Enhanced Flame Retardancy of Styrene-Acrylic Emulsion Based Damping Composites Based on an APP/EG Flame-Retardant System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
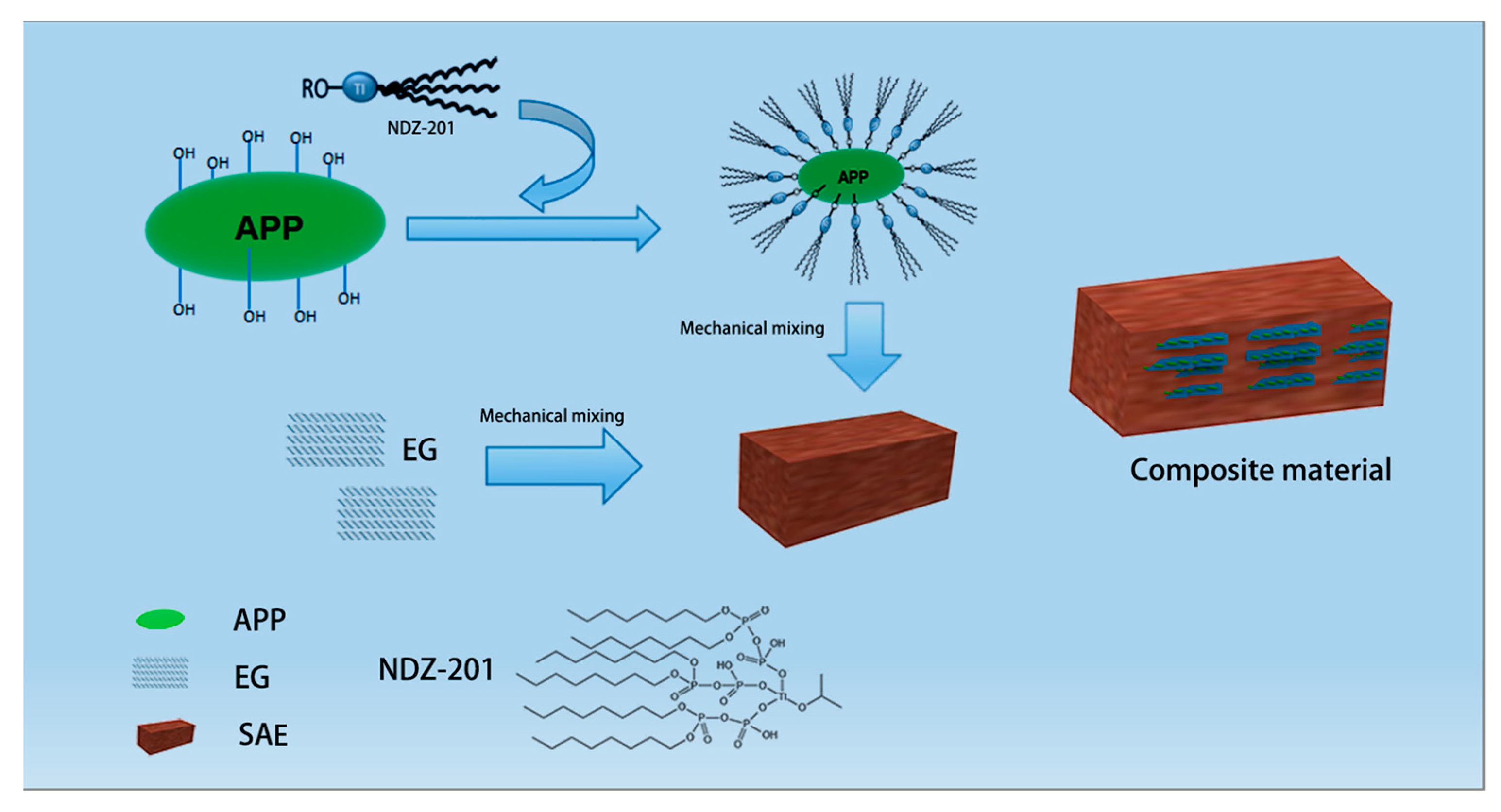
2.2. Preparation of Modified Ammonium Polyphosphate
2.3. Preparation of APP/EG/SAE Composites
2.4. Characterization
3. Results and Discussion
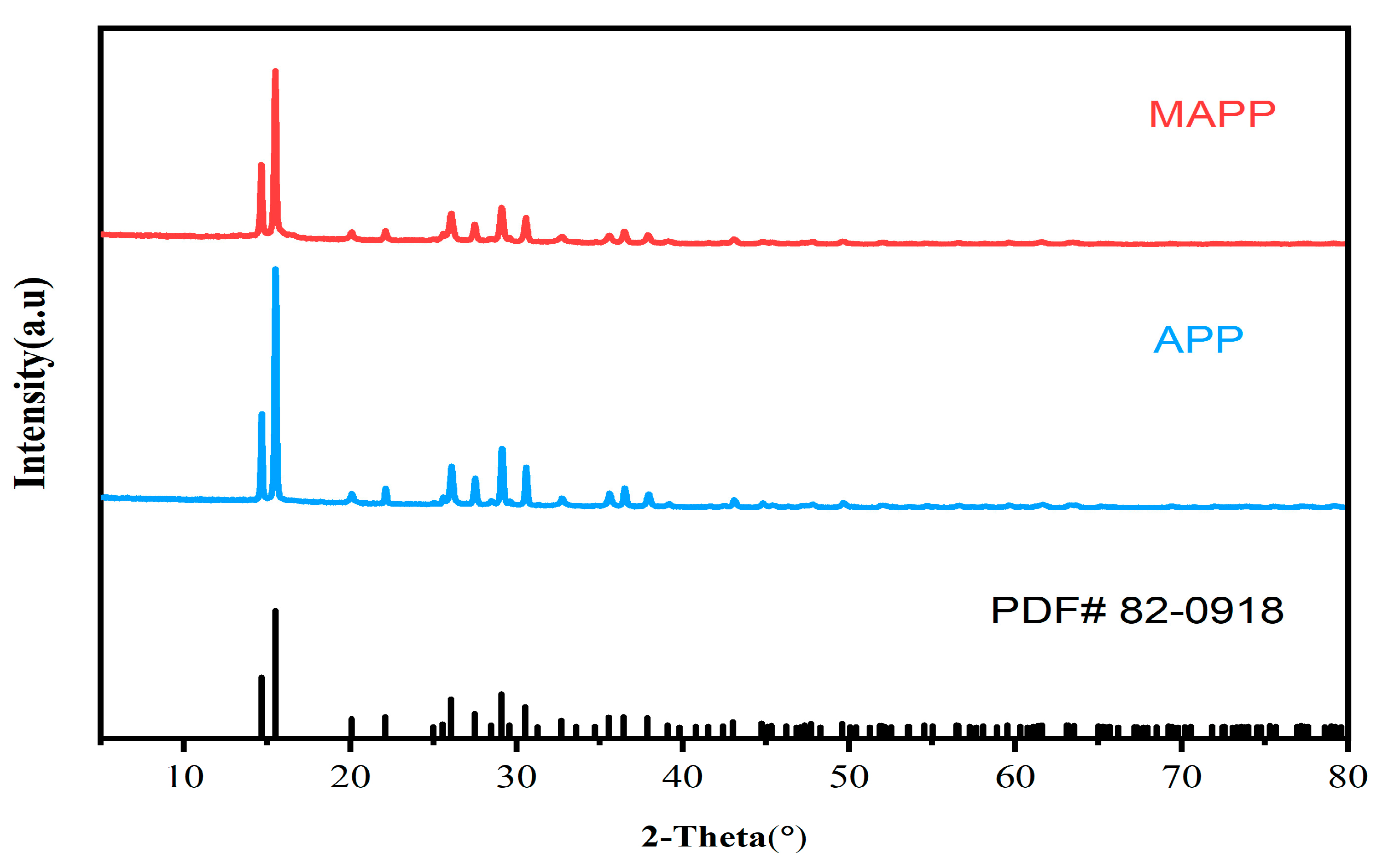
3.1. X-ray Diffraction Analysis
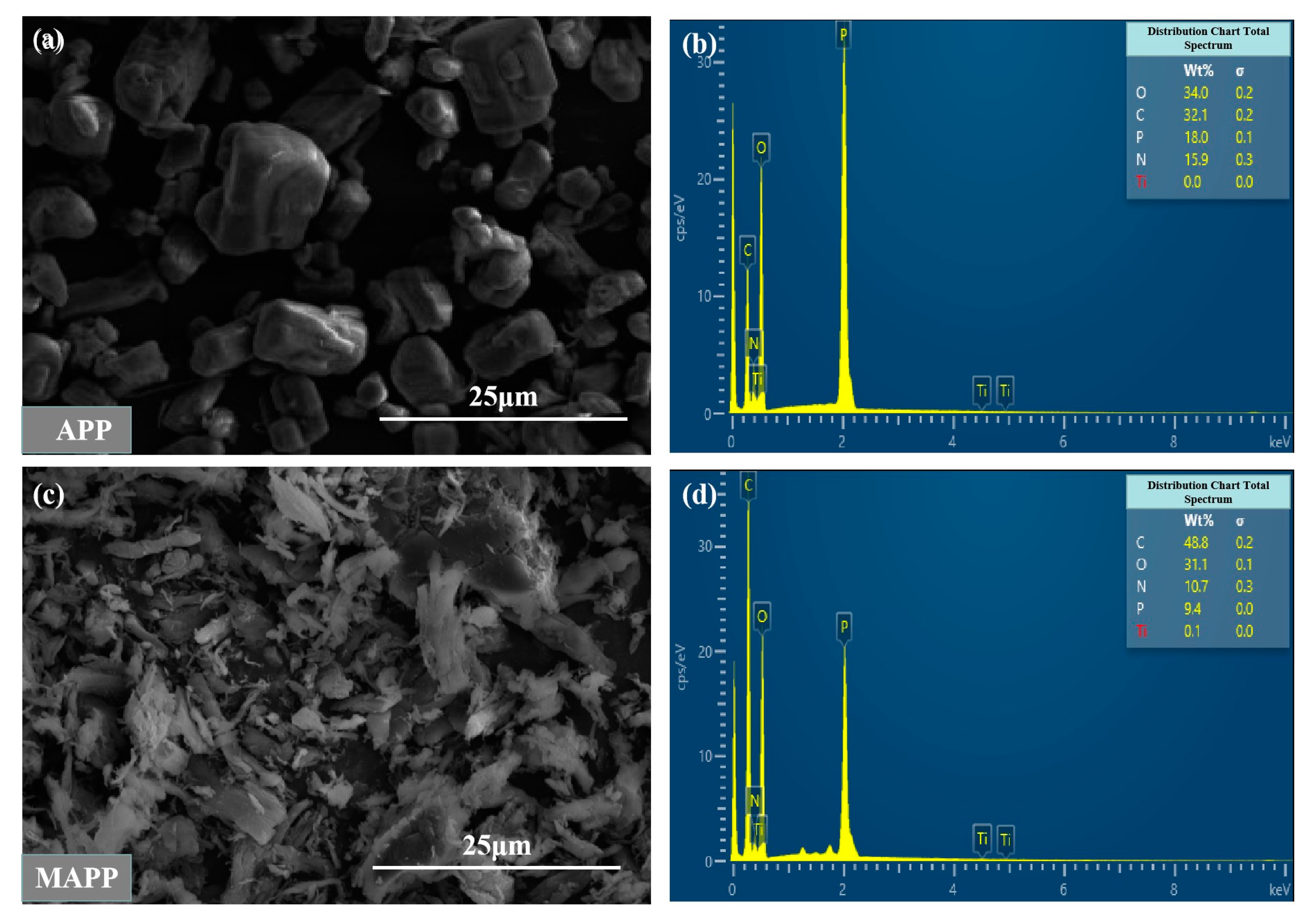
3.2. Scanning Electron Microscopy Energy Spectrum Analysis
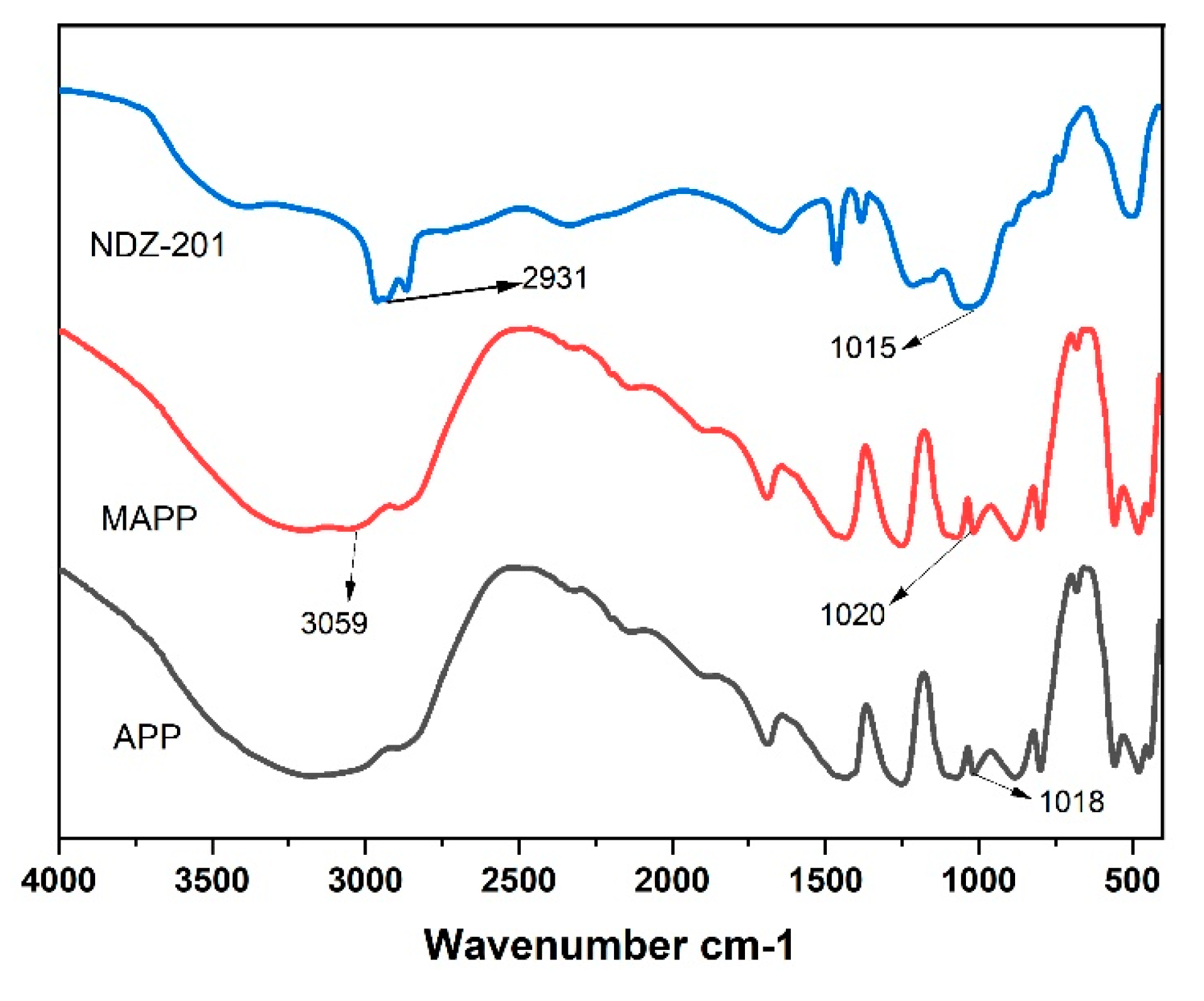
3.3. Fourier Transform Infrared Analysis
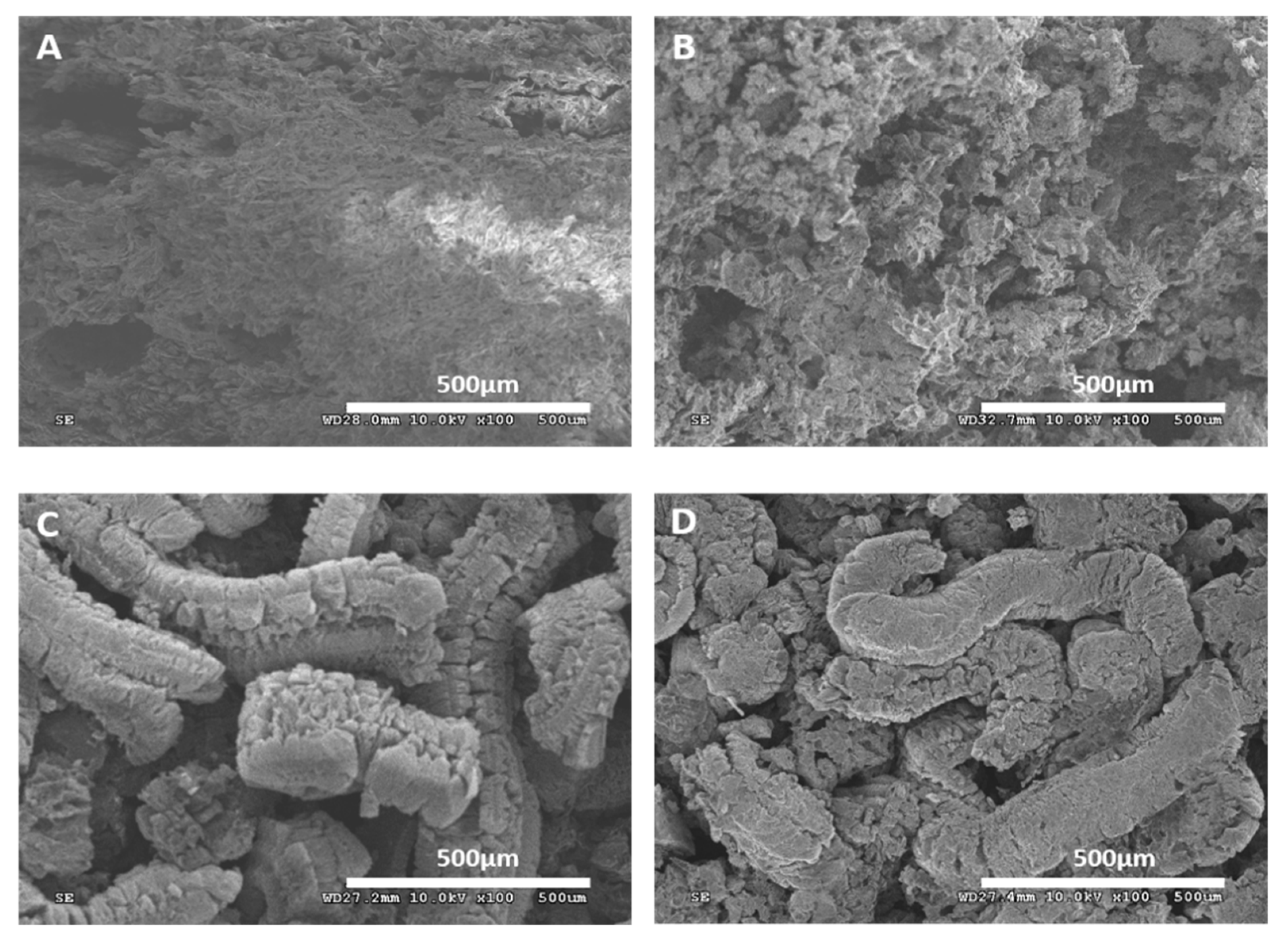
3.4. Scanning Microscope Analysis
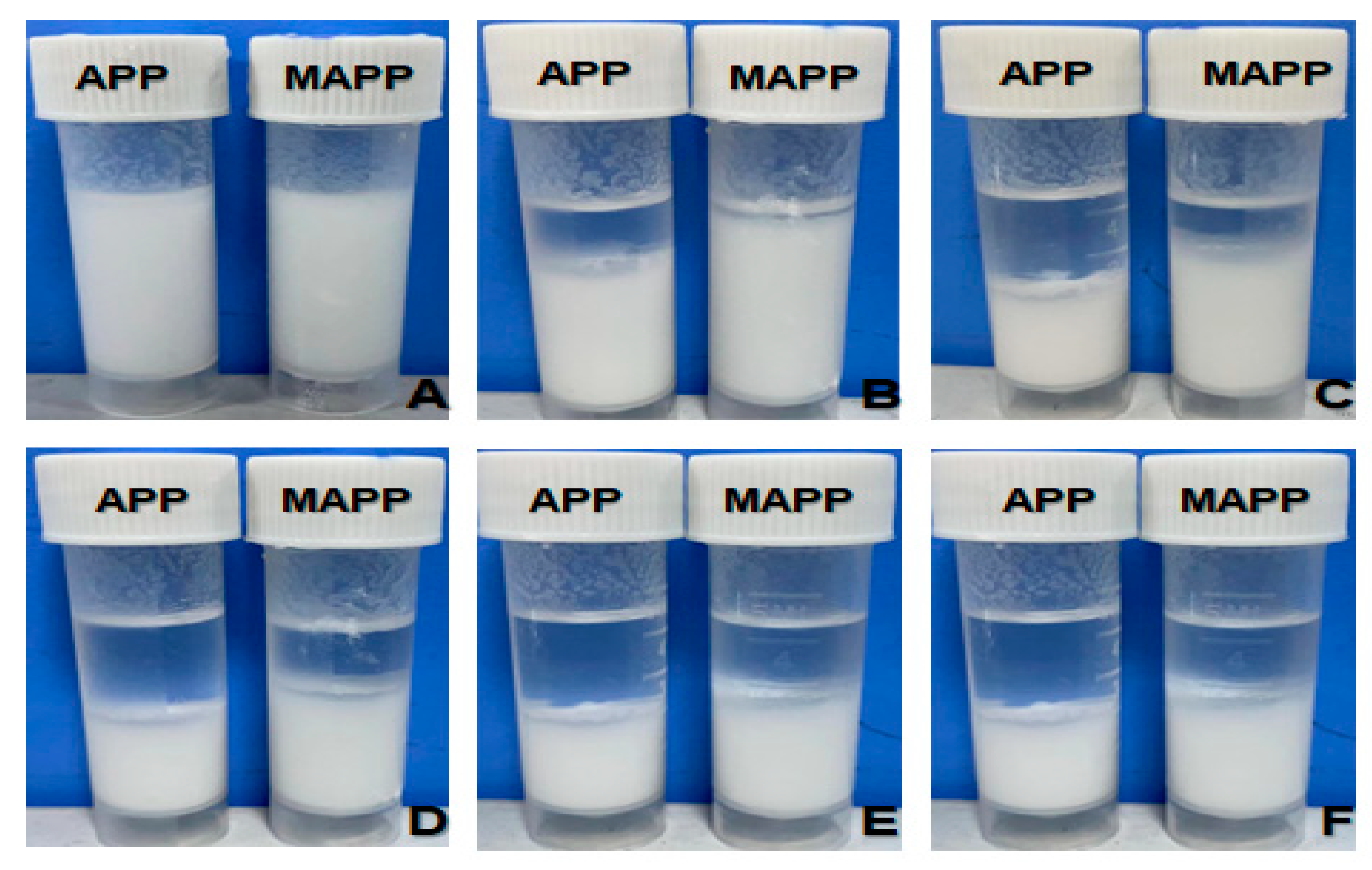
3.5. Contact Angle Analysis
3.6. Compatibility Analysis
3.7. Flame Retardancy of APP/EG Composites
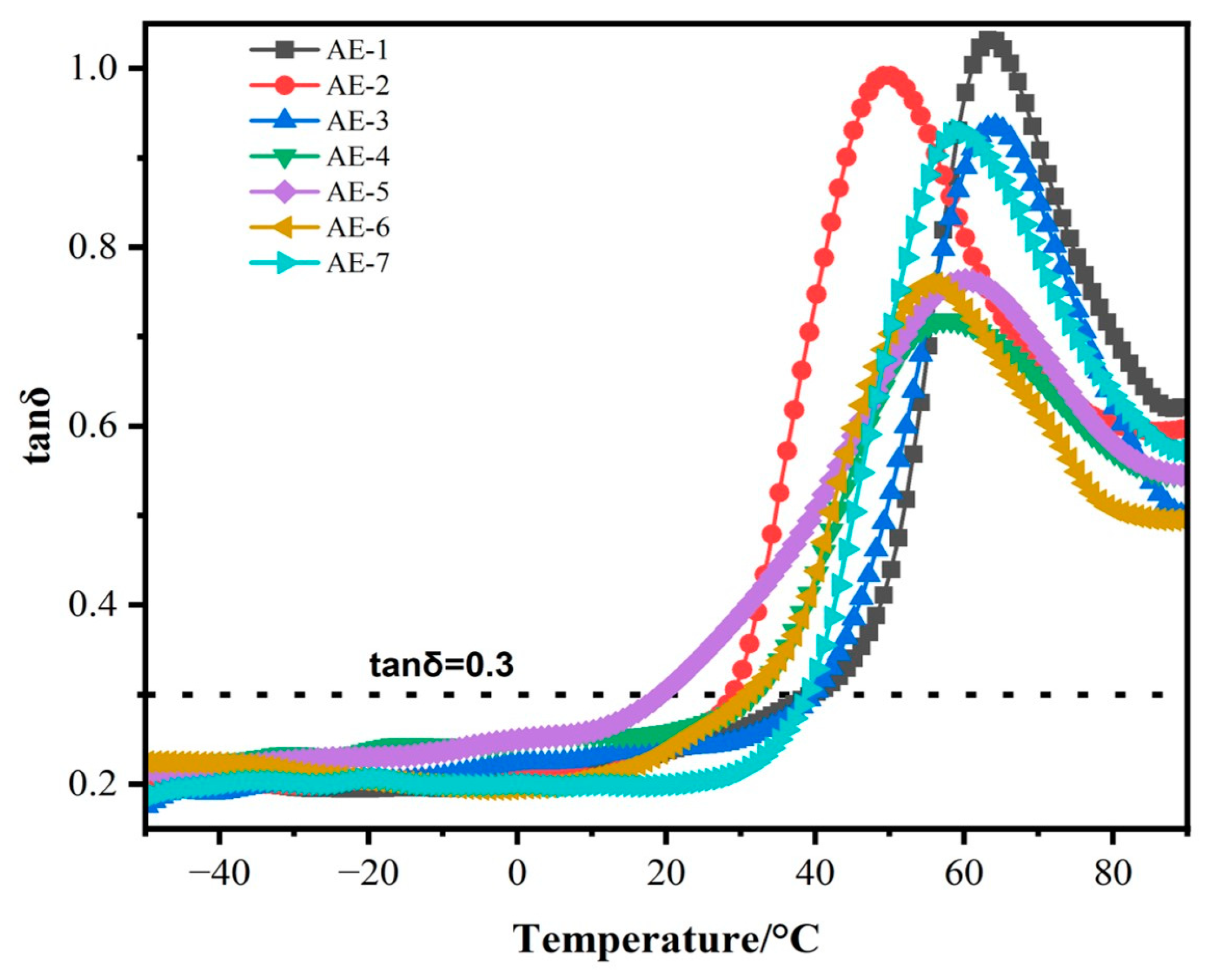
3.8. Dynamic Mechanical Properties of MAPP/EG Composites
3.9. Mechanical Properties of MAPP/EG Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, L.; Shen, Z.; Li, J.; Huang, A.; Lin, M.; Su, Z. Damping properties of expanded graphite filled fluorinated polyacrylate composites. Polym. Bull. 2021, 79, 4745–4759. [Google Scholar] [CrossRef]
- Gregorova, A.; Machovsky, M.; Wimmer, R. Viscoelastic Properties of Mineral-Filled Poly(lactic acid) Composites. Int. J. Polym. Sci. 2012, 2012, 252981. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, F.; Peng, X.; Scarpa, F.; Huang, Z.; Tao, G.; Liu, H.-Y.; Zhou, H.; Zhou, H. Improving the damping properties of carbon fiber reinforced polymer composites by interfacial sliding of oriented multilayer graphene oxide. Compos. Sci. Technol. 2022, 224, 109309. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, M.; Li, Y.; Shi, M.; Huang, Z. Mechanical and Damping Properties of Glass Fiber and Mica-Reinforced Epoxy Composites. Polym.-Plast. Technol. Eng. 2012, 51, 840–844. [Google Scholar] [CrossRef]
