Chemical Compatibility and Electrochemical Performance of Ba7Ta3.7Mo1.3O20.15 Electrolytes for Solid Oxide Fuel Cells
Abstract
:1. Introduction
2. Experimental
2.1. Material Synthesis
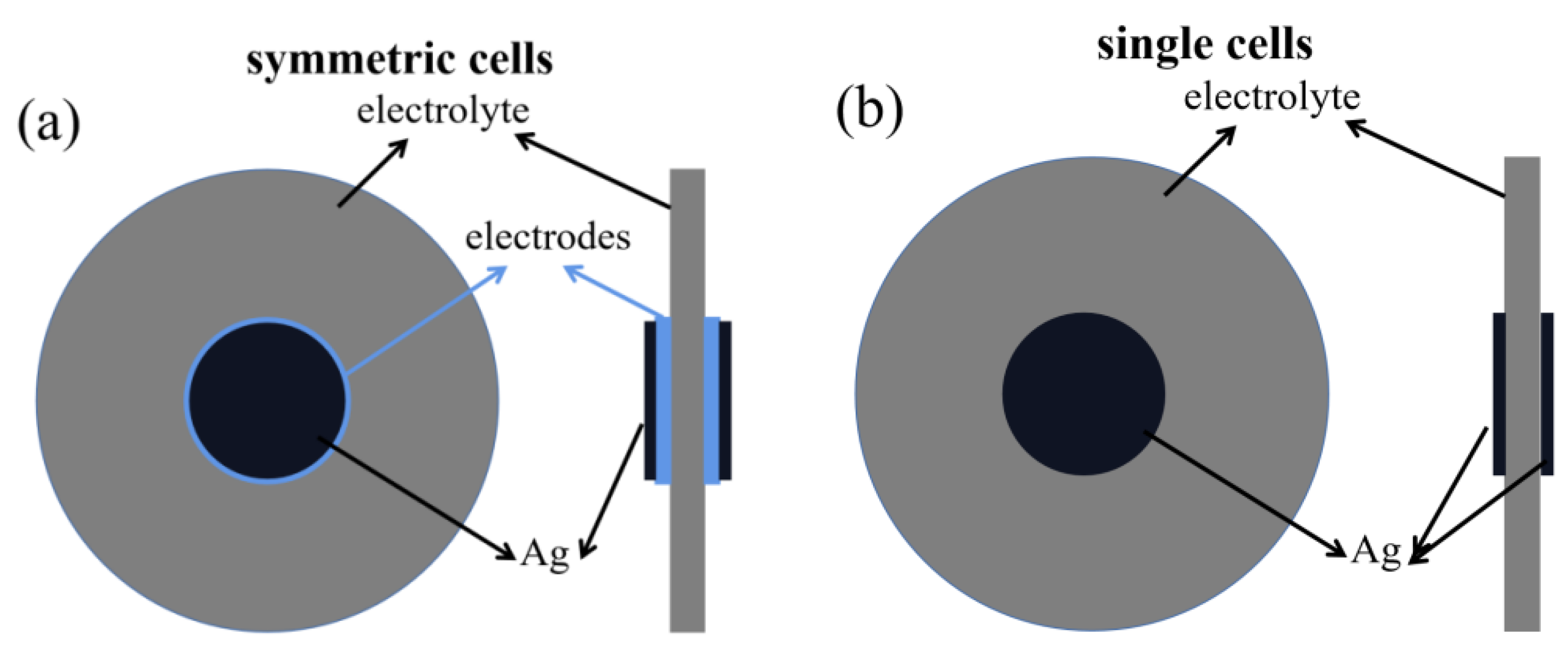
2.2. Preparation of Symmetric Cells and Single Cells
2.3. Characterization
3. Results and Discussion
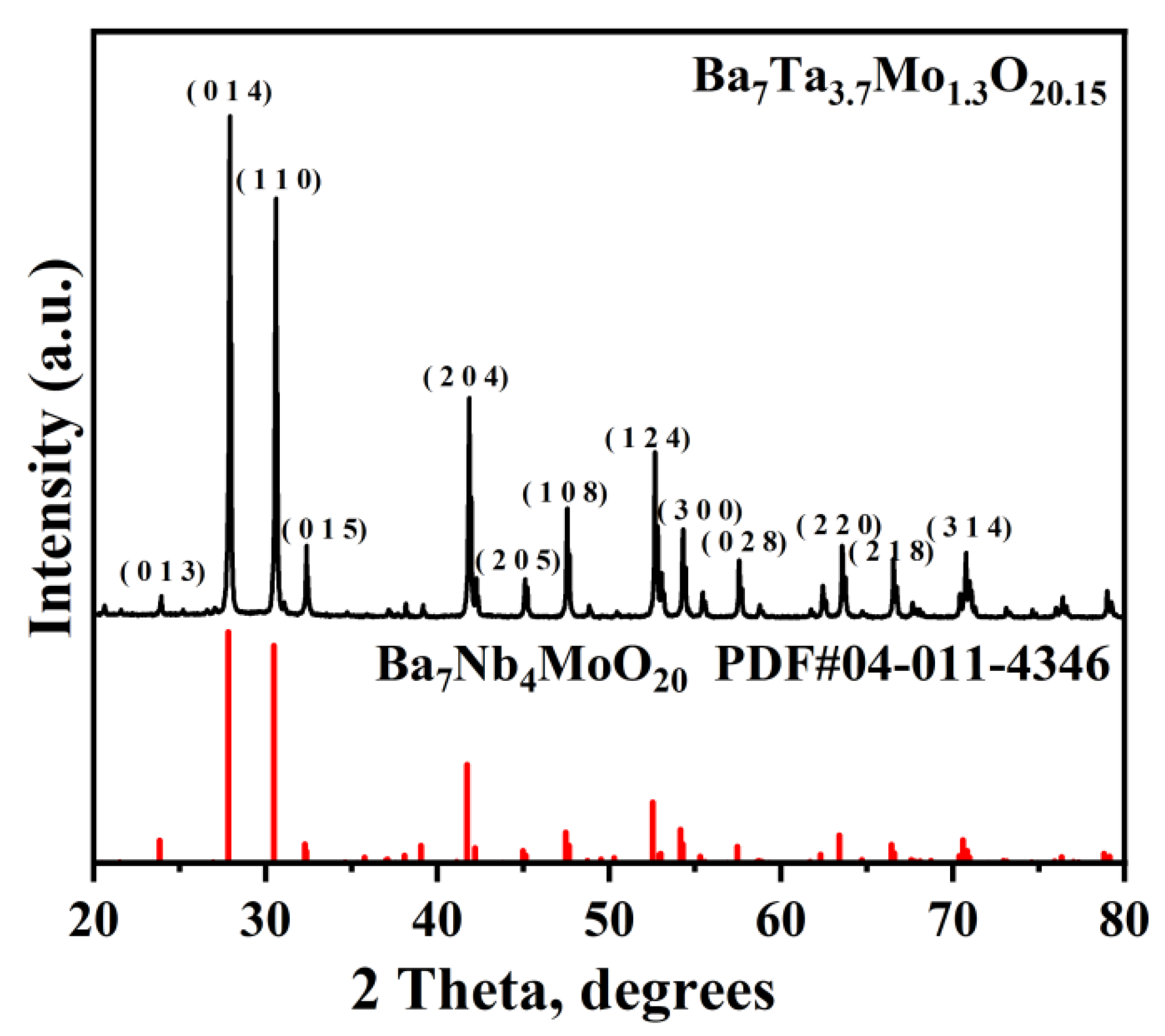
3.1. Characterization of the Ba7Ta3.7Mo1.3O20.15 Electrolyte
3.2. Chemical Compatibility of Ba7Ta3.7Mo1.3O20.15 with Different Electrodes
3.2.1. LSM Electrode
3.2.2. LSC, LSCF, BCFZY Electrodes
3.2.3. NiO, PBM, SFM Anodes
- -
- After the chemical reaction, the BTM phase usually disappeared completely while the electrode phase remained, more or less;
- -
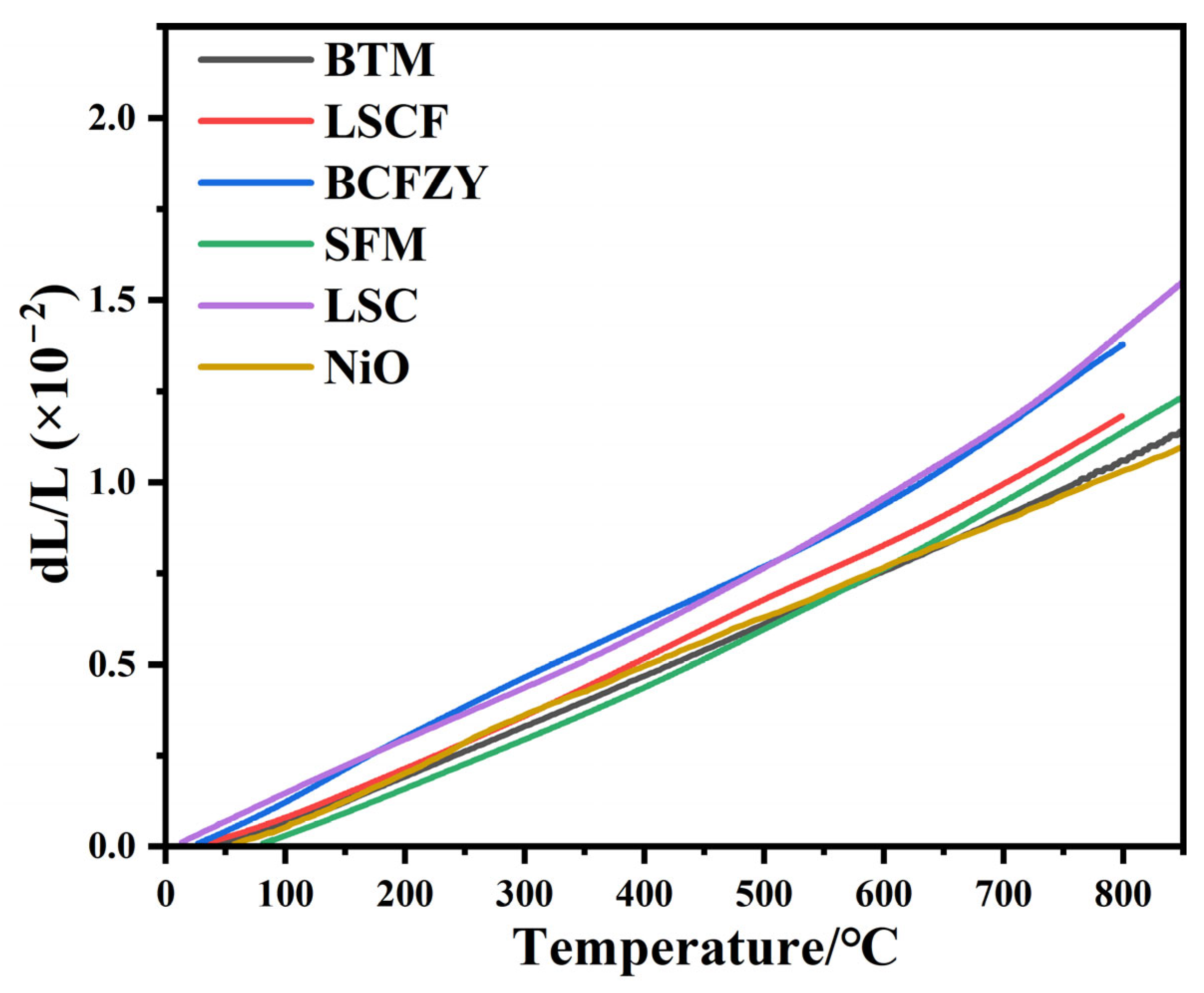
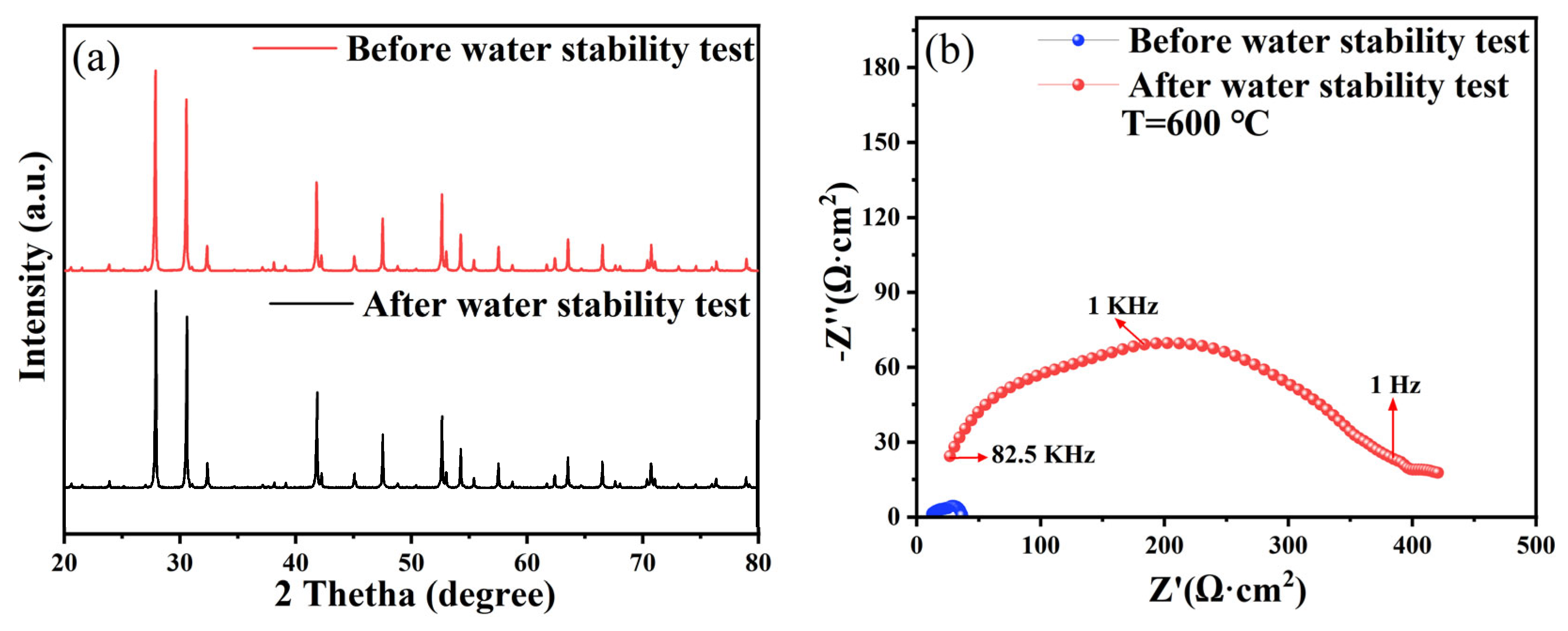
3.3. Chemical Stability Analysis
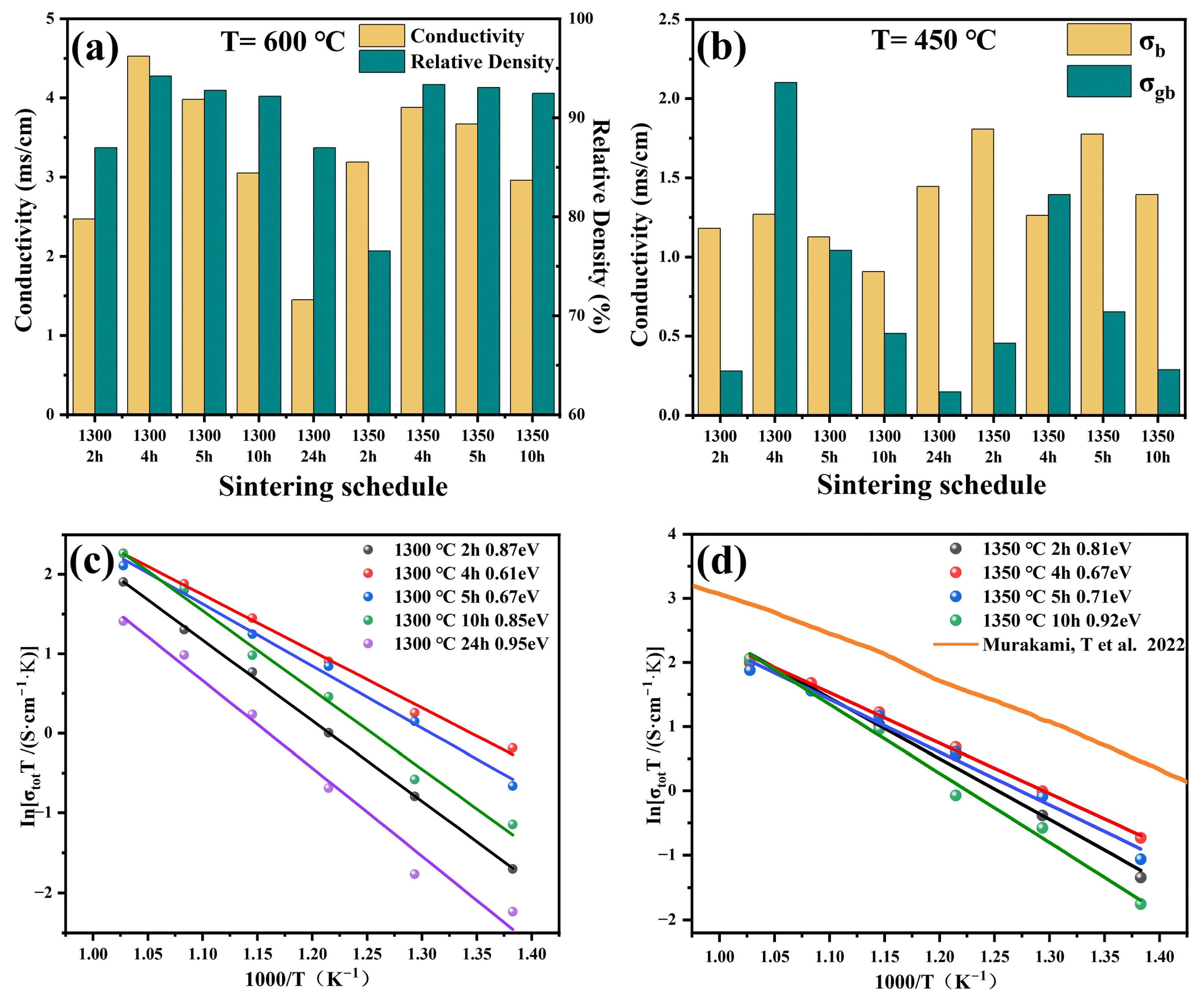
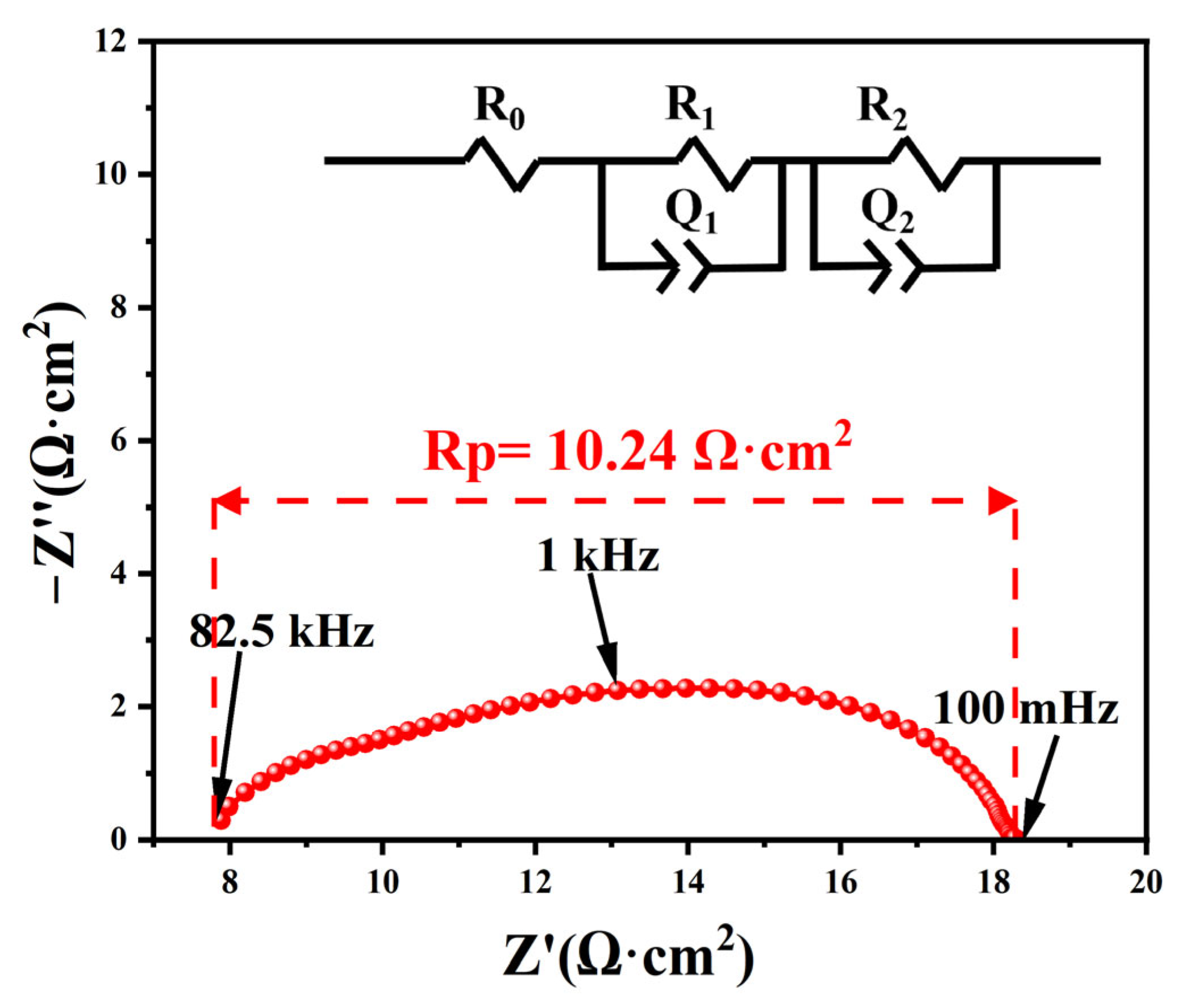
3.4. Electrochemical Impedance Spectroscopy Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Xu, X.; Bi, L. A high-entropy spinel ceramic oxide as the cathode for proton-conducting solid oxide fuel cells. J. Adv. Ceram. 2022, 11, 794–804. [Google Scholar] [CrossRef]
- Han, D.L.; Uda, T. The best composition of an Y-doped BaZrO3 electrolyte: Selection criteria from transport properties, microstructure, and phase behavior. J. Mater. Chem. A 2018, 6, 18571–18582. [Google Scholar]
- Bauerle, J.E. Study of solid electrolyte polarization by a complex admittance method. J. Phys. Chem. Solids 1969, 30, 2657–2670. [Google Scholar] [CrossRef]
