Performance Study of Diamond Powder-Filled Sodium Silicate-Based Thermal Conductive Adhesives
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Equipment
2.2. Diamond Powder Coupling Treatment
- (1)
- Hydrolysis of organosilanes to silanol:
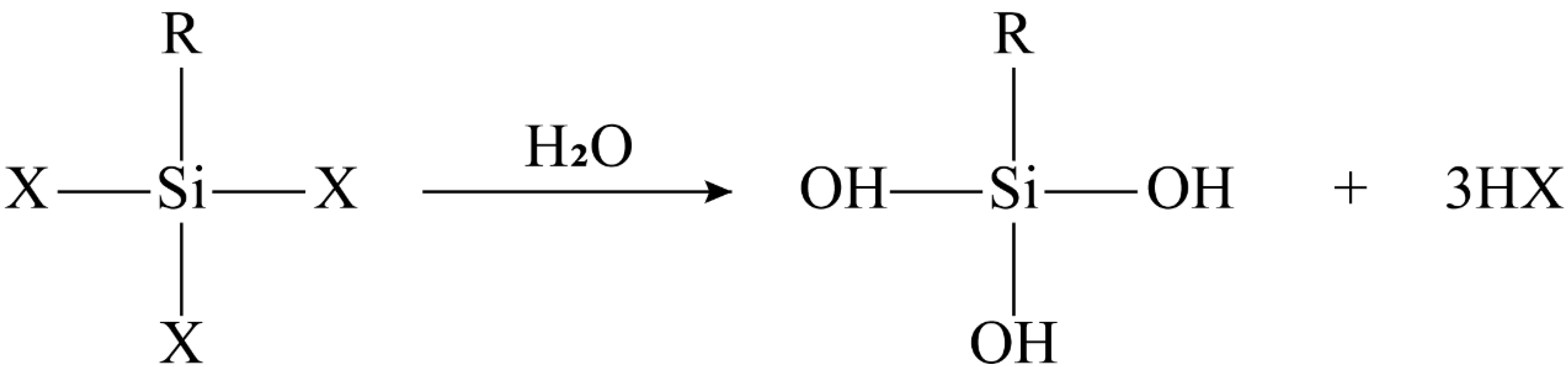
- (2)
- The silanol is bonded to the diamond surface: first, the silanol is hydrogen bonded to the diamond surface:

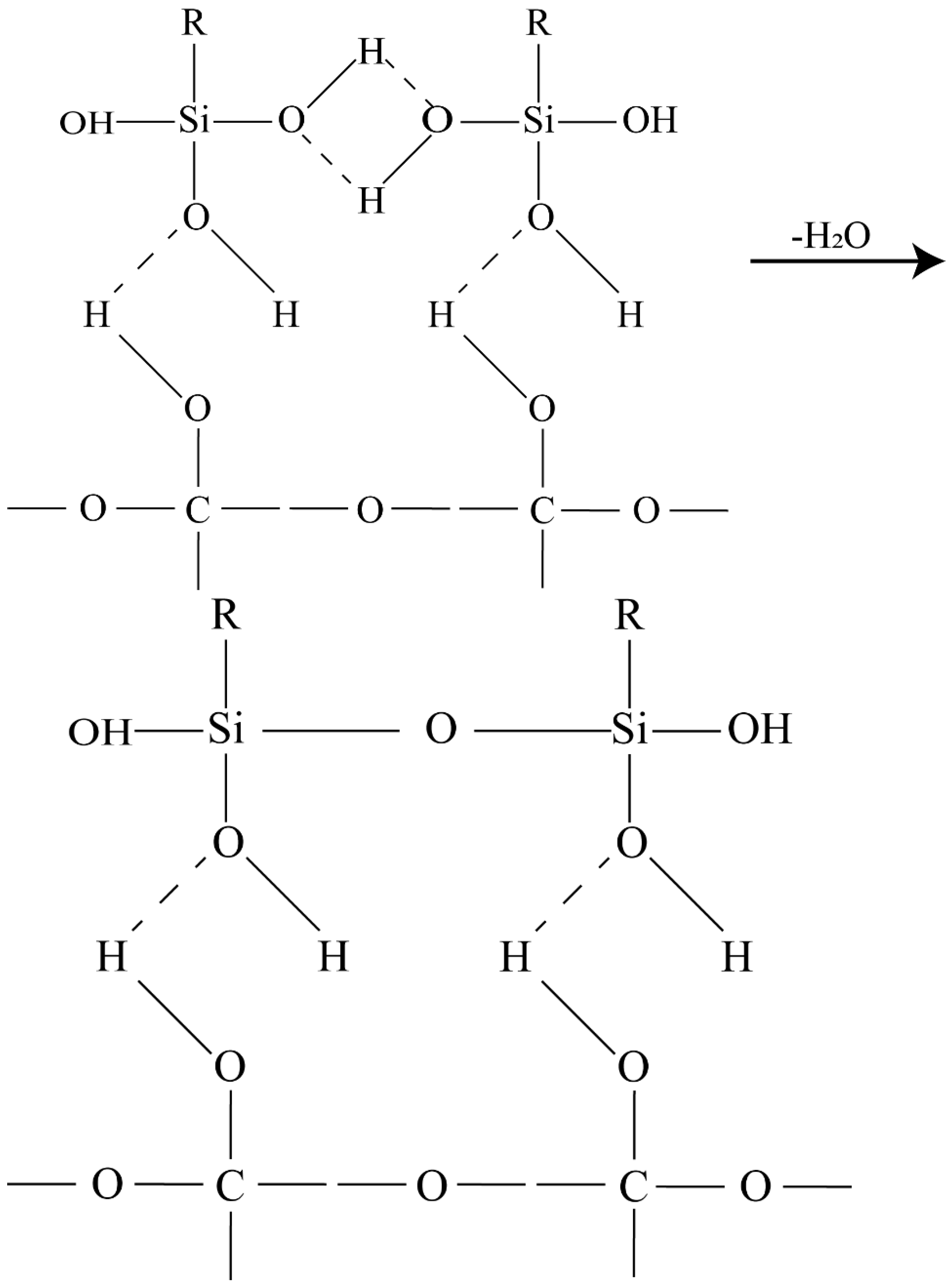
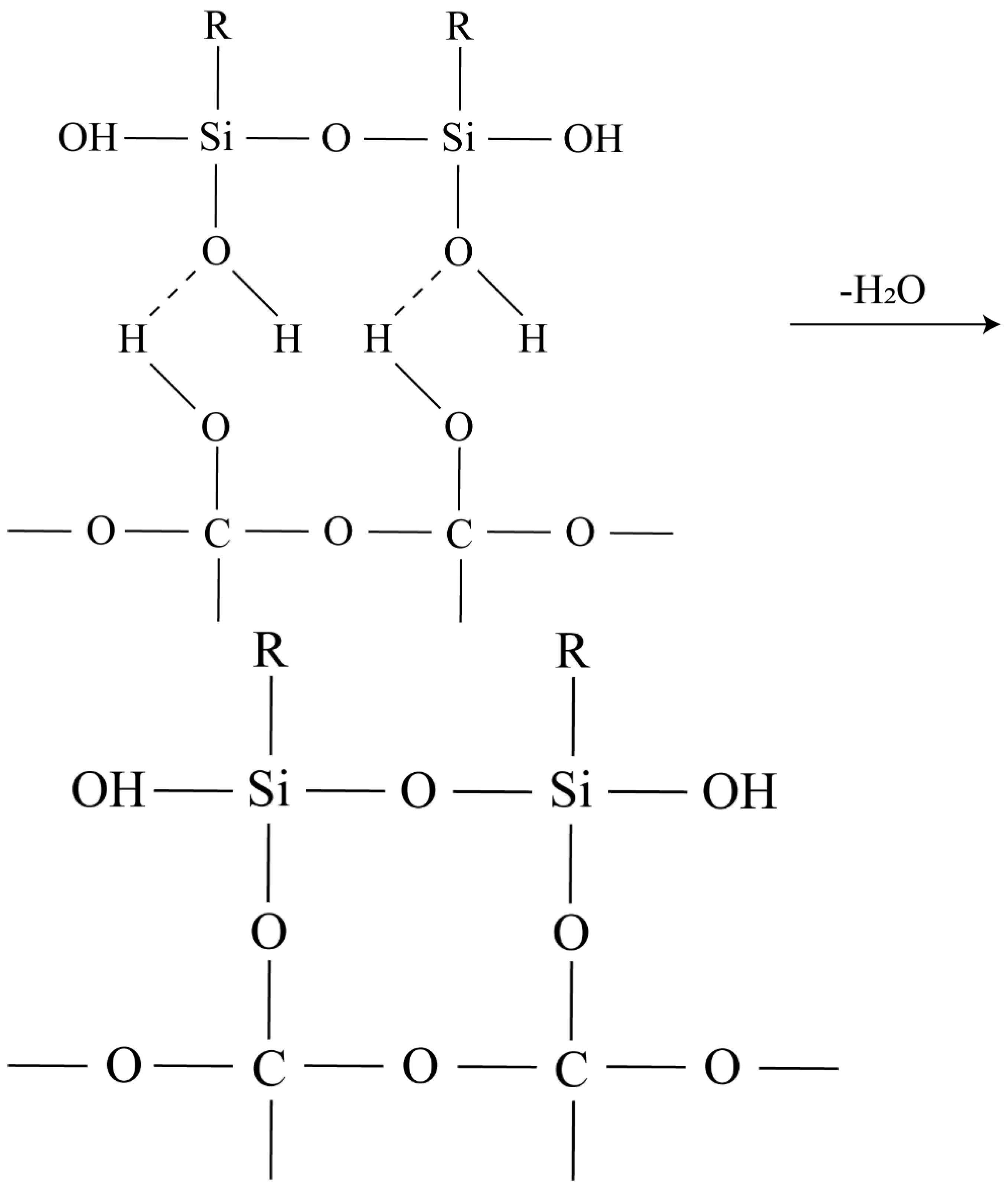
- (1)
- Weigh 1 g of diamond powder with a balance and measure 30 mL of anhydrous ethanol with a measuring cylinder, mix, add to a three-necked flask, and stir the sample at room temperature to disperse the diamond powder evenly in the anhydrous ethanol.
- (2)
- Diamond micro powder is measured according to the solid–liquid ratio of 5:1 mL/g with the silane coupling agent, and the silane coupling agent is mixed with water according to the ratio of 1:2.
- (3)
- Pour the mixed solution of silane coupling agent and water into the mixed solution of diamond micro powder and anhydrous ethanol, and stir thoroughly to mix it well.
- (4)
- Condensation reflux reaction in a constant temperature magnetic stirrer at 70 °C for 3 h.
- (5)
- After the reaction is completed, the reaction is repeatedly washed with anhydrous ethanol and filtered by extraction until the filtrate is clear and transparent, and the filter cake is placed in a vacuum drying oven and dried at 70 °C to obtain modified diamond particles.
2.3. Preparation of Sodium Silicate-Based Diamond Thermal Conductive Adhesive Specimens
- (1)
- The disc-shaped graphite mold is coated with liquid paraffin and placed in a blast drying oven for 3 h at 80 °C.
- (2)
- Prepare colloids with different filling contents using diamond powder as filler as shown in Table 1. Stirred magnetically for 1 h, poured into the treated disc mold, then placed in a drying oven and cured in the oven at a temperature of 50 °C for 1 h, cooled and demolded.
