Influence of Isocyanate Structure on Recyclable Shape Memory Poly(thiourethane)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
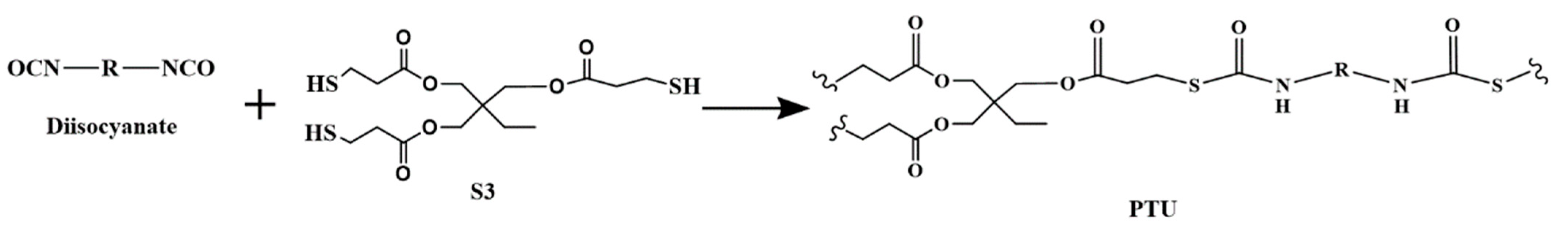
2.2. Sample Preparation
2.3. Characterization
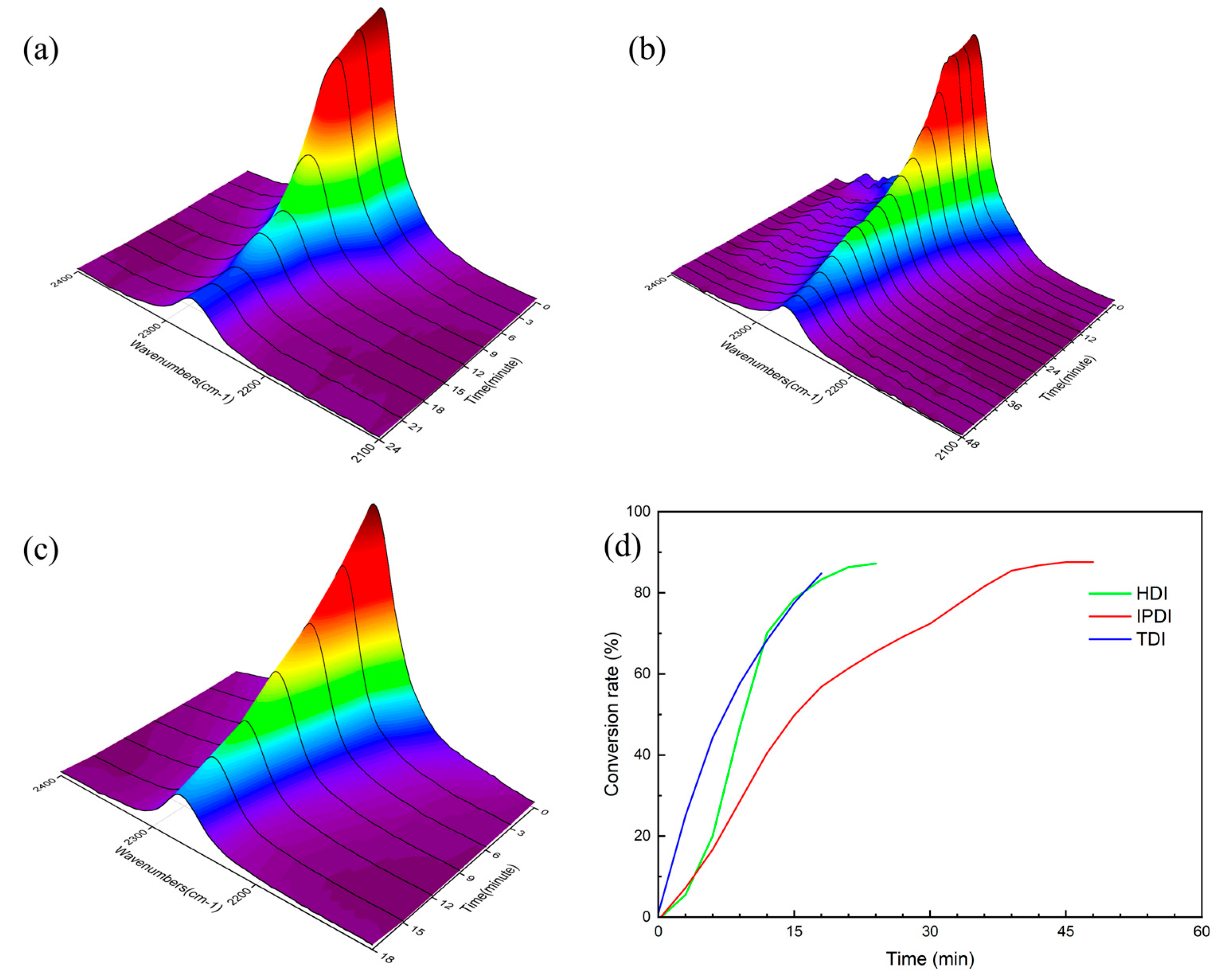
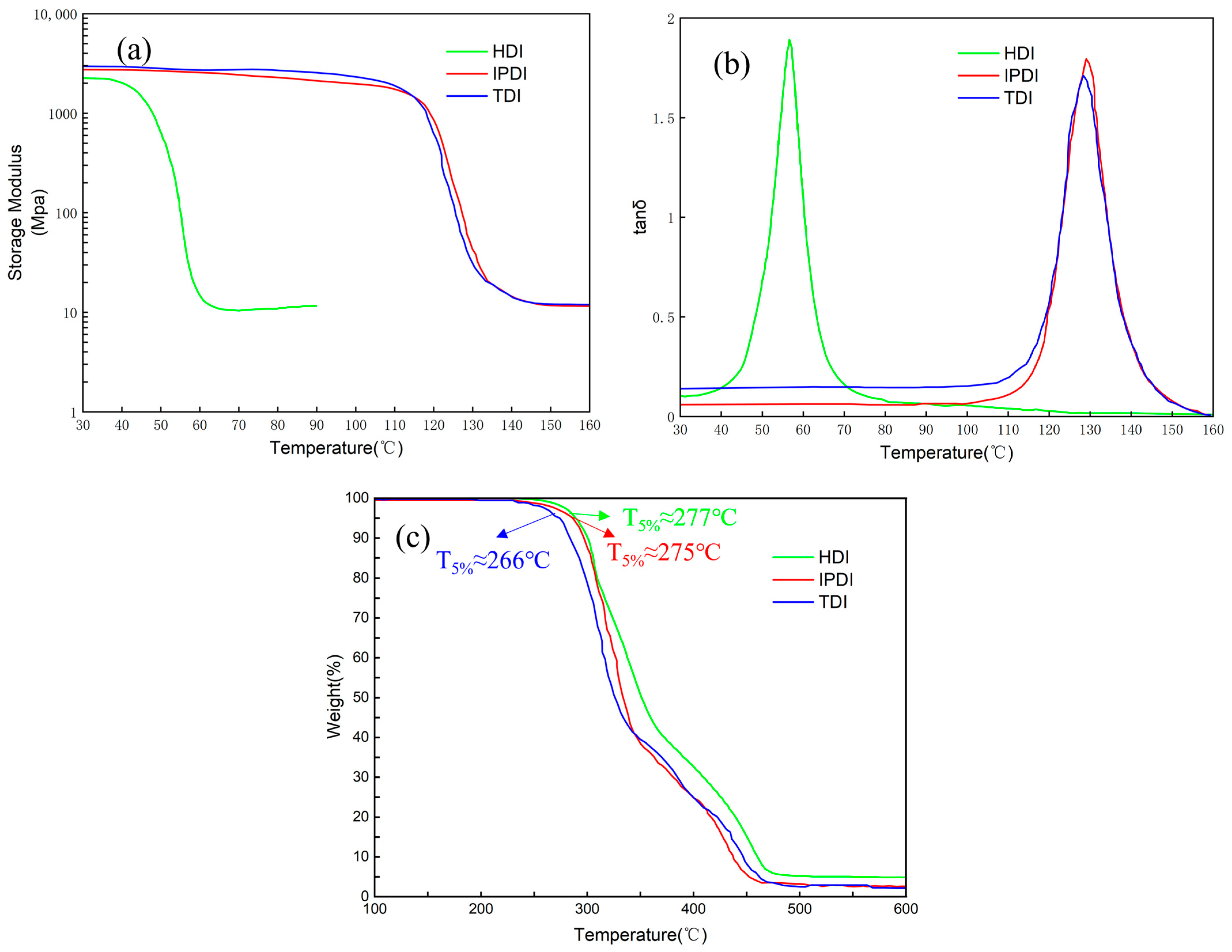
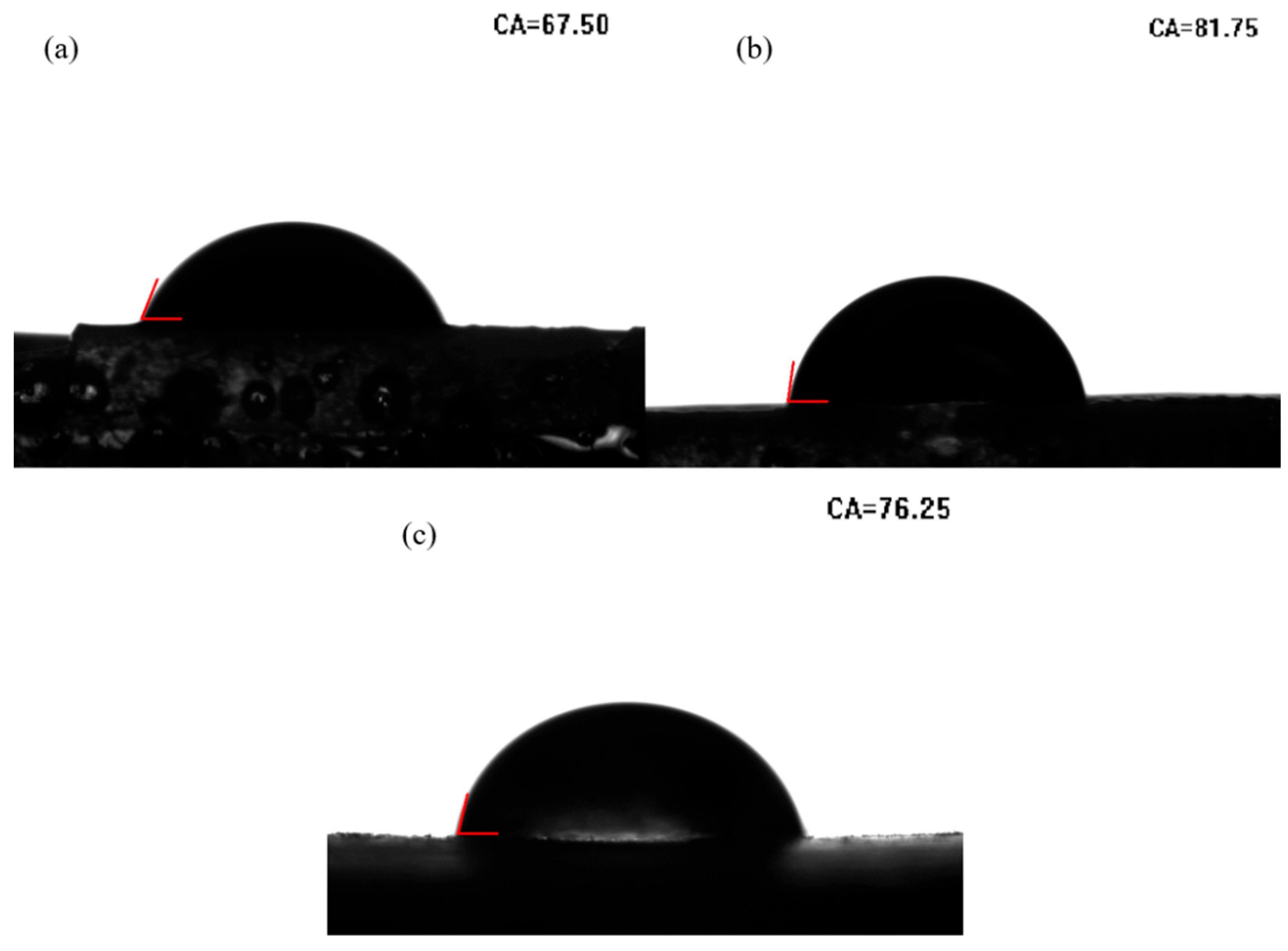
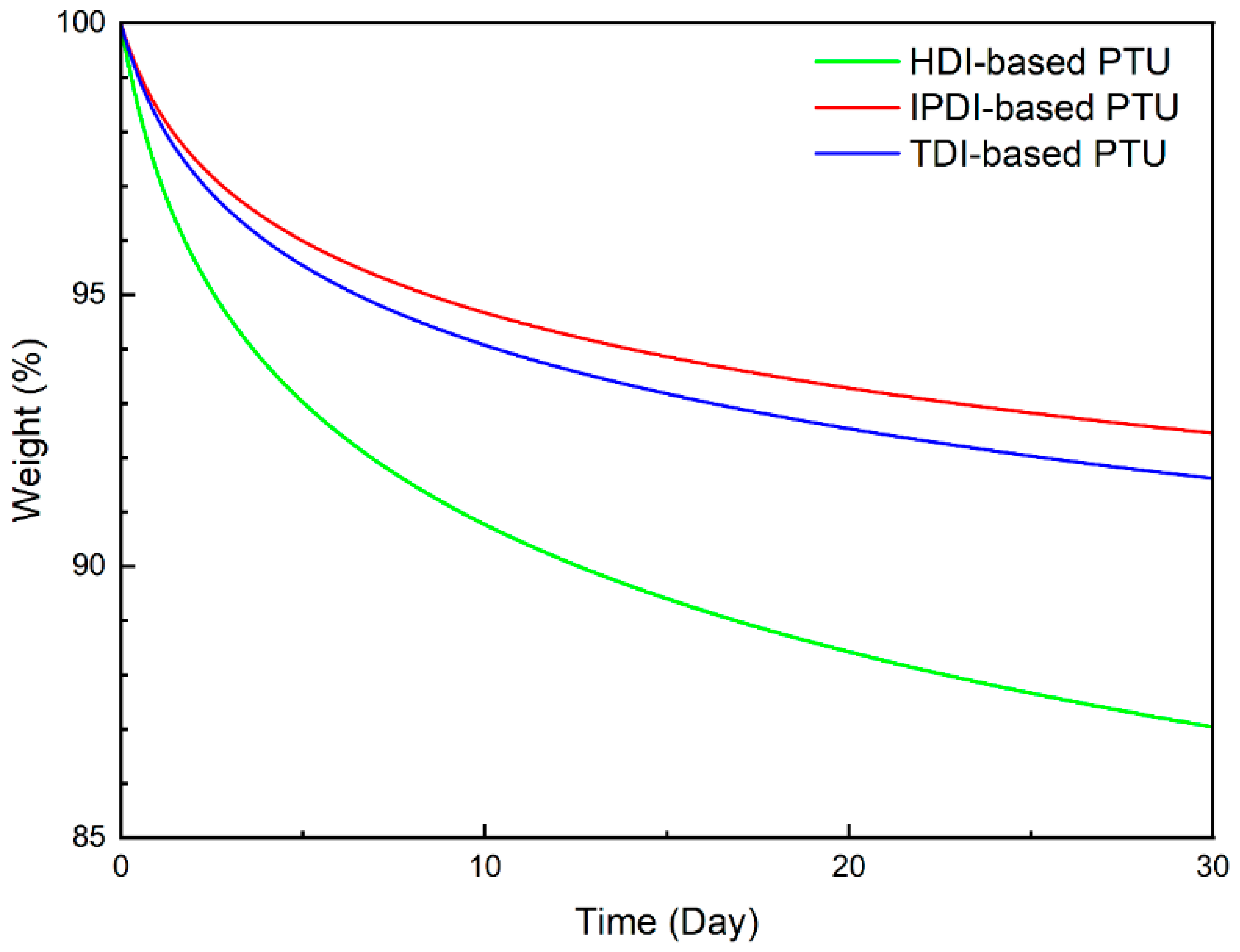
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Huang, W.M.; Yang, B.; An, L.; Li, C.; Chan, Y.S. Water-driven programmable polyurethane shape memory polymer: Demonstration and mechanism. Appl. Phys. Lett. 2005, 86, 114105. [Google Scholar] [CrossRef]
- Huang, W.M.; Zhao, Y.; Wang, C.C.; Ding, Z.; Purnawali, H.; Tang, C.; Zhang, J.L. Thermo/chemo-responsive shape memory effect in polymers: A sketch of working mechanisms, fundamentals and optimization. J. Polym. Res. 2012, 19, 9952. [Google Scholar] [CrossRef]
- Rose, A.; Zhu, Z.; Madigan, C.F.; Swager, T.M.; Bulović, V. Sensitivity gains in chemosensing by lasing action in organic polymers. Nature 2005, 434, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Qi, H.J.; Xie, T. Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding. Prog. Polym. Sci. 2015, 49–50, 79–120. [Google Scholar] [CrossRef]
- Lendlein, A.; Langer, R. Biodegradable, Elastic Shape-Memory Polymers for Potential Biomedical Applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef]
