The Combined Effects of Hydraulic Calcium Silicate Cement and Enamel Matrix Derivative Regarding Osteogenic and Dentinogenic Differentiation on Human Dental Pulp Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Dental Pulp Stem Cells (hDPSCs)
2.2. Production of Experimental MTA, HCSC Disks, or Eluates
2.3. Classification of the Groups
2.4. Cell Toxicity Measurement
2.5. Live/Dead Staining Assay
2.6. Alkaline Phosphatase (ALP) Activity
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT–PCR)
2.8. Alizarin Red-S (ARS) Staining Assay
2.9. Statistical Analysis
3. Results
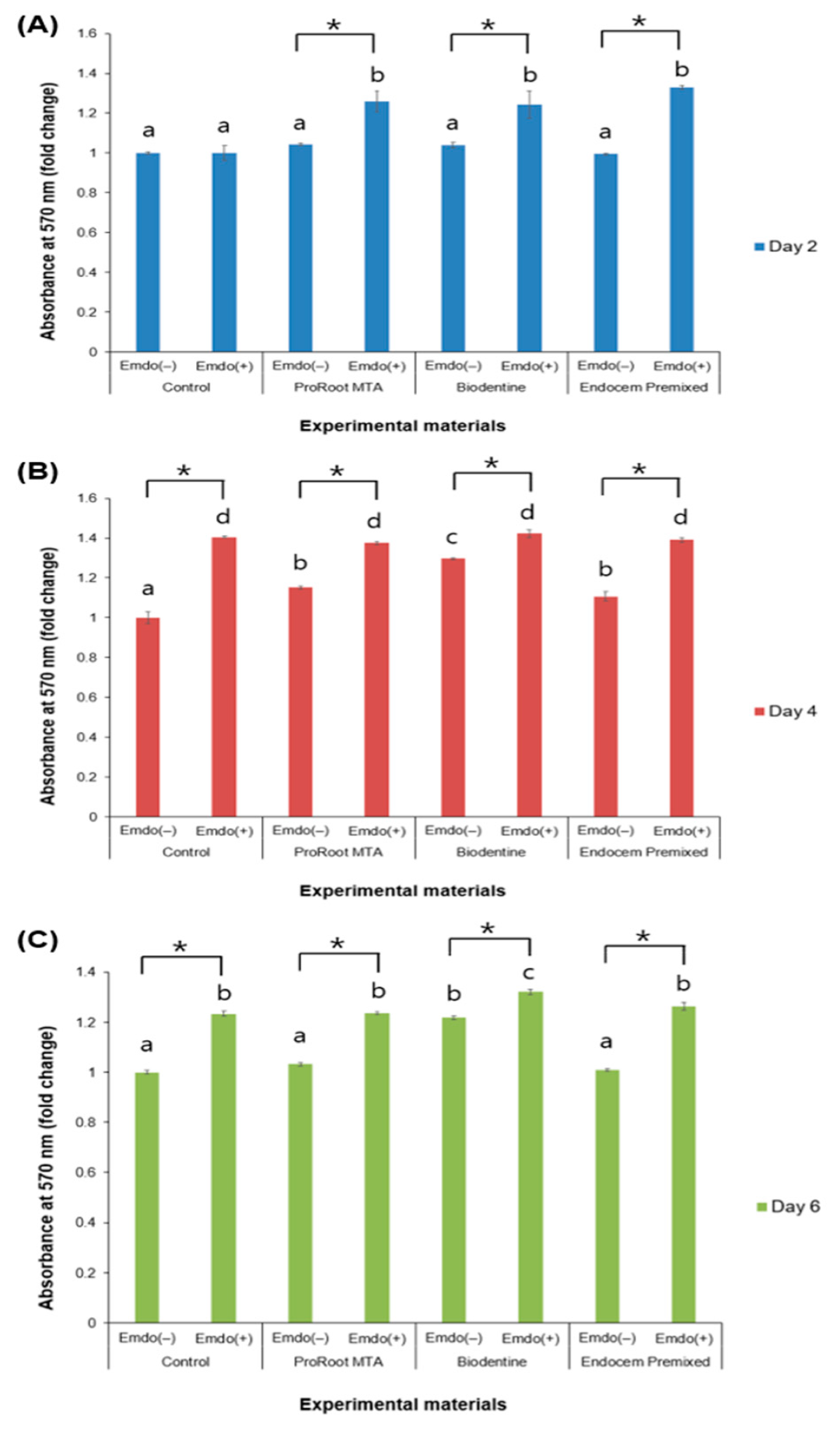
3.1. Cell Toxicity Measurement
3.2. Live/Dead Staining Analysis
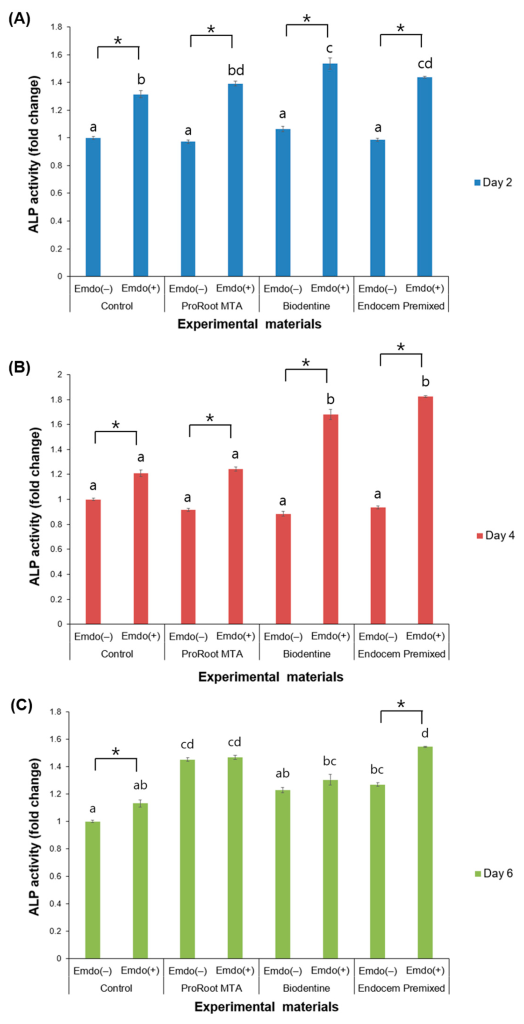
3.3. ALP Activity
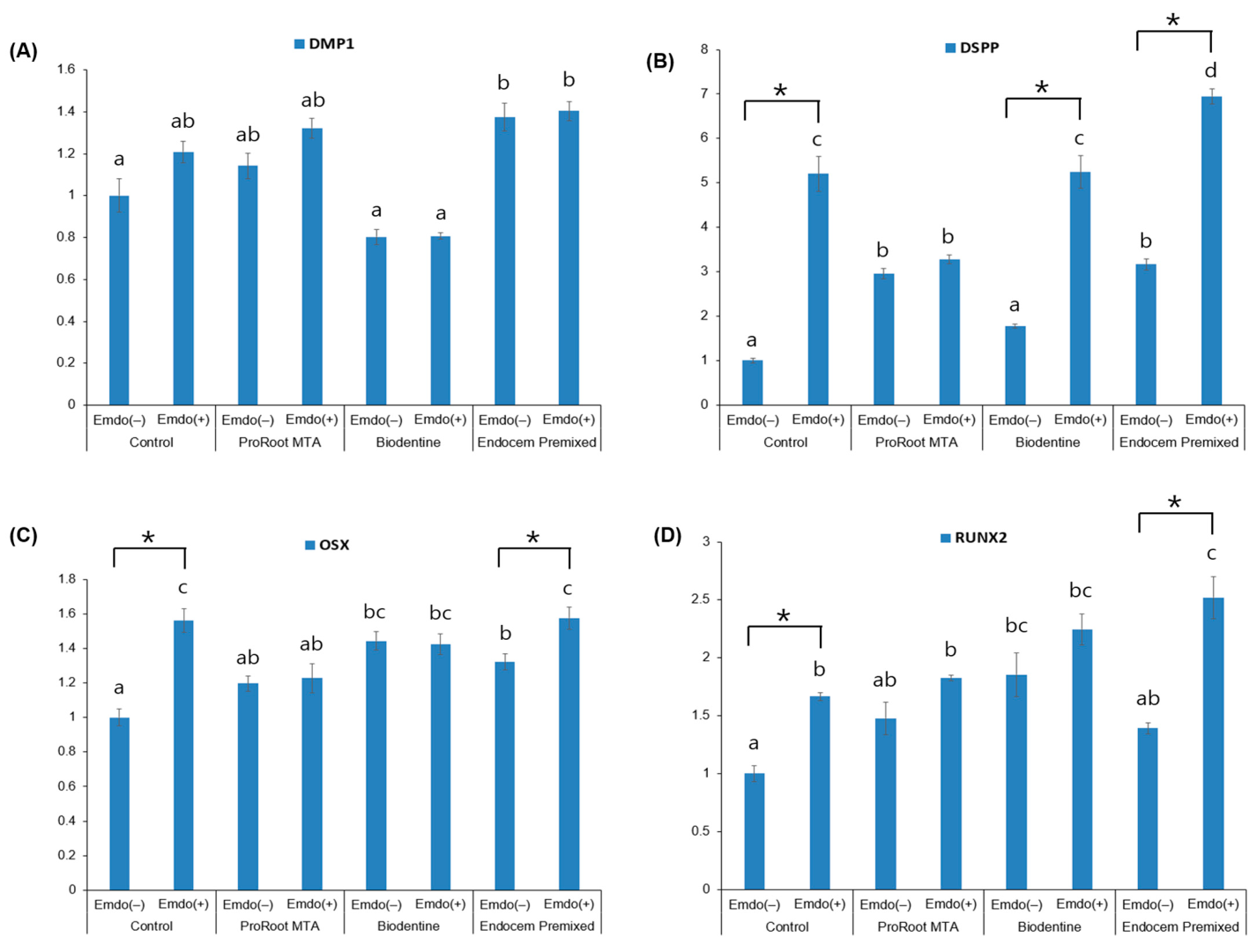
3.4. qRT–PCR
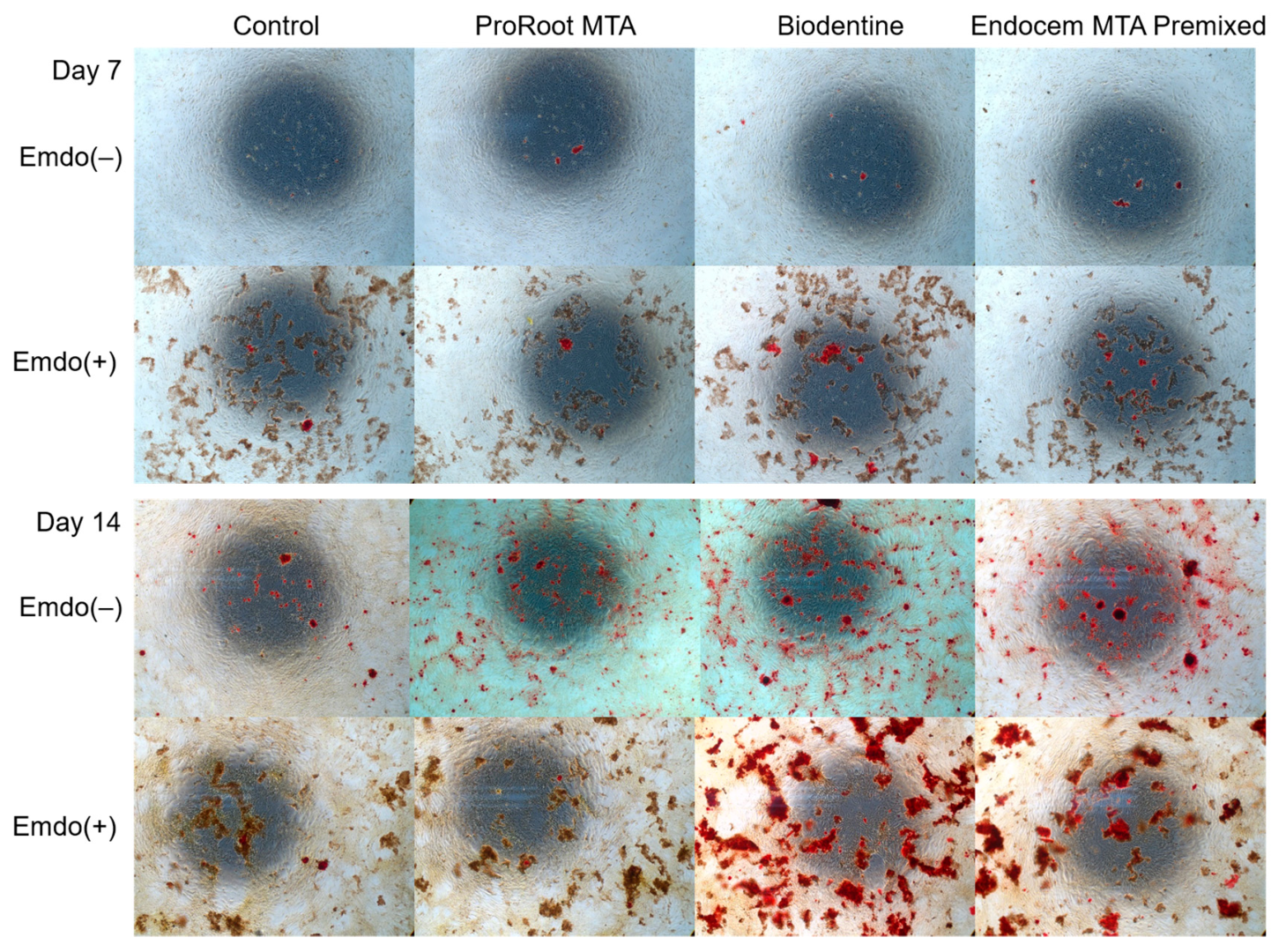
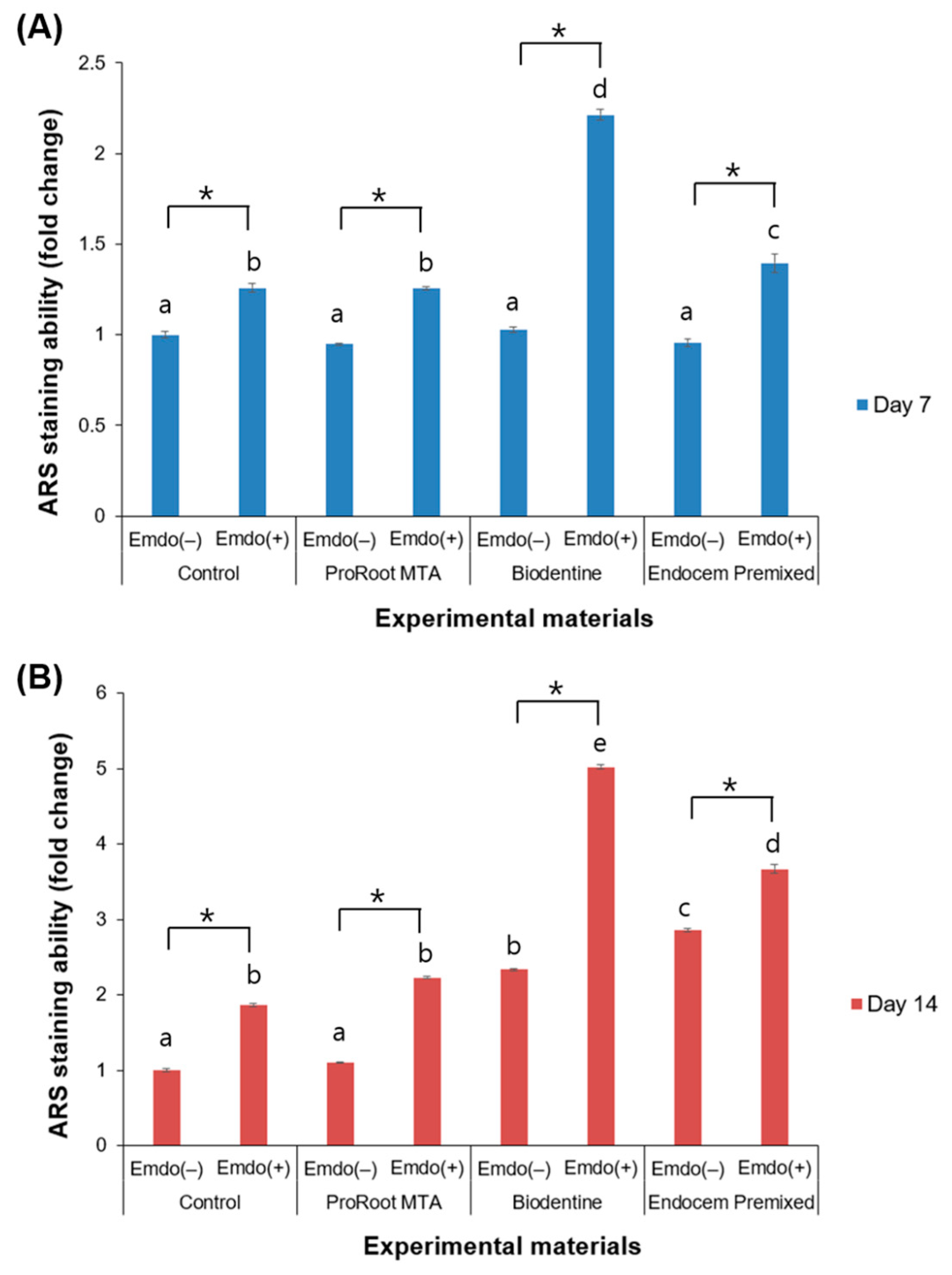
3.5. ARS Staining Assay
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Rossi-Fedele, G.; Chugal, N.; Lin, L.M. An evidence-based review of the efficacy of treatment approaches for immature permanent teeth with pulp necrosis. J. Endod. 2017, 43, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part I: Chemical, physical, and antibacterial properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.R.; Emara, R.; Taher, M.M.; Al-Allaf, F.A.; Almalki, M.; Almasri, M.A.; Siddiqui, S.S. Effects of mineral trioxide aggregate, calcium hydroxide, Biodentine and Emdogain on osteogenesis, odontogenesis, angiogenesis and cell viability of dental pulp stem cells. BMC Oral Health 2019, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Ber, B.S.; Hatton, J.F.; Stewart, G.P. Chemical modification of ProRoot MTA to improve handling characteristics and decrease setting time. J. Endod. 