. Sci. Technol. 2018, 163, 89–95. [Google Scholar] [CrossRef]
- Kausar, A. Role of Thermosetting Polymer in Structural Composite. Kausar A Am. J. Polym. Sci. Eng. 2017, 5, 1–12. [Google Scholar]
- Noor Azman, N.Z.; Siddiqui, S.A.; Ionescu, M.; Low, I.M. Synthesis and Characterisation of Ion-Implanted Epoxy Composites for X-ray Shielding. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2012, 287, 120–123. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Wang, H.; Mahmud, S.; Jahid, M.A.; Islam, M.; Jin, W.; Genyang, C. Colorful and Antibacterial Nylon Fabric via In-Situ Biosynthesis of Chitosan Mediated Nanosilver. J. Mater. Res. Technol. 2020, 9, 16135–16145. [Google Scholar] [CrossRef]
- Meyer, M.; Dietrich, S.; Schulz, H.; Mondschein, A. Comparison of the Technical Performance of Leather, Artificial Leather, and Trendy Alternatives. Coatings 2021, 11, 226. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Yan, C.; Li, H.; Zhu, Y.; Li, X.; Yu, L. Interfacial Microstructure and Properties of Carbon Fiber Composites Modified with Graphene Oxide. ACS Appl. Mater. Interfaces 2012, 4, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, M.; Palin, L.; Alberto, G.; Cantamessa, S.; Milanesio, M. Epoxy Resins Composites for X-ray Shielding Materials Additivated by Coated Barium Sulfate with Improved Dispersibility. Mater. Today Commun. 2021, 26, 101888. [Google Scholar] [CrossRef]
- Archer, B.R. Recent History of the Shielding of Medical X-ray Imaging Facilities. Health Phys. 2005, 88, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Künzel, R.; Okuno, E. Effects of the Particle Sizes and Concentrations on the X-ray Absorption by CuO Compounds. Appl. Radiat. Isot. 2012, 70, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Noor Azman, N.Z.; Musa, N.F.L.; Nik Ab Razak, N.N.A.; Ramli, R.M.; Mustafa, I.S.; Abdul Rahman, A.; Yahaya, N.Z. Effect of Bi2O3 Particle Sizes and Addition of Starch into Bi2O3–PVA Composites for X-ray Shielding. Appl. Phys. A Mater. Sci. Process. 2016, 122, 818. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Noh, Y.G.; Seo, H. Engineering Band Edge Properties of WO3 with Respect to Photoelectrochemical Water Splitting Potentials via a Generalized Doping Protocol of First-Row Transition Metal Ions. Appl. Surf. Sci. 2020, 509, 145253. [Google Scholar] [CrossRef]