2.4. Performance Testing and Characterization
3. Results and Discussion
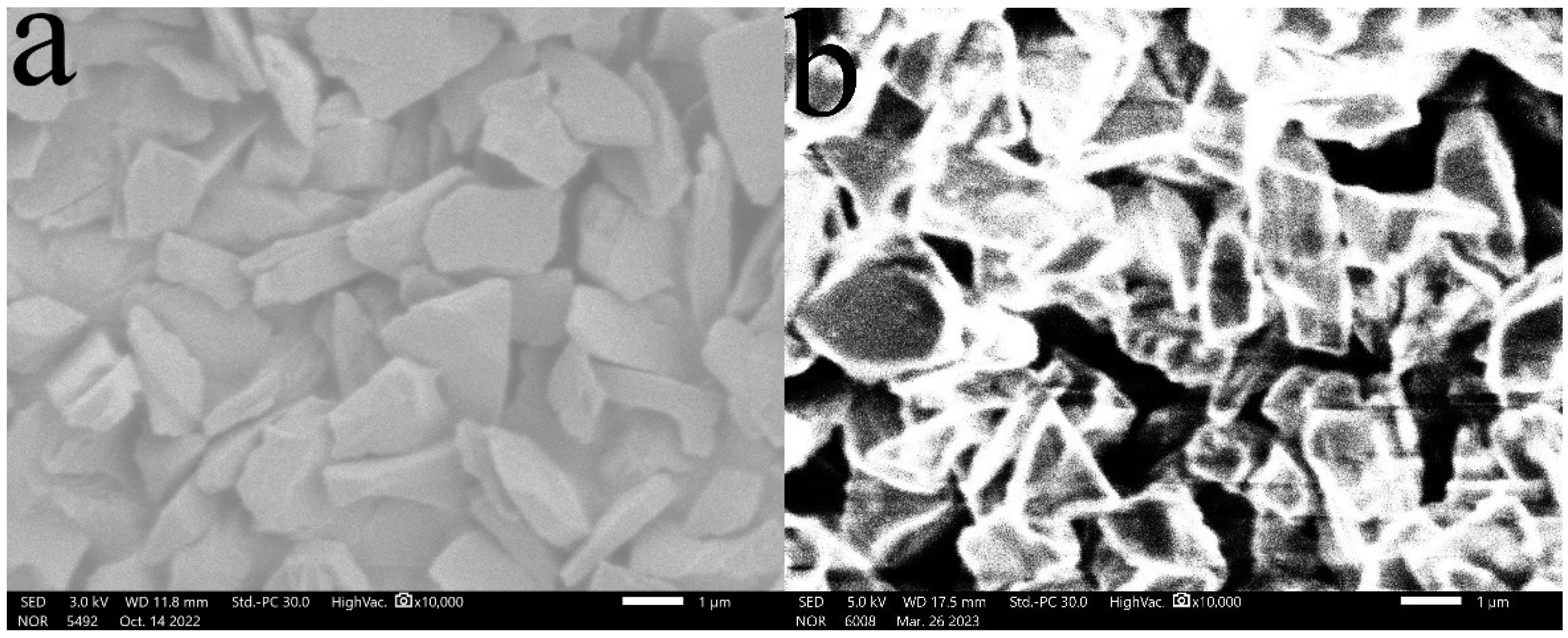
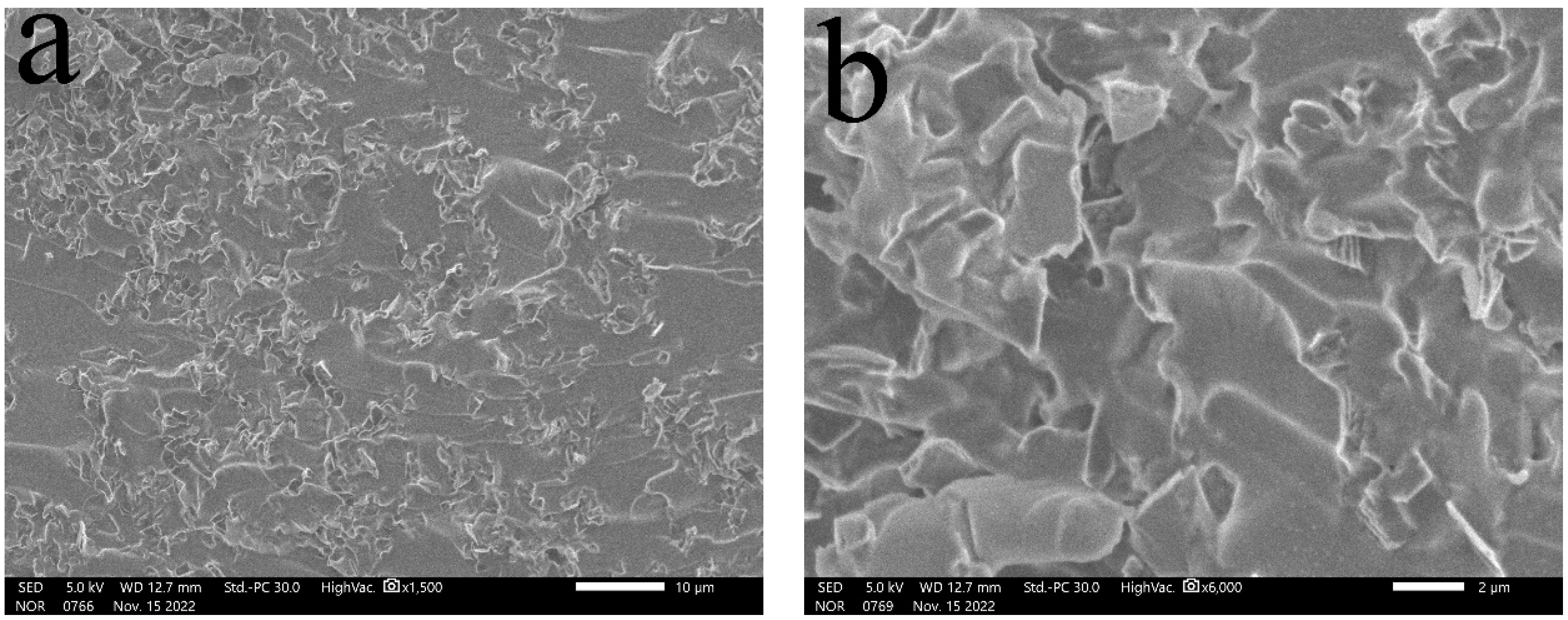
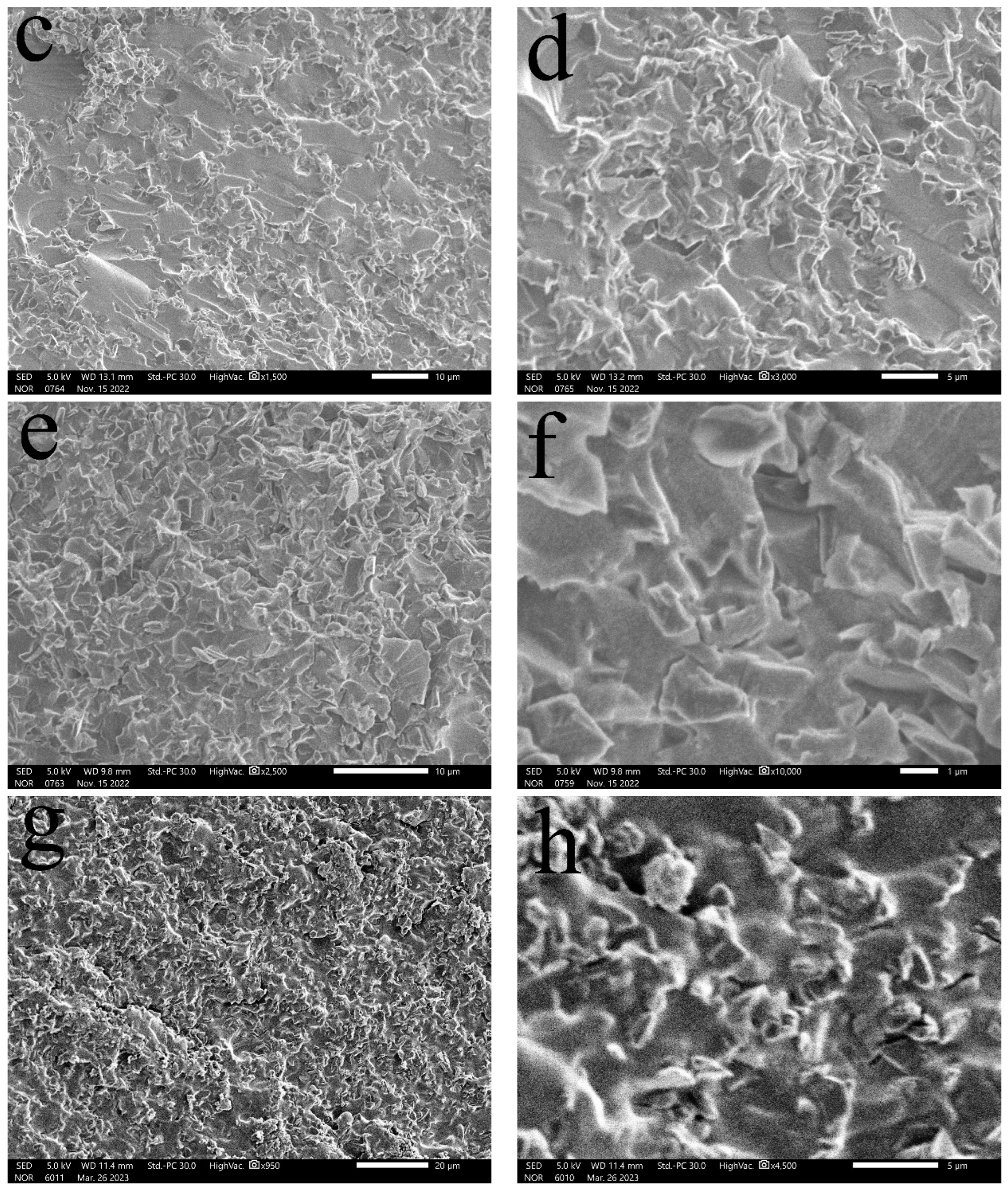
3.1. SEM Characterization and Analysis
- (1)
