Effects of ATP on the Physicochemical Properties and Cytocompatibility of Calcium Sulfate/Calcium Citrate Composite Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
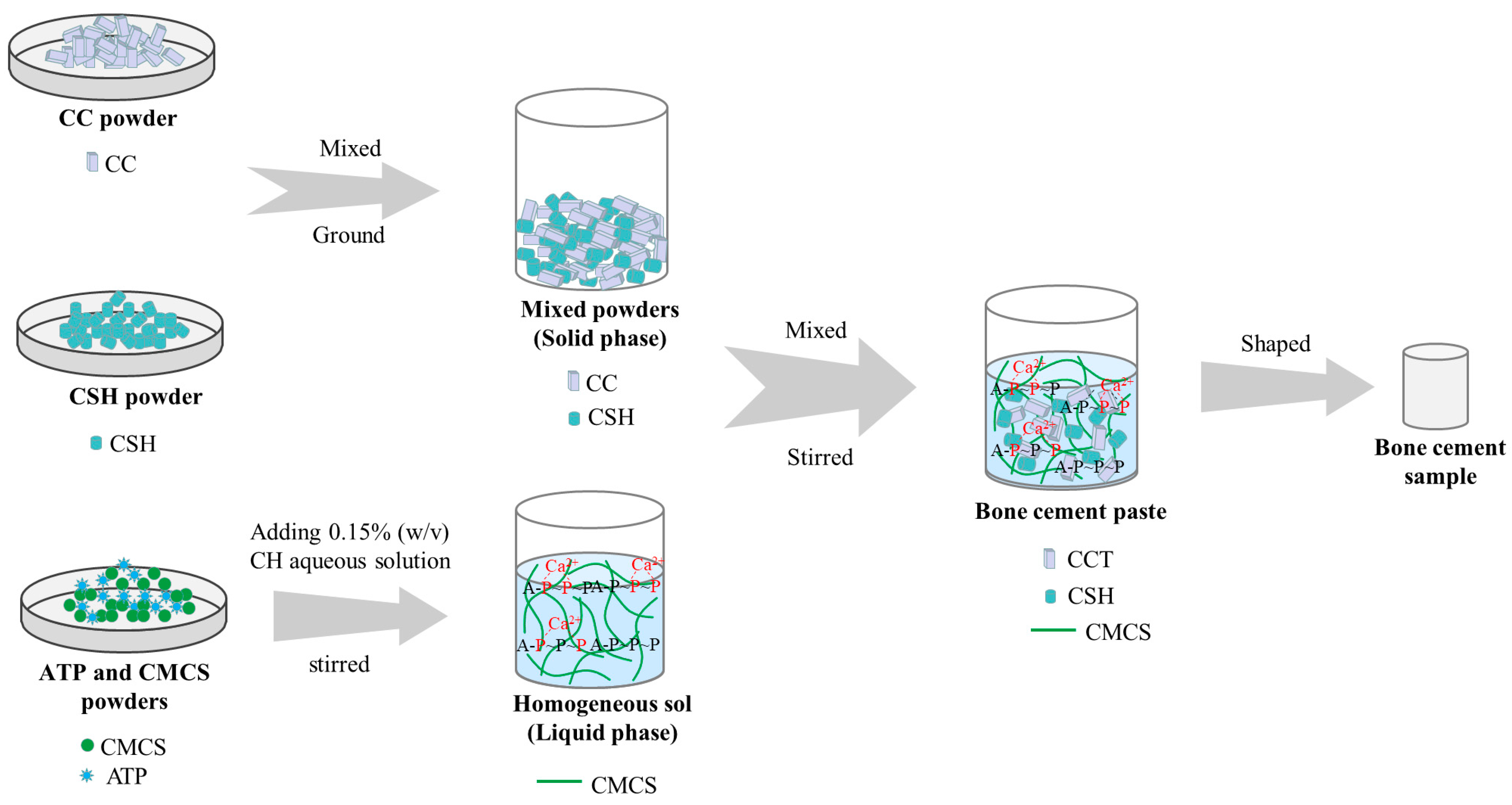
2.2. Preparation and Characterisation of ATP/CSH/CCT
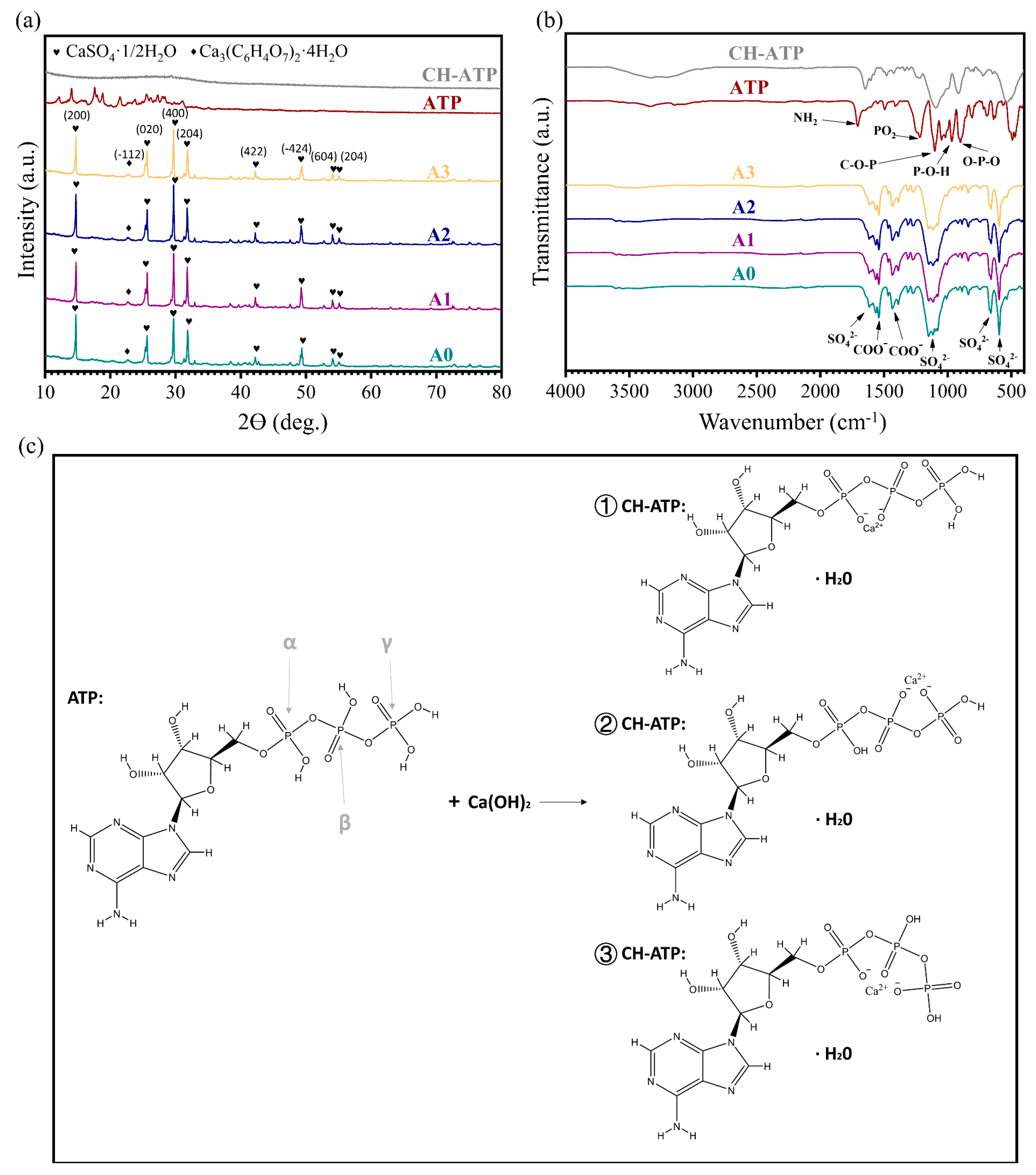
2.3. Preparation and Characterisation of CH-ATP
2.4. Compressive Strength
2.5. Weight Loss and pH Value
2.6. Release of ATP
2.7. Cell Proliferation and Fluorescence Staining
2.8. Osteogenic Differentiation
2.9. Statistical Methods
3. Results and Discussion
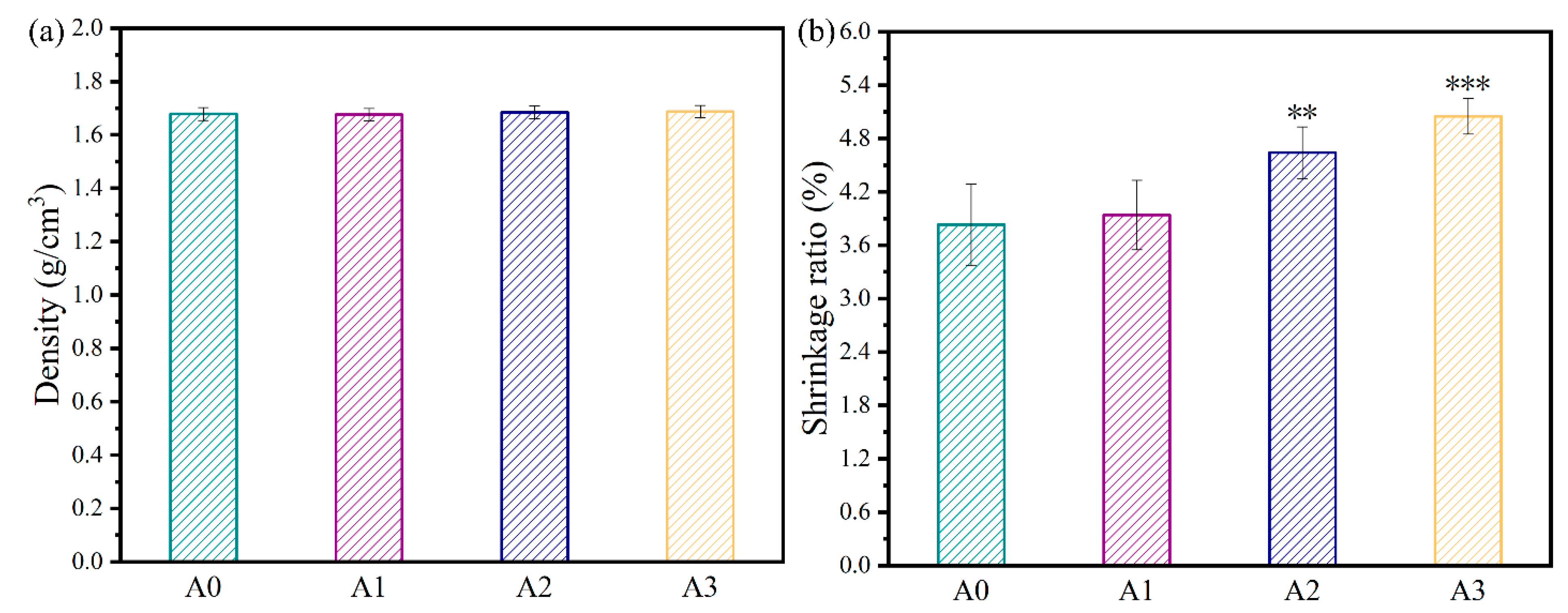
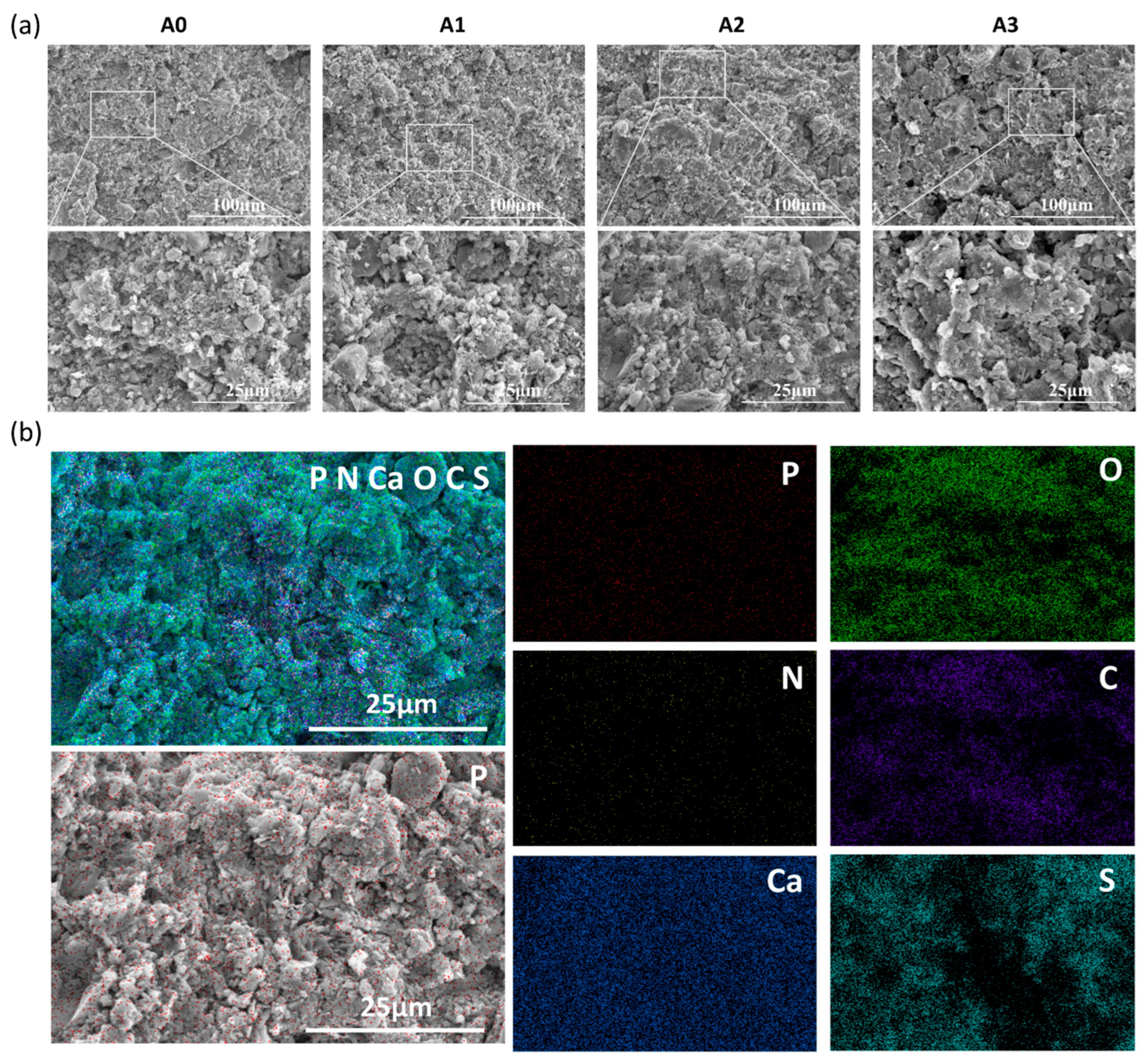
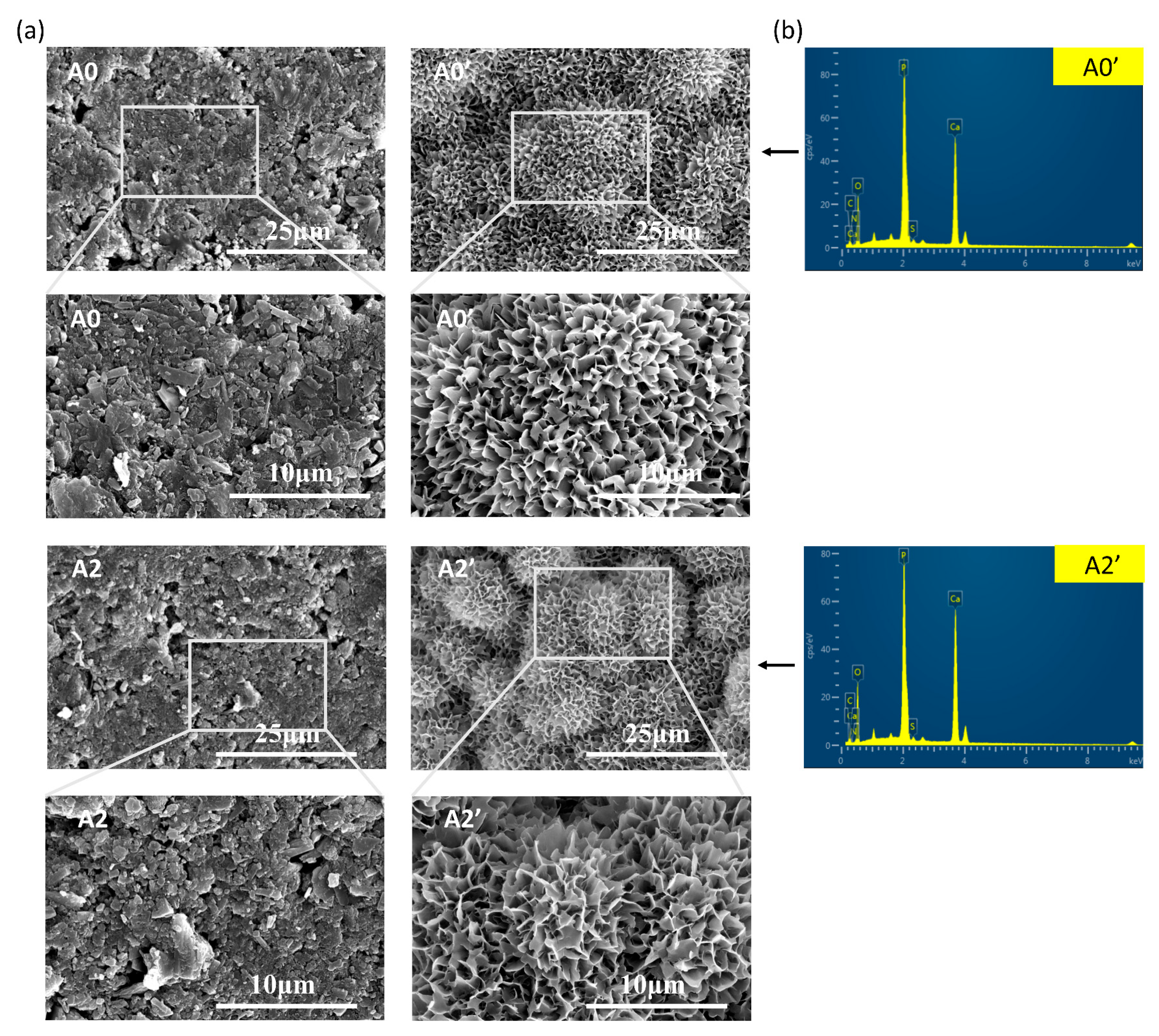
3.1. Characterization
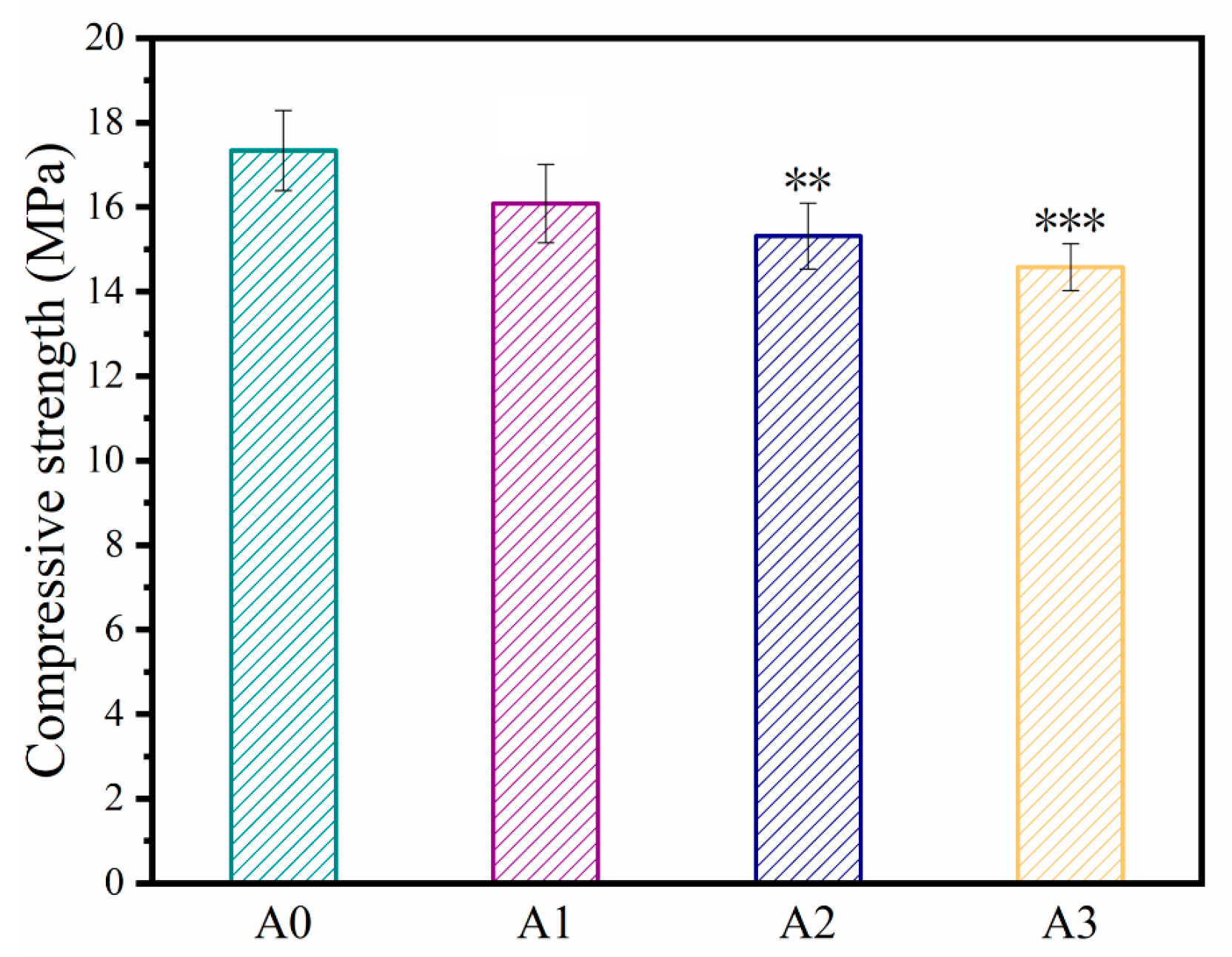
3.2. Compressive Strength
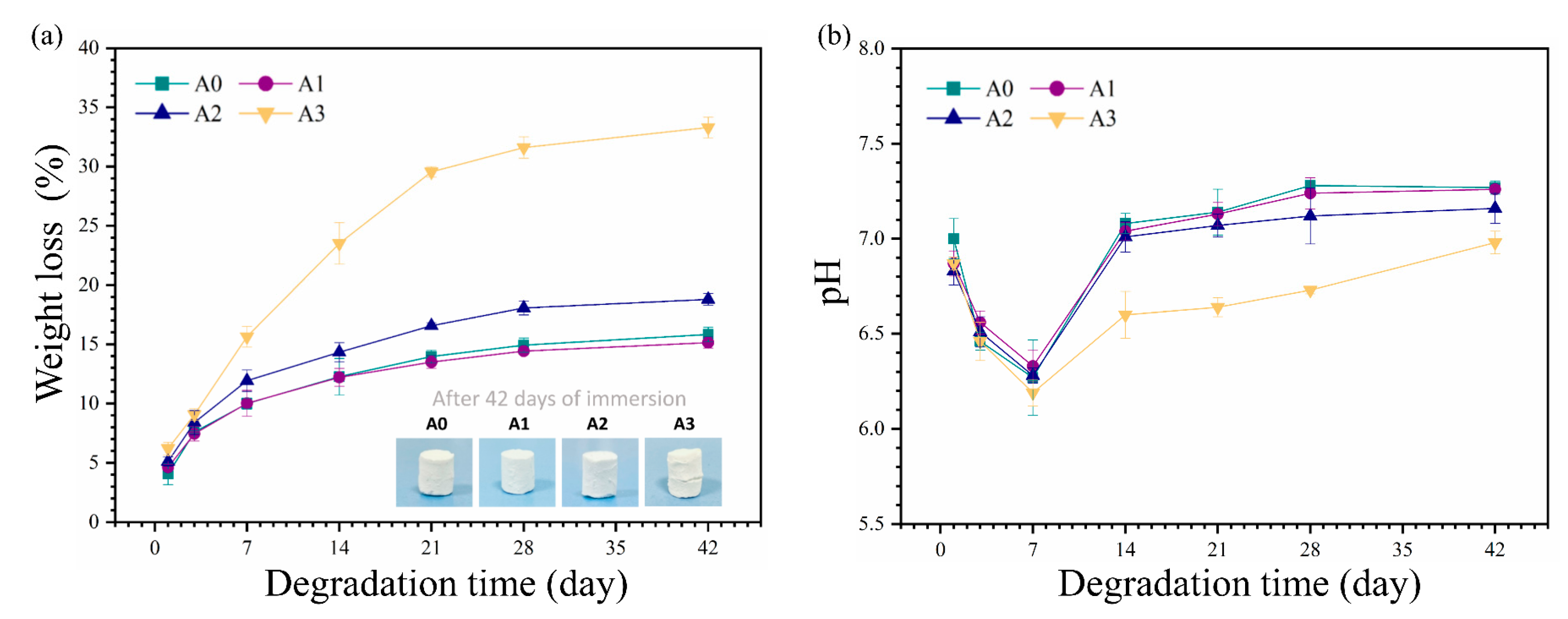
3.3. In Vitro Degradation
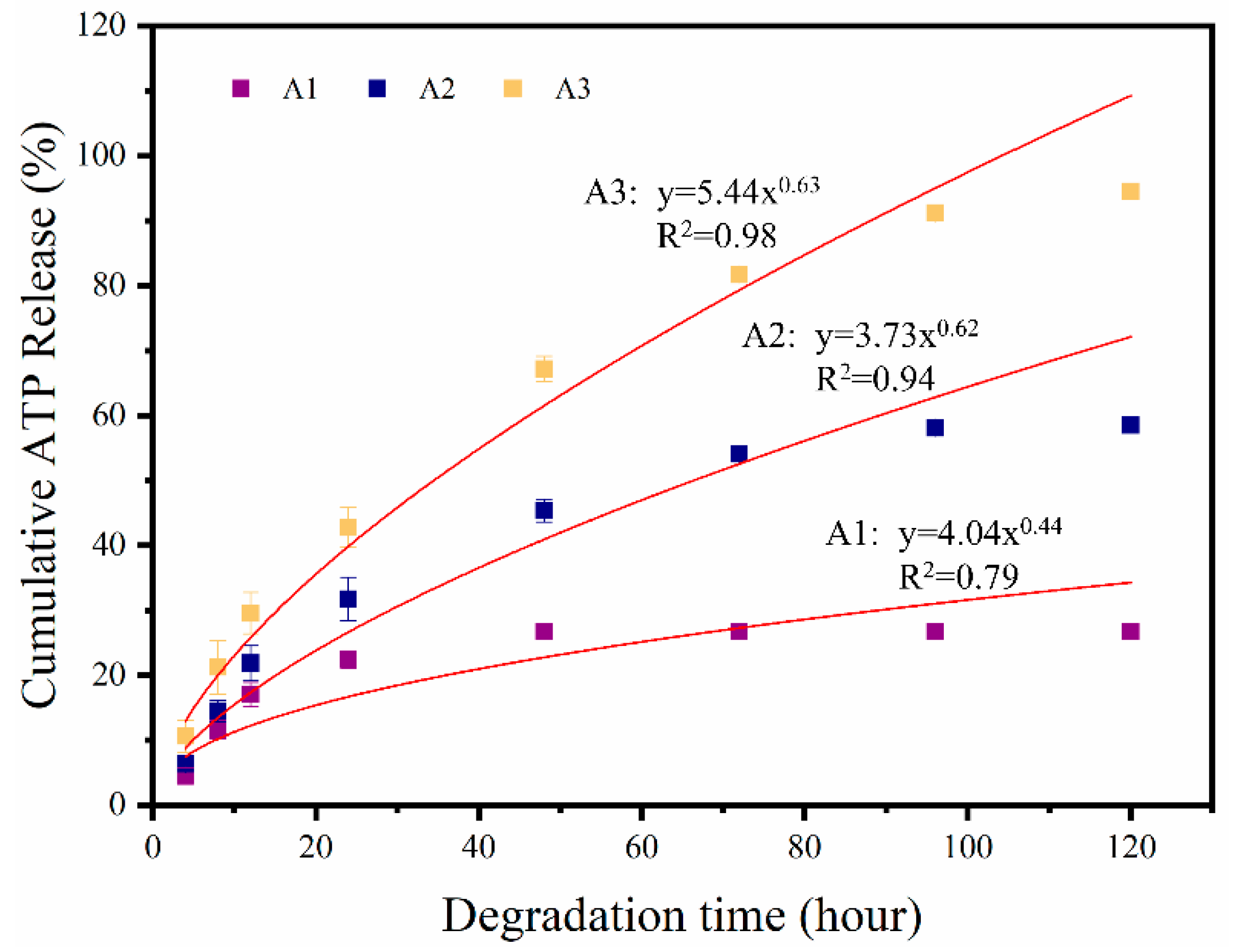
3.4. In Vitro Release of ATP
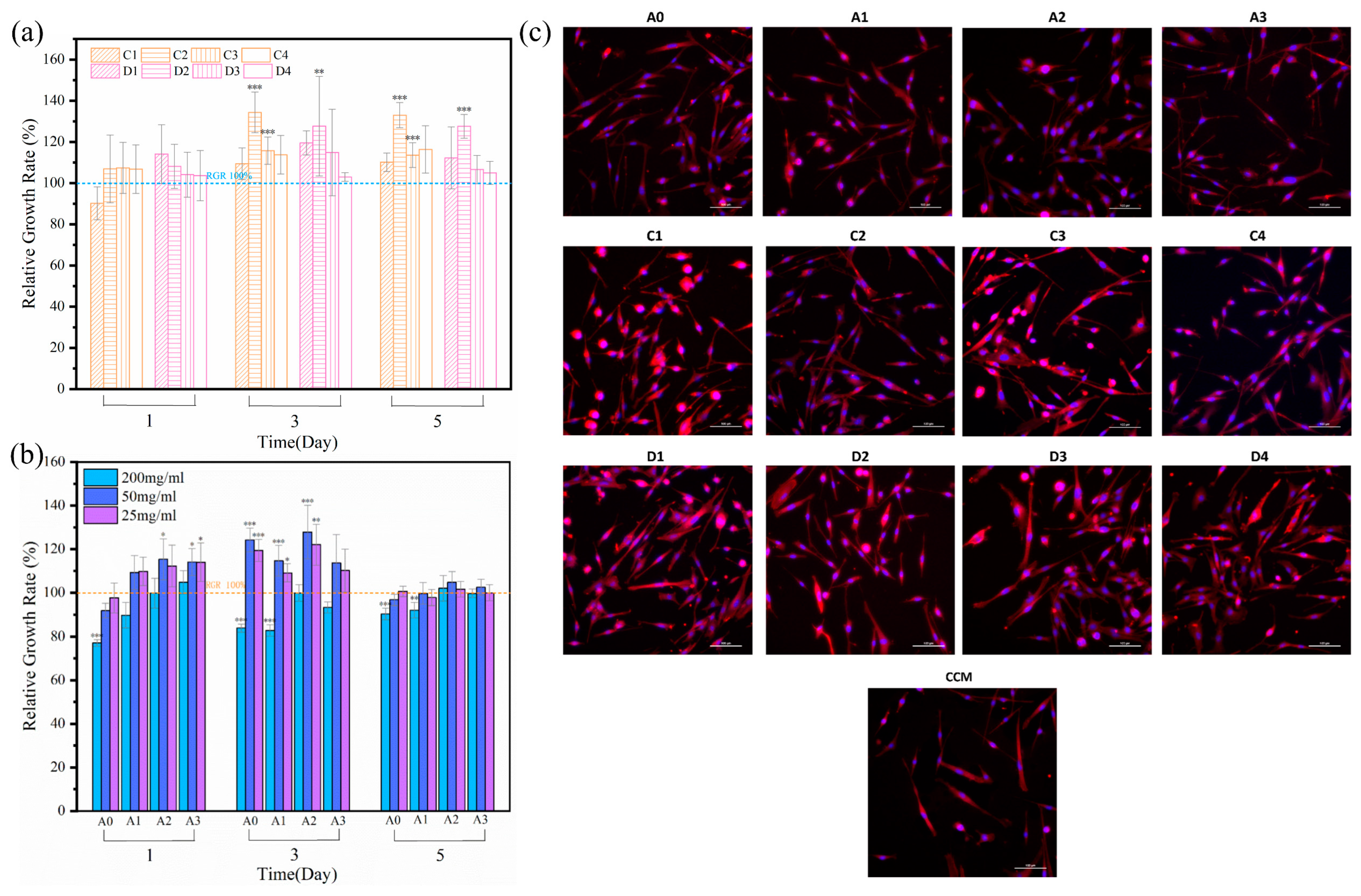
3.5. Cell Proliferation
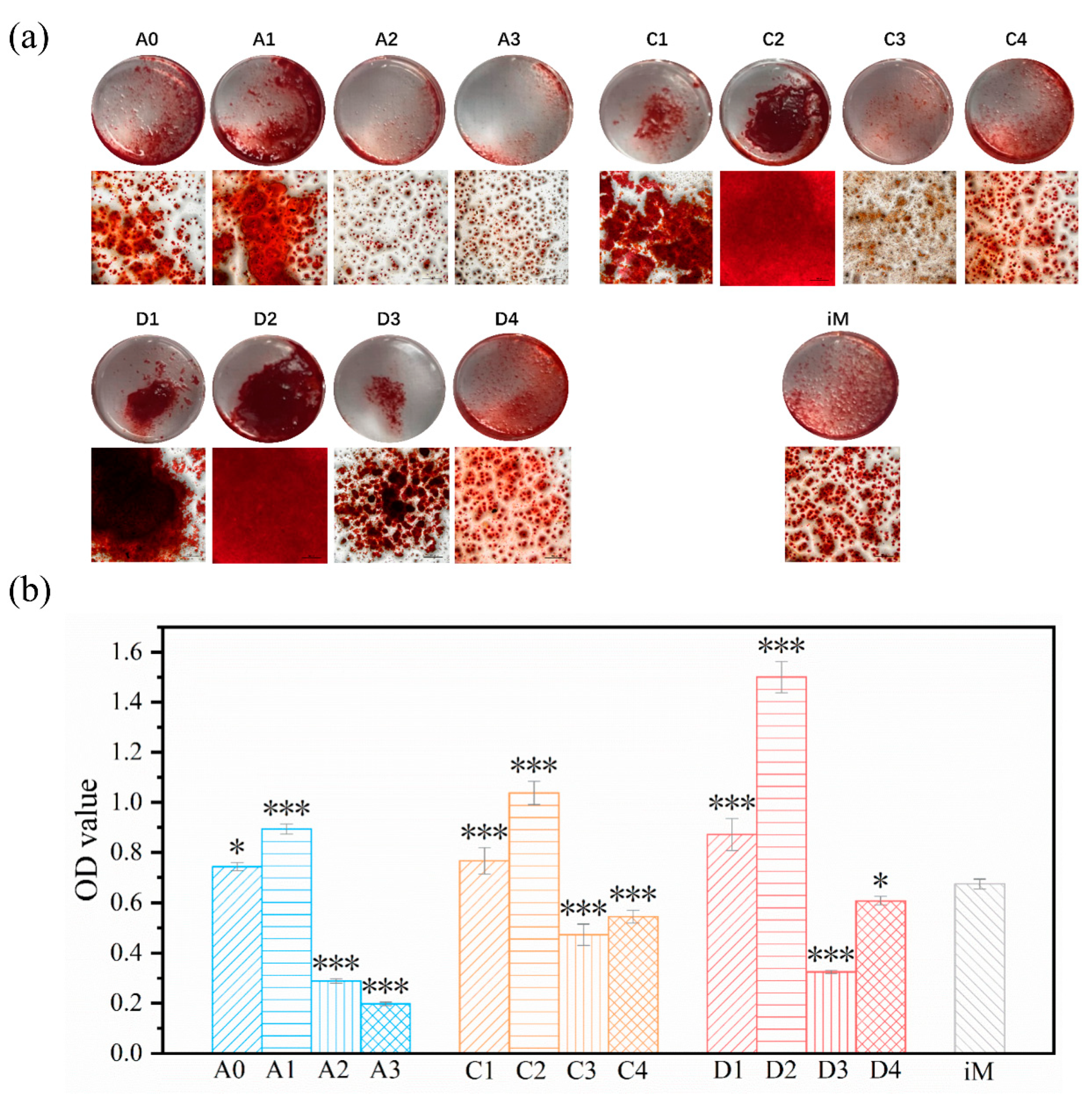
3.6. Cell Differentiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uskokovic, V.; Uskokovic, D.P. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96B, 152–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C. The use of bone-graft substitutes in large bone defects: Any specific needs? Inj. Int. J. Care Inj. 2011, 42, S56–S63. [Google Scholar] [CrossRef]
- Shi, Y.; Shen, D.; Zheng, H.; Wu, Z.; Shao, C.; Zhang, L.; Pan, H.; Tang, R.; Fu, B. Therapeutic Management of Demineralized Dentin Surfaces Using a Mineralizing Adhesive to Seal and Mineralize Dentin, Dentinal Tubules, and Odontoblast Processes. ACS Biomater. Sci. Eng. 2019, 5, 5481–5488. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Heredia, M.A.; Sohier, J.; Gaillard, C.; Quillard, S.; Dorget, M.; Layrolle, P. Rapid prototyped porous titanium coated with calcium phosphate as a scaffold for bone tissue engineering. Biomaterials 2008, 29, 2608–2615. [Google Scholar] [CrossRef]