2007, 33, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.W.; Toth, J.M.; Berzins, D.W.; Charlton, D.G. Mineral trioxide aggregate material use in endodontic treatment: A review of the literature. Dent. Mater. 2008, 24, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of human dental pulp capped with Biodentine and mineral trioxide aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef]
- Malkondu, Ö.; Kazandağ, M.K.; Kazazoğlu, E. A review on Biodentine, a contemporary dentine replacement and repair material. Biomed Res. Int. 2014, 2014, 160951. [Google Scholar] [CrossRef]
- Shin, M.; Chen, J.W.; Tsai, C.Y.; Aprecio, R.; Zhang, W.; Yochim, J.M.; Teng, N.; Torabinejad, M. Cytotoxicity and antimicrobial effects of a new fast-set MTA. Biomed Res. Int. 2017, 2017, 2071247. [Google Scholar] [CrossRef]
- Chung, H.; Yang, W.; Kim, M.; Ko, H. Comparison of the effects of enamel matrix derivative and mineral trioxide aggregate on the mineralization potential of human cementum-derived cells. J. Dent. Sci. 2011, 6, 153–157. [Google Scholar] [CrossRef]
- Matarasso, M.; Iorio-Siciliano, V.; Blasi, A.; Ramaglia, L.; Salvi, G.E.; Sculean, A. Enamel matrix derivative and bone grafts for periodontal regeneration of intrabony defects. A systematic review and meta-analysis. Clin. Oral Investig. 