N-Doped Graphene-like Film/Silicon Structures as Micro-Capacitor Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. N-GLF Deposition
2.2. Measurements
2.3. Characterization
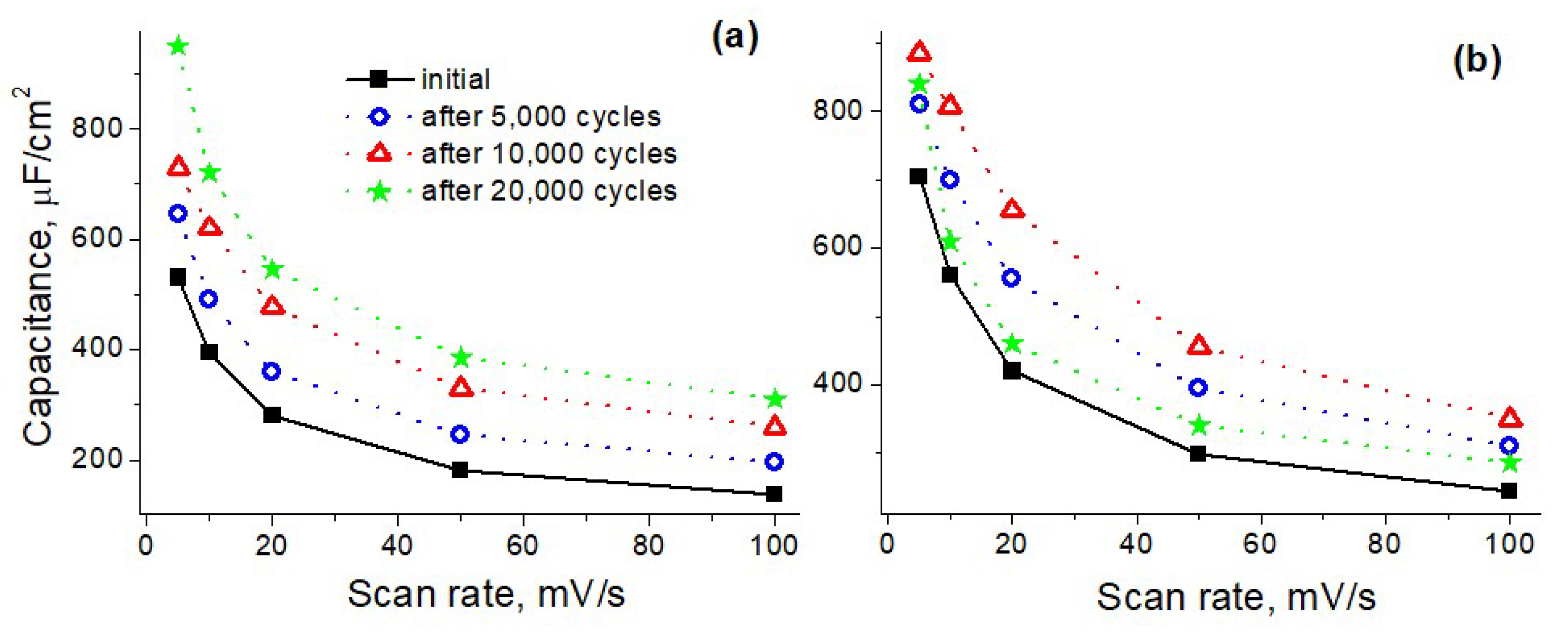
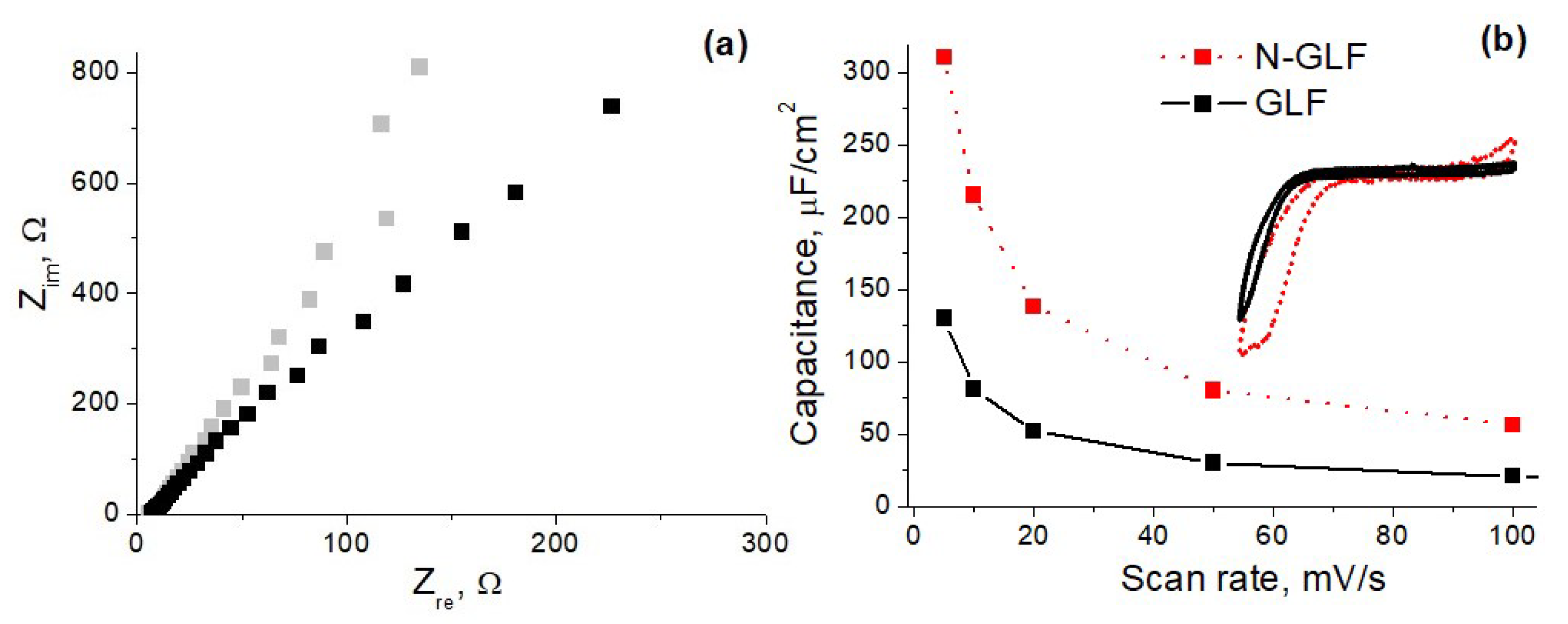
3. Results and Discussions
4. Results Evaluating
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H.; Mousavi, M.F.; Kaner, R.B. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.F.; Raza, M.A.; Rehman, Z.U.; Tayyeb, A.; Makhdoom, M.A.; Ghafoor, F.; Latif, U.; Khan, M.F. Role of solvent used in development of graphene oxide coating on az31b magnesium alloy: Corrosion behavior and biocompatibility analysis. Nanomaterials 2022, 12, 3745. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Zhao, Y.; Zhang, J.; Qu, L. Graphene materials for miniaturized energy harvest and storage devices. Small Struct. 2022, 3, 2100124. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Yu, H.; Liu, W.; Kuang, G.; Mei, L.; Wu, Z.; Wei, W.; Ji, X.; Qu, B. A multifunctional artificial interphase with fluorine-doped amorphous carbon layer for ultra-stable zn anode. Adv. Funct. Mater. 2022, 32, 2205600. [Google Scholar] [CrossRef]
- Wang, J.; Wang, N.; Xu, D.; Tang, L.; Sheng, B. Flexible humidity sensors composed with electrodes of laser induced graphene and sputtered sensitive films derived from poly (ether-ether-ketone). Sens. Actuators B Chem. 2023, 375, 132846. [Google Scholar] [CrossRef]
- Malik, M.T.U.; Sarker, A.; Rahat, S.S.M.; Shuchi, S.B. Performance enhancement of graphene/go/rgo based supercapacitors: A comparative review. Mater. Today Commun. 2021, 28, 102685. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef]
- Lee, M.S.; Choi, H.-J.; Baek, J.-B.; Chang, D.W. Simple solution-based synthesis of pyridinic-rich nitrogen-doped graphene nanoplatelets for supercapacitors. Appl. Energy 2017, 195, 1071–1078. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, Y.; Zou, K.; Ji, X. Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2016, 4, 1144–1173. [Google Scholar] [CrossRef]
- Lei, X.; Tang, Q.; Zheng, Y.; Kidkhunthod, P.; Zhou, X.; Ji, B.; Tang, Y. High-entropy single-atom activated carbon catalysts for sustainable oxygen electrocatalysis. Nat. Sustain. 2023, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.; Ji, H.; Stoller, M.D.; Lai, L.; Murali, S.; McDonnell, S.; Cleveger, B.; Wallace, R.M.; Ruoff, R.S. Nitrogen doping of graphene and its effect on quantum capacitance, and a new insight on the enhanced capacitance of n-doped carbon. Energy Environ. Sci. 2012, 5, 9618–9625. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y.; Yu, M.; Wang, Z.; Zhao, W.; Liu, P.; Tong, Y.; Lu, X. Nitrogen doped graphene paper as a highly conductive, and light-weight substrate for flexible supercapacitors. RSC Adv. 2014, 4, 51878–51883. [Google Scholar] [CrossRef]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-doped graphene for high-performance ultracapacitors and the importance of nitrogen-doped sites at basal planes. Nano Lett. 2011, 11, 2472–2477. [Google Scholar] [CrossRef]