- Du, J.; Zhou, Y.; Bao, X.; Kang, Z.; Huang, J.; Xu, G.; Yi, C.; Li, D. Surface polydopamine modification of bone defect repair materials: Characteristics and applications. Front. Bioeng. Biotechnol. 2022, 10, 974533. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Gonzalez-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef]
- Sopcak, T.; Medvecky, L.; Durisin, J. Hydrolysis, setting properties and invitro characterization of wollastonite/newberyite bone cement mixtures. J. Biomater. Appl. 2018, 32, 871–885. [Google Scholar] [CrossRef]
- Cai, Z.; Wu, Z.; Wan, Y.; Yu, T.; Zhou, C. Manipulation of the degradation behavior of calcium phosphate and calcium sulfate bone cement system by the addition of micro-nano calcium phosphate. Ceram. Int. 2021, 47, 29213–29224. [Google Scholar] [CrossRef]
- Hughes, E.; Yanni, T.; Jamshidi, P.; Grover, L.M. Inorganic cements for biomedical application: Calcium phosphate, calcium sulphate and calcium silicate. Adv. Appl. Ceram. 2015, 114, 65–76. [Google Scholar] [CrossRef]
- Xu, R.; Lian, X.; Zhao, L.; Wei, Y.; Hou, D.; Niu, B.; Zhang, Q.; Huang, D.; Li, F.; Gao, S. The effect of calcium phosphate and silk fibroin nanofiber tuning on properties of calcium sulfate bone cements. Biomed. Mater. 2021, 16, 015009. [Google Scholar] [CrossRef]
- Huan, Z.; Chang, J. Self-setting properties and in vitro bioactivity of calcium sulfate hemihydrate-tricalcium silicate composite bone cements. Acta Biomater. 2007, 3, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ji, M.; Ding, Z.; Yan, Y. Vitamin D3-loaded calcium citrate/calcium sulfate composite cement with enhanced physicochemical properties, drug release, and cytocompatibility. J. Biomater. Appl. 2020, 34, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Santos, C.; Almeida, M.M.; Costa, M.E.V. Hydroxyapatite micro- and nanoparticles: Nucleation and growth mechanisms in the presence of citrate species. J. Colloid Interface Sci. 2008, 318, 210–216. [Google Scholar] [CrossRef]