2015, 19, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Ferrarotti, F.; Mariani, G.M.; Romano, F. A novel flapless approach versus minimally invasive surgery in periodontal regeneration with enamel matrix derivative proteins: A 24-month randomized controlled clinical trial. Clin. Oral Investig. 2017, 21, 327–337. [Google Scholar] [CrossRef]
- Lossdörfer, S.; Sun, M.; Götz, W.; Dard, M.; Jäger, A. Enamel matrix derivative promotes human periodontal ligament cell differentiation and osteoprotegerin production in vitro. J. Dent. Res. 2007, 86, 980–985. [Google Scholar] [CrossRef]
- Wang, H.H.; Sarmast, N.D.; Shadmehr, E.; Angelov, N.; Shabahang, S.; Torabinejad, M. Application of enamel matrix derivative (Emdogain) in endodontic therapy: A comprehensive literature review. J. Endod. 2018, 44, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Ge, L. Effects of the enamel matrix derivative on the proliferation and odontogenic differentiation of human dental pulp cells. J. Dent. 2014, 42, 53–59. [Google Scholar] [CrossRef]
- Fransson, H.; Petersson, K.; Davies, J.R. Dentine sialoprotein and collagen I expression after experimental pulp capping in humans using Emdogain gel. Int. Endod. J. 2011, 44, 259–267. [Google Scholar] [CrossRef]
- Scarparo, R.K.; Dondoni, L.; Böttcher, D.E.; Grecca, F.S.; Figueiredo, J.A.; Batista, E.L., Jr. Apical periodontium response to enamel matrix derivative as an intracanal medication in rat immature teeth with pulp necrosis: Radiographic and histologic findings. J. Endod. 2012, 38, 449–453. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Kim, H.M.; Kye, M.; Kim, S.Y. Biological characteristics and odontogenic differentiation effects of calcium silicate-based pulp capping materials. Materials 2021, 14, 4661. [Google Scholar] [CrossRef]