- Pastorini, M.T.; Nunes, R.C.R. Mica as a filler for ABS/polycarbonate blends. J. Appl. Polym. Sci. 1999, 74, 1361–1365. [Google Scholar] [CrossRef]
- Ribeiro, S.P.d.S.; Cescon, L.d.S.; Ribeiro, R.Q.C.R.; Landesmann, A.; Estevão, L.R.d.M.; Nascimento, R.S.V. Effect of clay minerals structure on the polymer flame retardancy intumescent process. Appl. Clay Sci. 2018, 161, 301–309. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, S.; Zhao, C.; Hu, G.; Yang, M. Flame retardant mechanism of polymer/clay nanocomposites based on polypropylene. Polymer 2005, 46, 8386–8395. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Effect of Graphene on Flame Retardancy of Graphite Doped Intumescent Flame Retardant (IFR) Coatings: Synergy or Antagonism. Coatings 2019, 9, 94. [Google Scholar] [CrossRef]
- Kumar, R.; Chauhan, S. Effect of ammonium polyphosphate as synergist with nano silica dioxide on flammability of boron compound pretreated bamboo flour-HDPE composite. Fire Saf. J. 2022, 133, 103647. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Zhao, S.; Huang, R.Y.; Kong, X.J. A novel intumescent flame retardant imparts high flame retardancy to epoxy resin. Polym. Adv. Technol. 2019, 31, 932–940. [Google Scholar] [CrossRef]
- Mngomezulu, M.E.; Luyt, A.S.; John, M.J. Morphology, thermal and dynamic mechanical properties of poly(lactic acid)/expandable graphite (PLA/EG) flame retardant composites. J. Thermoplast. Compos. Mater. 2017, 32, 89–107. [Google Scholar] [CrossRef]
- Shen, M.-Y.; Chen, W.-J.; Kuan, C.-F.; Kuan, H.-C.; Yang, J.-M.; Chiang, C.-L. Preparation, characterization of microencapsulated ammonium polyphosphate and its flame retardancy in polyurethane composites. Mater. Chem. Phys. 2016, 173, 205–212. [Google Scholar] [CrossRef]