- Mitsionis, A.I.; Vaimakis, T.C.; Trapalis, C.C. The effect of citric acid on the sintering of calcium phosphate bioceramics. Ceram. Int. 2010, 36, 623–634. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Rawal, A.; Schmidt-Rohr, K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2010, 107, 22425–22429. [Google Scholar] [CrossRef] [Green Version]
- Costello, L.C.; Chellaiah, M.; Zou, J.; Franklin, R.B.; Reynolds, M.A. The status of citrate in the hydroxyapatite/collagen complex of bone; and Its role in bone formation. J. Regen. Med. Tissue Eng. 2014, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Davies, E.; Muller, K.H.; Wong, W.C.; Pickard, C.J.; Duer, M.J. Citrate bridges between mineral platelets in bone. Proc. Natl. Acad. Sci. USA 2014, 111, E1354–E1363. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.Y.; Shen, T.; Ye, H.Q. An absorption assessment of eggshell calcium citrate treated by PEF in mice. Adv. Mater. Res. 2013, 4, 634–638. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, W.; Chen, Q.-Y.; Lin, Z.-Q.; Cheng, S.-W.; Kou, D.-Q.; Ying, X.-Z.; Shen, Y.; Cheng, X.-J.; Nie, P.-F.; et al. Effect of calcium citrate on bone integration in a rabbit femur defect model. Asian Pac. J. Trop. Med. 2012, 5, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Abbracchio, M.P.; Saffrey, M.J.; Hopker, V.; Burnstock, G. Modulation of Astroglial Cell-Proliferation by Analogues of Adenosine and ATP in Primary Cultures of Rat Striatum. Neuroscience 1994, 59, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wilden, P.A.; Agazie, Y.M.; Kaufman, R.; Halenda, S.P. ATP-stimulated smooth muscle cell proliferation requires independent ERK and PI3K signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 1998, 275, H1209–H1215. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Rylova, Y.V.; Andreeva, E.R.; Kulikov, A.V.; Pogodina, M.V.; Zhivotovsky, B.; Gogvadze, V. Low ATP level is sufficient to maintain the uncommitted state of multipotent mesenchymal stem cells. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4418–4425. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Zhang, C.; Sun, J.; Feng, W.; Liang, X.-J.; Wang, S.; Zhang, J. Defect-Related Luminescent Hydroxyapatite-Enhanced Osteogenic Differentiation of Bone Mesenchymal Stem Cells Via an ATP-Induced cAMP/PKA Pathway. ACS Appl. Mater. Interfaces 2016, 8, 11262–11271. [Google Scholar] [CrossRef] [PubMed]
- Noronha-Matos, J.B.; Coimbra, J.; Sá-E-Sousa, A.; Rocha, R.; Marinhas, J.; Freitas, R.; Guerra-Gomes, S.; Ferreirinha, F.; Costa, M.A.; Correia-De-Sá, P. P2X7-induced zeiosis promotes osteogenic differentiation and mineralization of postmenopausal bone marrow-derived mesenchymal stem cells. FASEB J. 2014, 28, 5208–5222. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, S.; Chen, F.; Wang, Z.; Li, J.; Xie, Z.; Ma, M.; Wang, P.; Shen, H.; Wu, Y. Alpinetin alleviates osteoporosis by promoting osteogenic differentiation in BMSCs by triggering autophagy via PKA/mTOR/ULK1 signaling. Phytother. Res. 2023, 37, 252–270. [Google Scholar] [CrossRef]
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef] [Green Version]
- Diem, S.J.; Peters, K.W.; Gourlay, M.L.; Schousboe, J.T.; Taylor, B.C.; Orwoll, E.S.; Cauley, J.A.; Langsetmo, L.; Crandall, C.J.; Ensrud, K.R.; et al. Screening for Osteoporosis in Older Men: Operating Characteristics of Proposed Strategies for Selecting Men for BMD Testing. J. Gen. Intern. Med. 2017, 32, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern. Med. 2017, 167, ITC17–ITC31. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Xiao, J.; Zhou, R.; Li, C.-C.; Zheng, T.-T.; Dai, F. Clinical evaluation of the efficacy of a new bone cement-injectable cannulated pedicle screw in the treatment of spondylolysis-type lumbar spondylolisthesis with osteoporosis: A retrospective study. BMC Musculoskelet. Disord. 2022, 23, 951. [Google Scholar] [CrossRef]
