Tuning Nb Solubility, Electrical Properties, and Imprint through PbO Stoichiometry in PZT Films
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Solution Preparation
2.2. Coating Process
2.3. Materials Characterization
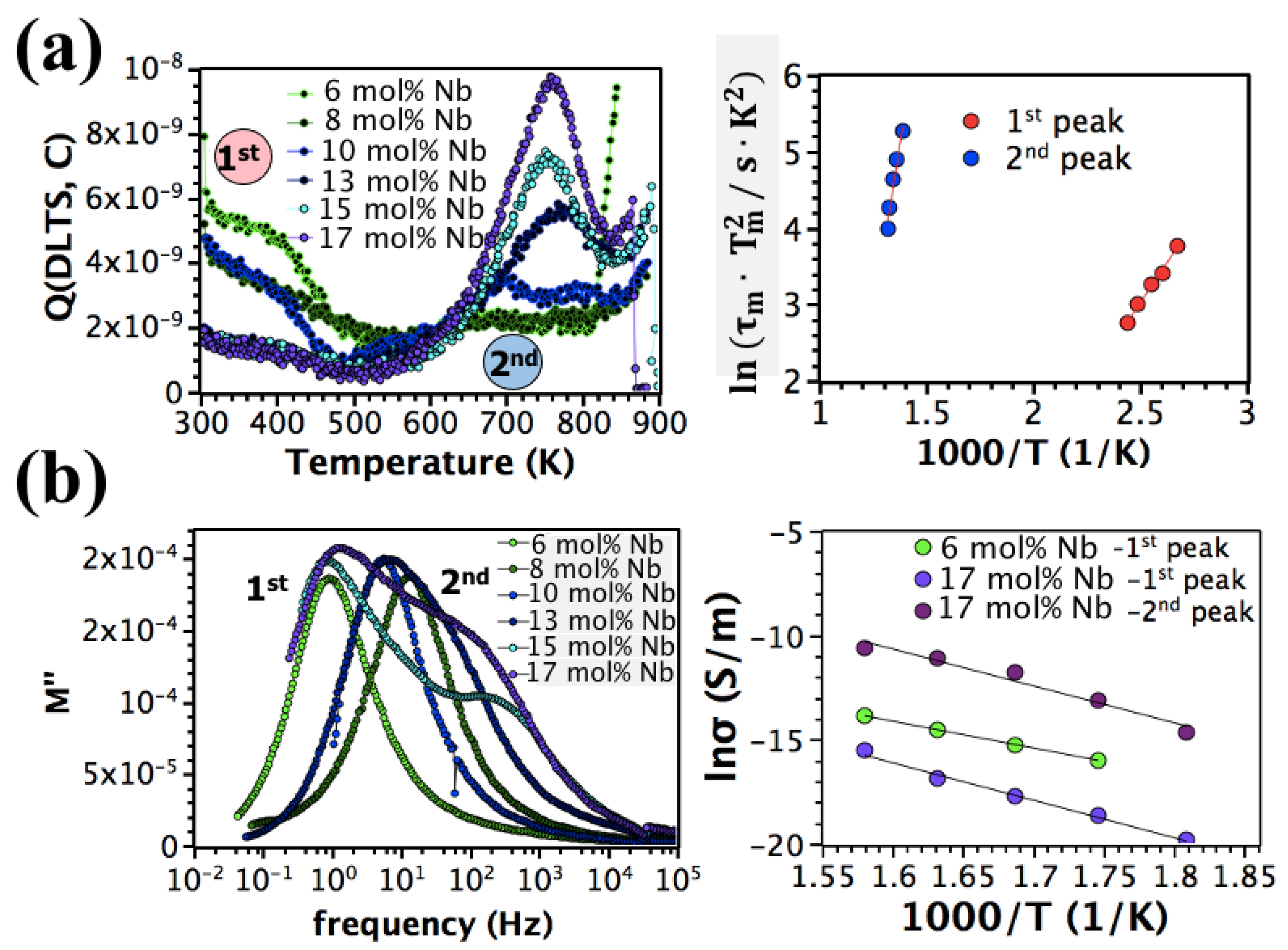
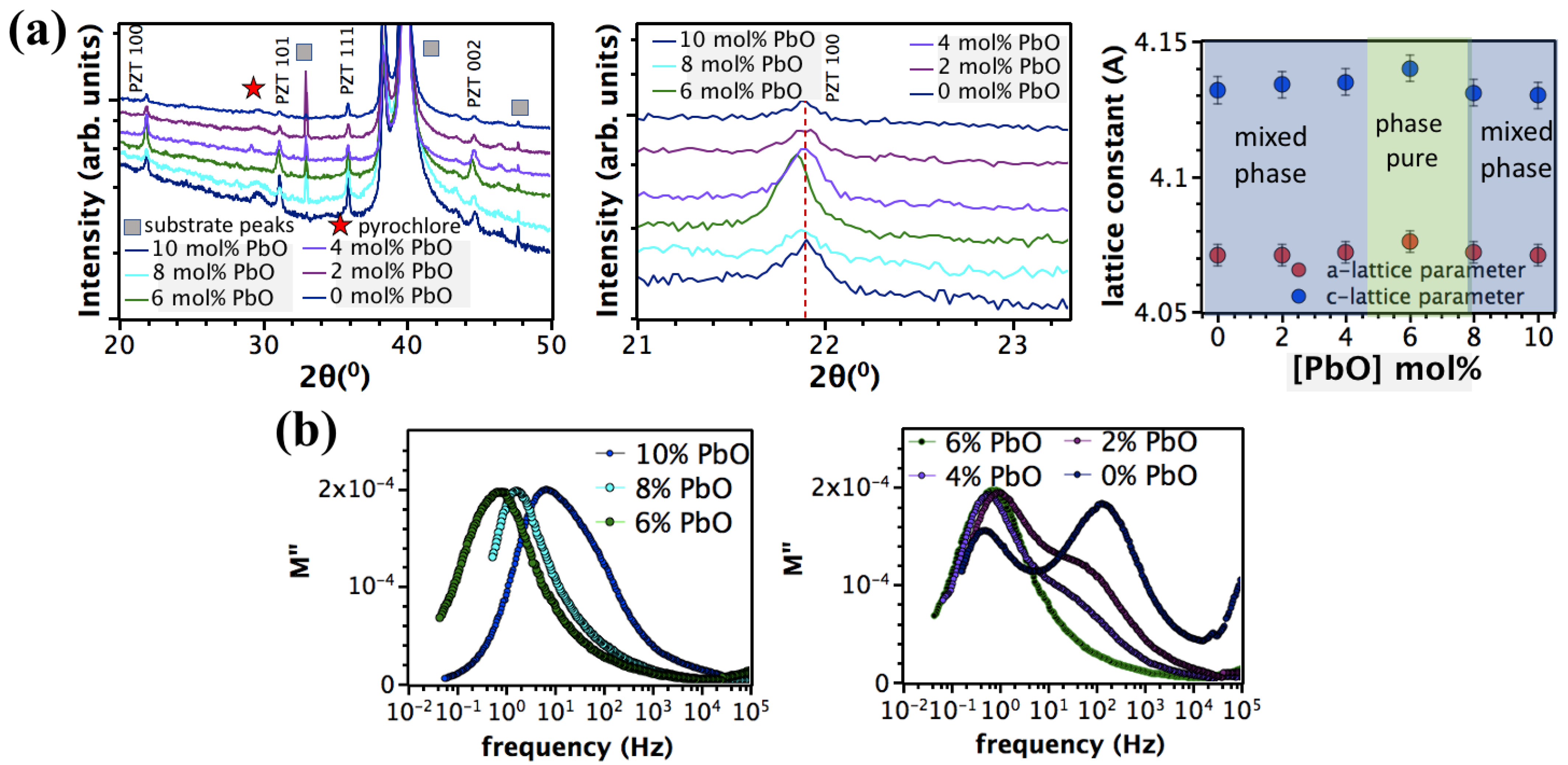
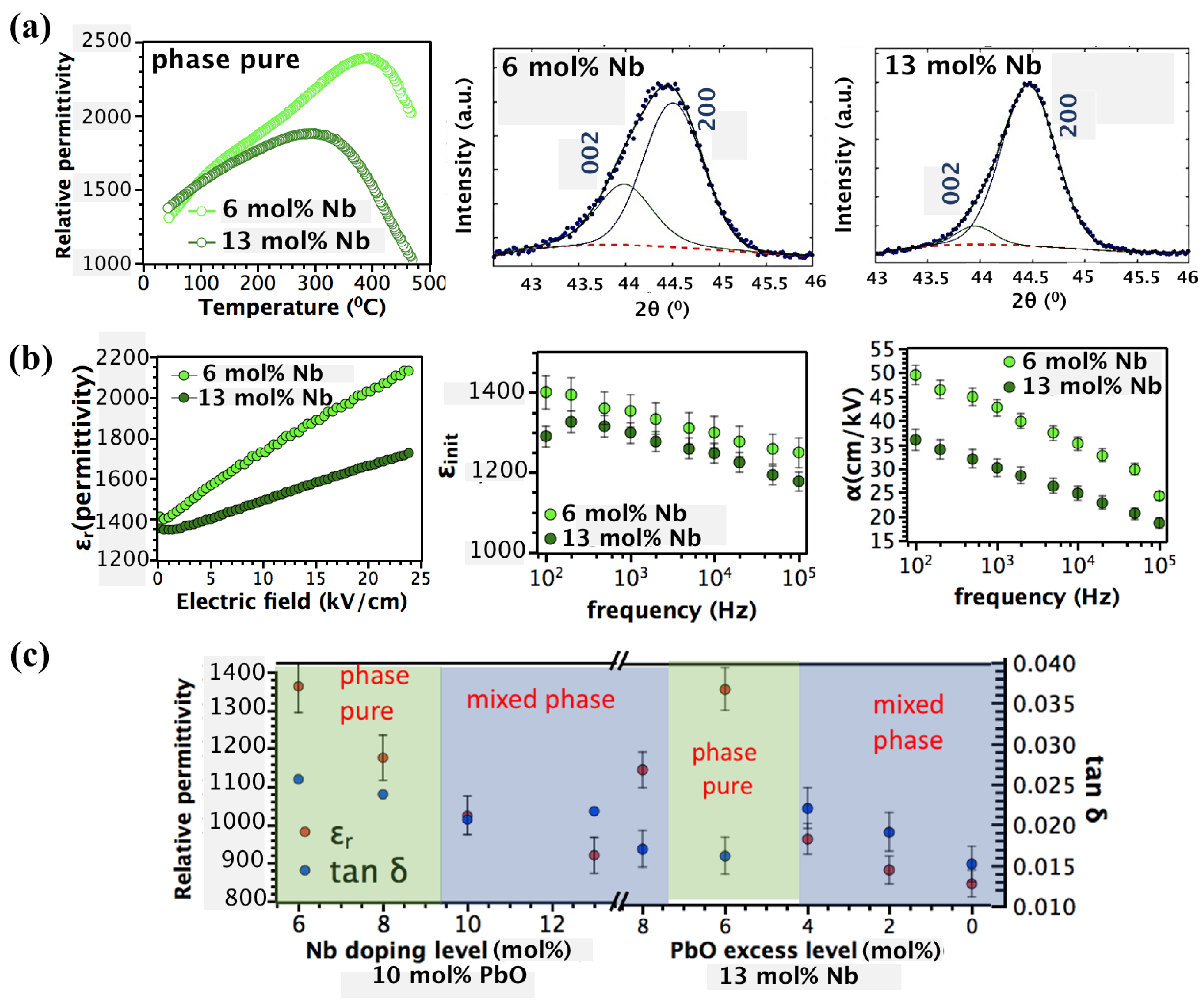
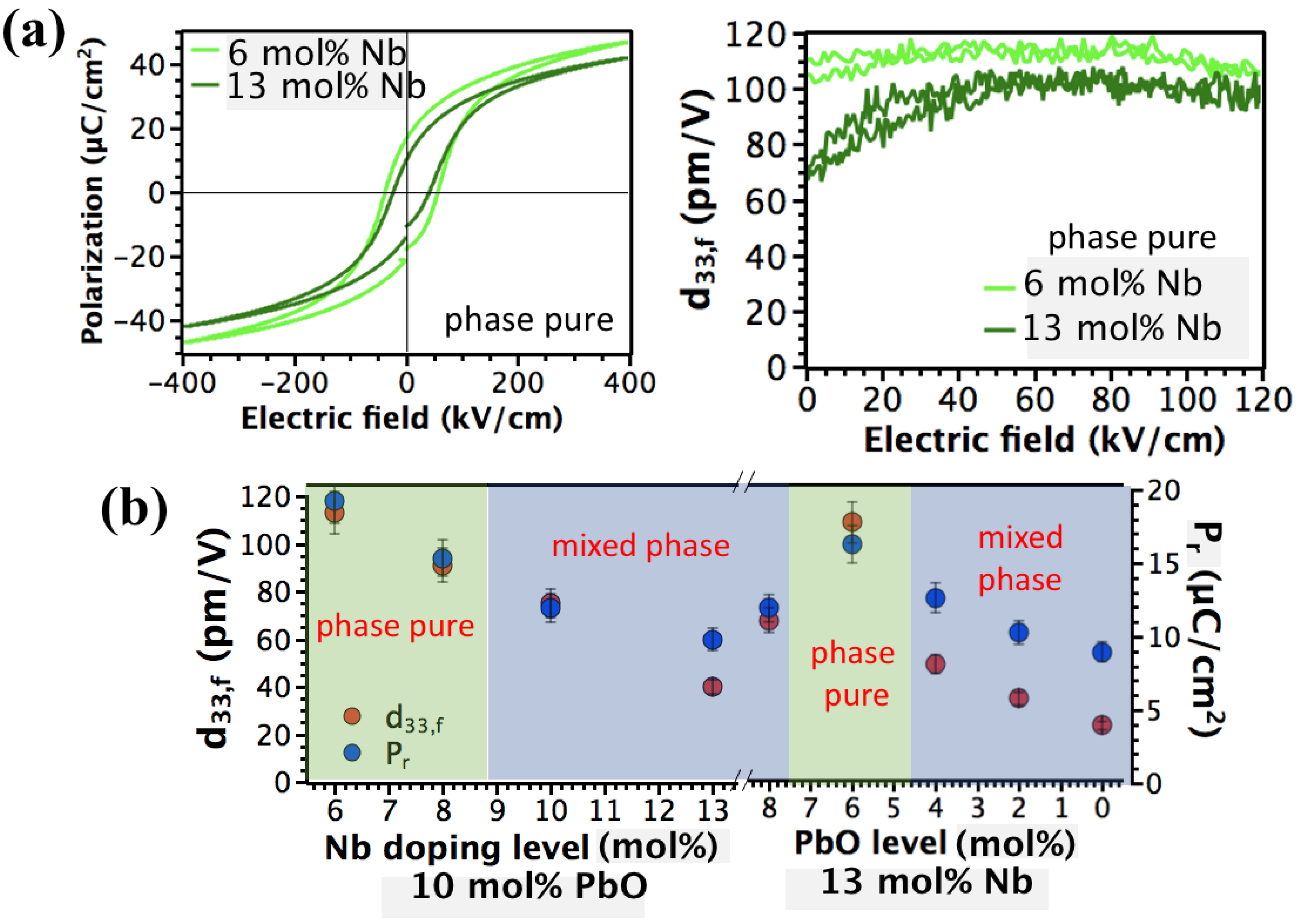
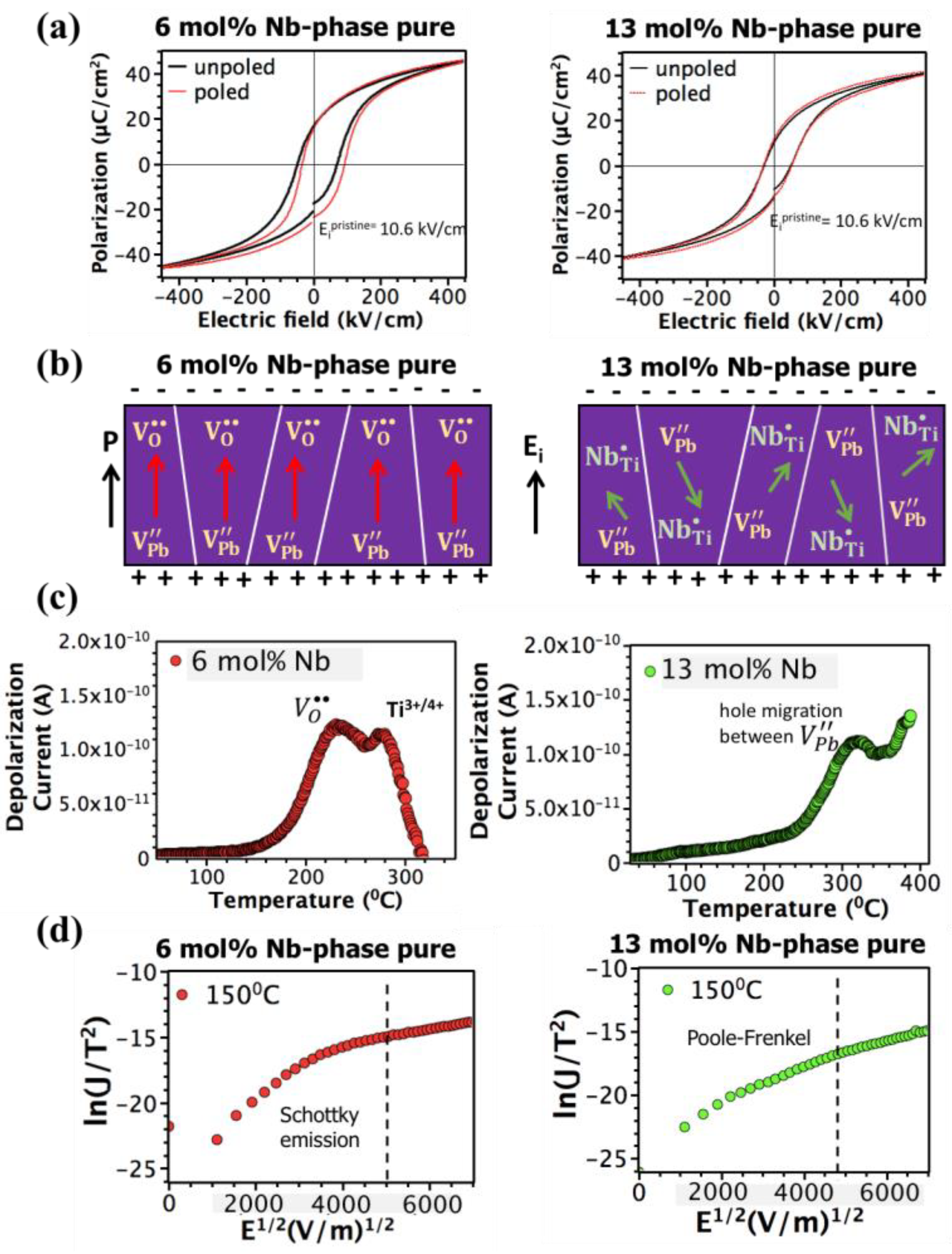
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muralt, P.; Polcawich, R.G.; Trolier-McKinstry, S. Piezoelectric thin films for sensors, actuators, and energy harvesting. MRS Bull. 2009, 34, 658–664. [Google Scholar] [CrossRef]
- Muralt, P.; Ledermann, N.; Baborowski, J.; Barzegar, A.; Gentil, S.; Belgacem, B.; Petitgrand, S.; Bosseboeuf, A.; Setter, N. Piezoelectric micromachined ultrasonic transducers based on PZT thin films. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Funakubo, H.; Dekkers, M.; Sambri, A.; Gariglio, S.; Shklyarevskiy, I.; Rijnders, G. Epitaxial PZT films for MEMS printing applications. MRS Bull. 2012, 37, 1030–1038. [Google Scholar] [CrossRef]
- Yeo, H.G.; Ma, X.; Rahn, C.; Trolier-McKinstry, S. Efficient piezoelectric energy harvesters utilizing (001) textured bimorph PZT films on flexible metal foils. Adv. Funct. Mater. 2016, 26, 5940–5946. [Google Scholar] [CrossRef]
- Jaffe, B.; Cook, W.R.; Jaffe, H. Piezoelectric Ceramics; Academic Press: New York, NY, USA, 1971; pp. 121–136. [Google Scholar]
- Tuttle, B.A.; Doughty, D.H.; Schwartz, R.W.; Garino, T.J.; Martinez, S.L.; Goodnow, D.; Tissot, R.G.; Hammetter, W.F. Chemically prepared PZT films with niobium additions. Ceram. Trans. 1999, 15, 179–191. [Google Scholar]
- Nguyen, M.D.; Houwman, E.P.; Dekkers, M.; Rijnders, G. Strongly enhanced piezoelectric response in lead zirconate titanate films with vertically aligned columnar grains. ACS Appl. Mater. Interfaces 2017, 9, 9849–9861. [Google Scholar] [CrossRef]