- Yan, H.; Dong, B.; Du, X.; Ma, S.; Wei, L.; Xu, B. Flame-Retardant Performance of Polystyrene Enhanced by Polyphenylene Oxide and Intumescent Flame Retardant. Polym. Plast. Technol. Eng. 2014, 53, 395–402. [Google Scholar] [CrossRef]
- Yuan, Z.G.; Shu, Z.H.; Qi, L.; Cai, W.A.; Liu, W.B.; Wang, J.; Derradji, M.; Wang, Y.H. Curing behavior, mechanical, and flame-retardant properties of epoxy-based composites filled by expandable graphite and ammonium polyphosphate. J. Appl. Polym. Sci. 2022, 140, e53267. [Google Scholar] [CrossRef]
- Lim, K.-S.; Bee, S.-T.; Sin, L.T.; Tee, T.-T.; Ratnam, C.T.; Hui, D.; Rahmat, A.R. A review of application of ammonium polyphosphate as intumescent flame retardant in thermoplastic composites. Compos. Part B Eng. 2016, 84, 155–174. [Google Scholar] [CrossRef]
- Chen, D.; Li, J.; Ren, J. Combustion properties and transference behavior of ultrafine microencapsulated ammonium polyphosphate in ramie fabric-reinforced poly(L-lactic acid) biocomposites. Polym. Int. 2011, 60, 599–606. [Google Scholar] [CrossRef]
- Ran, G.; Liu, X.; Guo, J.; Sun, J.; Li, H.; Gu, X.; Zhang, S. Improving the flame retardancy and water resistance of polylactic acid by introducing polyborosiloxane microencapsulated ammonium polyphosphate. Compos. Part B Eng. 2019, 173, 106772. [Google Scholar] [CrossRef]
- Meng, L.; Li, X.; Liu, M.; Li, C.; Meng, L.; Hou, S. Modified Ammonium Polyphosphate and Its Application in Polypropylene Resins. Coatings 2022, 12, 1738. [Google Scholar] [CrossRef]
- Zhaolu, Q.; Dinghua, L.; Rongjie, Y. Ammonium polyphosphate modified by titanate coupling agent and its application in flame retardant polypropylene. Polym. Mater. Sci. Eng. 2015, 31, 84–91. [Google Scholar]
