Effect of Heat Treatment on Crevice Corrosion Behavior of 304 Stainless Steel Clad Plate in Seawater Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Heat Treatments
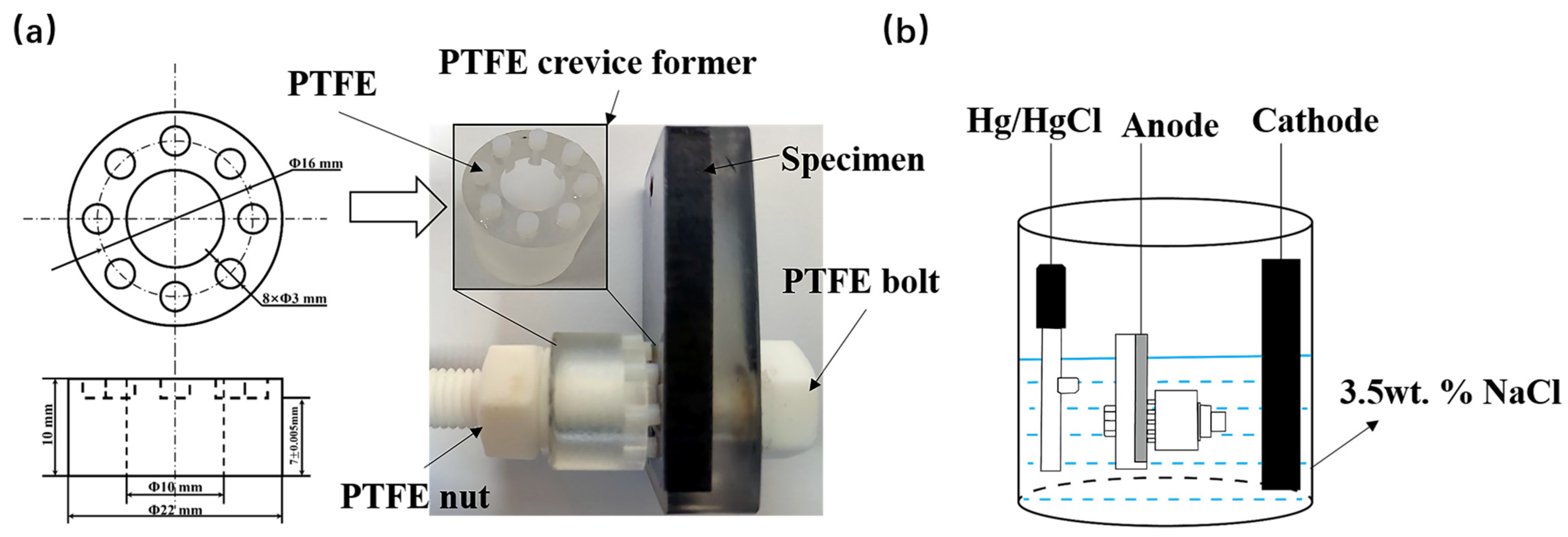
2.2. Crevice Preparation Procedure
2.3. Corrosion Performance
2.4. Morphology Observation
3. Results and Discussion
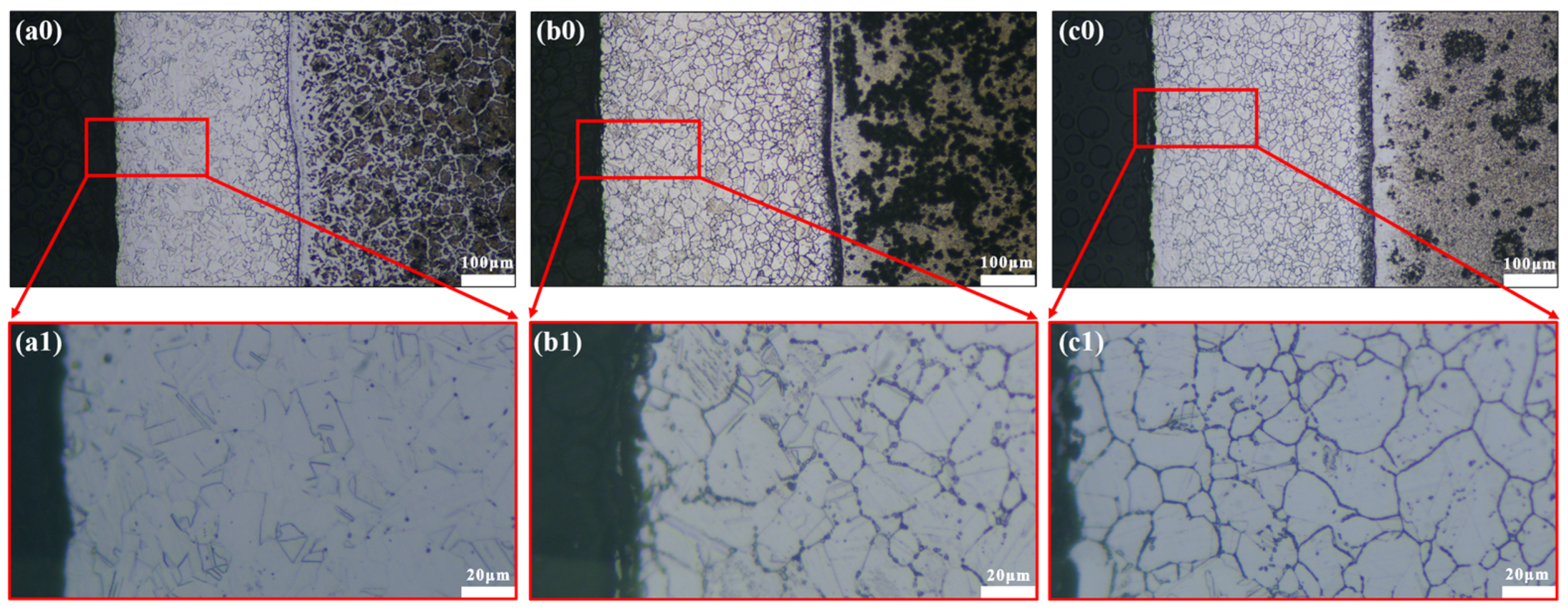
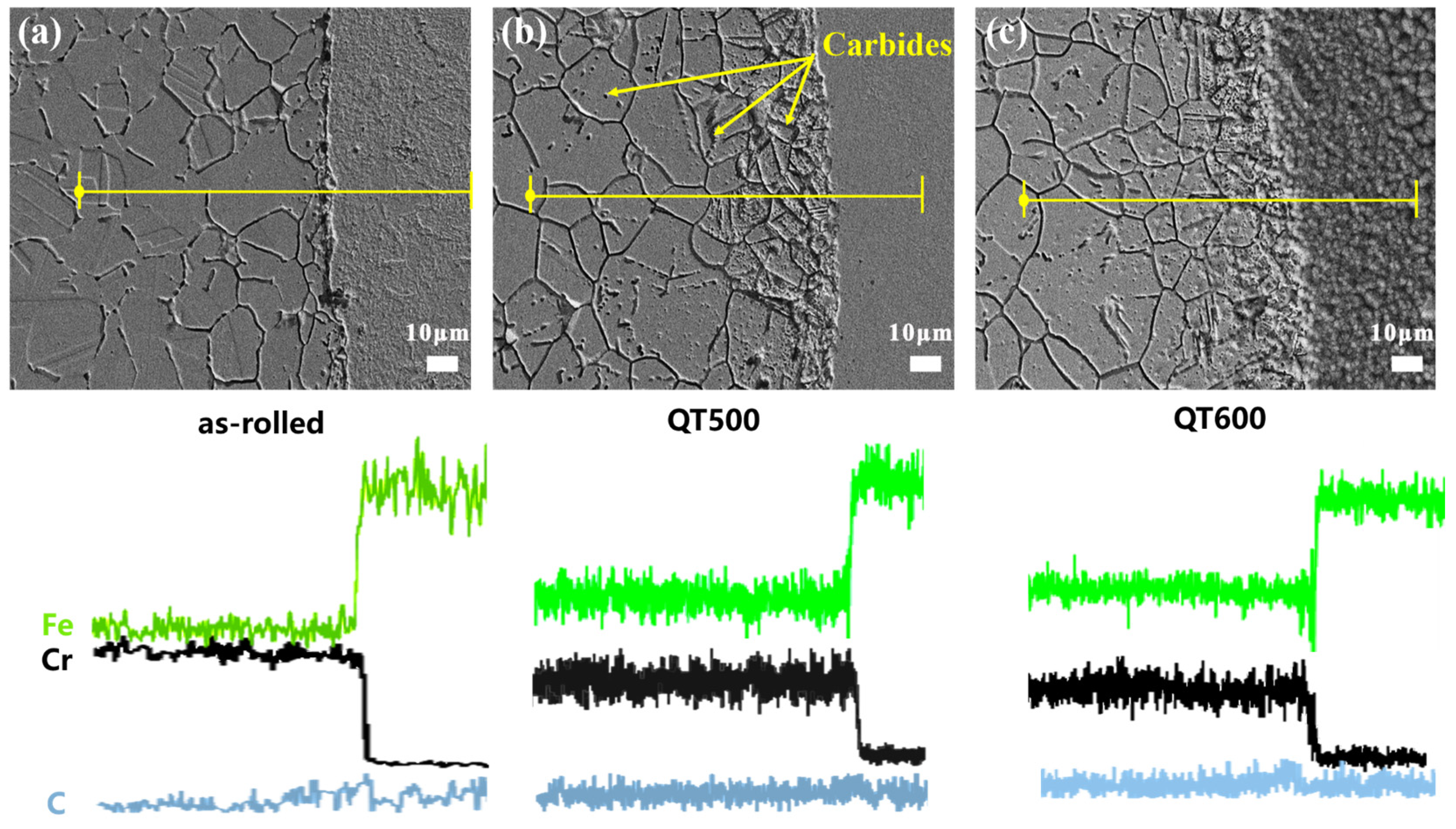
3.1. Microstructure of 304/45 SSCP Joints with Q-T Treatment
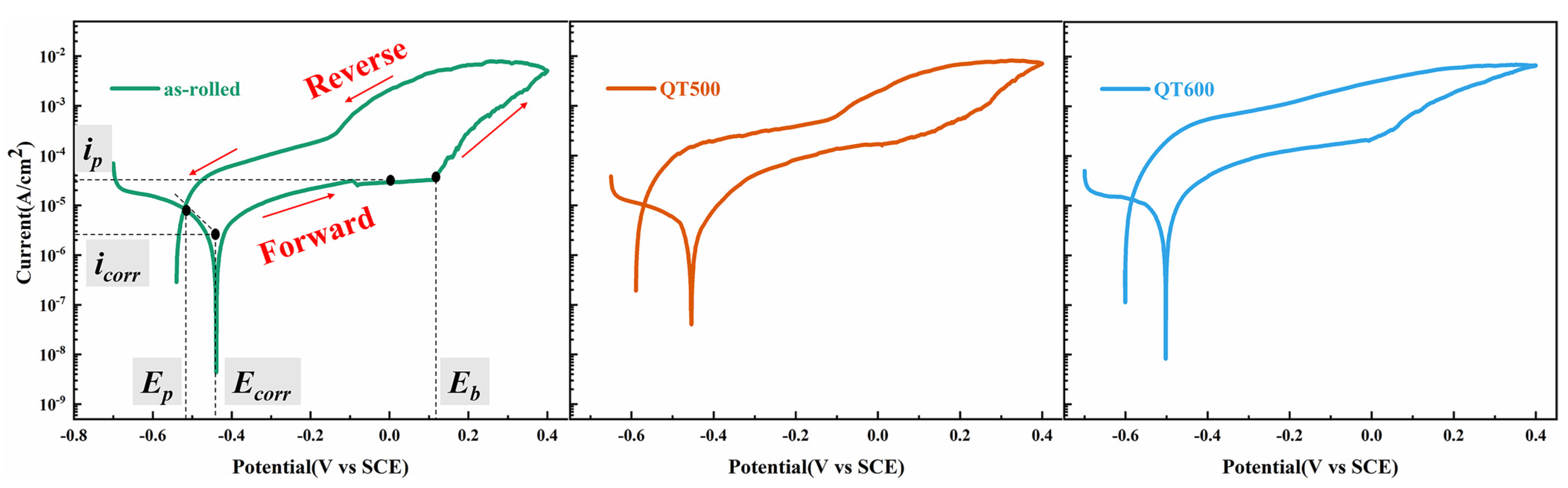
3.2. Electrochemical Behavior of SS Cladding
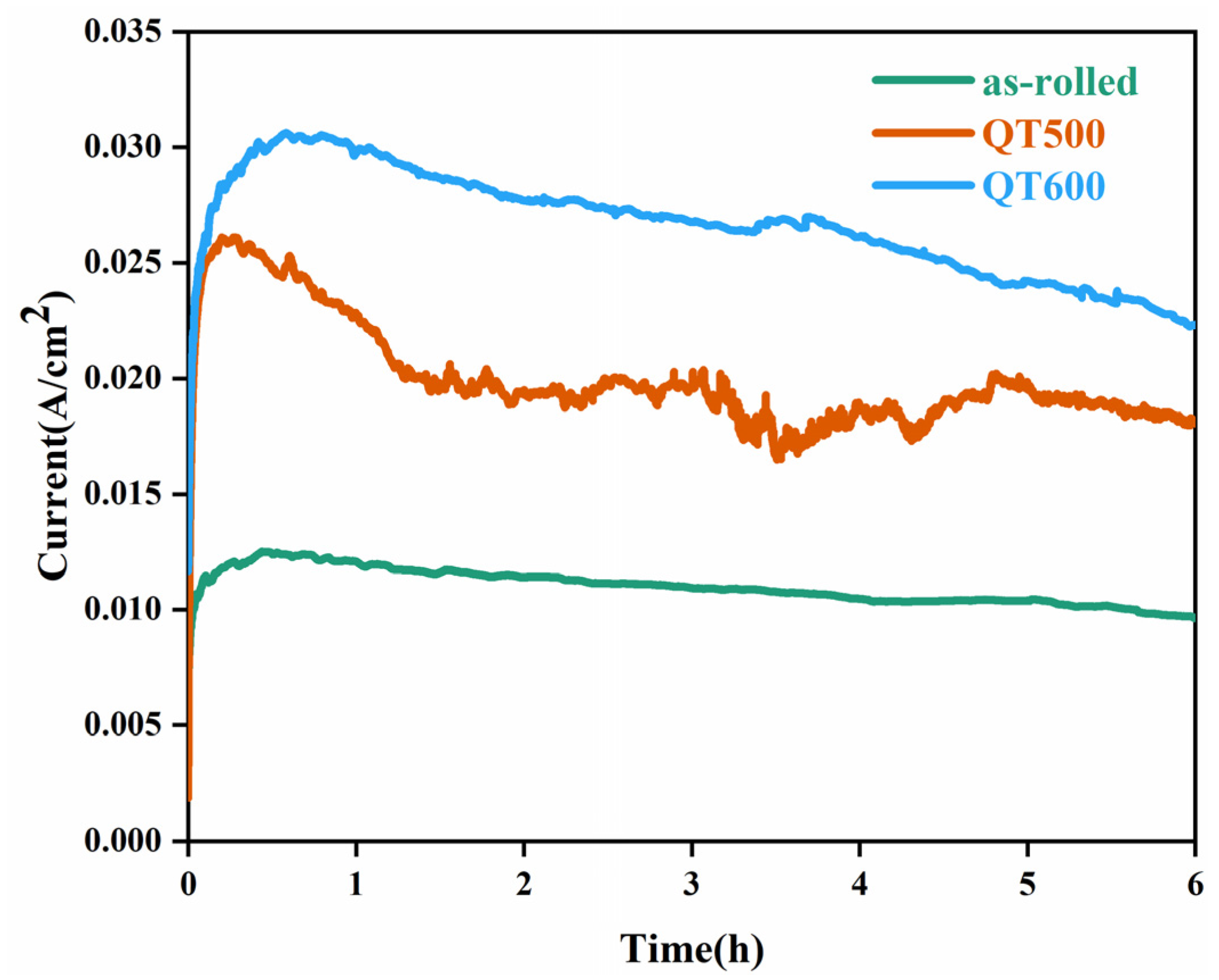
3.3. Electrochemical Behavior of SS Cladding with PTFE Crevice-Former
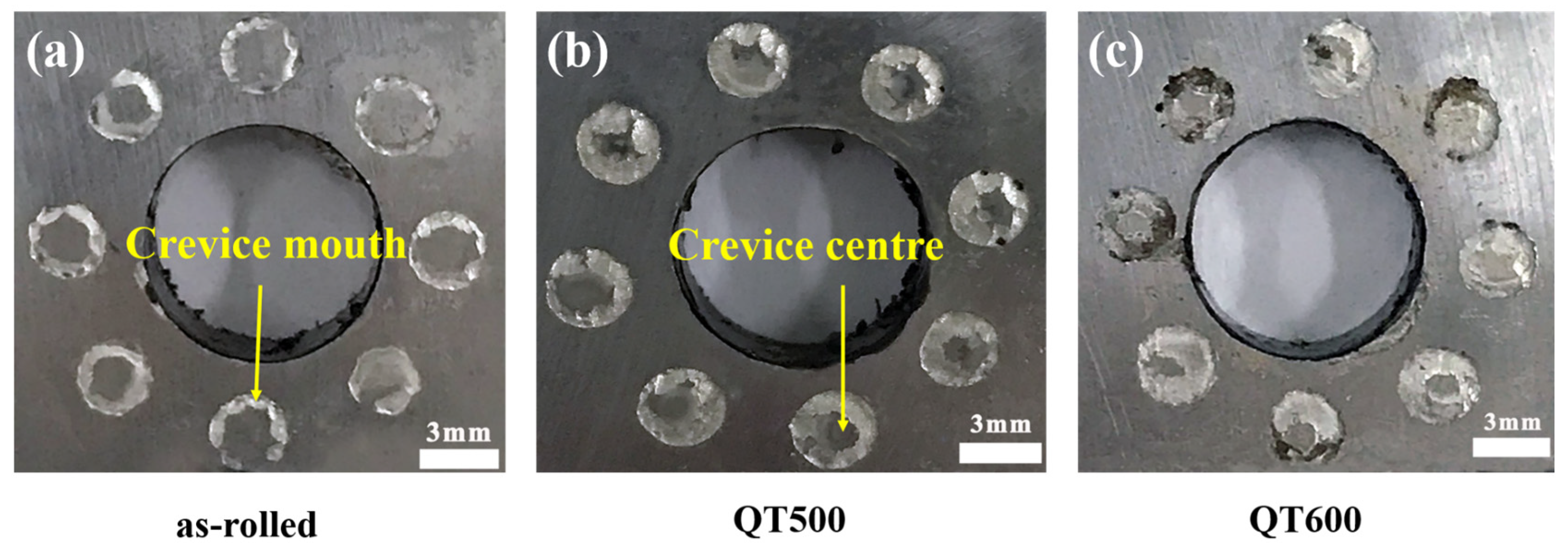
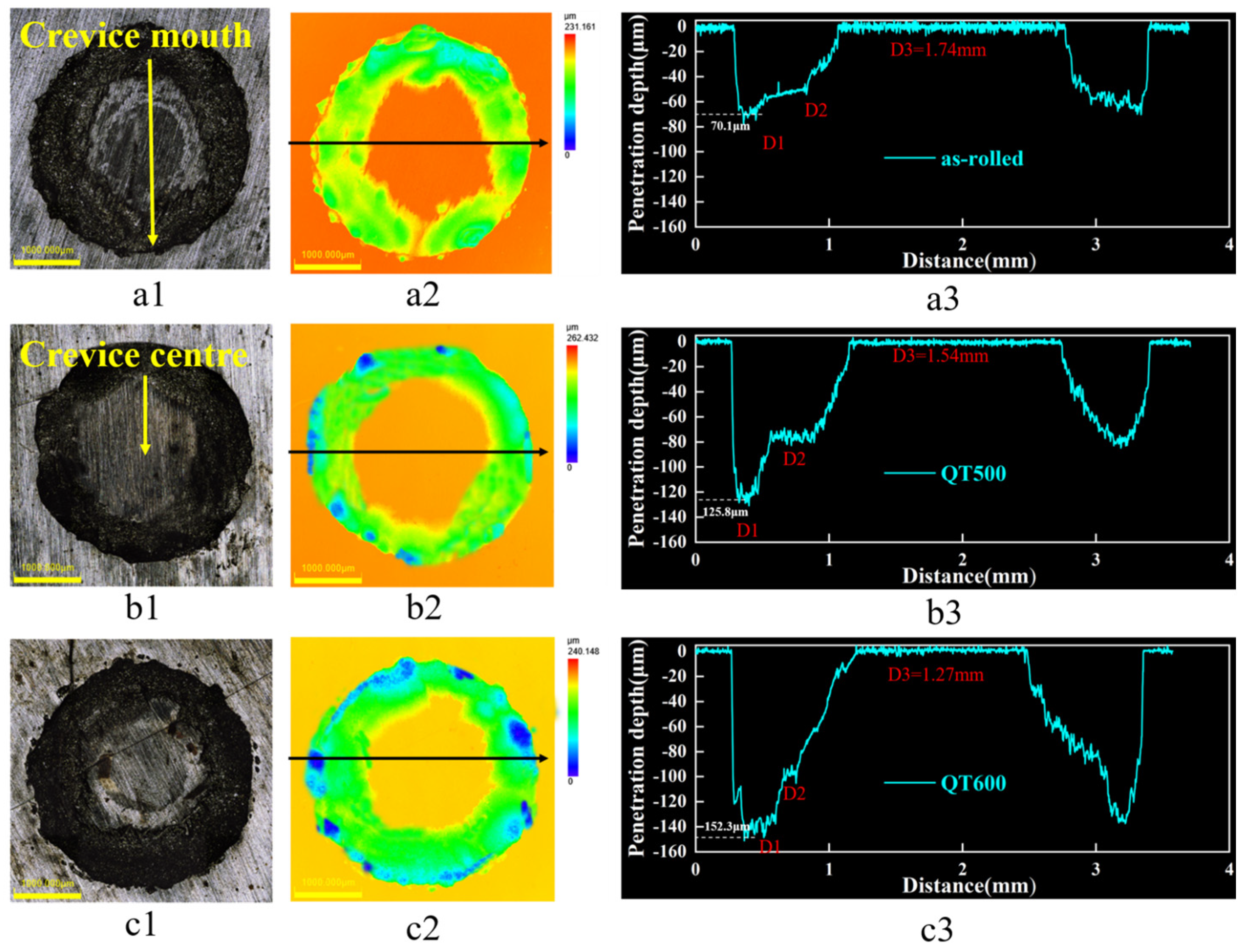
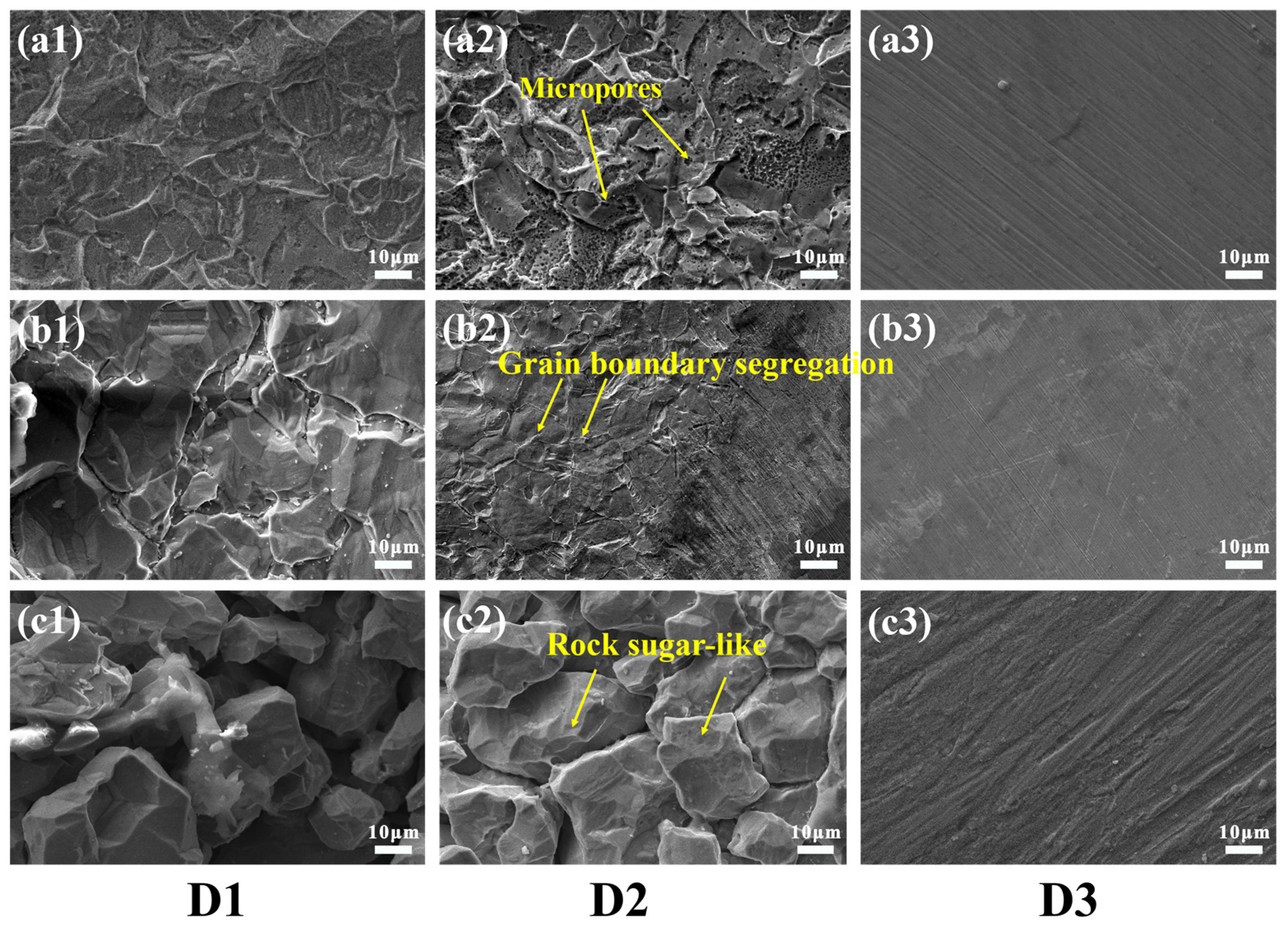
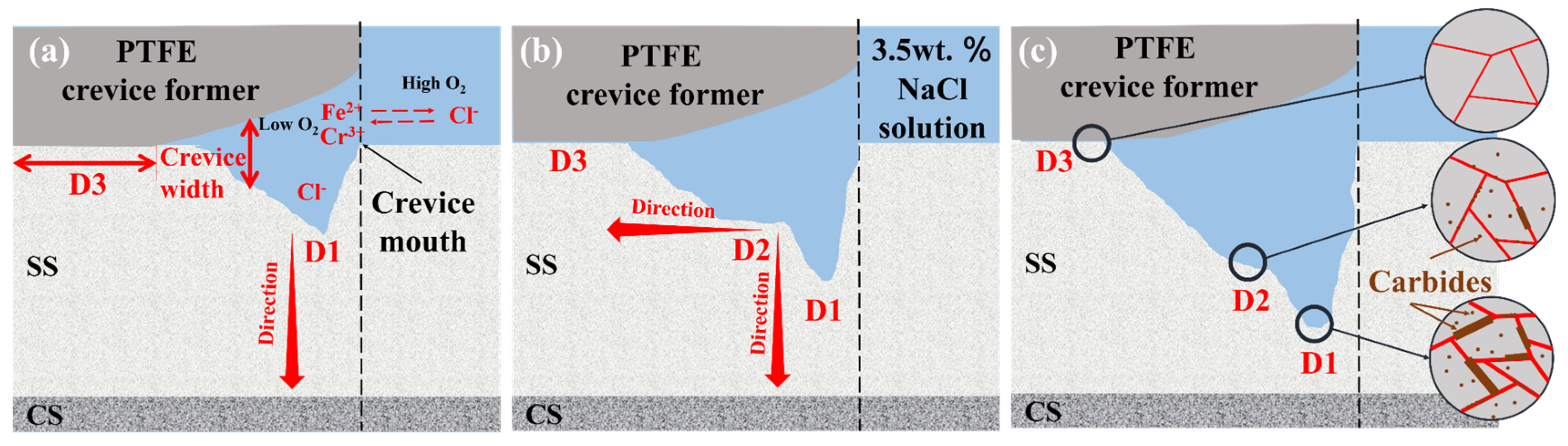
3.4. Corrosion Morphology of Stainless Steel Cladding
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, S.C.; Huang, M.N.; Tzou, G.Y.; Syu, S.W. Analysis of Asymmetrical Cold and Hot Bond Rolling of Unbounded Clad Sheet under Constant Shear Friction. J. Mater. Process. Technol. 2006, 177, 114–120. [Google Scholar] [CrossRef]
- Huang, Q.-X.; Yang, X.-R.; Ma, L.-F.; Zhou, C.-L.; Liu, G.-M.; Li, H.-B. Interface-Correlated Characteristics of Stainless Steel/Carbon Steel Plate Fabricated by AAWIV and Hot Rolling. J. Iron Steel Res. Int. 2014, 21, 931–937. [Google Scholar] [CrossRef]