- Nayak, A.K.; Ghosh, R.; Santra, S.; Guha, P.K.; Pradhan, D. Hierarchical Nanostructured WO3-SnO2 for Selective Sensing of Volatile Organic Compounds. Nanoscale 2015, 7, 12460–12473. [Google Scholar] [CrossRef]
- Gong, Q.; Cen, K.; Yu, C. Experimental Study on Supercritical Phenomena of WO3 Solubility in NaCl-H2O System. Sci. China Ser. D Earth Sci. 2003, 46, 664–671. [Google Scholar] [CrossRef]
- Kim, S.C.; Choi, J.R.; Jeon, B.K. Physical Analysis of the Shielding Capacity for a Lightweight Apron Designed for Shielding Low Intensity Scattering X-rays. Sci. Rep. 2016, 6, 27721. [Google Scholar] [CrossRef]
- Nambiar, S.; Osei, E.K.; Yeow, J.T.W. Polymer Nanocomposite-Based Shielding against Diagnostic X-rays. J. Appl. Polym. Sci. 2013, 127, 4939–4946. [Google Scholar] [CrossRef]
- Kumar, V.B.; Mohanta, D. Formation of Nanoscale Tungsten Oxide Structures. Bull. Mater. Sci. 2011, 34, 435–442. [Google Scholar] [CrossRef]
- Deepa, B.; Rajendran, V. Pure and Cu Metal Doped WO3 Prepared via Co-Precipitation Method and Studies on Their Structural, Morphological, Electrochemical and Optical Properties. Nano-Struct. Nano-Objects 2018, 16, 185–192. [Google Scholar] [CrossRef]
- Karthick, K.; Kathirvel, P.; Marnadu, R.; Chakravarty, S.; Shkir, M. Ultrafast One Step Direct Injection Flame Synthesis of Zinc Oxide Nanoparticles and Fabrication of P-Si/n-ZnO Photodiode and Characterization. Phys. B Condens. Matter 2021, 612, 412971. [Google Scholar] [CrossRef]
- Cheng, X.F.; Leng, W.H.; Liu, D.P.; Zhang, J.Q.; Cao, C.N. Enhanced Photoelectrocatalytic Performance of Zn-Doped WO3 Photocatalysts for Nitrite Ions Degradation under Visible Light. Chemosphere 2007, 68, 1976–1984. [Google Scholar] [CrossRef]