- Shah, A.; Peacock, R.; Eliyas, S. Pulp therapy and root canal treatment techniques in immature permanent teeth: An update. Br. Dent. J. 2022, 232, 524–530. [Google Scholar] [CrossRef]
- Gorduysus, M.; Avcu, N.; Gorduysus, O.; Pekel, A.; Baran, Y.; Avcu, F.; Ural, A.U. Cytotoxic effects of four different endodontic materials in human periodontal ligament fibroblasts. J. Endod. 2007, 33, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Jafarnia, B.; Jiang, J.; He, J.; Wang, Y.H.; Safavi, K.E.; Zhu, Q. Evaluation of cytotoxicity of MTA employing various additives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Hirschman, W.R.; Wheater, M.A.; Bringas, J.S.; Hoen, M.M. Cytotoxicity comparison of three current direct pulp-capping agents with a new bioceramic root repair putty. J. Endod. 2012, 38, 385–388. [Google Scholar] [CrossRef]
- Simon, S.R.; Tomson, P.L.; Berdal, A. Regenerative endodontics: Regeneration or repair? J. Endod. 2014, 40, S70–S75. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Ali, M.R.W.; Mustafa, M.; Bårdsen, A.; Bletsa, A. Tricalcium silicate cements: Osteogenic and angiogenic responses of human bone marrow stem cells. Eur. J. Oral Sci. 2019, 127, 261–268. [Google Scholar] [CrossRef]
- Chang, S.W.; Lee, S.Y.; Kum, K.Y.; Kim, E.C. Effects of ProRoot MTA, Bioaggregate, and Micromega MTA on odontoblastic differentiation in human dental pulp cells. J. Endod. 2014, 40, 113–118. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Viji Chandran, S.; Arumugam, B.; Saravanan, S.; Venkatasubbu, G.D.; Selvamurugan, N. Chitosan/nano-hydroxyapatite/nano-zirconium dioxide scaffolds with miR-590-5p for bone regeneration. Int. J. Biol. Macromol. 2018, 111, 953–958. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Kye, M.; Ha, Y.J.; Kim, S.Y. Biocompatible properties and mineralization potential of premixed calcium silicate-based cements and fast-set calcium silicate-based cements on human bone marrow-derived mesenchymal stem cells. Materials 2022, 15, 7595. [Google Scholar] [CrossRef]
- Jacob, A.; Zhang, Y.; George, A. Transcriptional regulation of dentin matrix protein 1 (DMP1) in odontoblasts and osteoblasts. Connect. Tissue Res. 2014, 55 (Suppl. 1), 107–112. [Google Scholar] [CrossRef]
- Simon, S.; Smith, A.J.; Lumley, P.J.; Berdal, A.; Smith, G.; Finney, S.; Cooper, P.R. Molecular characterization of young and mature odontoblasts. Bone 2009, 45, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Haruyama, N.; Nishimura, F.; Kulkarni, A.B. Dentin sialophosphoprotein and dentin matrix protein-1: Two highly phosphorylated proteins in mineralized tissues. Arch. Oral Biol. 2012, 57, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Wu, K.C.; Lee, A.; Saunders, T.L.; Ritchie, H.H. DSPP dosage affects tooth development and dentin mineralization. PLoS ONE 2021, 16, e0250429. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, N.; Fan, Y.; Wang, Y.; Gu, Y.; Li, Z.; Pan, Y.; Romila, G.; Zhou, Z.; Yu, J. The conditioned medium of calcined tooth powder promotes the osteogenic and odontogenic differentiation of human dental pulp stem cells via MAPK signaling pathways. Stem Cells Int. 2019, 2019, 4793518. [Google Scholar] [CrossRef]