- Kaya, Y.; Kahraman, N.; Durgutlu, A.; Gülenç, B. Investigation of the Microstructural, Mechanical and Corrosion Properties of Grade A Ship Steel-Duplex Stainless Steel Composites Produced via Explosive Welding. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 3721–3733. [Google Scholar] [CrossRef]
- Su, H.; Luo, X.-B.; Chai, F.; Shen, J.-C.; Sun, X.-J.; Lu, F. Manufacturing Technology and Application Trends of Titanium Clad Steel Plates. J. Iron Steel Res. Int. 2015, 22, 977–982. [Google Scholar] [CrossRef]
- Chen, D.; Wu, X.; Han, E.H.; Sun, H. Oxidation Behavior of 304 Stainless Steel during Crevice Corrosion in High-Temperature Pure Water. Corrosion 2015, 71, 1213–1223. [Google Scholar] [CrossRef]
- Larché, N.; Thierry, D.; Debout, V.; Blanc, J.; Cassagne, T.; Peultier, J.; Johansson, E.; Taravel-Condat, C. Crevice Corrosion of Duplex Stainless Steels in Natural and Chlorinated Seawater. Rev. Metall. Cah. D’Informations Tech. 2011, 108, 451–463. [Google Scholar] [CrossRef]
- Hornus, E.C.; Rodríguez, M.A.; Carranza, R.M.; Rebak, R.B. Comparative Study of the Crevice Corrosion Resistance of UNS S30400 and UNS S31600 Stainless Steels in the Context of Galvele’s Model. Corrosion 2017, 73, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Cui, Z.Y.; Cui, H.Z.; Wang, X.; Li, Y.Z. The Effect of Applied Stress on the Crevice Corrosion of 304 Stainless Steel in 3.5 wt% NaCl Solution. Corros. Sci. 2022, 196, 110039. [Google Scholar] [CrossRef]
- Palma Calabokis, O.; Núñez de la Rosa, Y.; Lepienski, C.M.; Perito Cardoso, R.; Borges, P.C. Crevice and Pitting Corrosion of Low Temperature Plasma Nitrided UNS S32750 Super Duplex Stainless Steel. Surf. Coat. Technol. 2021, 413, 17–20. [Google Scholar] [CrossRef]