- Thilagavathi, T.; Venugopal, D.; Marnadu, R.; Chandrasekaran, J.; Alshahrani, T.; Shkir, M. An Investigation on Microstructural, Morphological, Optical, Photoluminescence and Photocatalytic Activity of WO3 for Photocatalysis Applications: An Effect of Annealing. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1217–1230. [Google Scholar] [CrossRef]
- Singh, G.; Shrivastava, S.B.; Jain, D.; Pandya, S.; Shripathi, T.; Ganesan, V. Effect of Indium Dop Ing on Zinc Oxide Films Prepared by Chemical Spray Pyrolysis Technique. Bull. Mater. Sci. 2010, 33, 581–587. [Google Scholar] [CrossRef]
- Balaji, S.; Djaoued, Y.; Albert, A.S.; Brüning, R.; Beaudoin, N.; Robichaud, J. Porous Orthorhombic Tungsten Oxide Thin Films: Synthesis, Characterization, and Application in Electrochromic and Photochromic Devices. J. Mater. Chem. 2011, 21, 3940–3948. [Google Scholar] [CrossRef]
- Nonaka, K.; Takase, A.; Miyakawa, K. Raman Spectra of Sol-Gel-Derived Tungsten Oxides. J. Mater. Sci. Lett. 1993, 12, 274–277. [Google Scholar] [CrossRef]
- Nakahara, H.; Fukuda, K. Electronic Spectra and Molecular Orientation in Multilayers of Long-Chain Anthraquinone Derivatives. J. Colloid Interface Sci. 1981, 83, 401–409. [Google Scholar] [CrossRef]
- Wang, H.; Baek, S.; Lee, J.; Lim, S. High Photocatalytic Activity of Silver-Loaded ZnO-SnO2 Coupled Catalysts. Chem. Eng. J. 2009, 146, 355–361. [Google Scholar] [CrossRef]
- Height, M.J.; Pratsinis, S.E.; Mekasuwandumrong, O.; Praserthdam, P. Ag-ZnO Catalysts for UV-Photodegradation of Methylene Blue. Appl. Catal. B Environ. 2006, 63, 305–312. [Google Scholar] [CrossRef]
- Wang, W.W.; Zhu, Y.J.; Yang, L.X. ZnO-SnO2 Hollow Spheres and Hierarchical Nanosheets: Hydrothermal Preparation, Formation Mechanism, and Photocatalytic Properties. Adv. Funct. Mater. 2007, 17, 59–64. [Google Scholar] [CrossRef]
- Atheek, P.; Puviarasu, P.; Basha, S.M.; Bhujel, K. Micro Raman Analysis on the Impact of Light Ion Irradiation of Hydride Vapor-Phase Epitaxy Grown Gallium Nitride Epilayers. Thin Solid Films 2022, 761, 139526. [Google Scholar] [CrossRef]