- Zhu, H.; Xu, S. Preparation of Flame-Retardant Rigid Polyurethane Foams by Combining Modified Melamine Formaldehyde Resin and Phosphorus Flame Retardants. ACS Omega 2020, 5, 9658–9667. [Google Scholar] [CrossRef]
- Wu, K.; Wang, Z.; Hu, Y. Microencapsulated ammonium polyphosphate with urea-melamine-formaldehyde shell: Preparation, characterization, and its flame retardance in polypropylene. Polym. Adv. Technol. 2008, 19, 1118–1125. [Google Scholar] [CrossRef]
- Entezam, M.; Khonakdar, H.A.; Jafari, S.M.A.; Raji, S.; Otadi, M. Thermal stability and flammability of ethylene vinyl acetate copolymers in presence of nanoclay and a halogen-free flame retardant. J. Vinyl Addit. Technol. 2017, 23, E92–E98. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Yang, R. Polylactic acid flame-retarded by nano-compound of form II ammonium polyphosphate with montmorillonite. J. Fire Sci. 2021, 39, 495–511. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, X.; Tao, X. Intumescent flame retardant and anti-dripping of PET fabrics through layer-by-layer assembly of chitosan and ammonium polyphosphate. Prog. Org. Coat. 2019, 134, 162–168. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Z.; Zhu, J. Synergistic flame retardant effect of aluminum hydroxide and ammonium polyphosphate on epoxy resin. J. Appl. Polym. Sci. 2022, 139, e53168. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, M.; Li, S.-Y.; Zeng, H.-Y.; Du, J.-Z.; Chen, C.-R.; Wu, K.; Tian, X.-Y.; Pan, Y. The effect of ammonium polyphosphate on the mechanism of phosphorous-containing hydrotalcite synergism of flame retardation of polypropylene. Appl. Clay Sci. 2020, 185, 105348. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, S.; Hu, Y.; Chen, G.; Shi, X.; Peng, X. Hybrids of aluminum hypophosphite and ammonium polyphosphate: Highly effective flame retardant system for unsaturated polyester resin. Polym. Compos. 2018, 39, 1763–1770. [Google Scholar] [CrossRef]