- Machuca, L.L.; Bailey, S.I.; Gubner, R.; Watkin, E.L.J.; Ginige, M.P.; Kaksonen, A.H.; Heidersbach, K. Effect of Oxygen and Biofilms on Crevice Corrosion of UNS S31803 and UNS N08825 in Natural Seawater. Corros. Sci. 2013, 67, 242–255. [Google Scholar] [CrossRef]
- Yeh, C.P.; Tsai, K.C.; Huang, J.Y. The Effect of Deposited Dust on SCC and Crevice Corrosion of AISI 304L Stainless Steel in Saline Environment. Materials 2021, 14, 6834. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, E.; Moayed, M.H.; Mirjalili, M.; Pahlavan, S. Proposed Stability Product Criterion for Open Hemispherical Metastable Pits Formed in the Crevices of Different Aspect Ratios (l/d) on 316L Stainless Steel in 3.5% NaCl Solution. Corros. Sci. 2021, 184, 109389. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.H.; Kim, J.G.; Kim, W.C. Effect of the Crevice Former on the Corrosion Behavior of 316L Stainless Steel in Chloride-Containing Synthetic Tap Water. Met. Mater. Int. 2018, 24, 516–524. [Google Scholar] [CrossRef]
- Wu, B.; Guo, K.; Yang, X.; Gao, Y.; Jin, Y.; Gao, Y.; Wang, Q.; Zhang, F. Effect of Carbon Content of Substrate on the Microstructure Changes and Tensile Behavior of Clad Layer of Stainless Steel Composites. Mater. Sci. Eng. A 2022, 831, 142201. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Zhang, B.; Zhang, Q. Effect of Heat Treatment on the Microstructure and Corrosion Resistance of Stainless/Carbon Steel Bimetal Plate. Adv. Mater. Sci. Eng. 2020, 2020, 1280761. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, D.; Hu, W.; Cui, L.; Gao, Z.; Zhou, L. Effect of Interlayers on Microstructure and Properties of 2205/Q235B Duplex Stainless Steel Clad Plate. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 679–692. [Google Scholar] [CrossRef]
- Liu, B.X.; Wang, S.; Fang, W.; Yin, F.X.; Chen, C.X. Meso and Microscale Clad Interface Characteristics of Hot-Rolled Stainless Steel Clad Plate. Mater. Charact. 2019, 148, 17–25. [Google Scholar] [CrossRef]
- Liu, B.X.; Wang, S.; Fang, W.; Ma, J.L.; Yin, F.X.; He, J.N.; Feng, J.H.; Chen, C.X. Microstructure and Mechanical Properties of Hot Rolled Stainless Steel Clad Plate by Heat Treatment. Mater. Chem. Phys. 2018, 216, 460–467. [Google Scholar] [CrossRef]
- Jia, N.N.; Guo, K.; He, Y.M.; Wang, Y.H.; Peng, J.G.; Wang, T.S. A Thermomechanical Process to Achieve Mechanical Properties Comparable to Those of Quenched-Tempered Medium-C Steel. Mater. Sci. Eng. A 2017, 700, 175–182. [Google Scholar] [CrossRef]
- Song, H.; Shin, H.; Shin, Y. Heat-Treatment of Clad Steel Plate for Application of Hull Structure. Ocean Eng. 2016, 122, 278–287. [Google Scholar] [CrossRef]
- Cai, B.; Liu, Y.; Tian, X.; Wang, F.; Li, H.; Ji, R. An Experimental Study of Crevice Corrosion Behaviour of 316L Stainless Steel in Artificial Seawater. Corros. Sci. 2010, 52, 3235–3242. [Google Scholar] [CrossRef]
- Tao, Y.; Jing, Y.-A.; Yan, X.; Li, W.; Pang, Q.; Jing, G. Microstructures and Properties of Roll-Bonded Stainless /Medium Carbon Steel Clad Plates. J. Mater. Process. Technol. 2019, 266, 264–273. [Google Scholar] [CrossRef]