- Svitasheva, S.N.; Gilinsky, A.M. Influence of Doping Level on Shift of the Absorption Edge of Gallium Nitride Films (Burstein-Moss Effect). Appl. Surf. Sci. 2013, 281, 109–112. [Google Scholar] [CrossRef]
- Matalkeh, M.; Nasrallah, G.K.; Shurrab, F.M.; Al-Absi, E.S.; Mohammed, W.; Elzatahry, A.; Saoud, K.M. Visible light photocatalytic activity of Ag/WO3 nanoparticles and its antibacterial activity under ambient light and in the dark. Results Eng. 2022, 13, 100313. [Google Scholar] [CrossRef]
- Singh, J.; Verma, V.; Kumar, R.; Kumar, R. Influence of Mg2+-Substitution on the Optical Band Gap Energy of Cr2−xMg2−xO3 Nanoparticles. Results Phys. 2019, 13, 102106. [Google Scholar] [CrossRef]
- Mamat, M.H.; Sahdan, M.Z.; Amizam, S.; Rafaie, H.A.; Khusaimi, Z.; Rusop, M. Optical and Electrical Properties of Aluminum Doped Zinc Oxide Thin Films at Various Doping Concentrations. J. Ceram. Soc. Japan 2009, 117, 1263–1267. [Google Scholar] [CrossRef]
- Luo, J.Y.; Xu, N.S.; Zhao, F.L.; Deng, S.Z.; Tao, Y.T. Ultraviolet Superfluorescence from Oxygen Vacancies in WO3−x Nanowires at Room Temperature. J. Appl. Phys. 2011, 109, 024312. [Google Scholar] [CrossRef]
- Cho, H.D.; Yoon, I.T.; Chung, K.B.; Kim, D.Y.; Kang, T.W.; Yuldashev, S.U. Low-Temperature Photoluminescence of WO3 Nanoparticles. J. Lumin. 2018, 195, 344–347. [Google Scholar] [CrossRef]
- Hirano, M.; Okamoto, T. Synthesis, Morphology, and Luminescence of ZnNb2O6 Nanocrystals by Hydrothermal Method. Nano-Struct. Nano-Objects 2018, 13, 139–145. [Google Scholar] [CrossRef]
- Mohammadi, S.; Sohrabi, M.; Golikand, A.N.; Fakhri, A. Preparation and Characterization of Zinc and Copper Co-Doped WO3 Nanoparticles: Application in Photocatalysis and Photobiology. J. Photochem. Photobiol. B Biol. 2016, 161, 217–221. [Google Scholar] [CrossRef]
- Kavitha, V.S.; Reshmi Krishnan, R.; Sreeja Sreedharan, R.; Suresh, K.; Jayasankar, C.K.; Mahadevan Pillai, V.P. Tb3+-Doped WO3 Thin Films: A Potential Candidate in White Light Emitting Devices. J. Alloys Compd. 2019, 788, 429–445. [Google Scholar] [CrossRef]