- Wei, X.; Li, J.; Liu, H.; Niu, C.; Chen, D. Salidroside promotes the osteogenic and odontogenic differentiation of human dental pulp stem cells through the BMP signaling pathway. Exp. Ther. Med. 2022, 23, 55. [Google Scholar] [CrossRef]
- Camilleri, S.; McDonald, F. Runx2 and dental development. Eur. J. Oral Sci. 2006, 114, 361–373. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kanatani, N.; Rokutanda, S.; Yoshida, C.; Toyosawa, S.; Nakamura, R.; Takada, S.; Komori, T. Inhibition of the terminal differentiation of odontoblasts and their transdifferentiation into osteoblasts in Runx2 transgenic mice. Arch. Histol. Cytol. 2008, 71, 131–146. [Google Scholar] [CrossRef]
- Cohenca, N.; Paranjpe, A.; Berg, J. Vital pulp therapy. Dent. Clin. N Am. 2013, 57, 59–73. [Google Scholar] [CrossRef]
- Zhao, X.; He, W.; Song, Z.; Tong, Z.; Li, S.; Ni, L. Mineral trioxide aggregate promotes odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp stem cells. Mol. Biol. Rep. 2012, 39, 215–220. [Google Scholar] [CrossRef]
- Mounir, M.M.F.; Rashed, F.M.; Bukhary, S.M. Amelogenin as a regenerative endodontic molecule for immature teeth with apical periodontitis. An experimental study. J. Oral Biol. Craniofac. Res. 2022, 12, 721–726. [Google Scholar] [CrossRef]
- Kato, H.; Taguchi, Y.; Yamawaki, I.; Ruan, Y.; Wu, Q.; Nakano, Y.; Tsumori, N.; Nakata, T.; Noguchi, M.; Umeda, M. Amelogenin exon 5 peptide promotes cell proliferation and osteogenic differentiation in human dental pulp stem cells. Appl. Sci. 2019, 9, 4425. [Google Scholar] [CrossRef]