- Guo, C.; Niu, D.; Liu, J.; Bao, X.; Xu, G. Application of Biodegradable PLGA-PEG-PLGA/CPC Composite Bone Cement in the Treatment of Osteoporosis. Coatings 2021, 11, 827. [Google Scholar] [CrossRef]
- Shirosaki, Y.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Lopes, M.A.; Santos, J.D.; Costa, M.A.; Fernandes, M.H. Physical, chemical and in vitro biological profile of chitosan hybrid membrane and in vitro biological profile of chitosan hybrid membrane as a function of organosiloxane concentration. Acta Biomater. 2009, 5, 346–355. [Google Scholar] [CrossRef]
- Lee, E.J.; Shin, D.S.; Kim, H.E.; Kim, H.W.; Koh, Y.H.; Jang, J.H. Membrane of hybrid chitosan-silica xerogel for guided bone regeneration. Biomaterials 2009, 30, 743–750. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Mattioli-Belmonte, M.; Tietz, C.; Biagini, R.; Ferioli, G.; Brunelli, M.; Fini, M.; Giardino, R.; Ilari, P. Stimulatory effect on bone formation exerted by a modified chitosan. Biomaterials 1994, 15, 1075–1081. [Google Scholar] [CrossRef]
- Lin, Q.; Lan, X.; Li, Y.; Yu, Y.; Ni, Y.; Lu, C.; Xu, Z. Anti-washout carboxymethyl chitosan modified tricalcium silicate bone cement: Preparation, mechanical properties and in vitro bioactivity. J. Mater. Sci. Mater. Med. 2010, 21, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.A.A. Thermal-Decomposition of Calcium Citrate Tetrahydrate. Thermochim. Acta 1994, 233, 243–256. [Google Scholar] [CrossRef]