- Liu, B.; Yang, C.-M.; Liu, Z.; Lai, C.-S. N-doped graphene with low intrinsic defect densities via a solid source doping technique. Nanomaterials 2017, 7, 302. [Google Scholar] [CrossRef]
- Hsueh, H.-C.; Li, H.-C.; Chiang, D.; Lee, S. Effects of ammonia/methane mixtures on characteristics of plasma enhanced chemical vapor deposition n-type carbon films. J. Electrochem. Soc. 2011, 159, D77–D83. [Google Scholar] [CrossRef]
- Wei, D.; Peng, L.; Li, M.; Mao, H.; Niu, T.; Han, C.; Chen, W.; Wee, A.T.S. Low temperature critical growth of high quality nitrogen doped graphene on dielectrics by plasma-enhanced chemical vapor deposition. ACS Nano 2015, 9, 164–171. [Google Scholar] [CrossRef]
- Cui, L.; Chen, X.; Liu, B.; Chen, K.; Chen, Z.; Qi, Y.; Xie, H.; Zhou, F.; Rummeli, M.H.; Zhang, Y.; et al. Highly conductive nitrogen-doped graphene grown on glass toward electrochromic applications. ACS Appl. Mater. Interfaces 2018, 10, 32622–32630. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, W.; Jiang, Q.; Chen, Z.; Ju, H.; Xue, X.; Xu, Z.; Hu, P.; Yu, G. Hydrogen-dominated metal-free growth of graphitic-nitrogen doped graphene with n-type transport behaviors. Carbon 2020, 161, 123–131. [Google Scholar] [CrossRef]
- Zhai, Z.; Shen, H.; Chen, J.; Li, X.; Jiang, Y. Direct growth of nitrogen-doped graphene films on glass by plasma-assisted hot filament cvd for enhanced electricity generation. J. Mater. Chem. A 2019, 7, 12038–12049. [Google Scholar] [CrossRef]
- Sedlovets, D.M.; Naumov, A.P.; Korotitsky, V.I.; Starkov, V.V. Nanoporous silicon with graphene-like coating for pseudocapacitor application. Nanomaterials 2022, 12, 2191. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, J.M.; Kim, J.-D. Hydrothermal preparation of nitrogen-doped graphene sheets via hexamethylenetetramine for application as supercapacitor electrodes. Electrochim. Acta 2012, 85, 459–466. [Google Scholar] [CrossRef]
- Sedlovets, D.M.; Redkin, A.N.; Kabachkov, E.N.; Naumov, A.P.; Knyazev, M.A.; Moiseenko, A.V.; Korepanov, V.I. Transfer-and lithography-free cvd of n-doped graphenic carbon thin films on non-metal substrates. Mater. Res. Bull. 2022, 154, 111943. [Google Scholar] [CrossRef]