- Khalili, P.; Liu, X.; Tshai, K.Y.; Rudd, C.; Yi, X.; Kong, I. Development of fire retardancy of natural fiber composite encouraged by a synergy between zinc borate and ammonium polyphosphate. Compos. Part B Eng. 2019, 159, 165–172. [Google Scholar] [CrossRef]
- Feng, C.; Liang, M.; Jiang, J.; Huang, J.; Liu, H. Synergistic effect of a novel triazine charring agent and ammonium polyphosphate on the flame retardant properties of halogen-free flame retardant polypropylene composites. Thermochim. Acta 2016, 627–629, 83–90. [Google Scholar] [CrossRef]
- Wang, S.; Shi, M.; Yang, W.; Yan, H.; Zhang, C.; An, Y.; Zhang, F. Experimental investigation of flame retardancy and mechanical properties of APP/EG/TPU multilayer composites prepared by microlayer coextrusion technology. J. Appl. Polym. Sci. 2021, 138, 50219. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, J.; Wei, T.; Guo, W. Synergistic effect between ammonium polyphosphate and expandable graphite on flame-retarded poly(butylene terephthalate). Mater. Res. Express 2018, 5, 025310. [Google Scholar] [CrossRef]
- Wilke, A.; Langfeld, K.; Ulmer, B.; Andrievici, V.; Hörold, A.; Limbach, P.; Bastian, M.; Schartel, B. Halogen-Free Multicomponent Flame Retardant Thermoplastic Styrene–Ethylene–Butylene–Styrene Elastomers Based on Ammonium Polyphosphate–Expandable Graphite Synergy. Ind. Eng. Chem. Res. 2017, 56, 8251–8263. [Google Scholar] [CrossRef]
- Sun, L.; Xie, Y.; Ou, R.; Guo, C.; Hao, X.; Wu, Q.; Wang, Q. The influence of double-layered distribution of fire retardants on the fire retardancy and mechanical properties of wood fiber polypropylene composites. Constr. Build. Mater. 2020, 242, 118047. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, F.; Al-Sehemi, A.G.; Assiri, M.A.; Raza, M.R.; Irfan, A. Effect of expandable graphite and ammonium polyphosphate on the thermal degradation and weathering of intumescent fire-retardant coating. J. Appl. Polym. Sci. 2020, 138, 50310. [Google Scholar] [CrossRef]