- Otani, Y.; Fukuda, Y.; Okamura, S.; Nakamura, K.; Nishida, T.; Uchiyama, K.; Shiosaki, T. Formation mechanism of oriented Pb(Zr, Ti)O3 thin films on platinum bottom electrodes from amorphous films prepared by liquid delivery metalorganic chemical vapor deposition. Jpn. J. Appl. Phys. 2009, 48, 09KA021–09KA024. [Google Scholar] [CrossRef]
- Bai, W.; Meng, X.J.; Lin, T.; Tian, L.; Bing, C.; Liu, W.J.; Ma, J.H.; Sun, J.L.; Chu, J.H. Effect of Fe-doping concentration on microstructure, electrical, and magnetic properties of Pb(Zr0.5Ti0.5)O3 thin films prepared by chemical solution deposition. J. Appl. Phys. 2009, 106, 124908. [Google Scholar] [CrossRef]
- Zhang, Q.; Whatmore, R.W. Improved ferroelectric and pyroelectric properties in Mn-doped lead zirconate titanate thin films. J. Appl. Phys. 2003, 94, 5228–5233. [Google Scholar] [CrossRef]
- Koh, D.; Ko, S.W.; Yang, J.I.; Akkopru-Akgun, B.; Trolier-McKinstry, S. Effect of Mg-doping and Fe-doping in lead zirconate titanate (PZT) thin films on electrical reliability. J. Appl. Phys. 2022, 132, 174101. [Google Scholar] [CrossRef]
- Kozielski, L.; Adamczyk, M.; Erhart, J.; Pawełczyk, M. Application testing of Sr doping effect of PZT ceramics on the piezoelectric transformer gain and efficiency proposed for MEMS actuators driving. J. Electroceramics 2012, 29, 133–138. [Google Scholar] [CrossRef]
- Zhang, M.F.; Wang, Y.; Wang, K.F.; Zhu, J.S.; Liu, J.-M. Characterization of oxygen vacancies and their migration in Ba-doped Pb (Zr0.52Ti0.48)O3 ferroelectrics. J. Appl. Phys. 2009, 105, 061639. [Google Scholar] [CrossRef]
- Sheng, T.; Narayanan, M.; Beihai, M.; Shanshan, L.; Koritala, R.E.; Balachandran, U.; Donglu, S. Effect of lanthanum content and substrate strain on structural and electrical properties of lead lanthanum zirconate titanate thin films. Mater. Chem. Phys. 2013, 140, 427–430. [Google Scholar]
- Atkin, R.B.; Holman, R.L.; Fulrath, R.M. Substitution of Bi and Nb ions in lead zirconate–titanate. J. Am. Ceram. Soc. 1971, 54, 113–115. [Google Scholar] [CrossRef]