- Wu, T.-H.; Chang, C.-T.; Wang, C.-C.; Parwaiz, S.; Lai, C.-C.; Chen, Y.-Z.; Lu, S.-Y.; Chueh, Y.-L. Few-layer graphene sheet-passivated porous silicon toward excellent electrochemical double-layer supercapacitor electrode. Nanoscale Res. Lett. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Lei, Z.; Lu, L.; Zhao, X. The electrocapacitive properties of graphene oxide reduced by urea. Energy Environ. Sci. 2012, 5, 6391–6399. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.-L.; Simon, P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Kang, T.; Liu, H.; Zhang, J.; Wang, N.; Lu, N.; Ma, Y.; Umar, A.; Guo, Z. Potassium hydroxide activated and nitrogen doped graphene with enhanced supercapacitive behavior. Sci. Adv. Mater. 2018, 10, 937–949. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.; Shin, Y.; Yoon, Y.; Kim, D.; Lee, H. Highly transparent and flexible supercapacitors using graphene-graphene quantum dots chelate. Nano Energy 2016, 26, 746–754. [Google Scholar] [CrossRef]
- Li, L.; Secor, E.B.; Chen, K.S.; Zhu, J.; Liu, X.; Gao, T.Z.; Seo, J.W.T.; Zhao, Y.; Hersam, M.C. High-performance solid-state supercapacitors and microsupercapacitors derived from printable graphene inks. Adv. Energy Mater. 2016, 6, 1600909. [Google Scholar] [CrossRef]
- Wu, Z.S.; Parvez, K.; Feng, X.; Müllen, K. Graphene-based in-plane micro-supercapacitors with high power and energy densities. Nat. Commun. 2013, 4, 2487. [Google Scholar] [CrossRef]
- Hyun, W.J.; Secor, E.B.; Kim, C.H.; Hersam, M.C.; Francis, L.F.; Frisbie, C.D. Scalable, self-aligned printing of flexible graphene micro-supercapacitors. Adv. Energy Mater. 2017, 7, 1700285. [Google Scholar] [CrossRef]
- Yoo, J.J.; Balakrishnan, K.; Huang, J.; Meunier, V.; Sumpter, B.G.; Srivastava, A.; Conway, M.; Mohana Reddy, A.L.; Yu, J.; Vajtai, R. Ultrathin planar graphene supercapacitors. Nano Lett. 2011, 11, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Zhang, L.; Liu, L.; Zhu, B.; Dong, H.; Chen, X. All-solid-state flexible ultrathin micro-supercapacitors based on graphene. Adv. Mater. 2013, 25, 4035–4042. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.M.; Ci, L.; Vajtai, R.; Zhang, Q.; Wei, B.; Ajayan, P.M. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat. Nanotechnol. 2011, 6, 496–500. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Tan, Y.-Z.; Zheng, S.; Wang, S.; Parvez, K.; Qin, J.; Shi, X.; Sun, C.; Bao, X.; Feng, X. Bottom-up fabrication of sulfur-doped graphene films derived from sulfur-annulated nanographene for ultrahigh volumetric capacitance micro-supercapacitors. J. Am. Chem. Soc. 2017, 139, 4506–4512. [Google Scholar] [CrossRef]
- Li, J.; Sollami Delekta, S.; Zhang, P.; Yang, S.; Lohe, M.R.; Zhuang, X.; Feng, X.; Ostling, M. Scalable fabrication and integration of graphene microsupercapacitors through full inkjet printing. ACS Nano 2017, 11, 8249–8256. [Google Scholar] [CrossRef]