- Yu, K.; McClung, A.J.W.; Tandon, G.P.; Baur, J.W.; Jerry Qi, H. A thermomechanical constitutive model for an epoxy based shape memory polymer and its parameter identifications. Mech. Time-Depend. Mater. 2014, 18, 453–474. [Google Scholar] [CrossRef]
- Dong, Y.; Ni, Q.-Q.; Fu, Y. Preparation and characterization of water-borne epoxy shape memory composites containing silica. Compos. Part A Appl. Sci. Manuf. 2015, 72, 1–10. [Google Scholar] [CrossRef]
- Kausar, A. Review on Technological Significance of Photoactive, Electroactive, pH-sensitive, Water-active, and Thermoresponsive Polyurethane Materials. Polym. Plast. Technol. Eng. 2017, 56, 606–616. [Google Scholar] [CrossRef]
- Ahmad, M.; Luo, J.; Miraftab, M. Feasibility study of polyurethane shape-memory polymer actuators for pressure bandage application. Sci. Technol. Adv. Mater. 2012, 13, 015006. [Google Scholar] [CrossRef]
- Narayana, H.; Hu, J.; Kumar, B.; Shang, S.; Ying, M.; Young, R.J. Designing of advanced smart medical stocking using stress-memory polymeric filaments for pressure control and massaging. Mater. Sci. Eng. C 2018, 91, 263–273. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Zhao, X.; Xie, R.; Qin, T.; Ji, F. Mechanically robust shape memory polyurethane nanocomposites for minimally invasive bone repair. ACS Appl. Bio Mater. 2019, 2, 1056–1065. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Jeng, U.S.; Hsu, S.