- Choi, W.-Y.; Ahn, J.-H.; Lee, W.J.; Kim, H.-G. Electrical properties of Sb- doped PZT films deposited by d.c. reactive sputtering using multi-targets. Mater. Lett. 1998, 37, 119–127. [Google Scholar] [CrossRef]
- Robels, U.; Arlt, G. Domain wall clamping in ferroelectrics by orientation of defects. J. Appl. Phys. 1993, 73, 3454–3460. [Google Scholar] [CrossRef]
- Morozov, M.I.; Damjanovic, D. Charge migration in Pb(Zr,Ti)O3 ceramics and its relation to ageing, hardening, and softening. J. Appl. Phys. 2010, 107, 034106. [Google Scholar] [CrossRef]
- Grossmann, M.; Lohse, O.; Bolten, D.; Boettger, U.; Schneller, T. The interface screening model as origin of imprint in PbZrxTi1-xO3 thin films. I. dopant, illumination, and bias dependence. J. Appl. Phys. 2002, 92, 2680–2687. [Google Scholar] [CrossRef]
- Akkopru-Akgun, B.; Zhu, W.; Lanagan, M.T.; Trolier-McKinstry, S. The effect of imprint on remanent piezoelectric properties and ferroelectric aging of Mn or Nb doped PbZr0.52Ti0.48O3 thin films. J. Am. Ceram. Soc. 2019, 102, 5328–5341. [Google Scholar] [CrossRef]
- Akkopru-Akgun, B.; Bayer, T.; Tsuji, K.; Wang, K.; Randall, C.A.; Lanagan, M.T.; Trolier-McKinstry, S. Leakage current characteristics and DC resistance degradation mechanisms in Nb doped PZT films. J. Appl. Phys. 2021, 129, 174102. [Google Scholar] [CrossRef]
- Haccart, T.; Remiens, D.; Cattan, E. Substitution of Nb doping on the structural, microstructural and electrical properties in PZT films. Thin Solid Films 2003, 423, 235–242. [Google Scholar] [CrossRef]
- Kim, W.S.; Ha, S.-M.; Park, H.-H.; Kim, C.E. The effects of cation-substitution on the ferroelectric properties of sol-gel derived PZT thin film for FRAM application. Thin Solid Films 1999, 355–356, 531–535. [Google Scholar] [CrossRef]
- Zhu, W.; Fujii, I.; Ren, W.; Trolier-McKinstry, S. Domain wall motion in A and B site donor-doped Pb(Zr0.52Ti0.48)O3 Films. J. Am. Ceram. Soc. 2012, 95, 2906–2913. [Google Scholar] [CrossRef]