- Ng, Y.H.; Dasari, A.; Tan, K.H.; Qian, L. Intumescent fire-retardant acrylic coatings: Effects of additive loading ratio and scale of testing. Prog. Org. Coat. 2021, 150, 105985. [Google Scholar] [CrossRef]
- Lyu, X.; Zhang, H.; Yan, Y. Effect of expandable graphite and molybdenum trioxide in nitrogen/phosphorus synergistic system on acoustic performance and fire safety in rigid polyurethane foam. J. Appl. Polym. Sci. 2022, 139, e52488. [Google Scholar] [CrossRef]
- Li, J.; Mo, X.; Li, Y.; Zou, H.; Liang, M.; Chen, Y. Influence of expandable graphite particle size on the synergy flame retardant property between expandable graphite and ammonium polyphosphate in semi-rigid polyurethane foam. Polym. Bull. 2018, 75, 5287–5304. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, D.; Zhang, Y.; Fu, T.; Guan, D.; Dou, Y. Synergistic Flame Retardant Effects of Expandable Graphite and Ammonium Polyphosphate in Water-Blow Polyurethane Foam. Adv. Mater. Sci. Eng. 2019, 2019, 6921474. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, T.; Zhang, W.; Dou, Y. Influence of surface flame-retardant layer containing ammonium polyphosphate and expandable graphite on the performance of jute/polypropylene composites. J. Therm. Anal. Calorim. 2018, 135, 2367–2375. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Li, L.; Wang, Z. Microencapsulated ammonium polyphosphate with epoxy resin shell: Preparation, characterization, and application in EP system. Polym. Adv. Technol. 2011, 22, 2403–2408. [Google Scholar] [CrossRef]