-h. Biodegradable Water-Based Polyurethane Shape Memory Elastomers for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 1397–1406. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Huang, S.; Podgórski, M.; Han, X.; Bowman, C.N. Chemical recycling of poly(thiourethane) thermosets enabled by dynamic thiourethane bonds. Polym. Chem. 2020, 11, 6879–6883. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Torkelson, J.M. Reprocessable Polymer Networks via Thiourethane Dynamic Chemistry: Recovery of Cross-link Density after Recycling and Proof-of-Principle Solvolysis Leading to Monomer Recovery. Macromolecules 2019, 52, 8207–8216. [Google Scholar] [CrossRef]
- Fan, C.-J.; Wen, Z.-B.; Xu, Z.-Y.; Xiao, Y.; Wu, D.; Yang, K.-K.; Wang, Y.-Z. Adaptable Strategy to Fabricate Self-Healable and Reprocessable Poly(thiourethane-urethane) Elastomers via Reversible Thiol–Isocyanate Click Chemistry. Macromolecules 2020, 53, 4284–4293. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.; Jeong, H.M. Properties of polythiourethanes prepared by thiol–isocyanate click reaction. J. Appl. Polym. Sci. 2018, 135, 46070. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef]
- Yue, H.; Zhou, J.; Huang, M.; Hao, C.; Hao, R.; Dong, C.; He, S.; Liu, H.; Liu, W.; Zhu, C. Recyclable, reconfigurable, thermadapt shape memory polythiourethane networks with multiple dynamic bonds for recycling of carbon fiber-reinforced composites. Polymer 2021, 237, 124358. [Google Scholar] [CrossRef]