- Kwok, C.K.; Desu, S.B. Pyrochlore to perovskite phase transformation in sol-gel derived lead- zirconate-titanate thin films. Appl. Phys. Lett. 1992, 60, 1430–1432. [Google Scholar] [CrossRef]
- Souza, E.C.F.; Simões, A.Z.; Cilense, M.; Longo, E.; Varela, J.A. The effect of Nb doping on ferroelectric properties of PZT thin films prepared from polymeric precursors. Mater. Chem. Phys. 2004, 88, 155–159. [Google Scholar] [CrossRef]
- Paiva-Santos, C.O.; Oliveira, C.F.; Las, W.C.; Zaghete, M.A.; Varela, J.A.; Cilense, M. Effect of niobia on the crystal structure and dielectric characteristics of Pb(Zr0.45Ti0.55)O3 prepared from polymeric precursor. Mater. Res. Bull. 2000, 35, 15–24. [Google Scholar] [CrossRef]
- Kurchnia, R.; Milne, S.J. Effect of niobium modifications to PZT (53/47) thin films made by a sol-gel route. J. Sol-Gel Sci. Technol. 2003, 28, 143–150. [Google Scholar] [CrossRef]
- Araujo, E.B.; Eiras, J.A. Structural, dielectric and ferroelectric properties of Nb-doped PZT thin films produced by oxide precursor method. Ferroelectrics 2002, 270, 1237–1242. [Google Scholar] [CrossRef]
- Pereira, M.; Peixoto, A.G.; Gomes, M.J.M. Effect of Nb doping on the microstructural and electrical properties of the PZT ceramics. J. Eur. Ceram. Soc. 2001, 21, 1353–1356. [Google Scholar] [CrossRef]
- Mohiddon, M.A.; Kumar, R.; Goel, P.; Yadav, K.L. Effect of Nb doping on structural and electric properties of PZT (65/35) ceramic. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 204–211. [Google Scholar] [CrossRef]
- Chu, S.-Y.; Chen, T.-Y.; Tsai, I.-T.; Water, W. Doping effects of Nb additives on the piezoelectric and dielectric properties of PZT ceramics and its application on SAW device. Sens. Actuators A Phys. 2004, 113, 198–203. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Wang, F.; Dou, J.; Xu, W.; Zou, H. Effect of Nb doping on crystalline orientation, electric and fatigue properties of PZT thin films prepared by sol-gel process. J. Ceram. Sci. Technol. 2017, 08, 519–524. [Google Scholar]
