Study on the Properties of Carbon Nanotube (CNTs) Reinforced AlSi10Mg Composites Fabricated by Powder Metallurgy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation Methods
2.2. Material Characterization
3. Results and Discussion
3.1. Effects of Different Ball-Grinding Times on Composite Powders
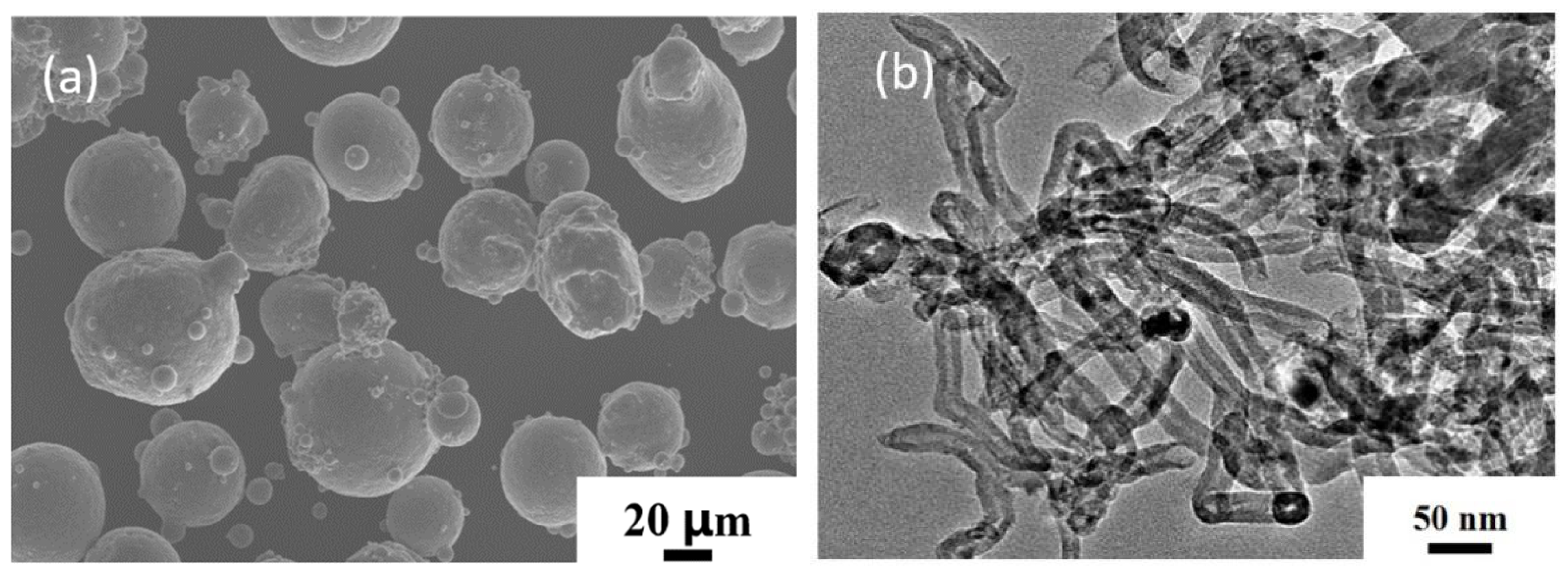
3.1.1. Morphology of CNT/AlSi10Mg Composite Powders at Various Ball-Milling Times
3.1.2. Characterization of CNTs Integrity at Different Ball-Milling Times
3.1.3. Analysis of Phase Composition of CNT/AlSi10Mg Composite Powder at Different Ball-Milling Times
3.1.4. Mechanical Properties of CNT/AlSi10Mg Composite Powder at Different Ball-Milling Times
3.2. Effect of Different Carbon Nanotube Content on the Mechanical Properties of the Composites
3.2.1. Analysis of SAED TEM Images Corresponding to 1.1 wt.% CNT/AlSi10Mg Composites
3.2.2. Mechanical Properties of CNT/AlSi10Mg Composites with Different CNT Content
3.3. Effect of Different Carbon Nanotube Content on the Corrosion Resistance of CNT/AlSi10Mg Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Popov, V.V.; Pismenny, A.; Larionovsky, N.; Lapteva, A.; Safranchik, D. Corrosion Resistance of Al-CNT Metal Matrix Composites. Materials 2021, 14, 3530. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Xiao, B.L.; Ji, Z.H.; Liu, B.S.; Liu, Z.Y.; Ma, Z.Y. Dispersion and Damage of Carbon Nanotubes in Carbon Nanotube/7055Al Composites During High-Energy Ball Milling Process. Acta Met. Sin. Engl. Lett. 2020, 34, 196–204. [Google Scholar] [CrossRef]
- Tjong, S.C. Recent progress in the development and properties of novel metal matrix nanocomposites reinforced with carbon nanotubes and graphene nanosheets. Mater. Sci. Eng. R. Rep. 2013, 74, 281–350. [Google Scholar] [CrossRef]
- Geng, H.; Chen, B.; Wan, J.; Shen, J.; Kondoh, K.; Li, J.S. Matrix effect on strengthening behavior of carbon nanotubes in aluminum matrix composites. Mater. Charact. 2023, 195, 112484. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R. Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Peng, B.; Locascio, M.; Zapol, P.; Li, S.; Mielke, S.L.; Schatz, G.C.; Espinosa, H.D. Measurements of near-ultimate strength for multiwalled carbon nanotubes and irradiation-induced crosslinking improvements. Nat. Nanotechnol. 2008, 3, 626–631. [Google Scholar] [CrossRef]
- Jagannatham, M.; Chandran, P.; Sankaran, S.; Haridoss, P.; Nayan, N.; Bakshi, S.R. Tensile properties of carbon nanotubes reinforced aluminum matrix composites: A review. Carbon 2020, 160, 14–44. [Google Scholar] [CrossRef]
- Yang, X.; Zou, T.; Shi, C.; Liu, E.; He, C.; Zhao, N. Effect of carbon nanotube (CNT) content on the properties of in-situ synthesis CNT reinforced Al composites. Mater. Sci. Eng. A 2016, 660, 11–18. [Google Scholar] [CrossRef]