- Zhou, J.; Yue, H.; Huang, M.; Hao, C.; He, S.; Liu, H.; Liu, W.; Zhu, C.; Dong, X.; Wang, D. Arbitrarily Reconfigurable and Thermadapt Reversible Two-Way Shape Memory Poly(thiourethane) Accomplished by Multiple Dynamic Covalent Bonds. ACS Appl. Mater. Interfaces 2021, 13, 43426–43437. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.T.; Truong, T.T.; Nguyen, H.T.; Le, L.; Nguyen, V.Q.; Van Le, T.; Luu, A.T. Healable shape memory (thio)urethane thermosets. Polym. Chem. 2015, 6, 3143–3154. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, T.; Li, C.; Xiao, X.; Tang, Y.; Fang, X.; Peng, H.; Liu, X.; Dong, Y.; Cai, Y.; et al. Shape-reconfigurable transparent wood based on solid-state plasticity of polythiourethane for smart building materials with tunable light guiding, energy saving, and fire alarm actuating functions. Compos. Part B Eng. 2022, 246, 110260. [Google Scholar] [CrossRef]
- Dailing, E.A.; Nair, D.P.; Setterberg, W.K.; Kyburz, K.A.; Yang, C.; D’Ovidio, T.; Anseth, K.S.; Stansbury, J.W. Combined, independent small molecule release and shape memory via nanogel-coated thiourethane polymer networks. Polym. Chem. 2016, 7, 816–825. [Google Scholar] [CrossRef]
- Cui, C.; An, L.; Zhang, Z.; Ji, M.; Chen, K.; Yang, Y.; Su, Q.; Wang, F.; Cheng, Y.; Zhang, Y. Reconfigurable 4D Printing of Reprocessable and Mechanically Strong Polythiourethane Covalent Adaptable Networks. Adv. Funct. Mater. 2022, 32, 2203720. [Google Scholar] [CrossRef]