- Bourdin, M.; Gaudon, M.; Weill, F.; Duttine, M.; Bourdin, M.; Gaudon, M.; Weill, F.; Duttine, M.; Gayot, M.; Bourdin, M.; et al. Nanoparticles (NPs) of WO3−x Compounds by Polyol Route with Enhanced Photochromic Properties. Nanomaterials 2020, 9, 1555. [Google Scholar] [CrossRef]
- Wang, F.; Di Valentin, C.; Pacchioni, G. Semiconductor-to-Metal Transition in WO3−x: Nature of the Oxygen Vacancy. Phys. Rev. B—Condens. Matter Mater. Phys. 2011, 84, 073103. [Google Scholar] [CrossRef]
- Peters, S.M.B.; Zweers, D.; de Lange, F.; Mourik, J.E.M. Lead Composite vs. Nonlead Protective Garments: Which Are Better? A Multivendor Comparison. Radiat. Prot. Dosim. 2017, 175, 460–465. [Google Scholar] [CrossRef]
- Reda, A.M.; El-Daly, A.A. Gamma ray Shielding Characteristics of Sn-20Bi and Sn-20Bi-0.4Cu Lead-Free Alloys. Prog. Nucl. Energy 2020, 123, 103304. [Google Scholar] [CrossRef]
- Spierings, A.B.; Schneider, M.; Eggenberger, R. Comparison of Density Measurement Techniques for Additive Manufactured Metallic Parts. Rapid Prototyp. J. 2011, 17, 380–386. [Google Scholar] [CrossRef]
- Buchtela, K.; Oesterreichischen, A. Der Gamma ray Spectrometer. Anal. Chem. 2005, 27, 59. [Google Scholar] [CrossRef]
- Issa, S.A.M.; Sayyed, M.I.; Zaid, M.H.M.; Matori, K.A. Photon Parameters for Gamma-rays Sensing Properties of Some Oxide of Lanthanides. Results Phys. 2018, 9, 206–210. [Google Scholar] [CrossRef]
- Li, Q.; Zhong, R.; Xiao, X.; Liao, J.; Liao, X.; Shi, B. Lightweight and Flexible Bi@Bi-La Natural Leather Composites with Superb X-ray Radiation Shielding Performance and Low Secondary Radiation. ACS Appl. Mater. Interfaces 2020, 12, 54117–54126. [Google Scholar] [CrossRef]
- Mesbahi, A.; Ghiasi, H. Shielding Properties of the Ordinary Concrete Loaded with Micro- and Nano-Particles against Neutron and Gamma Radiations. Appl. Radiat. Isot. 2018, 136, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.Z.; Künzel, R.; Okuno, E.; Levenhagen, R.S.; Basegio, T.; Bergmann, C.P. X-ray Transmission through Nanostructured and Microstructured CuO Materials. Appl. Radiat. Isot. 2011, 69, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, S.; Seo, Y. Enhanced X-ray Shielding Ability of Polymer-Nonleaded Metal Composites by Multilayer Structuring. Ind. Eng. Chem. Res. 2015, 54, 5968–5973. [Google Scholar] [CrossRef]