- Klissurska, R.D.; Brooks, K.G.; Reaney, I.M.; Pawlaczyk, C.; Kosec, M.; Setter, N. Effect of Nb doping on the microstructure of sol-gel derived PZT thin films. J. Am. Ceram. Soc. 1995, 78, 1513–1520. [Google Scholar] [CrossRef]
- Fan, H.; Park, G.-T.; Choi, J.-J.; Kim, H.-E. Preparation and characterization of sol–gel-derived lead magnesium niobium titanate thin films with pure perovskite phase and lead oxide cover coat. J. Am. Ceram. Soc. 2002, 85, 2001–2004. [Google Scholar] [CrossRef]
- Lee, C.; Kawano, S.; Itoh, T.; Suga, T. Characteristics of sol-gel derived PZT thin films with lead oxide cover layers and lead titanate interlayers. J. Mater. Sci. 1996, 31, 4559–4568. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Ardvamudan, G.; Subba Rao, G.V. Oxide oyrochlores—A review. Prog. Solid State Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- Klissurska, R.D.; Tagantsev, A.K.; Brooks, K.G.; Setter, N. Use of ferroelectric hysteresis parameters for evaluation of niobium effects in lead zirconate titanate thin films. J. Am. Ceram. Soc. 1997, 80, 336–342. [Google Scholar] [CrossRef]
- M’peko, J.-C.; Peixoto, A.G.; Jiménez, E.; Gaggero-Sager, L.M. Electrical properties of Nb-doped PZT 65/35 ceramics: Influence of Nb and excess PbO. J. Electroceramics 2005, 15, 167–176. [Google Scholar] [CrossRef]
- Es-Souni, M.; Piorra, A.; Solterbeck, C.-H.; Iakovlev, S.; Abed, M. Microstructure and properties of solution deposited Nb-doped PZT thin films. J. Electroceramics 2002, 9, 125–135. [Google Scholar] [CrossRef]
- Fujii, T.; Hishinuma, Y.; Mita, T.; Arakawa, T. Preparation of Nb doped PZT film by RF sputtering. Solid State Commun. 2009, 149, 1799–1802. [Google Scholar] [CrossRef]
- Berenov, A.; Petrov, P.; Moffat, B.; Phair, J.; Allers, L.; Whatmore, R.W. Pyroelectric and photovoltaic properties of Nb-doped PZT thin films. APL Mater. 2021, 9, 041108. [Google Scholar] [CrossRef]
