Study on the Stability of Low-Carbon Magnesium Cementitious Materials in Sulfate Erosion Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Test Process
2.3. Test Method
3. Results and Discussion
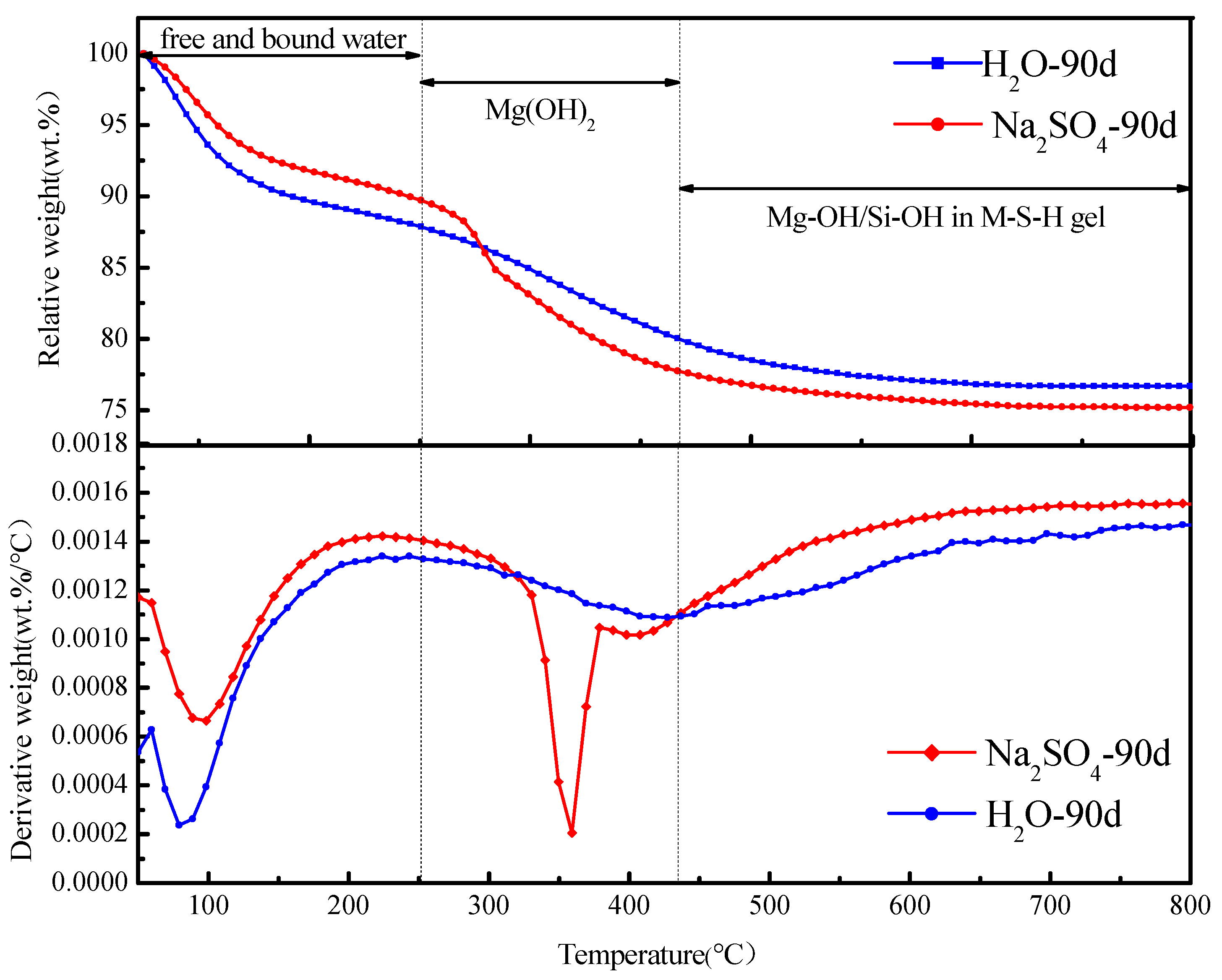
3.1. Effect of Sulfate Erosion Environment on Hydration Mechanism of Magnesium-Based Cementitious Materials
3.2. Effects of Long-Term Sulfate Erosion