- Dong, Y.; Chang, S.Q.; Zhang, H.X.; Ren, C.; Kang, B.; Dai, M.Z.; Dai, Y.D. Effects of WO3 Particle Size in WO3/Epoxy Resin Radiation Shielding Material. Chinese Phys. Lett. 2012, 29, 108102. [Google Scholar] [CrossRef]
- Dejangah, M.; Ghojavand, M.; Poursalehi, R.; Gholipour, P.R. X-ray Attenuation and Mechanical Properties of Tungsten-Silicone Rubber Nanocomposites. Mater. Res. Express 2019, 6, 085045. [Google Scholar] [CrossRef]
- Abdolahzadeh, T.; Morshedian, J.; Ahmadi, S. Novel Polyethylene/Tungsten Oxide/Bismuth Trioxide/Barium Sulfate/Graphene Oxide Nanocomposites for Shielding against X-ray Radiations. Int. J. Radiat. Res. 2023, 21, 79–87. [Google Scholar] [CrossRef]
- Muthamma, M.V.; Gudennavar, B.S.; Gudennavar, S.B. Attenuation Parameters of Polyvinyl Alcohol-Tungsten Oxide Composites at the Photon Energies 5.895, 6.490, 59.54 and 662 KeV. Polish J. Med. Phys. Eng. 2020, 26, 77–85. [Google Scholar] [CrossRef]
- Morshedian, J.; Reza, M.; Darounkola, R.; Mansoori, E.; Keshvari, R. New Flexible Non-Toxic X-ray Shielding Hybrid Materials Based on X-SBR Liquid Rubber. Radiat. Phys. Chem. 2023, 207, 110852. [Google Scholar] [CrossRef]
- Hosseini, M.A.; Malekie, S.; Kazemi, F. Experimental Evaluation of Gamma Radiation Shielding Characteristics of Polyvinyl Alcohol/Tungsten Oxide Composite: A Comparison Study of Micro and Nano Sizes of the Fillers. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2022, 1026, 166214. [Google Scholar] [CrossRef]
- Yun, J.; Hou, J.; Jang, W.; Kim, S.Y.; Byun, H. Electrospun Tungsten-Polyurethane Composite Nanofiber Mats for Medical Radiation-Shielding Applications. ChemNanoMat 2022, 8, e202100387. [Google Scholar] [CrossRef]

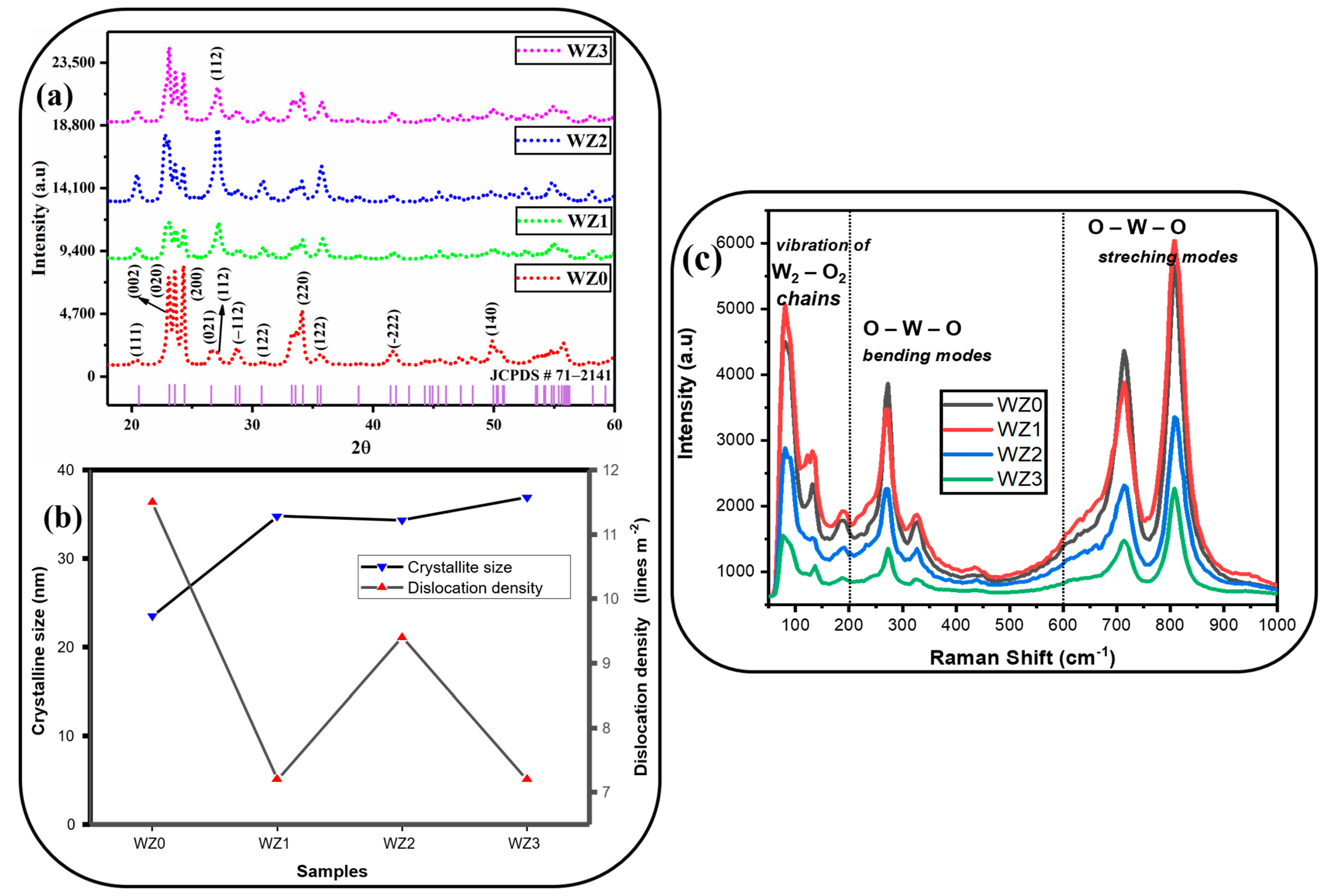
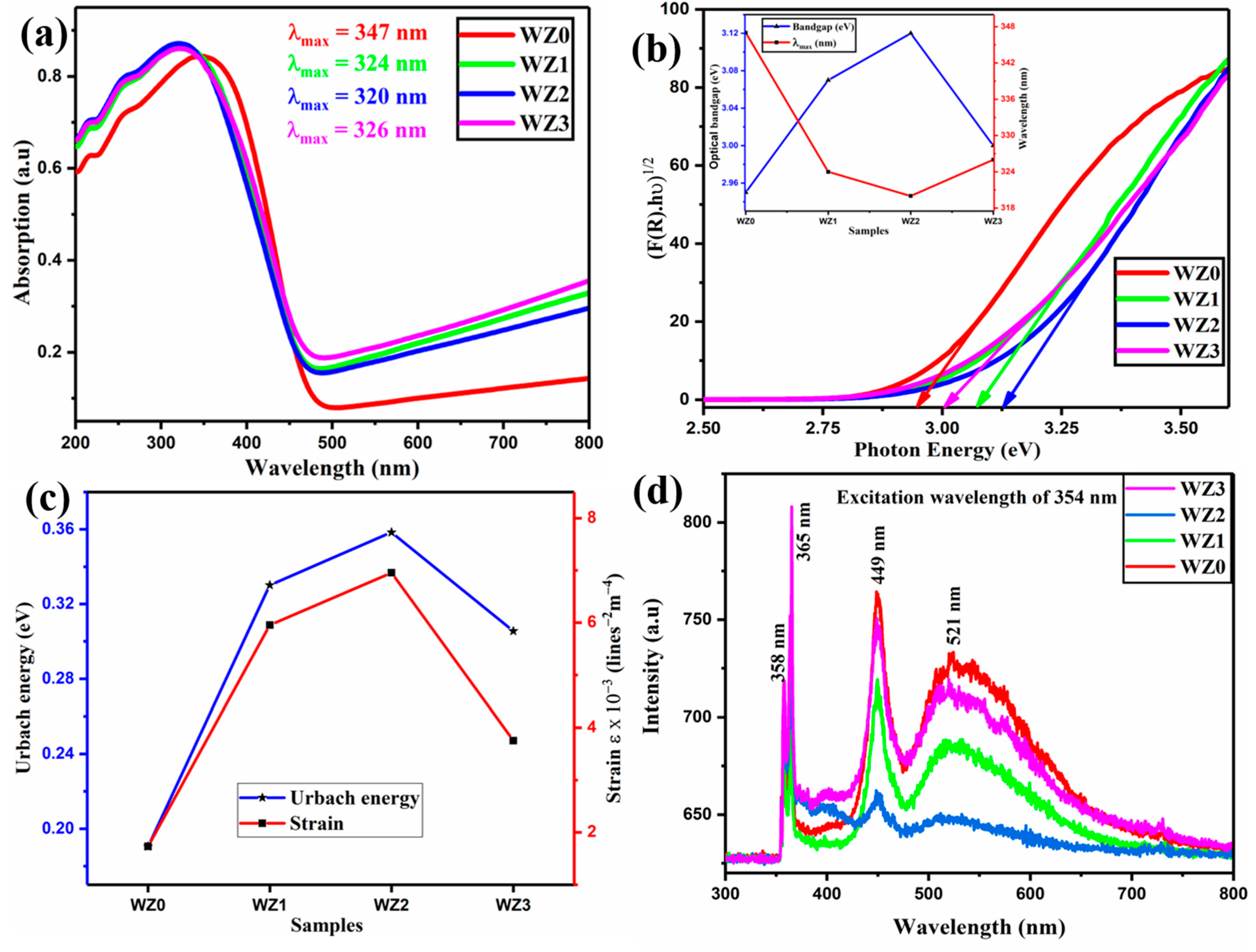
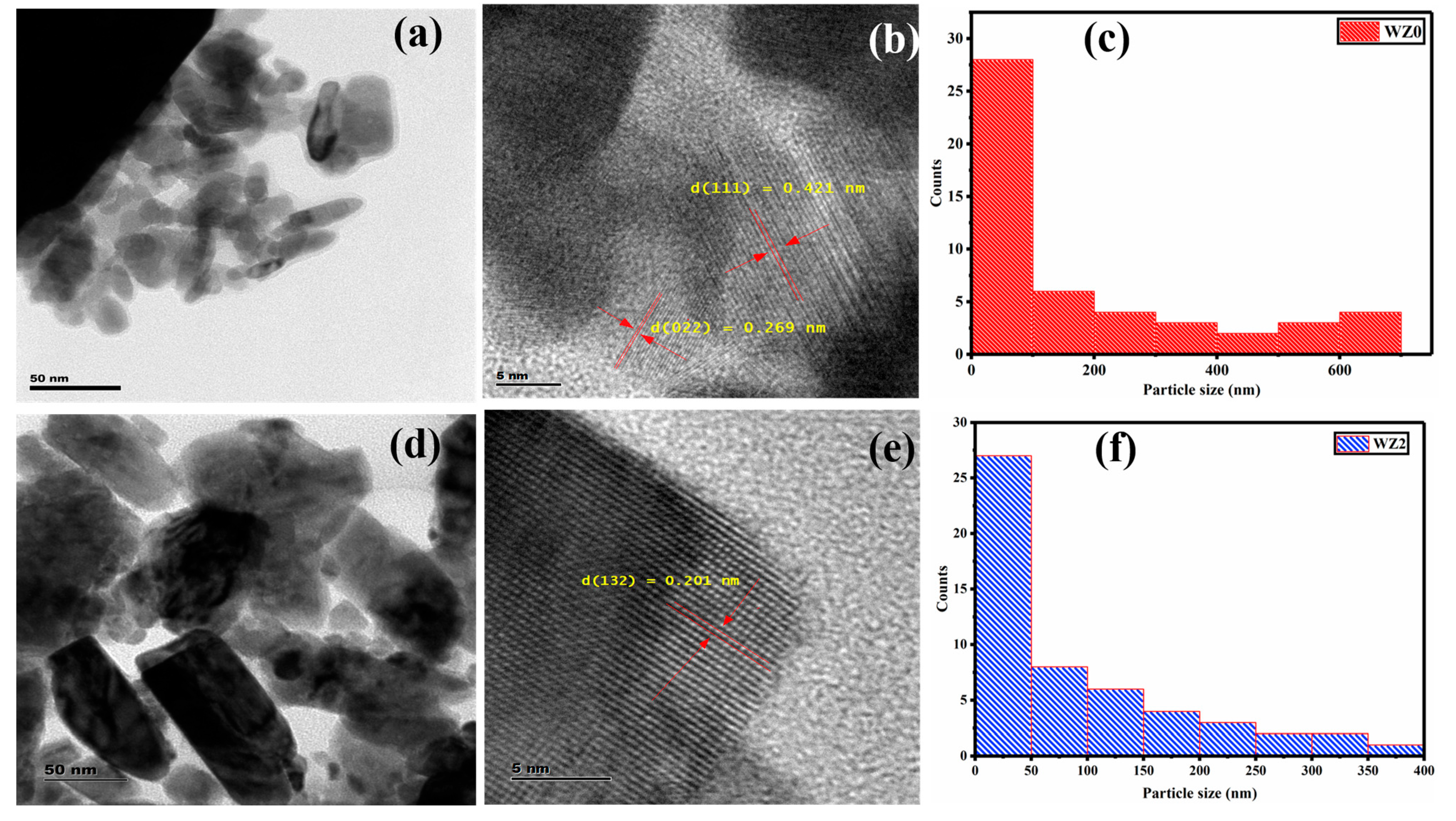
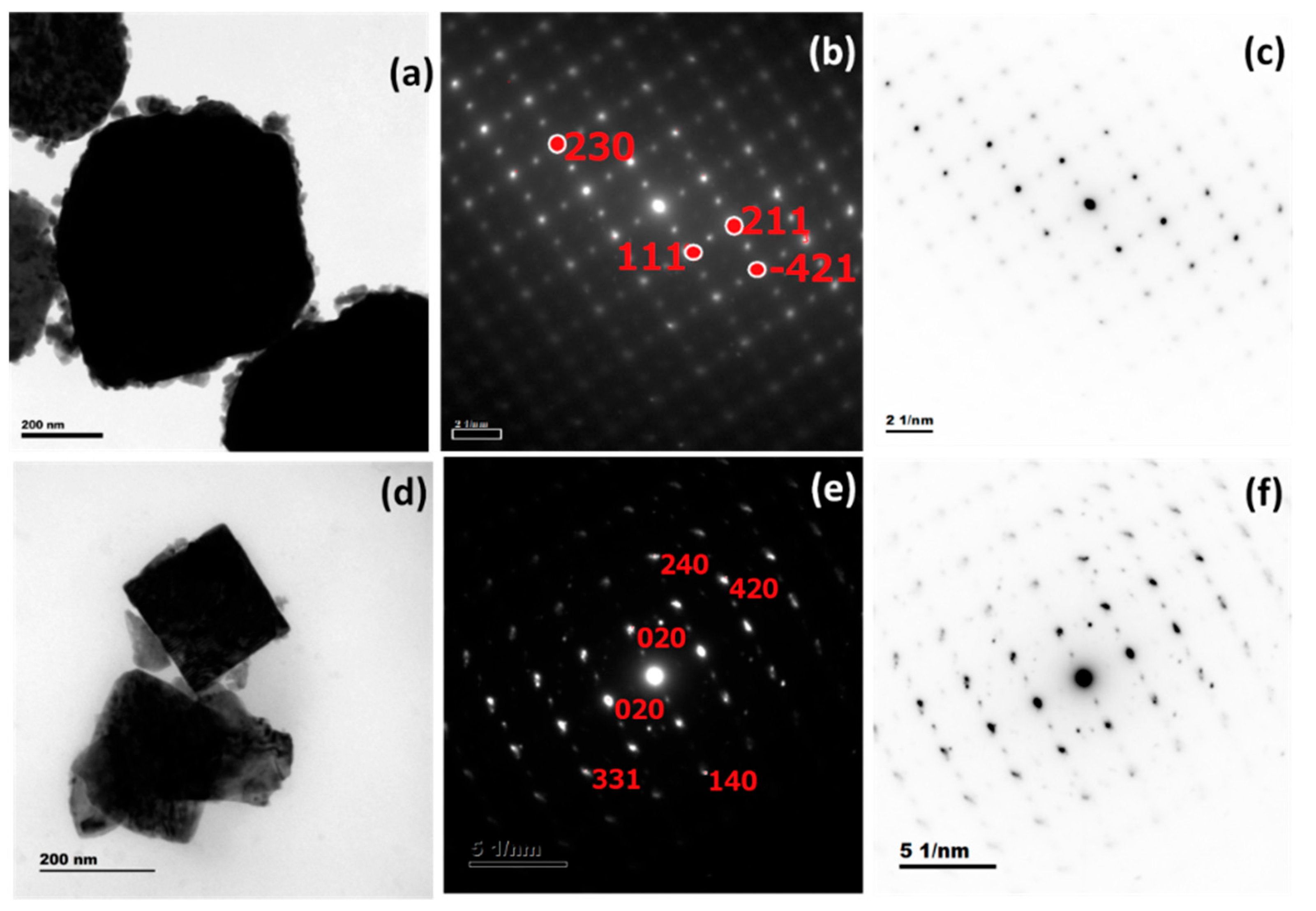