- Dolci, L.S.; Panzavolta, S.; Torricelli, P.; Albertini, B.; Sicuro, L.; Fini, M.; Bigi, A.; Passerini, N. Modulation of Alendronate release from a calcium phosphate bone cement: An in vitro osteoblast-osteoclast co-culture study. Int. J. Pharm. 2019, 554, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.Z.; Ding, Z.W.; Chen, H.; Peng, H.T.; Yan, Y.G. Design of novel organic-inorganic composite bone cements with high compressive strength, in vitro bioactivity and cytocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2365–2377. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, K.; Chang, J. Preparation and characterization of Sr-hardystonite (Sr2ZnSi2O7) for bone repair applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 184–188. [Google Scholar] [CrossRef]
- Wang, B.; Chen, H.; Peng, S.; Li, X.-D.; Liu, X.; Ren, H.; Yan, Y.; Zhang, Q. Multifunctional magnesium-organic framework doped biodegradable bone cement for antibacterial growth, inflammatory regulation and osteogenic differentiation. J. Mater. Chem. B 2023, 11, 2872–2885. [Google Scholar] [CrossRef]
- Shen, Z.; Yu, T.; Ye, J. Microstructure and properties of alendronate-loaded calcium phosphate cement. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 303–311. [Google Scholar] [CrossRef]
- Uskoković, V.; Lee, P.P.; Walsh, L.A.; Fischer, K.E.; Desai, T.A. PEGylated silicon nanowire coated silica microparticles for drug delivery across intestinal epithelium. Biomaterials 2012, 33, 1663–1672. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Lee, E.-J.; Park, C.-S.; Yoon, B.-H.; Shin, D.-S.; Kim, H.-E.; Koh, Y.-H.; Park, S.-H. Calcium sulfate hemihydrate powders with a controlled morphology for use as bone cement. J. Am. Ceram. Soc. 2008, 91, 2039–2042. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.B.; Singh, A.K.; Singh, S.P. Effect of citric-acid on the hydration of Portland-Cement. Cem. Concr. Res. 1986, 16, 911–920. [Google Scholar] [CrossRef]
- Takeuchi, H.; Murata, H.; Harada, I. Interaction of Adenosine 5′-Triphosphate with Mg2+: Vibrational Study of Coordination Sites by Use of 18O-Labeled Triphosphates. J. Am. Chem. Soc. 1988, 110, 392–397. [Google Scholar] [CrossRef]