- Sun, L.; Chen, S.; Qiu, J.; Zhao, T. Research on the Mechanism and Detection Method of Intergranular Corrosion of AISI 304 Stainless Steel by Electrochemical Techniques in Heat Exchanger Equipment. J. Mater. Eng. Perform. 2022, 32, 534–543. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, Z.; Chen, Y.; Liu, X.; Sun, J.; Wang, W. Interfacial Microstructure and Strengthening Mechanism of Stainless Steel/Carbon Steel Laminated Composite Fabricated by Liquid-Solid Bonding and Hot Rolling. Mater. Charact. 2022, 191, 112122. [Google Scholar] [CrossRef]
- Ernst, F.; Cao, Y.; Michal, G.M.; Heuer, A.H. Carbide Precipitation in Austenitic Stainless Steel Carburized at Low Temperature. Acta Mater. 2007, 55, 1895–1906. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, H.; Zhang, B.; Lu, J.; Du, L. Effect of Cr-Rich Carbide Precipitates on Austenite Stability and Consequent Corrosion Behavior of Ultrafine-Grained 304 Stainless Steel Produced by Cryogenic Rolling and Annealing Treatment. Mater. Charact. 2023, 195, 112553. [Google Scholar] [CrossRef]
- Jiang, S.; Chai, F.; Su, H.; Yang, C. Influence of Chromium on the Flow-Accelerated Corrosion Behavior of Low Alloy Steels in 3.5% NaCl Solution. Corros. Sci. 2017, 123, 217–227. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Abe, M.; Ishii, T. The Effect of Impurity Concentration and Cr Content on the Passive Oxide Films in Ferritic Stainless Steels. J. Electrochem. Soc. 2015, 162, C528–C535. [Google Scholar] [CrossRef]