| Sample | Lattice Parameters (Å) | Cell Volume (Å3) | Crystallite Size (nm) | Dislocation Density (1015) Lines m−2 | Micro Strain (Ԑ) × 10−3 Lines−2 m−4 | Texture Coefficient (TC(hkl)) | Stacking Fault | ||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | |||||||
| WZ0 | 7.32 | 7.50 | 7.68 | 422 | 29.2 | 1.19 | 1.73 | 1.08 | 0.37 |
| WZ1 | 7.27 | 7.56 | 7.70 | 424 | 12.9 | 5.96 | 2.65 | 1.58 | 0.75 |
| WZ2 | 7.29 | 7.47 | 7.72 | 421 | 11.9 | 6.95 | 2.86 | 2.08 | 0.82 |
| WZ3 | 7.28 | 7.48 | 7.71 | 420 | 16.3 | 3.75 | 2.09 | 0.63 | 0.70 |
| Samples | Peak Position (cm−1) | ||||||
|---|---|---|---|---|---|---|---|
| WZ0 | 81.8 | 131.8 | 188.8 | 272.3 | 326.8 | 713.5 | 807.7 |
| WZ1 | 80.8 | 131.5 | 188.3 | 270.4 | 326.3 | 713.7 | 807.7 |
| WZ2 | 80.8 | 133 | 190.7 | 270.4 | 326.8 | 715.9 | 809.7 |
| WZ3 | 76.5 | 136 | 186.7 | 272.5 | 326.3 | 713.7 | 807.7 |
| Samples (with Epoxy Composite) | Thickness (10−3 m) | Density (g/cm3) |
|---|---|---|
| WZO | 4.36 | 1.29 |
| WZ1 | 4.33 | 1.27 |
| WZ2 | 4.29 | 1.28 |
| WZ3 | 4.35 | 1.27 |
| PbO (reference material) | 4.50 | 1.30 |
| Pure epoxy (Polymer) | 4.15 | 1.05 |
| Material | Density (g/cm3) | X-ray Energy (kVp) | Major Results | Reference |
|---|---|---|---|---|
| SRM-50 (dimethyl polysiloxane + wt 50% PbO) | 2.038 | 59.51 and 80.99 | HVL ≈ 2 and 4 | [4] |
| SRN-50 (dimethyl polysiloxane + wt 50% PbO) | 2.086 | HVL ≈ 2.5 and 3 | ||
| Portland Cement (89%) + PU-Plaster (1%) + Lead Oxide (10%) | - | 121.78–1408.01 | HVL ≈ 30 and 125 | [5] |
| PS/PbO (A) 35 | 1.4015 | 40–100 | μ = 9.918 − 1.062 | [7] |
| PS/PbO (B) 35 | 1.3560 | μ = 9.829 − 1.440 | ||
| B5 [(10% PVA) (WO3 Filler loading 40%)] | ≈1.29 | 8.64–25.20, 57.53 | μm ≈ 155 − 25.20, 130 | [9] |
| EPVC/micro WO3 40 wt% | - | 40 and 100 | μm = 11.20 and 5.49 HVL = 0.5 and 0.9 | [8] |
| EPVC/nano WO3 40 wt% | μm = 11.81 and 5.68 HVL = 0.5 and 1 | |||
| WP1(W/polymer ration 300%) | - | 150 | μm ≈ 3.5 | [64] |
| Micro WO3/E44 & Nano WO3/E44 | 2.18, 2.29 | 59.6 | μm = 1.0196, 1.0706 | [65] |
| W-SR (W wt 80%) | - | 59.5 | μm ≈ 2.05 | [66] |
| W0.115at% | - | 40–100 | μm ≈ 0.34 − 0.24 | [19] |