- Suemoto, T.; Ishigame, M. Composition dependence of the ionic diffusion coefficients in yttria-stabilized zirconias. Solid State Ion. 1986, 21, 225–229. [Google Scholar] [CrossRef]
- Ge, L.; Guo, K.; Guo, L. Sinterability, reducibility, and electrical conductivity of fast oxide-ion conductors La1.8R0.2MoWO9 (R = Pr, Nd, Gd and Y). Ceram. Int. 2015, 41, 10208–10215. [Google Scholar] [CrossRef]
- Pergolesi, D.; Fabbri, E.; D’Epifanio, A.; Di Bartolomeo, E.; Tebano, A.; Sanna, S.; Licoccia, S.; Balestrino, G.; Traversa, E. High proton conduction in grain-boundary-free yttrium-doped barium zirconate films grown by pulsed laser deposition. Nat. Mater. 2010, 9, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Kreuer, K.D. Proton-conducting oxides. Annu. Rev. Mater. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Bi, L.; Fang, S.M.; Tao, Z.T.; Zhang, S.Q.; Peng, R.R.; Liu, W. Influence of anode pore forming additives on the densification of supported BaCe0.7Ta0.1Y0.2O3−δ electrolyte membranes based on a solid state reaction. J. Eur. Ceram. Soc. 2009, 29, 2567–2573. [Google Scholar] [CrossRef]
- Ge, L.; Jiao, J.; Zhu, Z.; Zhang, Q.; Zheng, Y.; Chen, H.; Guo, L. A facile method to fabricate proton-conducting BaZr0.85Y0.15O3−δ electrolyte with a large grain size and high conductivity. Ceram. Int. 2019, 45, 24946–24952. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Q.; Gu, Y.; Luo, Y.; Ge, L.; Zheng, Y.; Chen, H.; Guo, L. Effect of BaO-B2O3 composite sintering aid on sinterability and electrical property of BaZr0.85Y0.15O3−δ ceramic. Ceram. Int. 2019, 45, 13679–13684. [Google Scholar] [CrossRef]