- Wang, T.; Yang, J.; Yan, J.; Xu, G.; Li, Z. Preparation of modified ammonium polyphosphate and its application in flame retardant air filter paper. Polym. Bull. 2021, 79, 3085–3098. [Google Scholar] [CrossRef]
- Liu, X.; Ma, X.; Zhu, L.; Zhu, L. Fabrication of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) biocomposite reinforced wood fiber modified with mixed coupling agents CS-201 and KH550. Ind. Crops Prod. 2021, 164, 113352. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.; Lu, S.; Wang, Z.; Li, J.; Liu, B.; Fang, X.; Ding, T.; Xu, Y. Synergistic Flame Retardant Properties of Polyoxymethylene with Surface Modified Intumescent Flame Retardant and Calcium Carbonate. Polymers 2023, 15, 537. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Geng, J.; Sun, B.; Xu, Q.; Li, Y.; Xie, S.; Xue, Y.; Yan, H. Great enhancement of efficiency of intumescent flame retardants by titanate coupling agent and polysiloxane. Polym. Adv. Technol. 2020, 32, 41–53. [Google Scholar] [CrossRef]
- Yan, J.; Xu, P.; Zhang, P.; Fan, H. Surface-modified ammonium polyphosphate for flame-retardant and reinforced polyurethane composites. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127092. [Google Scholar] [CrossRef]
- Tang, M.; Qi, F.; Chen, M.; Sun, Z.; Xu, Y.; Chen, X.; Zhang, Z.; Shen, R. Synergistic effects of ammonium polyphosphate and red phosphorus with expandable graphite on flammability and thermal properties of HDPE/EVA blends. Polym. Adv. Technol. 2016, 27, 52–60. [Google Scholar] [CrossRef]
- Wang, J.; Xue, L.; Zhao, B.; Lin, G.; Jin, X.; Liu, D.; Zhu, H.; Yang, J.; Shang, K. Flame Retardancy, Fire Behavior, and Flame Retardant Mechanism of Intumescent Flame Retardant EPDM Containing Ammonium Polyphosphate/Pentaerythrotol and Expandable Graphite. Materials 2019, 12, 4035. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, Q.; Li, J.; Tao, K.; Xue, L.; Yan, Q. Synergistic effect between expandable graphite and ammonium polyphosphate on flame retarded polylactide. Polym. Degrad. Stab. 2011, 96, 183–189. [Google Scholar] [CrossRef]