- Almubarak, A.; Abuhaimed, W.; Almazrouee, A. Corrosion Behavior of the Stressed Sensitized Austenitic Stainless Steels of High Nitrogen Content in Seawater. Int. J. Electrochem. 2013, 2013, 970835. [Google Scholar] [CrossRef]
- Evans, K.J.; Yilmaz, A.; Day, S.D.; Wong, L.L.; Estill, J.C.; Rebak, R.B. Using Electrochemical Methods to Determine Alloy 22′s Crevice Corrosion Repassivation Potential. JOM 2005, 57, 56–61. [Google Scholar] [CrossRef]
- He, X.; Dunn, D.S.; Csontos, A.A. Corrosion of Similar and Dissimilar Metal Crevices in the Engineered Barrier System of a Potential Nuclear Waste Repository. Electrochim. Acta 2007, 52, 7556–7569. [Google Scholar] [CrossRef]
- Wu, X.Y.; Sun, J.K.; Wang, J.M.; Jiang, Y.M.; Li, J. Crevice Corrosion Behaviors Between CFRP and Stainless Steel 316L for Automotive Applications. Acta Metall. Sin. (Engl. Lett.) 2019, 32, 1219–1226. [Google Scholar] [CrossRef]
- Li, X.; Dou, W.; Tian, L.; Dong, H. Combating the Tribo-Corrosion of LDX2404 Lean Duplex Stainless Steel by Low Temperature Plasma Nitriding. Lubricants 2018, 6, 93. [Google Scholar] [CrossRef]
- Chen, D.; Han, E.H.; Wu, X. Effects of Crevice Geometry on Corrosion Behavior of 304 Stainless Steel during Crevice Corrosion in High Temperature Pure Water. Corros. Sci. 2016, 111, 518–530. [Google Scholar] [CrossRef]
- Yeh, C.P.; Tsai, K.C.; Huang, J.Y. Influence of Chloride Concentration on Stress Corrosion Cracking and Crevice Corrosion of Austenitic Stainless Steel in Saline Environments. Materials 2020, 13, 5640. [Google Scholar] [CrossRef]