- Kuang, X.; Green, M.A.; Niu, H.; Zajdel, P.; Dickinson, C.; Claridge, J.B.; Jantsky, L.; Rosseinsky, M.J. Interstitial oxide ion conductivity in the layered tetrahedral network melilite structure. Nat. Mater. 2008, 7, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Marrero-Lopez, D.; Martin-Sedeno, M.C.; Pena-Martinez, J.; Ruiz-Morales, J.C.; Nunez, P.; Aranda, M.A.G.; Ramos-Barrado, J.R. Evaluation of apatite silicates as solid oxide fuel cell electrolytes. J. Power Sources 2010, 195, 2496–2506. [Google Scholar] [CrossRef]
- Shi, H.G.; Su, C.; Ran, R.; Cao, J.F.; Shao, Z.P. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Prog. Nat. Sci. 2020, 30, 764–774. [Google Scholar] [CrossRef]
- Singh, K.; Kannan, R.; Thangadurai, V. Perspective of perovskite-type oxides for proton conducting solid oxide fuel cells. Solid State Ion. 2019, 339, 13. [Google Scholar] [CrossRef]
- Rajendran, S.; Thangavel, N.K.; Alkatie, S.; Ding, Y.; Arava, L.M.R. Y, Gd, and Pr tri-doped perovskite-type proton conducting electrolytes with improved sinterability and chemical stability. J. Alloys Compd. 2021, 870, 7. [Google Scholar] [CrossRef]
- Fukui, T.; Ohara, S.; Murata, K.; Yoshida, H.; Miura, K.; Inagaki, T. Performance of intermediate temperature solid oxide fuel cells with La(Sr)Ga(Mg)O3 electrolyte film. J. Power Sources 2002, 106, 142–145. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takita, Y. Doped LaGaO3 perovskite type oxide as a new oxide ionic conductor. J. Am. Chem. Soc. 1994, 116, 3801–3803. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Ono, K.; Ogaki, K. Proton conduction in sintered oxides based on BaCeO3. J. Electrochem. Soc. 1988, 135, 529–533. [Google Scholar] [CrossRef]