- Mostapha, S.; Fontaine-Vive, F.; Berthon, L.; Boubals, N.; Zorz, N.; Solari, P.L.; Charbonnel, M.C.; Auwer, C.D. On the structure of thorium and americium adenosine triphosphate complexes. Int. J. Radiat. Biol. 2014, 90, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Zhu, C.F.; Lanctot, D.R.; Agrawal, C.M.; Wang, X. Fundamentals of biomechanics in tissue engineering of bone. Tissue Eng. 2000, 6, 361–381. [Google Scholar] [CrossRef]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deng, Y. Supersaturation control in aragonite synthesis using sparingly soluble calcium sulfate as reactants. J. Colloid Interface Sci. 2003, 266, 359–365. [Google Scholar] [CrossRef]
- Cheng, H.; Garcia, A.C.; Tang, N.; Danielsen, B.P.; Skibsted, L.H. Combinations of isocitrate and citrate enhance calcium salt solubility and supersaturation robustness. Int. Dairy J. 2018, 85, 225–236. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Adluri, R.S.; Zhan, L.; Bagchi, M.; Maulik, N.; Maulik, G. Comparative effects of a novel plant-based calcium supplement with two common calcium salts on proliferation and mineralization in human osteoblast cells. Mol. Cell. Biochem. 2010, 340, 73–80. [Google Scholar] [CrossRef]
- Berlier, J.L.; Rigutto, S.; Valle, A.D.; Lechanteur, J.; Soyfoo, M.S.; Gangji, V.; Rasschaert, J. Adenosine Triphosphate Prevents Serum Deprivation-Induced Apoptosis in Human Mesenchymal Stem Cells via Activation of the MAPK Signaling Pathways. Stem Cells 2015, 33, 211–218. [Google Scholar] [CrossRef]
- Ciciarello, M.; Zini, R.; Rossi, L.; Salvestrini, V.; Ferrari, D.; Manfredini, R.; Lemoli, R.M. Extracellular Purines Promote the Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells to the Osteogenic and Adipogenic Lineages. Stem Cells Dev. 2013, 22, 1097–1111. [Google Scholar] [CrossRef] [Green Version]