- Wu, Z.-K.; Lin, Z.; Li, L.; Song, B.; Moon, K.-S.; Bai, S.-L.; Wong, C.-P. Flexible micro-supercapacitor based on in-situ assembled graphene on metal template at room temperature. Nano Energy 2014, 10, 222–228. [Google Scholar] [CrossRef]
- Bellani, S.; Petroni, E.; Del Rio Castillo, A.E.; Curreli, N.; Martín-García, B.; Oropesa-Nuñez, R.; Prato, M.; Bonaccorso, F. Scalable production of graphene inks via wet-jet milling exfoliation for screen-printed micro-supercapacitors. Adv. Funct. Mater. 2019, 29, 1807659. [Google Scholar] [CrossRef]
- Aradilla, D.; Delaunay, M.; Sadki, S.; Gérard, J.-M.; Bidan, G. Vertically aligned graphene nanosheets on silicon using an ionic liquid electrolyte: Towards high performance on-chip micro-supercapacitors. J. Mater. Chem. A 2015, 3, 19254–19262. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Z.-S.; Zheng, S.; Zhou, F.; Sun, C.; Cheng, H.-M.; Bao, X. Scalable fabrication of photochemically reduced graphene-based monolithic micro-supercapacitors with superior energy and power densities. ACS Nano 2017, 11, 4283–4291. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Kaner, R.B. Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage. Nat. Commun. 2013, 4, 1475. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, J.; Li, H.; Wang, Y.; Yang, H.Y.; Zhu, T.; Zhang, S.; Cao, G.; Zhao, X. Nitrogen-doped reduced graphene oxide for high-performance flexible all-solid-state micro-supercapacitors. J. Mater. Chem. A 2014, 2, 18125–18131. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, C.; Yan, Z.; Zhu, Y.; Peng, Z.; Hauge, R.H.; Natelson, D.; Tour, J.M. 3-dimensional graphene carbon nanotube carpet-based microsupercapacitors with high electrochemical performance. Nano Lett. 2013, 13, 72–78. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, Z.S.; Yang, S.; Dong, R.; Feng, X.; Müllen, K. Ultraflexible in-plane micro-supercapacitors by direct printing of solution-processable electrochemically exfoliated graphene. Adv. Mater. 2016, 28, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Nolan, H.; Mendoza-Sanchez, B.; Ashok Kumar, N.; McEvoy, N.; O’Brien, S.; Nicolosi, V.; Duesberg, G.S. Nitrogen-doped reduced graphene oxide electrodes for electrochemical supercapacitors. Phys. Chem. Chem. Phys. 2014, 16, 2280–2284. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Gao, J.; Shao, C.; Xiao, Y.; Zhao, Y.; Qu, L. Versatile origami micro-supercapacitors array as a wind energy harvester. J. Mater. Chem. A 2018, 6, 19750–19756. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, L.; Li, X.; Zuo, P.; Xu, C.; Tian, M.; Zhang, X.; Wang, S.; Lu, B.; Shao, C. Laser photonic-reduction stamping for graphene-based micro-supercapacitors ultrafast fabrication. Nat. Commun. 2020, 11, 6185. [Google Scholar] [CrossRef]