- Du, W.P.; Zhang, Y.; Tan, L.J.; Chen, H.F. Cure-Reaction Kinetics of Crosslinked Polythiourethane Network for Optical Applications Using FTIR Spectroscopy. Polym. Sci. Ser. B 2019, 61, 247–253. [Google Scholar] [CrossRef]
- Lü, C.; Cui, Z.; Li, Z.; Yang, B.; Shen, J. High refractive index thin films of ZnS/polythiourethane nanocomposites. J. Mater. Chem. 2003, 13, 526–530. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Pal, U.; Choudhary, P.; Singh, H.; Reiter, R.J.; Ethirajan, A.; Swarnakar, S.; Das, A. Stimuli-Responsive Nanocapsules for the Spatiotemporal Release of Melatonin: Protection against Gastric Inflammation. ACS Appl. Bio Mater. 2019, 2, 5218–5226. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Seneca, S.; Peters, M.; D’Olieslaeger, L.; Reekmans, G.; Vanderzande, D.; Adriaensens, P.; Ethirajan, A. Morphology-dependent pH-responsive release of hydrophilic payloads using biodegradable nanocarriers. RSC Adv. 2018, 8, 36869–36878. [Google Scholar] [CrossRef]
- Eglin, D.; Griffon, S.; Alini, M. Thiol-Containing Degradable Poly(thiourethane-urethane)s for Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2010, 21, 477–491. [Google Scholar] [CrossRef]




| Sample | Weight | |
|---|---|---|
| Diisocyanate | S3 | |
| HDI + S3 | 1.69 g | 4.69 g |
| IPDI + S3 | 3.37 g | |