- Kim, J.; Shin, S.; Lee, S. Correlation between Microstructural Evolution and Corrosion Resistance of Hypoeutectic Al–Si–Mg Alloy: Influence of Corrosion Product Layer. Mater. Charact. 2022, 193, 112276. [Google Scholar] [CrossRef]
- Amadeh, A.; Pahlevani, B.; Heshmati-Manesh, S. Effects of Rare Earth Metal Addition on Surface Morphology and Corrosion Resistance of Hot-Dipped Zinc Coatings. Corros. Sci. 2002, 44, 2321–2331. [Google Scholar] [CrossRef]
- Frankel, G.S. Fundamentals of Corrosion Kinetics. In Active Protective Coatings; Springer Series in Materials Science; Springer: Dordrecht, The Netherlands, 2016; Volume 233, p. 17. [Google Scholar]

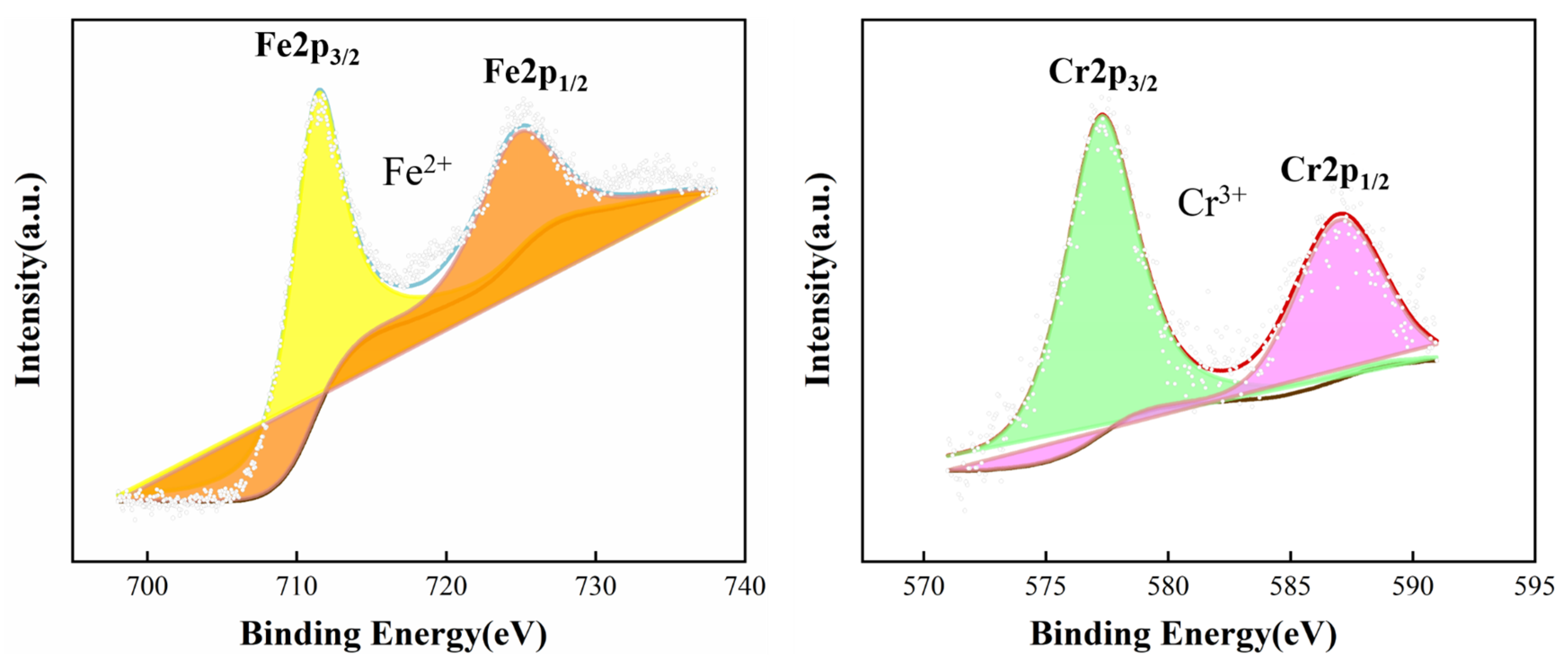
| Materials | C | Si | Mn | P | S | Ni | Cr | Fe |
|---|---|---|---|---|---|---|---|---|
| SS (AISI 304) | 0.055 | 0.44 | 1.21 | 0.028 | 0.003 | 8.13 | 18.28 | bal. |
| CS (45 STEEL) | 0.432 | 0.35 | 0.55 | 0.013 | 0.003 | 0.16 | 0.21 | bal. |
| Specimen Name | Bonding Parameter | Processing Conditions |
|---|---|---|
| as-rolled | hot rolling at 1150 °C for 1 h | Control group |
| QT500 | Quenching at 850 °C for 0.5 h and WC + tempering at 500 °C for 1 h and AC | |
| QT600 | Quenching at 850 °C for 0.5 h and WC + tempering at 600 °C for 1 h and AC |
| Ia (A/cm2) | Ir (A/cm2) | DOS (Ir/Ia × 100%) | Ecorr/V | Icorr (A/cm2) | Eb (V) | |
|---|---|---|---|---|---|---|
| as-rolled | 7.6 × 10−2 | 9.35 × 10−4 | 0.012 | −0.16 | 2.7 × 10−8 | 0.35 |
| QT500 | 8.22 × 10−2 | 5.93 × 10−3 | 0.072 | −0.20 | 8.5 × 10−8 | 0.23 |