| Synthesis Temperature, °C | 800 | 850 | 900 | 950 | 1000 | |
|---|---|---|---|---|---|---|
| N/C, % | 2.1 | 2.0 | 2.8 | |||
| N configuration 2 | Pyrrolic, % | 13.8 | 9.2 | 10 | 4 | 2.1 |
| Pyridinic, % | 7.3 | 11.3 | 4.1 | 1.8 | <0.005 | |
| Graphitic, % | 78.9 | 79.5 | 85.9 | 93.8 | 97.9 | |
| Material 1 | Method | Electrolyte 1 | C, mF/cm2 | Thickness | Ref. |
|---|---|---|---|---|---|
| G-GQDs | Electrophoretic deposition | PVA/H3PO4 | 0.009 | - | [28] |
| G/EC | Inkjet printing | PVA/H3PO4 | 0.028 | 40 nm | [29] |
| Graphene | Plasma etching | PVA/H2SO4 | 0.081 | 15 nm | [30] |
| Graphene | Self-aligned printing | Ionic liquid | 0.268 | 300 nm | [31] |
| rGO | Self-assembly | PVA/H3PO4 | 0.394 | - | [32] |
| rGO | Electrophoretic deposition | PVA/H3PO4 | 0.462 | 25 nm | [33] |
| rGO/GO/rGO | Direct laser writing | 1 M Na2SO4 | 0.51 | ≈25 μm | [34] |
| SG | Plasma etching | PVA/H2SO4 | 0.582 | 10 nm | [35] |
| EEG aerogel | Inkjet printing | PSSH | 0.7 | 0.7 μm | [36] |
| rGO | Self-assembly | PVA/H2SO4 | 0.95 | 27 μm | [37] |
| N-GLFs | Direct CVD | 0.5 M Na2SO4 | 0.96 | 50 nm | This work |
| WJW-G+SWCNTs | Screen printing | PVA/H3PO4 | 1.324 | 27 μm | [38] |
| PRG | Photo reduction | PVA/H2SO4 | 1.5 | 2–4.5 μm | [40] |
| VGN | CVD | Ionic liquid | 2 | 1–2 μm | [39] |
| rGO | Laser writing | PVA/H2SO4 | 2.32 | ≈8 μm | [41] |
| N-rGO | Inkjet printing | PVA/H3PO4 | 3.4 | 10 μm | [42] |
| G/CNTs | CVD | Ionic liquid | 3.93 | tens μm | [43] |
| LIG | Laser irradiation | 1 M H2SO4 | 4 | 25 μm | [44] |
| EEG | Spray coating | PVA/H3PO4 | 5.4 | 2 μm | [45] |
| N-rGO | Spray coating | 1M H2SO4 | 9.5 | 700 nm | [46] |
| PPyG | - | PVA/H2SO4 | 22 | 20 μm | [47] |
| LIG/MnO2 | Laser irradiation | 0.5 M Na2SO4 | 128 | - | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlovets, D.M. N-Doped Graphene-like Film/Silicon Structures as Micro-Capacitor Electrodes. Materials 2023, 16, 4007. https://doi.org/10.3390/ma16114007
Sedlovets DM. N-Doped Graphene-like Film/Silicon Structures as Micro-Capacitor Electrodes. Materials. 2023; 16(11):4007. https://doi.org/10.3390/ma16114007
Chicago/Turabian StyleSedlovets, Daria M. 2023. "N-Doped Graphene-like Film/Silicon Structures as Micro-Capacitor Electrodes" Materials 16, no. 11: 4007. https://doi.org/10.3390/ma16114007
APA StyleSedlovets, D. M. (2023). N-Doped Graphene-like Film/Silicon Structures as Micro-Capacitor Electrodes. Materials, 16(11), 4007. https://doi.org/10.3390/ma16114007