- Yang, J.-S.; Kang, Y.; Kang, I.; Lim, S.; Shin, S.-J.; Lee, J.; Hur, K.H. Comparison of the thermal degradation of heavily Nb-doped and normal PZT thin films. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Han, C.S.; Park, K.S.; Choi, H.J.; Cho, Y.S. Origin of in situ domain formation of heavily Nb-doped Pb(Zr,Ti)O3 thin films sputtered on Ir/TiW/SiO2/Si substrates for mobile sensor applications. ACS Appl. Mater. Interfaces 2017, 9, 18904–18910. [Google Scholar] [CrossRef] [PubMed]
- Akkopru-Akgun, B.; Wang, K.; Trolier-McKinstry, S. Links between defect chemistry, conduction, and lifetime in heavily Nb doped lead zirconate titanate films. Appl. Phys. Lett. 2022, 121, 162903. [Google Scholar] [CrossRef]
- Ozer, N.; Sands, T. Preparation and optical characterization of sol-gel deposited Pb(Zr0.45Ti0.55)O3 Films. J. Sol-Gel Sci. Technol. 2000, 19, 157–162. [Google Scholar] [CrossRef]
- Benkler, M.; Paul, F.; Schott, J.; Hanemann, T. Ferroelectric thin film fabrication by direct UV-lithography. Microsyst. Technol. 2014, 20, 1859–1867. [Google Scholar] [CrossRef]
- Lang, D.V. Deep-level transient spectroscopy: A new method to characterize traps in Semiconductors. J. Appl. Phys. 1974, 45, 3023–3032. [Google Scholar] [CrossRef]
- Pintilie, L.; Pereira, M.; Gomes, M.J.M.; Boerasu, I. Pyroelectric current spectroscopy: Example of application on Nb doped Pb (Zr0.92Ti0.08)O3 ceramic for infrared detection. Sens. Actuators A Phys. 2004, 115, 185–190. [Google Scholar] [CrossRef]
- Muralt, P. Piezoelectric thin films for MEMS. Integr. Ferroelectr. 1997, 17, 297–307. [Google Scholar] [CrossRef]
- Fox, G.R.; Krupanidhi, S.B. Dependence of perovskite/pyrochlore phase formation on oxygen stoichiometry in PLT thin films. J. Mater. Res. 1994, 9, 699–711. [Google Scholar] [CrossRef]
- Chen, S.; Chen, I. Phase transformations of oriented Pb (Zr1−xTix)O3 thin films from Metallo-Organic Precursors. Ferroelectrics 1994, 152, 25–30. [Google Scholar] [CrossRef]