- Jafari, M.; Abbasi, M.H.; Enayati, M.H.; Karimzadeh, F. Mechanical properties of nanostructured Al2024–MWCNT composite prepared by optimized mechanical milling and hot pressing methods. Adv. Powder Technol. 2012, 23, 205–210. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Z.; Fan, G.; Cao, L.; Zhang, D. The use of flake powder metallurgy to produce carbon nanotube (CNT)/aluminum composites with a homogenous CNT distribution. Carbon 2012, 50, 1993–1998. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Xiao, B.L.; Wang, W.G.; Ma, Z.Y. Analysis of carbon nanotube shortening and composite strengthening in carbon nanotube/aluminum composites fabricated by multi-pass friction stir processing. Carbon 2014, 69, 264–274. [Google Scholar] [CrossRef]
- Nam, D.H.; Cha, S.I.; Lim, B.K.; Park, H.M.; Han, D.S.; Hong, S.H. Synergistic strengthening by load transfer mechanism and grain refinement of CNT/Al–Cu composites. Carbon 2012, 50, 2417–2423. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, Z.-Y.; Xiao, B.-L.; Ni, D.-R.; Ma, Z.-Y. Origin of Insignificant Strengthening Effect of CNTs in T6-Treated CNT/6061Al Composites. Acta Met. Sin. Engl. Lett. 2017, 31, 134–142. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhao, K.; Xiao, B.L.; Wang, W.G.; Ma, Z.Y. Fabrication of CNT/Al composites with low damage to CNTs by a novel solution-assisted wet mixing combined with powder metallurgy processing. Mater. Des. 2016, 97, 424–430. [Google Scholar] [CrossRef]
- Kuzumaki, T.; Miyazawa, K.; Ichinose, H.; Ito, K. Processing of Carbon Nanotube Reinforced Aluminum Composite. J. Mater. Res. 1998, 13, 2445–2449. [Google Scholar] [CrossRef]
- Singh, L.K.; Bhadauria, A.; Laha, T. Comparing the strengthening efficiency of multiwalled carbon nanotubes and graphene nanoplatelets in aluminum matrix. Powder Technol. 2019, 356, 1059–1076. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Xu, S.J.; Xiao, B.L.; Xue, P.; Wang, W.G.; Ma, Z.Y. Effect of ball-milling time on mechanical properties of carbon nanotubes reinforced aluminum matrix composites. Compos. Part A: Appl. Sci. Manuf. 2012, 43, 2161–2168. [Google Scholar] [CrossRef]
- Nie, J.-H.; Jia, C.-C.; Shi, N.; Zhang, Y.-F.; Li, Y.; Jia, X. Aluminum matrix composites reinforced by molybdenum-coated carbon nanotubes. Int. J. Miner. Met. Mater. 2011, 18, 695–702. [Google Scholar] [CrossRef]
- Xu, R.; Tan, Z.; Xiong, D.; Fan, G.; Guo, Q.; Zhang, J.; Su, Y.; Li, Z.; Zhang, D. Balanced strength and ductility in CNT/Al composites achieved by flake powder metallurgy via shift-speed ball milling. Compos. Part A: Appl. Sci. Manuf. 2017, 96, 57–66. [Google Scholar] [CrossRef]