- Zhang, B.; Xiao, M.; Cheng, X.; Bai, Y.; Chen, H.; Yu, Q.; Qui, L. Enamel matrix derivative enhances the odontoblastic differentiation of dental pulp stem cells via activating MAPK signaling pathways. Stem Cell Int. 2022, 2022, 2236250. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Grandin, H.M.; Gemperli, A.C.; Dard, M. Enamel matrix derivative: A review of cellular effects in vitro and a model of molecular arrangement and functioning. Tissue Eng. Part B Rev. 2012, 18, 181–202. [Google Scholar] [CrossRef] [PubMed]


| Material | Manufacturer | Composition | Final Setting Time | Batch Number |
|---|---|---|---|---|
| ProRoot MTA | Dentsply Tulsa Dental Specialties, Tulsa, OK, USA | Portland cement (tricalcium silicate, dicalcium silicate, and tricalcium aluminate) 75% | 261 min | 0000176943 |
| Calcium sulfate dehydrate (gypsum) 5% | ||||
| Bismuth oxide 20% | ||||
| Biodentine | Septodont, Saint-Maur-des-Fosses Cedex, France | Tricalcium silicate 80.1% | 12 min | B24553 |
| Calcium carbonate 14.9% | ||||
| Zirconium oxide 5% | ||||
| Calcium chloride | ||||
| Soluble polymer as an aqueous liquid | ||||
| Endocem MTA Premixed | Maruchi, Wonju, Republic of Korea | Zirconium dioxide 45–55% | 27 min | FS200831A |
| Calcium silicate 20–25% | ||||
| Calcium aluminate 1–5% | ||||
| Calcium sulfate 1–5% | ||||
| Dimethyl sulfoxide 20–25% | ||||
| Thickening agent 1–5% | ||||
| Emdogain gel | Straumann, Basel, Switzerland | Amelogenin 90% | FMN92 | |