| QT600 | 8.61 × 10−2 | 2.97 × 10−2 | 0.345 | −0.23 | 2.1 × 10−7 | 0.11 |
| Ecorr (V) | Icorr (A/cm2) | Ip (A/cm2) | Eb (V) | Ep (V) | |
|---|---|---|---|---|---|
| as-rolled | −0.44 | 1.6 × 10−6 | 2.9 × 10−5 | 0.11 | −0.52 |
| QT500 | −0.45 | 5.7 × 10−6 | 1.7 × 10−4 | 0.025 | −0.57 |
| QT600 | −0.50 | 1.1 × 10−5 | 2.2 × 10−4 | −0.003 | −0.59 |
| (a) as-rolled (at%) | (b) QT500 (at%) | (c) QT600 (at%) | |
|---|---|---|---|
| Fe | 52.9 | 47.39 | 56.14 |
| Cr | 14.65 | 7.93 | 4.15 |
| C | 12.58 | 11.71 | 10.31 |
| O | 17.57 | 25.38 | 29.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hang, P.; Zhao, B.; Zhou, J.; Ding, Y. Effect of Heat Treatment on Crevice Corrosion Behavior of 304 Stainless Steel Clad Plate in Seawater Environment. Materials 2023, 16, 3952. https://doi.org/10.3390/ma16113952
Hang P, Zhao B, Zhou J, Ding Y. Effect of Heat Treatment on Crevice Corrosion Behavior of 304 Stainless Steel Clad Plate in Seawater Environment. Materials. 2023; 16(11):3952. https://doi.org/10.3390/ma16113952
Chicago/Turabian StyleHang, Pengwei, Boshen Zhao, Jiaming Zhou, and Yi Ding. 2023. "Effect of Heat Treatment on Crevice Corrosion Behavior of 304 Stainless Steel Clad Plate in Seawater Environment" Materials 16, no. 11: 3952. https://doi.org/10.3390/ma16113952
APA StyleHang, P., Zhao, B., Zhou, J., & Ding, Y. (2023). Effect of Heat Treatment on Crevice Corrosion Behavior of 304 Stainless Steel Clad Plate in Seawater Environment. Materials, 16(11), 3952. https://doi.org/10.3390/ma16113952