| TDI + S3 | 1.78 g | |
| Diisocyanate | N1 | C1 | O1 | N2 | C2 | O2 |
|---|---|---|---|---|---|---|
| HDI | −0.430 | 0.603 | −0.412 | −0.430 | 0.603 | −0.412 |
| IPDI | −0.430 | 0.595 | −0.415 | −0.420 | 0.697 | −0.410 |
| 2,4-TDI | −0.494 | 0.588 | −0.400 | −0.505 | 0.603 | −0.392 |
| 2,6-TDI | −0.649 | 0.750 | −0.495 | −0.652 | 0.773 | −0.486 |
| Sample | Rf (%) | Rr (%) |
|---|---|---|
| HDI-based PTU | 96.9 | 97.7 |
| IPDI-based PTU | 93.4 | 95.1 |
| TDI-based PTU | 92.2 | 93.2 |
| Recycled HDI-based PTU | 95.5 | 95.3 |
| Recycled IPDI-based PTU | 92.0 | 91.8 |
| Recycled TDI-based PTU | 89.7 | 91.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Song, J.; Li, J.; Yuan, C. Influence of Isocyanate Structure on Recyclable Shape Memory Poly(thiourethane). Materials 2023, 16, 4040. https://doi.org/10.3390/ma16114040
Zeng Y, Song J, Li J, Yuan C. Influence of Isocyanate Structure on Recyclable Shape Memory Poly(thiourethane). Materials. 2023; 16(11):4040. https://doi.org/10.3390/ma16114040
Chicago/Turabian StyleZeng, Yu, Jiale Song, Jinfu Li, and Chi Yuan. 2023. "Influence of Isocyanate Structure on Recyclable Shape Memory Poly(thiourethane)" Materials 16, no. 11: 4040. https://doi.org/10.3390/ma16114040
APA StyleZeng, Y., Song, J., Li, J., & Yuan, C. (2023). Influence of Isocyanate Structure on Recyclable Shape Memory Poly(thiourethane). Materials, 16(11), 4040. https://doi.org/10.3390/ma16114040