- Wienstroer, S.; Wiemhofer, H.D. Investigation of the influence of zirconium substitution on the properties of neodymium-doped barium cerates. Solid State Ion. 1997, 101, 1113–1117. [Google Scholar] [CrossRef]
- Fop, S.; McCombie, K.S.; Wildman, E.J.; Skakle, J.M.S.; Irvine, J.T.S.; Connor, P.A.; Savaniu, C.; Ritter, C.; McLaughlin, A.C. High oxide ion and proton conductivity in a disordered hexagonal perovskite. Nat. Mater. 2020, 19, 752–757. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Cava, R.J. Hexagonal Perovskites as Quantum Materials. Chem. Rev. 2021, 121, 2935–2965. [Google Scholar] [CrossRef] [PubMed]
- Yashima, M.; Tsujiguchi, T.; Sakuda, Y.; Yasui, Y.; Zhou, Y.; Fujii, K.; Torii, S.; Kamiyama, T.; Skinner, S.J. High oxide-ion conductivity through the interstitial oxygen site in Ba7Nb4MoO20-based hexagonal perovskite related oxides. Nat. Commun. 2021, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Murakami, T.; Fujii, K.; Hester, J.R.; Yasui, Y.; Yashima, M. Simultaneous Reduction of Proton Conductivity and Enhancementof Oxide-Ion Conductivity by Aliovalent Doping in Ba7Nb4MoO20. Inorg. Chem. 2022, 61, 7537–7545. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Shibata, T.; Yasui, Y.; Fujii, K.; Hester, J.R.; Yashima, M. High Oxide-Ion Conductivity in a Hexagonal Perovskite-Related Oxide Ba7Ta3.7Mo1.3O20.15 with Cation Site Preference and Interstitial Oxide Ions. Small 2022, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Yin, Y.R.; Xu, Y.S.; Yu, S.F.; Bi, L. Tailoring Sr2Fe1.5Mo0.5O6−δ with Sc as a new single-phase cathode for proton-conducting solid oxide fuel cells. Sci. China Mater. 2022, 65, 1485–1494. [Google Scholar] [CrossRef]