| PE-A-25 (PE 75%, TBG 25%) (TBG − nano WO3 + BI2O3 + GO) | 1.43 | 50–120 | μm = 0.26 − 0.15 | [67] |
| Wt 40% WO3 + PVA | 1.79 | 5.89–59.54 | μm = 127.60 − 1.33 | [68] |
| W | 4.18 | 50 and 120 | μm = 8.74 and 5.25 | [69] |
| Sn-W | 4.2 | μm = 9.89 and 5.71 | ||
| W-Ba | 3.11 | μm = 9.51 and 5.55 | ||
| PVA + wt 40% WO3 (micro) | - | 201Tl-radioactivesources | μm ≈ 0.98 HVL = 3 | [70] |
| PVA + wt 40% WO3 (nano) | μm ≈ 1.28 HVL = 2 | |||
| WN400m | ≈1.9 | 60–120 | μm ≈ 14.2 − 8 | [71] |
| Epoxy + wt 50% PbO | 1.30 | 40–100 | μm = 5.85 − 2.76 HVL = 0.09 − 0.19 | Our work |
| Epoxy + wt 50% WZ0 (undoped WO3 NPs) | 1.29 | μm = 4.73 − 2.24 HVL = 0.12 − 0.24 | ||
| Epoxy + wt 50% WZ2 (2% Zn doped WO3) | 1.28 | μm = 6.54 − 2.66 HVL = 0.08 − 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palanisami, S.; Dhandapani, V.S.; Jayachandran, V.; Muniappan, E.; Park, D.; Kim, B.; Govindasami, K. Investigation on Physico Chemical and X-ray Shielding Performance of Zinc Doped Nano-WO3 Epoxy Composite for Light Weight Lead Free Aprons. Materials 2023, 16, 3866. https://doi.org/10.3390/ma16103866
Palanisami S, Dhandapani VS, Jayachandran V, Muniappan E, Park D, Kim B, Govindasami K. Investigation on Physico Chemical and X-ray Shielding Performance of Zinc Doped Nano-WO3 Epoxy Composite for Light Weight Lead Free Aprons. Materials. 2023; 16(10):3866. https://doi.org/10.3390/ma16103866
Chicago/Turabian StylePalanisami, Sanjeevi, Vishnu Shankar Dhandapani, Varuna Jayachandran, Elango Muniappan, Dongkyou Park, Byungki Kim, and Kalpana Govindasami. 2023. "Investigation on Physico Chemical and X-ray Shielding Performance of Zinc Doped Nano-WO3 Epoxy Composite for Light Weight Lead Free Aprons" Materials 16, no. 10: 3866. https://doi.org/10.3390/ma16103866
APA StylePalanisami, S., Dhandapani, V. S., Jayachandran, V., Muniappan, E., Park, D., Kim, B., & Govindasami, K. (2023). Investigation on Physico Chemical and X-ray Shielding Performance of Zinc Doped Nano-WO3 Epoxy Composite for Light Weight Lead Free Aprons. Materials, 16(10), 3866. https://doi.org/10.3390/ma16103866