- Bouregba, R.; Poullain, G.; Vilquin, B.; Murray, H. Orientation control of textured PZT thin films sputtered on silicon substrate with TiOx seeding. Mater. Res. Bull. 2000, 35, 1381–1390. [Google Scholar] [CrossRef]
- Brooks, K.G.; Reaney, I.M.; Klissurska, R.; Huang, Y.; Bursill, L.; Setter, N. Orientation of rapid thermally annealed lead zirconate titanate thin films on (111) Pt substrates. J. Mater. Res. 1994, 9, 2540–2553. [Google Scholar] [CrossRef]
- Sreenivas, K.; Reaney, I.; Maeder, T.; Setter, N.; Jagadish, C.; Elliman, R.G. Investigation of Pt/Ti bilayer metallization on silicon for ferroelectric thin film integration. J. Appl. Phys. 1994, 75, 232–239. [Google Scholar] [CrossRef]
- Ryder, D.F.; Raman, N.K. Sol-gel processing of Nb-doped Pb(Zr, Ti)O3 thin films for ferroelectric memory applications. J. Electron. Mater. 1992, 21, 971–975. [Google Scholar] [CrossRef]
- Shakeri, A.; Abdizadeh, H.; Golobostanfard, M.R. Fabrication of Nb-doped lead zirconate titanate thick films synthesized by sol–gel dip coating method. J. Mater. Sci. Mater. Electron. 2016, 27, 5654–5664. [Google Scholar] [CrossRef]
- Robertson, J.; Warren, W.L.; Tuttle, B.A.; Dimos, D.; Smyth, D.M. Shallow Pb3+ hole traps in lead zirconate titanate ferroelectrics. Appl. Phys. Lett. 1993, 63, 1519–1521. [Google Scholar] [CrossRef]
- Dih, J.J.; Fulrath, R.M. Electrical conductivity in lead zirconate-titanate ceramics. J. Am. Ceram. Soc. 1978, 61, 448–451. [Google Scholar] [CrossRef]
- Raymond, M.V.; Smyth, D.M. Defects and charge transport in perovskite ferroelectrics. J. Phys. Chem. Solids 1996, 57, 1507–1511. [Google Scholar] [CrossRef]
- Akkopru-Akgun, B.; Zhu, W.; Randall, C.A.; Lanagan, M.T.; Trolier-McKinstry, S. Polarity dependent DC resistance degradation and electrical breakdown in Nb doped PZT films. APL Mater. 2019, 7, 120901. [Google Scholar] [CrossRef]