- Surface morphology of diamond powder before and after surface modification
- (2)
- Distribution of diamond powder with different content in the matrix
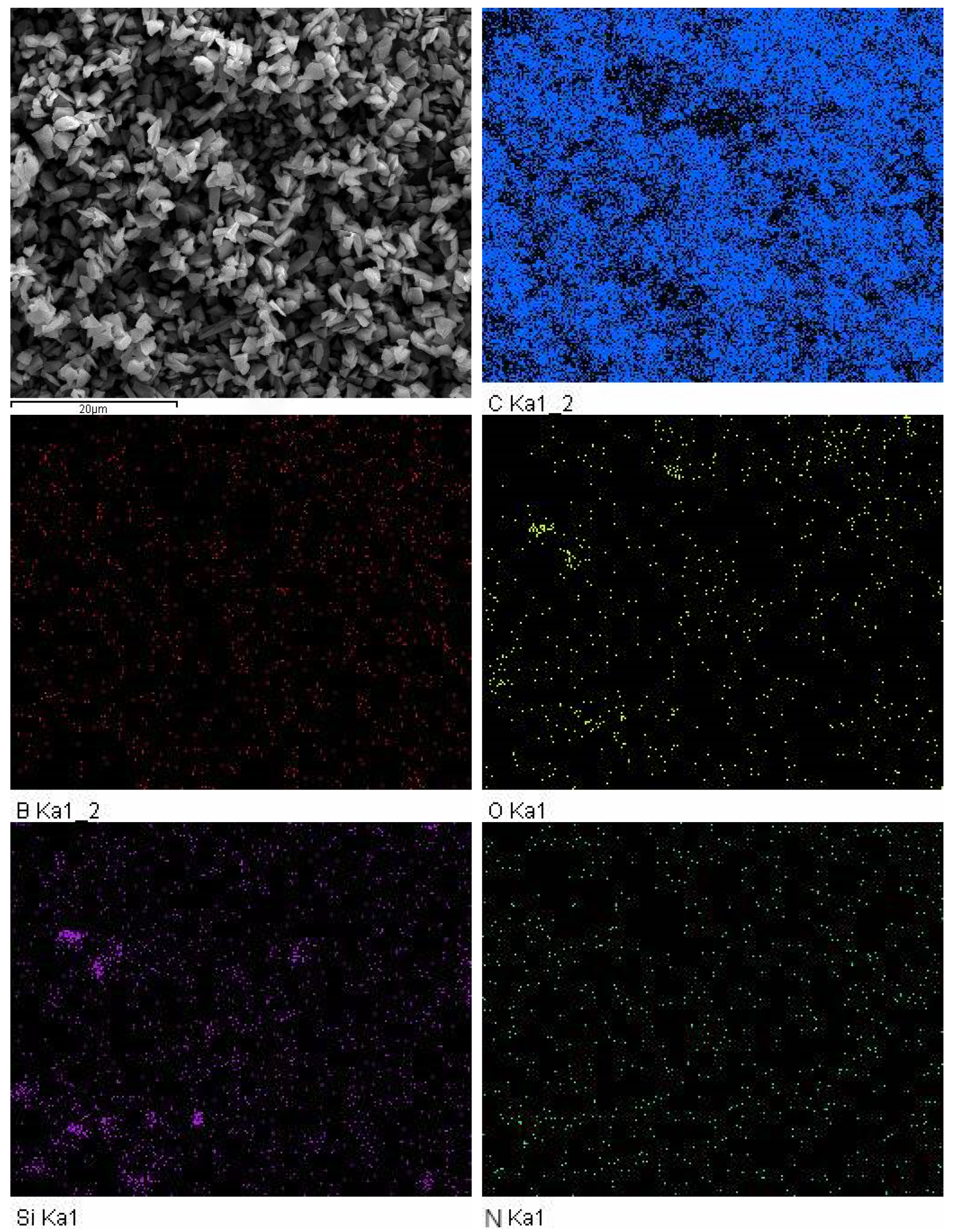
3.2. EDS Characterization and Analysis