- Kim, H.; Ida, S.; Ju, Y.W.; Matsuda, J.; Kim, G.; Ishihara, T. Mixing effects of Cr2O3-PrBaMn2O5 for increased redox cycling properties of Fe powder for a solid-oxide Fe-air rechargeable battery. J. Mater. Chem. A 2017, 5, 364–371. [Google Scholar] [CrossRef]
- Wei, K.; Li, N.; Wu, Y.; Song, W.; Wang, X.; Guo, L.; Khan, M.; Wang, S.; Zhou, F.; Ling, Y. Characterization and optimization of highly active and Ba-deficient BaCo0.4Fe0.4Zr0.1Y0.1O3−δ-based cathode materials for protonic ceramics fuel cells. Ceram. Int. 2019, 45, 18583–18591. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, K.; Xu, D.; Gu, Y.; Ni, Q.; Zheng, Y.; Chen, H.; Ge, L.; Huang, X.; Guo, L. Enhancing the performance of symmetrical solid oxide fuel cells with Sr2Fe1.5Mo0.5O6−δ electrodes via infiltration of Pr6O11 bifunctional catalyst. Electrochim. Acta 2022, 402, 139569. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Zheng, Y.; Chen, H.; Ge, L.; Guo, L. PrBaMn2O5+δ with praseodymium oxide nano-catalyst as electrode for symmetrical solid oxide fuel cells. Appl. Catal. B Environ. 2019, 257, 117868. [Google Scholar] [CrossRef]
- Ge, L.; Sun, K.; Gu, Y.; Ni, Q.; Huang, X. Boosting the performance of conventional air electrodes for solid oxide cells by in-situ loading of nano praseodymium oxide. Energy Convers. Manag. 2021, 249, 114873. [Google Scholar] [CrossRef]
- Kostogloudis, G.C.; Ftikos, C. Properties of A-site-deficient La0.6Sr0.4Co0.2Fe0.8O3−δ-based perovskite oxides. Solid State Ion. 1999, 126, 143–151. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.Z.; Khan, K.; Wu, H.D.; Zhang, D.D.; Yang, Y.; Jiang, Y.H.; Lin, B. Enhanced ORR activity of A-site deficiency engineered BaCo0.4Fe0.4Zr0.1Y0.1O3−δ cathode in practical YSZ fuel cells. Int. J. Hydrogen Energy 2021, 46, 5593–5603. [Google Scholar] [CrossRef]