- Esteves, G.; Ramos, K.; Fancher, C.M.; Jones, J.L. LIPRAS: Line-Profile Analysis Software. 2017. Available online: https://www.researchgate.net/publication/316985889_LIPRAS_Line-Profile_Analysis_Software?channel=doi&linkId=592f83000f7e9beee7619156&showFulltext=true (accessed on 24 April 2023).
- Sun, H.; Zhang, Y.; Liu, X.; Guo, S.; Liu, Y.; Chen, W. The effect of Mn/Nb doping on dielectric and ferroelectric properties of PZT thin films prepared by sol–gel process. J. Sol-Gel Sci. Technol. 2015, 74, 378–386. [Google Scholar] [CrossRef]
- Kayasu, V.; Ozenbas, M. The effect of Nb doping on dielectric and ferroelectric properties of PZT thin films prepared by solution deposition. J. Eur. Ceram. Soc. 2009, 29, 1157–1163. [Google Scholar] [CrossRef]
- Erdem, E.; Eichel, R.-A.; Kungl, H.; Hoffmann, M.J.; Ozarowski, A.; Van Tol, J.; Brunel, L.C. Characterization of defect dipoles in (La,Fe)-codoped PZT 52.5/47.5 piezoelectric ceramics by multifrequency electron paramagnetic resonance spectroscopy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 1061–1068. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.J.; Hong, J.G.; Streiffer, S.K.; Kingon, A.I. Imprint and fatigue properties of chemical solution derived Pb1−xLax(ZryTi1−y)1−x/4O3 thin films. J. Mater. Res. 1999, 14, 1371–1377. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkopru-Akgun, B.; Trolier-McKinstry, S. Tuning Nb Solubility, Electrical Properties, and Imprint through PbO Stoichiometry in PZT Films. Materials 2023, 16, 3970. https://doi.org/10.3390/ma16113970
Akkopru-Akgun B, Trolier-McKinstry S. Tuning Nb Solubility, Electrical Properties, and Imprint through PbO Stoichiometry in PZT Films. Materials. 2023; 16(11):3970. https://doi.org/10.3390/ma16113970
Chicago/Turabian StyleAkkopru-Akgun, Betul, and Susan Trolier-McKinstry. 2023. "Tuning Nb Solubility, Electrical Properties, and Imprint through PbO Stoichiometry in PZT Films" Materials 16, no. 11: 3970. https://doi.org/10.3390/ma16113970