- Kameda, T.; Mano, H.; Yamada, Y.; Takai, H.; Amizuka, N.; Kobori, M.; Izumi, N.; Kawashima, H.; Ozawa, H.; Ikeida, K.; et al. Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochem. Biophys. Res. Commun. 1998, 245, 419–422. [Google Scholar] [CrossRef] [PubMed]
| Cement Label | Solid Phase | Liquid Phase | Liquid–Solid Ratio | |||
|---|---|---|---|---|---|---|
| CSH (g) | CC (g) | 0.15% CH (mL) | CMCS (mg) | ATP (mg) | (mL/g) | |
| A0 | 6 | 4 | 3 | 200 | 0 | 0.3 |
| A1 | 6 | 4 | 3 | 200 | 10 | 0.3 |
| A2 | 6 | 4 | 3 | 200 | 50 | 0.3 |
| A3 | 6 | 4 | 3 | 200 | 100 | 0.3 |
| ATP | ATP/CH | |||||||
|---|---|---|---|---|---|---|---|---|
| Extract Solutions | C1 | C2 | C3 | C4 | D1 | D2 | D3 | D4 |
| ATP (μg/mL) | 1250 | 312.5 | 78.13 | 19.53 | 1250 | 312.5 | 78.13 | 19.53 |
| CH (μg/mL) | 0 | 0 | 0 | 0 | 112.5 | 28.13 | 7.03 | 1.76 |
| P (%) | N (%) | Ca (%) | O (%) | C (%) | S (%) | Ca/P | |
|---|---|---|---|---|---|---|---|
| A0 | 16.39 | 0 | 24.99 | 42.35 | 16.14 | 0.13 | 1.52 |
| A2 | 15.99 | 0 | 25.94 | 45.56 | 12.27 | 0.24 | 1.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chen, H.; Ren, H.; Wang, B.; Li, X.; Peng, S.; Zhang, Q.; Yan, Y. Effects of ATP on the Physicochemical Properties and Cytocompatibility of Calcium Sulfate/Calcium Citrate Composite Cement. Materials 2023, 16, 3947. https://doi.org/10.3390/ma16113947
Liu X, Chen H, Ren H, Wang B, Li X, Peng S, Zhang Q, Yan Y. Effects of ATP on the Physicochemical Properties and Cytocompatibility of Calcium Sulfate/Calcium Citrate Composite Cement. Materials. 2023; 16(11):3947. https://doi.org/10.3390/ma16113947
Chicago/Turabian StyleLiu, Xiangyue, Hong Chen, Haohao Ren, Bo Wang, Xiaodan Li, Suping Peng, Qiyi Zhang, and Yonggang Yan. 2023. "Effects of ATP on the Physicochemical Properties and Cytocompatibility of Calcium Sulfate/Calcium Citrate Composite Cement" Materials 16, no. 11: 3947. https://doi.org/10.3390/ma16113947