- Hou, M.Y.; Sun, W.; Li, P.F.; Feng, J.; Yang, G.Q.; Qiao, J.S.; Wang, Z.H.; Rooney, D.; Feng, J.S.; Sun, K.N. Investigation into the effect of molybdenum-site substitution on the performance of Sr2Fe1.5Mo0.5O6−δ for intermediate temperature solid oxide fuel cells. J. Power Sources 2014, 272, 759–765. [Google Scholar] [CrossRef]
- Huang, K.Q.; Goodenough, J.B. Oxygen permeation through cobalt-containing perovskites—Surface oxygen exchange vs. lattice oxygen diffusion. J. Electrochem. Soc. 2001, 148, E203–E214. [Google Scholar] [CrossRef]
- Marrero-Lopez, D.; Pena-Martinez, J.; Ruiz-Morales, J.C.; Perez-Coll, D.; Martin-Sedeno, M.C.; Nunez, P. Applicability of La2Mo2−yWyO9 materials as solid electrolyte for SOFCs. Solid State Ion. 2007, 178, 1366–1378. [Google Scholar] [CrossRef]
- Zhu, Z.; Gong, Z.; Qu, P.; Li, Z.; Rasaki, S.A.; Liu, Z.; Wang, P.; Liu, C.; Lao, C.; Chen, Z. Additive manufacturing of thin electrolyte layers via inkjet printing of highly-stable ceramic inks. J. Adv. Ceram. 2021, 10, 279–290. [Google Scholar] [CrossRef]
- Li, P.; Yang, W.; Tian, C.; Zhao, W.; Lu, Z.; Xie, Z.; Wang, C.-A. Electrochemical performance of La2NiO4+δ-Ce0.55La0.45O2−δ as a promising bifunctional oxygen electrode for reversible solid oxide cells. J. Adv. Ceram. 2021, 10, 328–337. [Google Scholar] [CrossRef]
- Ling, Y.; Guo, T.; Guo, Y.; Yang, Y.; Tian, Y.; Wang, X.; Ou, X.; Feng, P. New two-layer Ruddlesden-Popper cathode materials for protonic ceramics fuel cells. J. Adv. Ceram. 2021, 10, 1052–1060. [Google Scholar] [CrossRef]