Environment on Different Cementification Systems
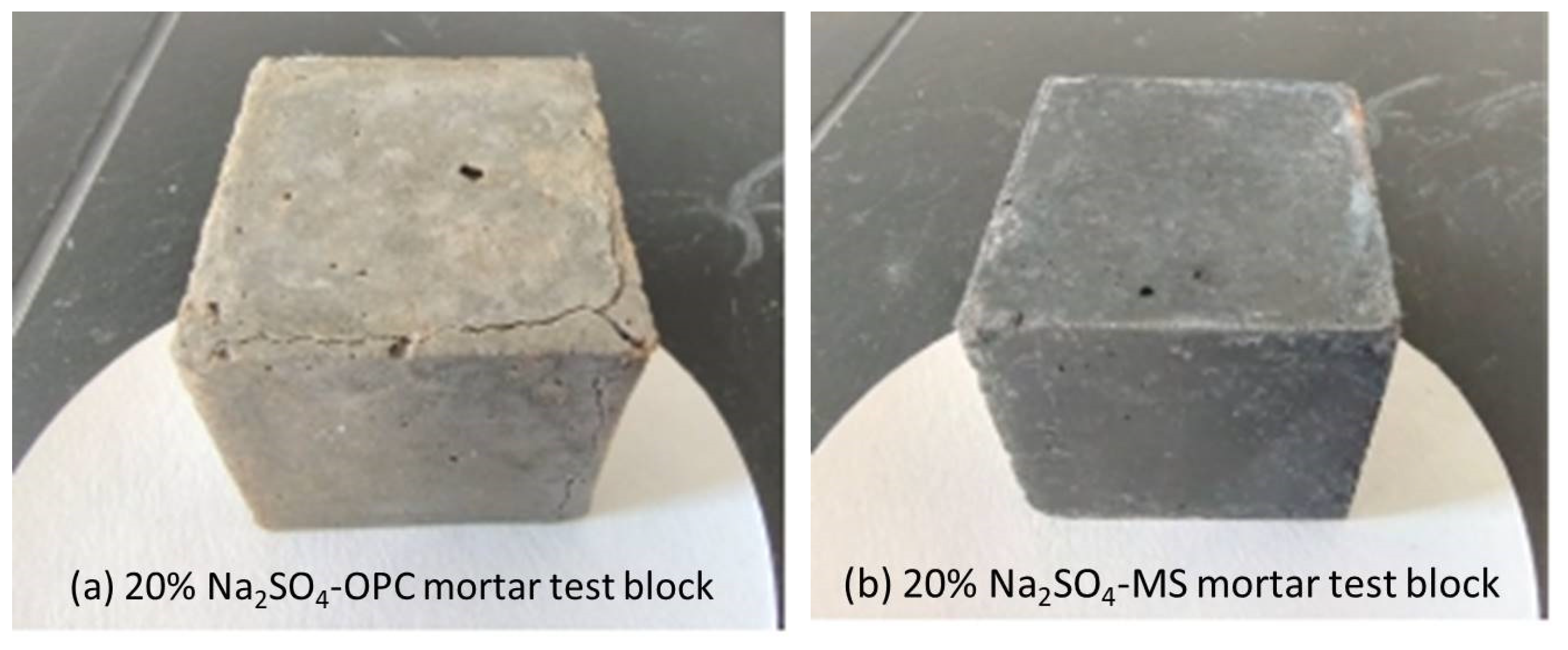
3.2.1. Comparison of Macroscopic Morphology
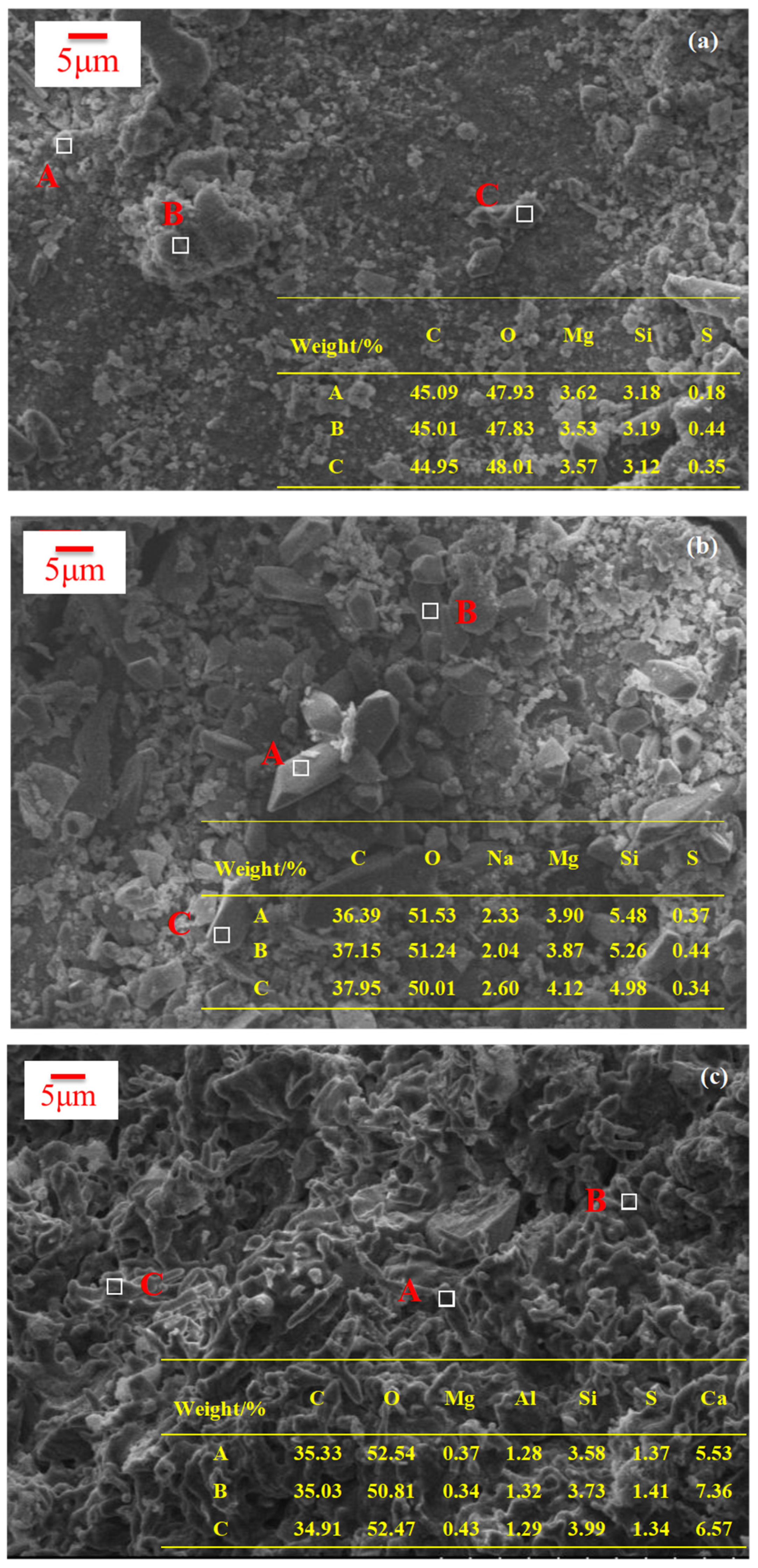