- Thijs, L.; Kempen, K.; Kruth, J.-P.; Van Humbeeck, J. Fine-structured aluminium products with controllable texture by selective laser melting of pre-alloyed AlSi10Mg powder. Acta Mater. 2013, 61, 1809–1819. [Google Scholar] [CrossRef]
- Trevisan, F.; Calignano, F.; Lorusso, M.; Pakkanen, J.; Aversa, A.; Ambrosio, E.P.; Lombardi, M.; Fino, P.; Manfredi, D. On the Selective Laser Melting (SLM) of the AlSi10Mg Alloy: Process, Microstructure, and Mechanical Properties. Materials 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, B.; Zhao, Z.; Li, X.; Liu, B.; Bai, P. Effect of chloride ion concentration on corrosion process of selective laser melted AlSi10Mg with different heat treatments studied by electrochemical noise. J. Mater. Res. Technol. 2022, 16, 1597–1609. [Google Scholar] [CrossRef]
- Wenyi, L.; Xiaohui, H.; Yapeng, L.; Ling, T.; Hui, Z. Research progress on preparation technology and strengthening mechanism of graphene reinforced aluminum matrix composites. J. Aeronaut. Mater. 2023, 43, 51–59. [Google Scholar] [CrossRef]
- Wang, L.-z.; Chen, T.; Wang, S. Microstructural characteristics and mechanical properties of carbon nanotube reinforced AlSi10Mg composites fabricated by selective laser melting. Optik 2017, 143, 173–179. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Liu, T.T.; Zhang, C.D.; Zhang, K.; Li, M.C.; Ma, T.; Liao, W.H. Preparation and mechanical properties of CNTs-AlSi10Mg composite fabricated via selective laser melting. Mater. Sci. Eng. A 2018, 734, 171–177. [Google Scholar] [CrossRef]
- Jiang, Y.; Tan, Z.; Xu, R.; Fan, G.; Xiong, D.-B.; Guo, Q.; Su, Y.; Li, Z.; Zhang, D. Tailoring the structure and mechanical properties of graphene nanosheet/aluminum composites by flake powder metallurgy via shift-speed ball milling. Compos. Part A: Appl. Sci. Manuf. 2018, 111, 73–82. [Google Scholar] [CrossRef]
- Vatankhah, A.R.; Hosseini, M.A.; Malekie, S. The characterization of gamma-irradiated carbon-nanostructured materials carried out using a multi-analytical approach including Raman spectroscopy. Appl. Surf. Sci. 2019, 488, 671–680. [Google Scholar] [CrossRef]
- Cha, S.I.; Kim, K.T.; Lee, K.H.; Mo, C.B.; Hong, S.H. Strengthening and toughening of carbon nanotube reinforced alumina nanocomposite fabricated by molecular level mixing process. Scr. Mater. 2005, 53, 793–797. [Google Scholar] [CrossRef]
- Wang, L.; Choi, H.; Myoung, J.-M.; Lee, W. Mechanical alloying of multi-walled carbon nanotubes and aluminium powders for the preparation of carbon/metal composites. Carbon 2009, 47, 3427–3433. [Google Scholar] [CrossRef]
- George, R.; Kashyap, K.T.; Rahul, R.; Yamdagni, S. Strengthening in carbon nanotube/aluminium (CNT/Al) composites. Scr. Mater. 2005, 53, 1159–1163. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Zhang, X. Processing map of Al2O3 particulate reinforced Al alloy matrix composites. Mater. Sci. Eng. A 2012, 558, 112–118. [Google Scholar] [CrossRef]