- Zvonareva, I.A.; Mineev, A.M.; Tarasova, N.A.; Fu, X.-Z.; Medvedev, D.A. High-temperature transport properties of BaSn1−xScxO3−δ ceramic materials as promising electrolytes for protonic ceramic fuel cells. J. Adv. Ceram. 2022, 11, 1131–1143. [Google Scholar] [CrossRef]
- Xiong, D.; Rasaki, S.A.; Li, Y.; Fan, L.; Liu, C.; Chen, Z. Enhanced cathodic activity by tantalum inclusion at B-site of La0.6Sr0.4Co0.4Fe0.6O3 based on structural property tailored via camphor-assisted solid-state reaction. J. Adv. Ceram. 2022, 11, 1330–1342. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, S.; Yin, Y.; Bi, L. Taking advantage of Li-evaporation in LiCoO2 as cathode for proton-conducting solid oxide fuel cells. J. Adv. Ceram. 2022, 11, 1849–1859. [Google Scholar] [CrossRef]
- Luo, J.; Zhao, K.; Zhao, J.; Li, J.; Liu, Y.; Chen, D.; Xu, Q.; Chen, M. Functional ceramic support as an independent catalyst layer for direct liquid fuel solid oxide fuel cells. J. Adv. Ceram. 2023, 12, 474–486. [Google Scholar] [CrossRef]
- Yin, Y.R.; Zhou, Y.B.; Gu, Y.Y.; Bi, L. Successful preparation of BaCo0.5Fe0.5O3−δ cathode oxide by rapidly cooling allowing for high-performance proton-conducting solid oxide fuel cells. J. Adv. Ceram. 2023, 12, 587–597. [Google Scholar] [CrossRef]
- Qiu, P.; Liu, B.; Wu, L.; Qi, H.; Tu, B.; Li, J.; Jia, L. K-doped BaCo0.4Fe0.4Zr0.2O3−δ as a promising cathode material for protonic ceramic fuel cells. J. Adv. Ceram. 2022, 11, 1988–2000. [Google Scholar] [CrossRef]