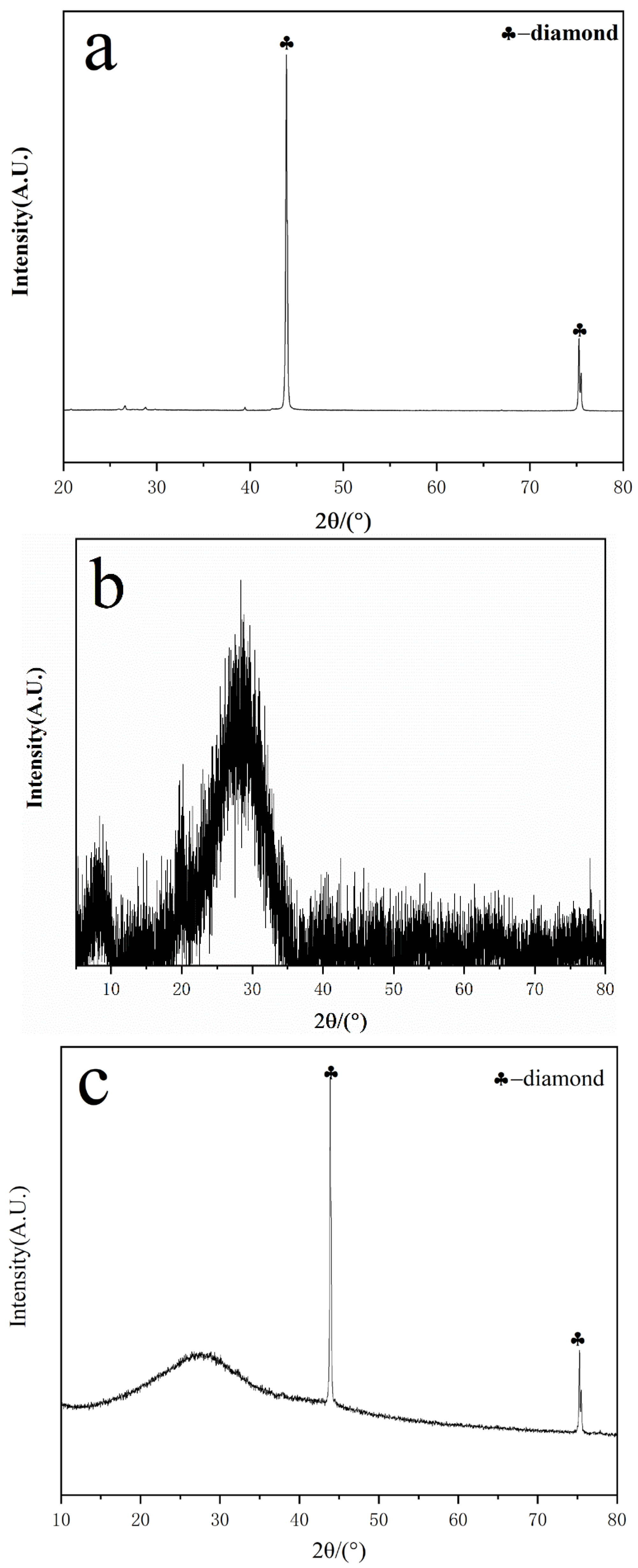
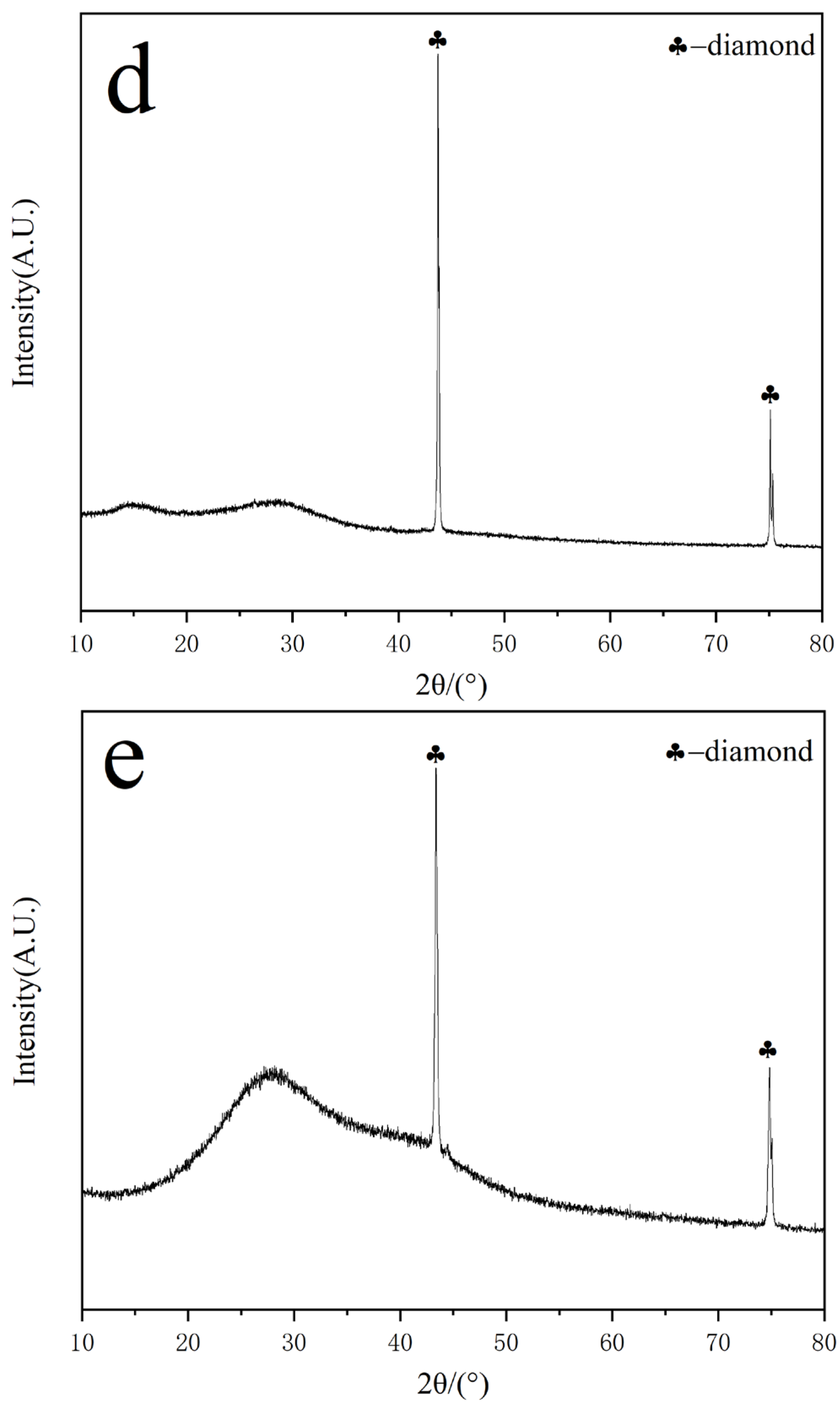
3.3. XRD Characterization and Analysis
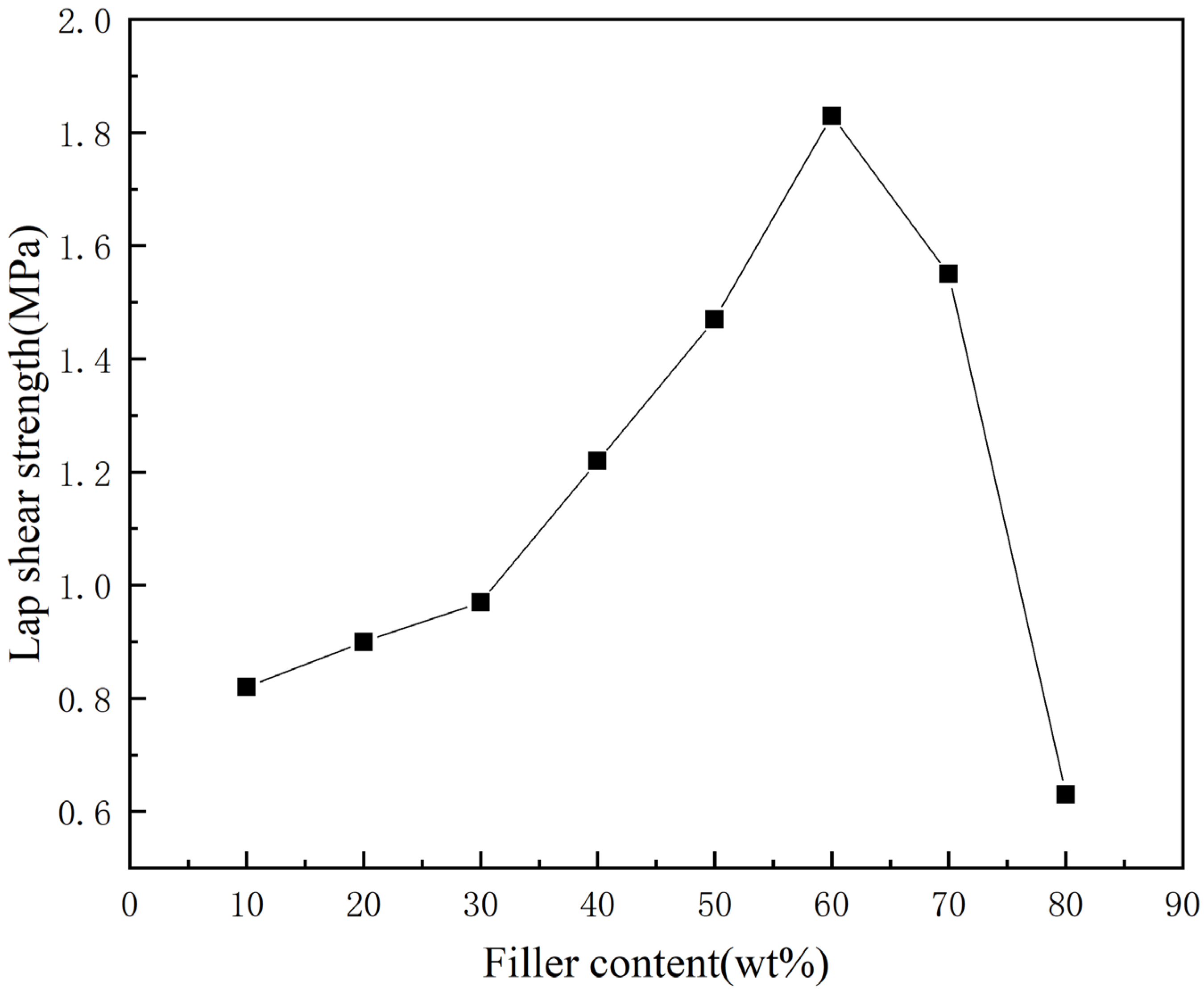
3.4. Characterization and Analysis of Bonding Properties
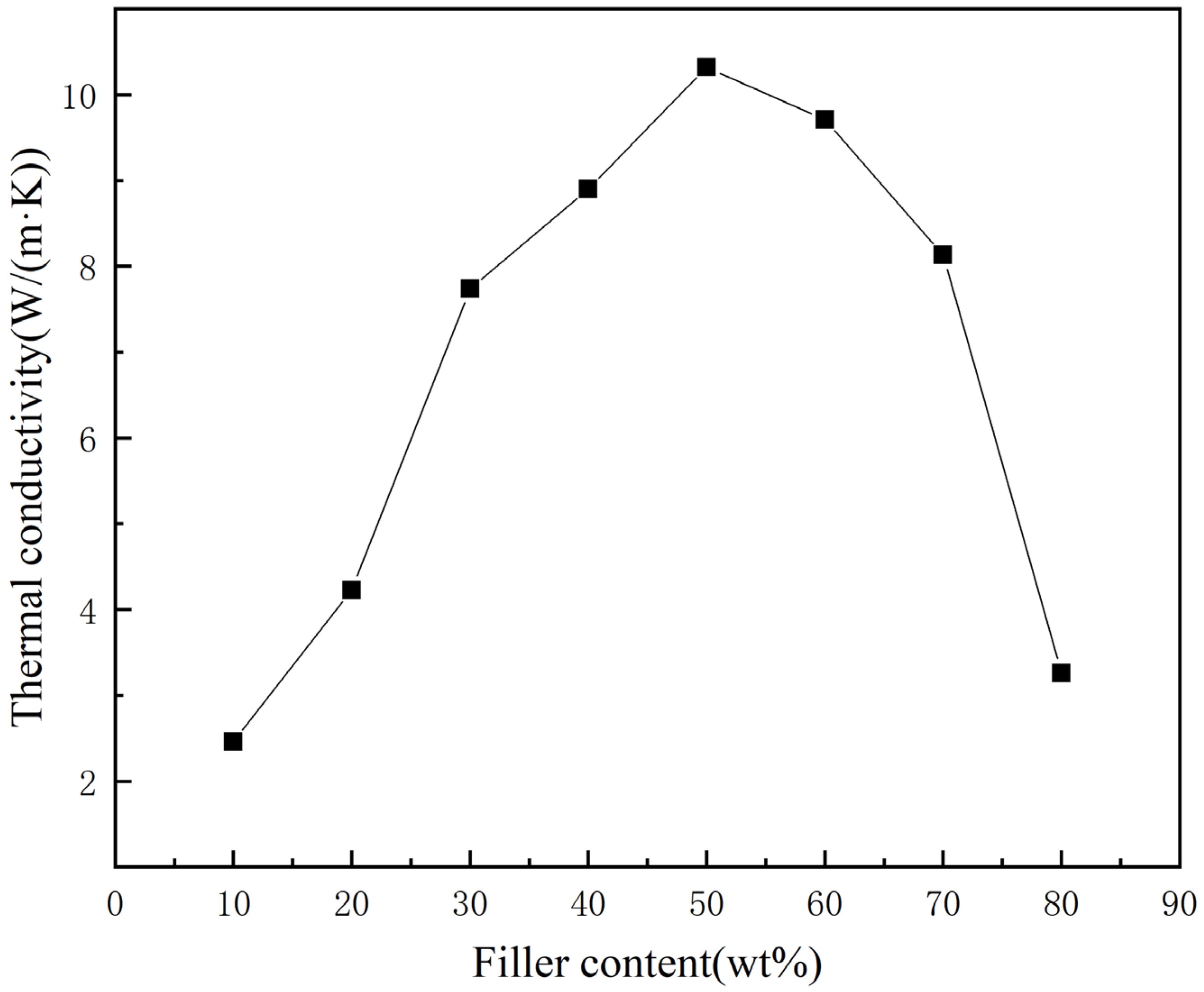
3.5. Characterization and Analysis of Thermal Conductivity
4. Conclusions
- (1)
- At low filler levels, the bonding performance of thermal conductive adhesives can be effectively improved with the increase of diamond content. After that, with the increase of diamond content, the bonding performance of thermal conductive adhesive starts to decrease, especially when the mass fraction of diamond is 70–80%, the bonding performance of thermal conductive adhesive decreases sharply.