- Fan, J.-P.; Zhuang, D.-M.; Zhao, D.-Q.; Zhang, G.; Wu, M.-S.; Wei, F.; Fan, Z.-J. Toughening and reinforcing alumina matrix composite with single-wall carbon nanotubes. Appl. Phys. Lett. 2006, 89, 121910. [Google Scholar] [CrossRef]
- Salazar, J.M.G.D.; Ureña, A.; Manzanedo, S.; Barrena, M.I. Corrosion behaviour of AA6061 and AA7005 reinforced with Al2O3 particles in aerated 3.5% chloride solutions: Potentiodynamic measurements and microstructure evaluation. Corros. Sci. 1998, 41, 529–545. [Google Scholar] [CrossRef]
- El Shayeb, H.; El Wahab, F.A.; El Abedin, S.Z. Effect of gallium ions on the electrochemical behaviour of Al, Al–Sn, Al–Zn and Al–Zn–Sn alloys in chloride solutions. Corros. Sci. 2001, 43, 643–654. [Google Scholar] [CrossRef]
- Deepa, P.; Padmalatha, R. Corrosion behaviour of 6063 aluminium alloy in acidic and in alkaline media. Arab. J. Chem. 2017, 10, S2234–S2244. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Li, X. Design materials based on simulation results of silicon induced segregation at AlSi10Mg interface fabricated by selective laser melting. J. Mater. Sci. Technol. 2020, 46, 145–155. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]

















| Alloy | UTS (MPa) | YS (MPa) | EL (%) |
|---|---|---|---|
| 0% | 151 ± 2.1 | 82 ± 2.0 | 9.16 ± 0.5 |
| 0.2% | 182 ± 2.5 | 115 ± 1.2 | 8.7 ± 0.3 |
| 0.5% | 220 ± 3.4 | 132 ± 2.1 | 5.2 ± 0.2 |
| 0.8% | 256 ± 3.2 | 154 ± 3.4 | 4.04 ± 0.2 |
| 1.1% | 305 ± 4.4 | 206 ± 1.8 | 3.73 ± 0.3 |
| 1.5% | 337 ± 5.2 | 241 ± 2.3 | 1.9 ± 0.1 |
| Alloy | 0 wt.% | 0.2 wt.% | 0.5 wt.% | 0.8 wt.% | 1.1 wt.% |
|---|---|---|---|---|---|
| Ecorr (V) | −0.695 | −0.654 | −0.686 | −0.677 | −0.719 |
| Icorr (A/cm2) | −6.312 | −6.301 | −6.361 | −6.346 | −6.322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.; Zou, L.; Liao, Z.; Lin, Z.; Chen, J. Study on the Properties of Carbon Nanotube (CNTs) Reinforced AlSi10Mg Composites Fabricated by Powder Metallurgy. Materials 2023, 16, 3905. https://doi.org/10.3390/ma16113905
Pan J, Zou L, Liao Z, Lin Z, Chen J. Study on the Properties of Carbon Nanotube (CNTs) Reinforced AlSi10Mg Composites Fabricated by Powder Metallurgy. Materials. 2023; 16(11):3905. https://doi.org/10.3390/ma16113905
Chicago/Turabian StylePan, Jian, Linchi Zou, Zengxiang Liao, Zhijie Lin, and Junfeng Chen. 2023. "Study on the Properties of Carbon Nanotube (CNTs) Reinforced AlSi10Mg Composites Fabricated by Powder Metallurgy" Materials 16, no. 11: 3905. https://doi.org/10.3390/ma16113905
APA StylePan, J., Zou, L., Liao, Z., Lin, Z., & Chen, J. (2023). Study on the Properties of Carbon Nanotube (CNTs) Reinforced AlSi10Mg Composites Fabricated by Powder Metallurgy. Materials, 16(11), 3905. https://doi.org/10.3390/ma16113905