- Li, Z.; Yang, B.; Qian, B.; Wang, S.; Zheng, Y.; Ge, L.; Chen, H. Evaluation of Fe-doped Pr1.8Ba0.2NiO4 as a high-performance air electrode for reversible solid oxide cell. Sep. Purif. Technol. 2023, 308, 123002. [Google Scholar] [CrossRef]

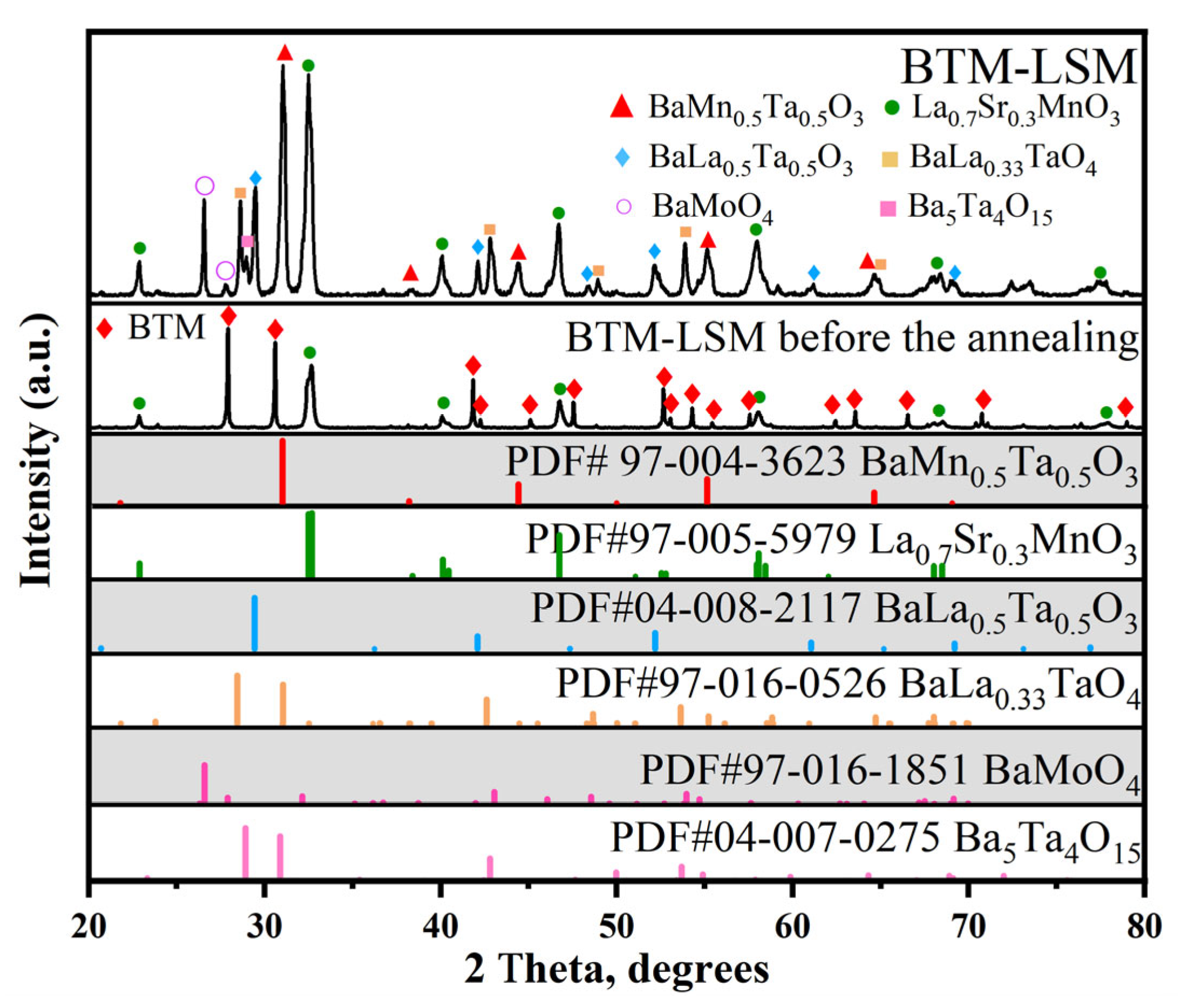
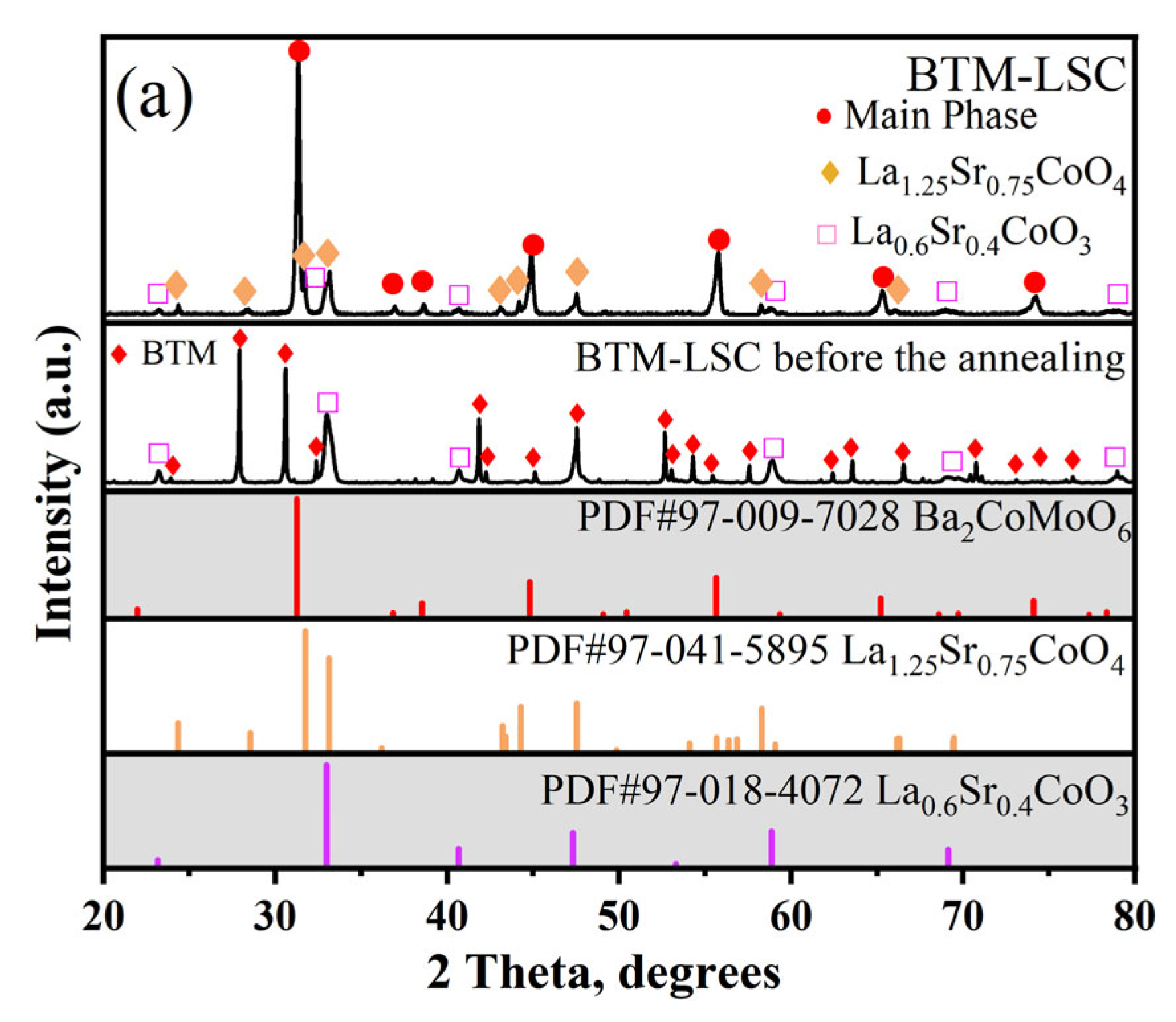
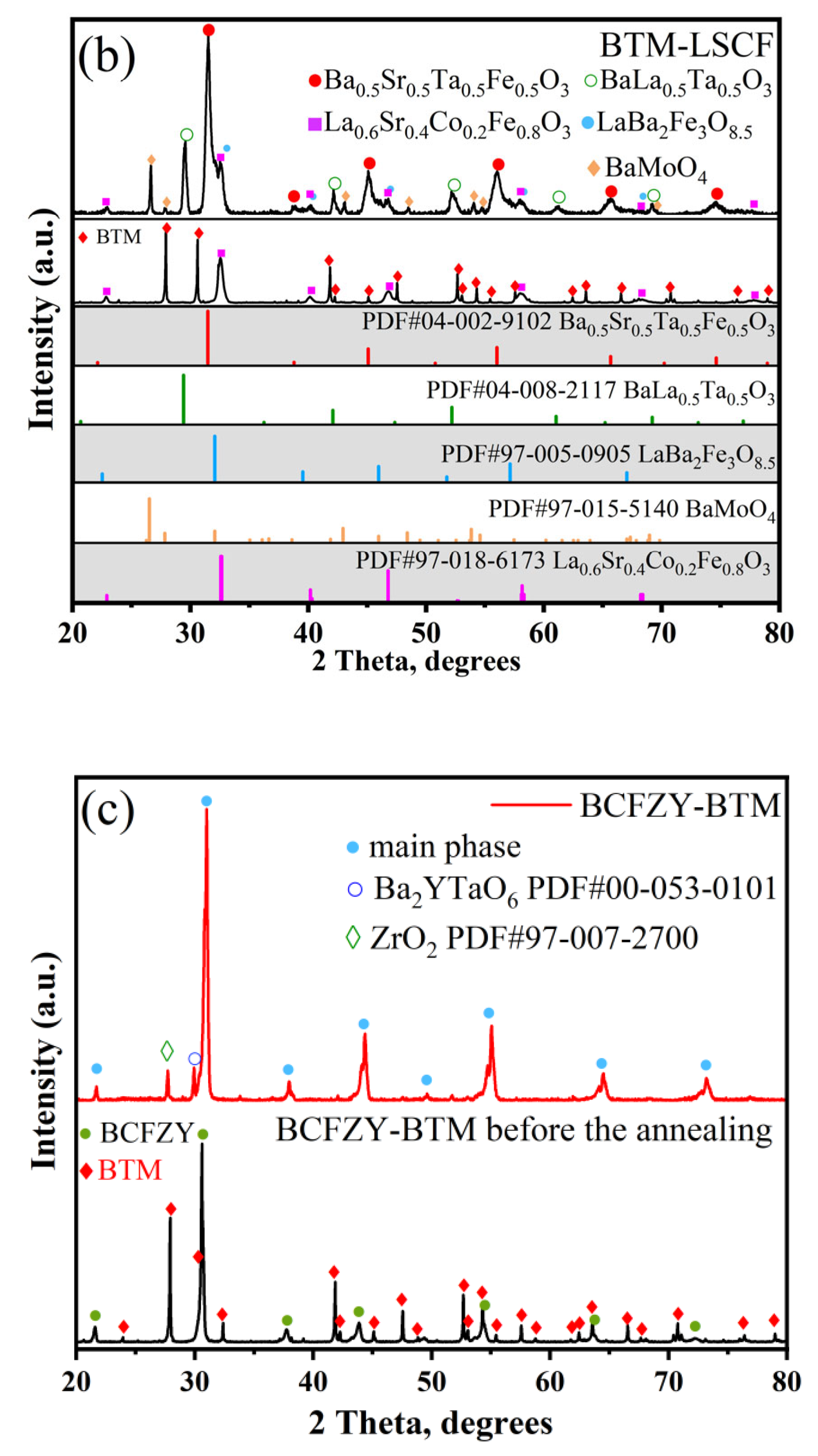
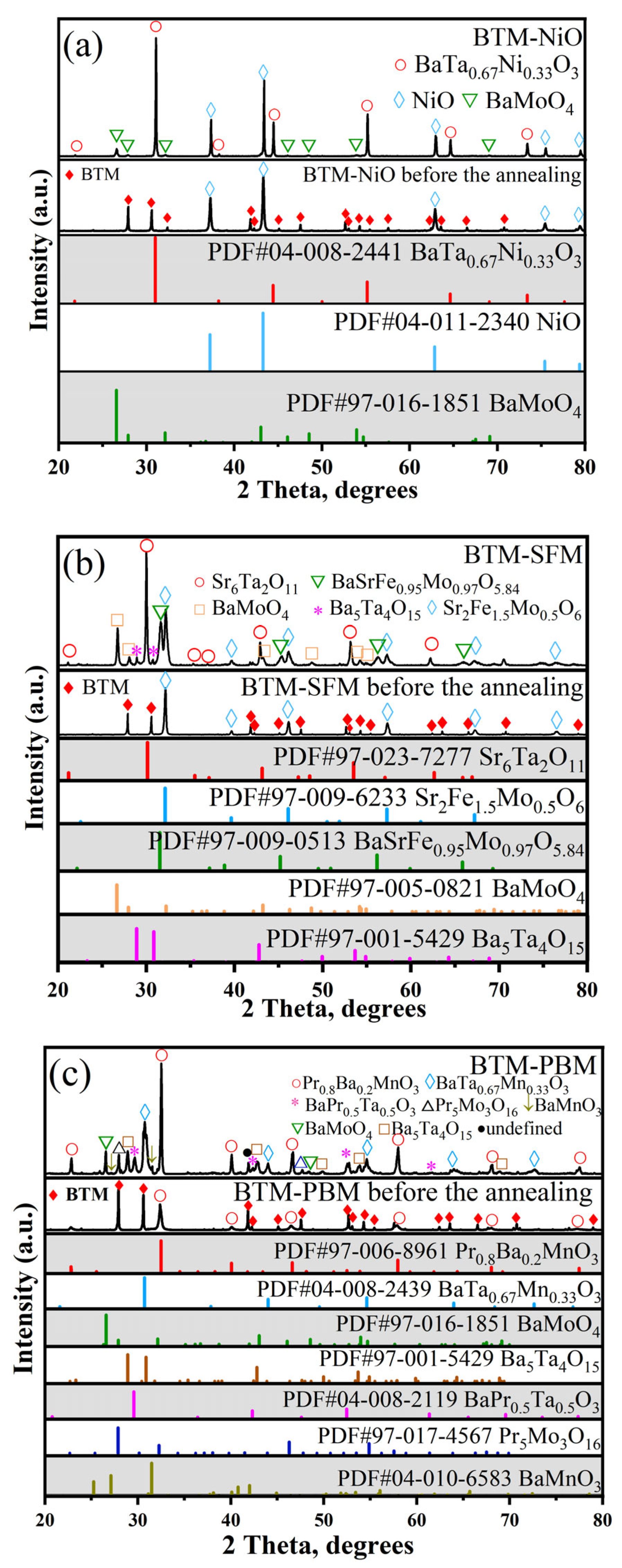
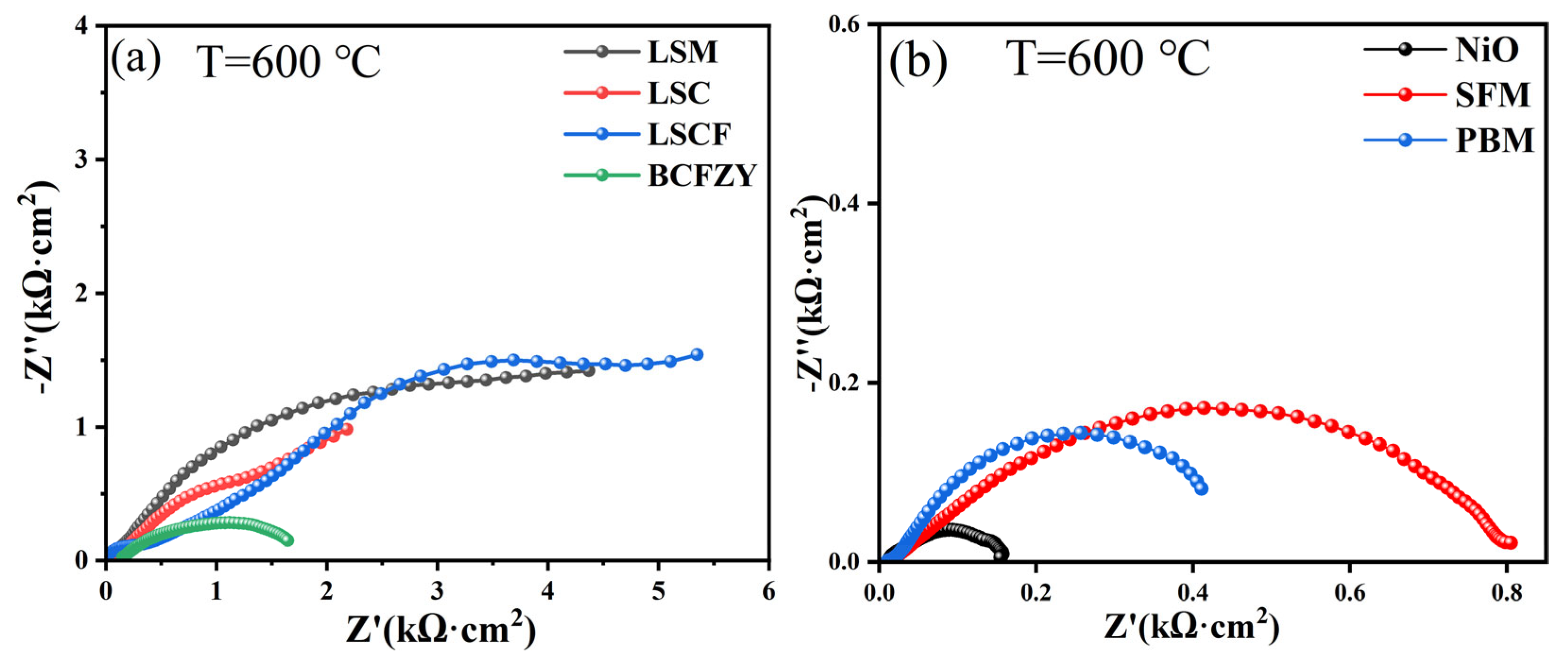
| Electrode | LSM | LSC | LSCF | BCFZY | NiO | SFM | PBM |
|---|---|---|---|---|---|---|---|
| Polarization impedance (kΩ·cm−2) | >4.26 | >2.15 | >5.31 | ~1.49 | ~0.14 | ~0.78 | ~0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Zhou, X.; Li, Y.; Yu, X.; Yu, Z.; Shi, B.; Mi, Y.; Wu, B.; Ge, L. Chemical Compatibility and Electrochemical Performance of Ba7Ta3.7Mo1.3O20.15 Electrolytes for Solid Oxide Fuel Cells. Materials 2023, 16, 3919. https://doi.org/10.3390/ma16113919
Xu D, Zhou X, Li Y, Yu X, Yu Z, Shi B, Mi Y, Wu B, Ge L. Chemical Compatibility and Electrochemical Performance of Ba7Ta3.7Mo1.3O20.15 Electrolytes for Solid Oxide Fuel Cells. Materials. 2023; 16(11):3919. https://doi.org/10.3390/ma16113919
Chicago/Turabian StyleXu, Dong, Xingkai Zhou, Yu Li, Xiaole Yu, Zhexiang Yu, Bochang Shi, Yaowei Mi, Bangze Wu, and Lin Ge. 2023. "Chemical Compatibility and Electrochemical Performance of Ba7Ta3.7Mo1.3O20.15 Electrolytes for Solid Oxide Fuel Cells" Materials 16, no. 11: 3919. https://doi.org/10.3390/ma16113919
APA StyleXu, D., Zhou, X., Li, Y., Yu, X., Yu, Z., Shi, B., Mi, Y., Wu, B., & Ge, L. (2023). Chemical Compatibility and Electrochemical Performance of Ba7Ta3.7Mo1.3O20.15 Electrolytes for Solid Oxide Fuel Cells. Materials, 16(11), 3919. https://doi.org/10.3390/ma16113919