- Tang, X.; Yan, X. A review on the damping properties of fiber reinforced polymer composites. J. Ind. Text. 2018, 49, 693–721. [Google Scholar] [CrossRef]



| Sample | Formulation (wt%) | LOI (%) | UL-94 Test | |||
|---|---|---|---|---|---|---|
| Mica Powder | MAPP | EG | Flaming Dripping | UL-94 Rating | ||
| AE-1 | 50 | / | / | 21.7 | Yes | No rating |
| AE-2 | 25 | 25 | / | 31.2 | NO | V-0 |
| AE-3 | 25 | / | 25 | 44.5 | NO | V-0 |
| AE-4 | 25 | 20 | 5 | 45.7 | NO | V-0 |
| AE-5 | 25 | 15 | 10 | 54.3 | NO | V-0 |
| AE-6 | 25 | 10 | 15 | 50.7 | NO | V-0 |
| AE-7 | 25 | 5 | 20 | 52.5 | NO | V-0 |
| Samples | Adhesion Strength/MPa | Impact Strength/kg·cm | Tensile Strength/MPa |
|---|---|---|---|
| AE-1 | 0.35 | 50 | 5.75 |
| AE-2 | 0.23 | 35 | 0.88 |
| AE-3 | 0.32 | 50 | 2.07 |
| AE-4 | 0.14 | 40 | 3.24 |
| AE-5 | 0.15 | 45 | 3.27 |
| AE-6 | 0.27 | 50 | 3.05 |
| AE-7 | 0.29 | 50 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Bi, J.; Xu, B.; Fu, L.; Hao, W. Enhanced Flame Retardancy of Styrene-Acrylic Emulsion Based Damping Composites Based on an APP/EG Flame-Retardant System. Materials 2023, 16, 3894. https://doi.org/10.3390/ma16113894
Wu J, Bi J, Xu B, Fu L, Hao W. Enhanced Flame Retardancy of Styrene-Acrylic Emulsion Based Damping Composites Based on an APP/EG Flame-Retardant System. Materials. 2023; 16(11):3894. https://doi.org/10.3390/ma16113894
Chicago/Turabian StyleWu, Jingxing, Jianhua Bi, Baoluo Xu, Lisha Fu, and Wanjun Hao. 2023. "Enhanced Flame Retardancy of Styrene-Acrylic Emulsion Based Damping Composites Based on an APP/EG Flame-Retardant System" Materials 16, no. 11: 3894. https://doi.org/10.3390/ma16113894