| Remaining percentage is proline-rich non-amelogenin, tuftelins, tuft proteins, ameloblastin, amelins |
| Runt-related transcription factor 2 (RUNX2) | Forward: 5′-AAG TGC GGT GCA AAC TTT CT-3′ |
| Reverse: 5′-TCT CGG TGG CTG CTA GTG A-3 | |
| Osterix (OSX) | Forward: 5′-AGC CTC TGG CTA TGC AAA TGA-3′ |
| Reverse: 5′-TGT AGA CAC TAG GCA GGC AGT CA-3 | |
| Dentin matrix protein-1 (DMP-1) | Forward: TGG TCC CAG CAG TGA GTC CA |
| Reverse: TGT GTG CGA GCT GTC CTC CT | |
| Dentin sialophosphoprotein (DSPP) | Forward: 5′-GGG AAT ATT GAG GGC TGG AA-3′ |
| Reverse: 5′-TCA TTG TGA CCT GCA TCG CC-3 ′ | |
| GAPDH | Forward: GAA GGT GAA GGT CGG AGT C |
| Reverse: GAG ATG GTG ATG GGA TTT C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yune, J.-Y.; Lee, D.; Kim, S.-Y. The Combined Effects of Hydraulic Calcium Silicate Cement and Enamel Matrix Derivative Regarding Osteogenic and Dentinogenic Differentiation on Human Dental Pulp Stem Cells. Materials 2023, 16, 4003. https://doi.org/10.3390/ma16114003
Yune J-Y, Lee D, Kim S-Y. The Combined Effects of Hydraulic Calcium Silicate Cement and Enamel Matrix Derivative Regarding Osteogenic and Dentinogenic Differentiation on Human Dental Pulp Stem Cells. Materials. 2023; 16(11):4003. https://doi.org/10.3390/ma16114003
Chicago/Turabian StyleYune, Ji-Young, Donghee Lee, and Sin-Young Kim. 2023. "The Combined Effects of Hydraulic Calcium Silicate Cement and Enamel Matrix Derivative Regarding Osteogenic and Dentinogenic Differentiation on Human Dental Pulp Stem Cells" Materials 16, no. 11: 4003. https://doi.org/10.3390/ma16114003