- (2)
- At low filling levels, the thermal conductivity of the thermal conductive adhesive gradually increases as the diamond content increases, and when the diamond mass fraction reaches 20–30%, the thermal conductivity increases rapidly, and the thermal conductivity reaches its highest value of 10.32 W/(m·K) at 50% diamond mass fraction. After that, the thermal conductivity of the thermal conductive adhesive starts to decrease as the diamond content increases.
- (3)
- The suitable diamond mass fraction for this thermal conductive adhesive is 50–60%. Within this range, there is an optimum value for the thermal conductivity and adhesive performance of the adhesive, and the diamond content can be adjusted within this range according to the different requirements for thermal conductivity and adhesive performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, S. Preparation and Properties of Diamond-Reinforced Polymer Based Thermal Conductivity Composites. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2020. [Google Scholar]
- Li, Y. Research on Sodium Silicate Based Thermal Conductive Adhesive for LED Packaging. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2011. [Google Scholar]
- Meng, Q.; Han, S.; Araby, S.; Zhao, Y.; Liu, Z.; Lu, S. Mechanically robust, electrically and thermally conductive graphene-based epoxy adhesives. J. Adhes. Sci. Technol. 2019, 33, 1337–1356. [Google Scholar] [CrossRef]
- Aradhana, A.; Mohanty, S.; Nayak, S.N. Synergistic effect of polypyrrole and reduced graphene oxide on mechanical, electrical and thermal properties of epoxy adhesives. Polymer 2019, 166, 215–228. [Google Scholar] [CrossRef]
- Kumar, R.; Mohanty, S.; Nayak, S.K. Thermal conductive epoxy adhesive composites filled with carbon-based particulate fillers: A comparative study. J. Adhes. Sci. Technol. 2020, 34, 807–827. [Google Scholar] [CrossRef]
- Xiong, W. Study on Preparation and Properties of Epoxy Resin Composite Insulation Adhesive with High Thermal Conductivity. Ph.D. Thesis, Beijing Jiaotong University, Beijing, China, 2020. [Google Scholar]
- Guan, Y. Preparation and Properties of H-BN Powder Thermal Conductive Adhesive Containing Multi-Particle Size. Ph.D. Thesis, Nanjing University of Technology, Nanjing, China, 2017. [Google Scholar]
- Gojny, F.H.; Wichmann, M.H.G.; Fiedler, B.; Kinloch, I.A.; Bauhofer, W.; Windle, A.H.; Shulte, K. Evaluation and identification of electrical and thermal conduction mechanisms in carbon nanotube/epoxy composites. Polymer 2006, 47, 2036–2045. [Google Scholar] [CrossRef]
- Nithikarnjanatharn, J.; Ueda, H.; Tanoue, S.; Uematsu, H.; Iemoto, Y. The rheological behavior and thermal conductivity of melt-compounded polycarbonate/vapor-grown carbon fibercomposites. Polym. J. 2012, 44, 427–432. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.D.; Nam, D.G.; Bae, J.-S.; Yeum, J.H.; Oh, W. Thermal Properties of Epoxy Composites with Silicon Carbide and/or Graphite. J. Korean Phys. Soc. 2016, 68, 551–556. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Z.; Luo, D. Preparation of epoxy resin/diamond thermal conductive adhesive for LED packaging. Plast. Sci. Technol. 2015, 43, 59–62. [Google Scholar]
- Guo, H.; Sheng, H.; Peng, X.; Yu, X.; Naito, K.; Qu, X.; Zhang, Q. Preparation and mechanical properties of epoxy/diamond nanocomposites. Polym. Compos. 2014, 35, 2144–2149. [Google Scholar] [CrossRef]
- Ghim, D.; Kim, J.H. Fabrication of acrylic copolymer with aluminum nitride fillers and its physical and thermal properties. Korean J. Chem. Eng. 2017, 34, 245–248. [Google Scholar] [CrossRef]
- Gao, B. Preparation and Mechanical Properties of Polycarbonate Composite Resin Reinforced with Diamond Powder. Ph.D. Thesis, Beijing Institute of Technology, Beijing, China, 2015. [Google Scholar]
- Zhao, Y. Preparation and Properties of Phosphate-Based Inorganic Thermal Conductive Adhesive. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2021. [Google Scholar]
- GB/T 7124-2008; Adhesives—Determination of Tensile Lap-Shear Strength of Rigid-to-RIGID bonded Assemblies. China Petroleum and Chemical Industry Association: Beijing, China, 2008.
| Diamond Grain Size (μm) | Diamond Powder as a Percentage of Overall Dry Weight (%) | ||||
|---|---|---|---|---|---|
| 1 | 10 | 20 | 30 | 40 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Zhou, Z.; Wang, X.; Zhao, Y.; Zhou, Y. Performance Study of Diamond Powder-Filled Sodium Silicate-Based Thermal Conductive Adhesives. Materials 2023, 16, 3937. https://doi.org/10.3390/ma16113937
Chen M, Zhou Z, Wang X, Zhao Y, Zhou Y. Performance Study of Diamond Powder-Filled Sodium Silicate-Based Thermal Conductive Adhesives. Materials. 2023; 16(11):3937. https://doi.org/10.3390/ma16113937
Chicago/Turabian StyleChen, Ming, Zhihao Zhou, Xu Wang, Yangchun Zhao, and Yongmin Zhou. 2023. "Performance Study of Diamond Powder-Filled Sodium Silicate-Based Thermal Conductive Adhesives" Materials 16, no. 11: 3937. https://doi.org/10.3390/ma16113937
APA StyleChen, M., Zhou, Z., Wang, X., Zhao, Y., & Zhou, Y. (2023). Performance Study of Diamond Powder-Filled Sodium Silicate-Based Thermal Conductive Adhesives. Materials, 16(11), 3937. https://doi.org/10.3390/ma16113937