3.2.2. Comparison of Microscopic Morphology
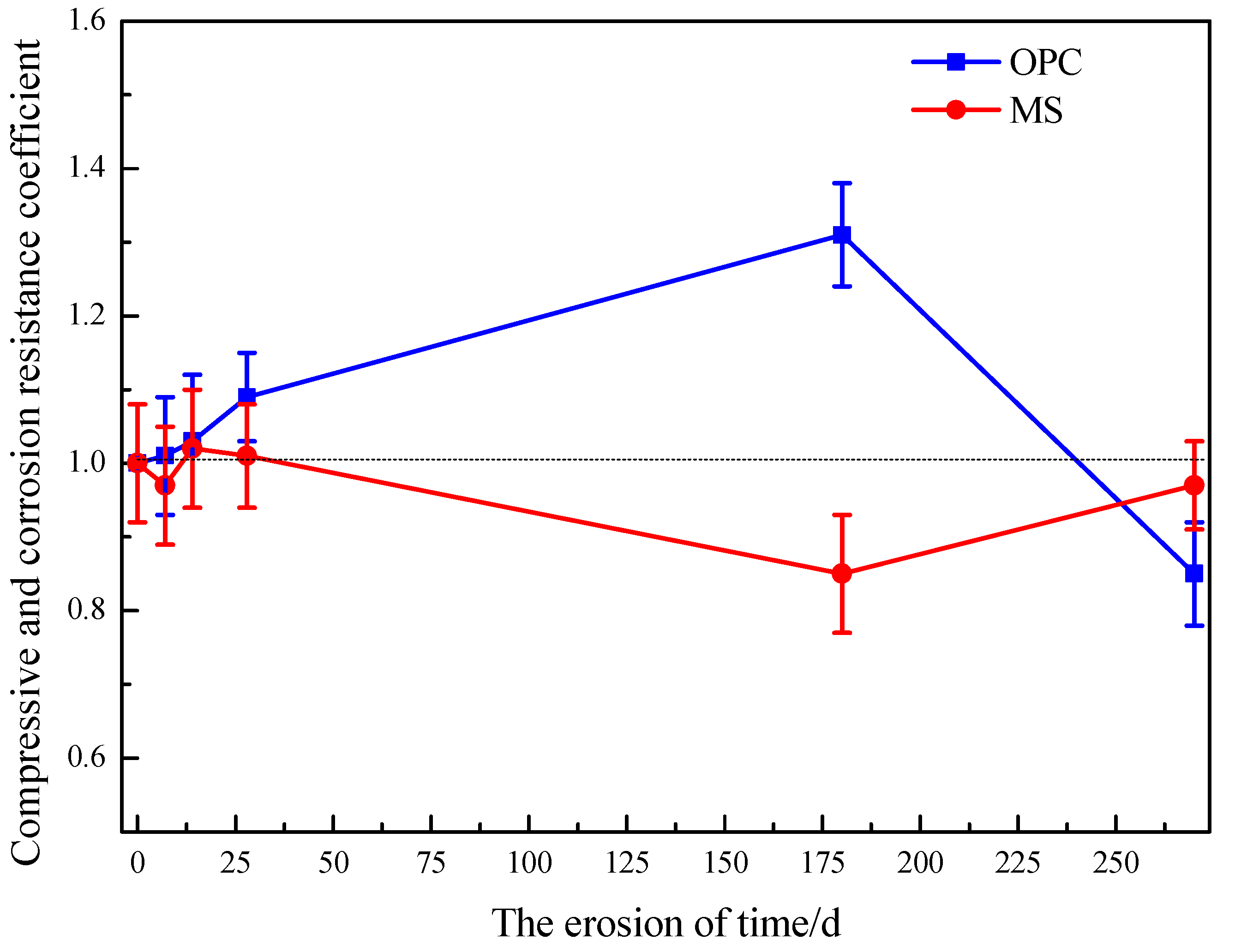
3.2.3. Mechanical Properties Analysis
3.3. Effect of Sulfate Dry-Wet Cycle Erosion Environment on Different Cementitious Systems
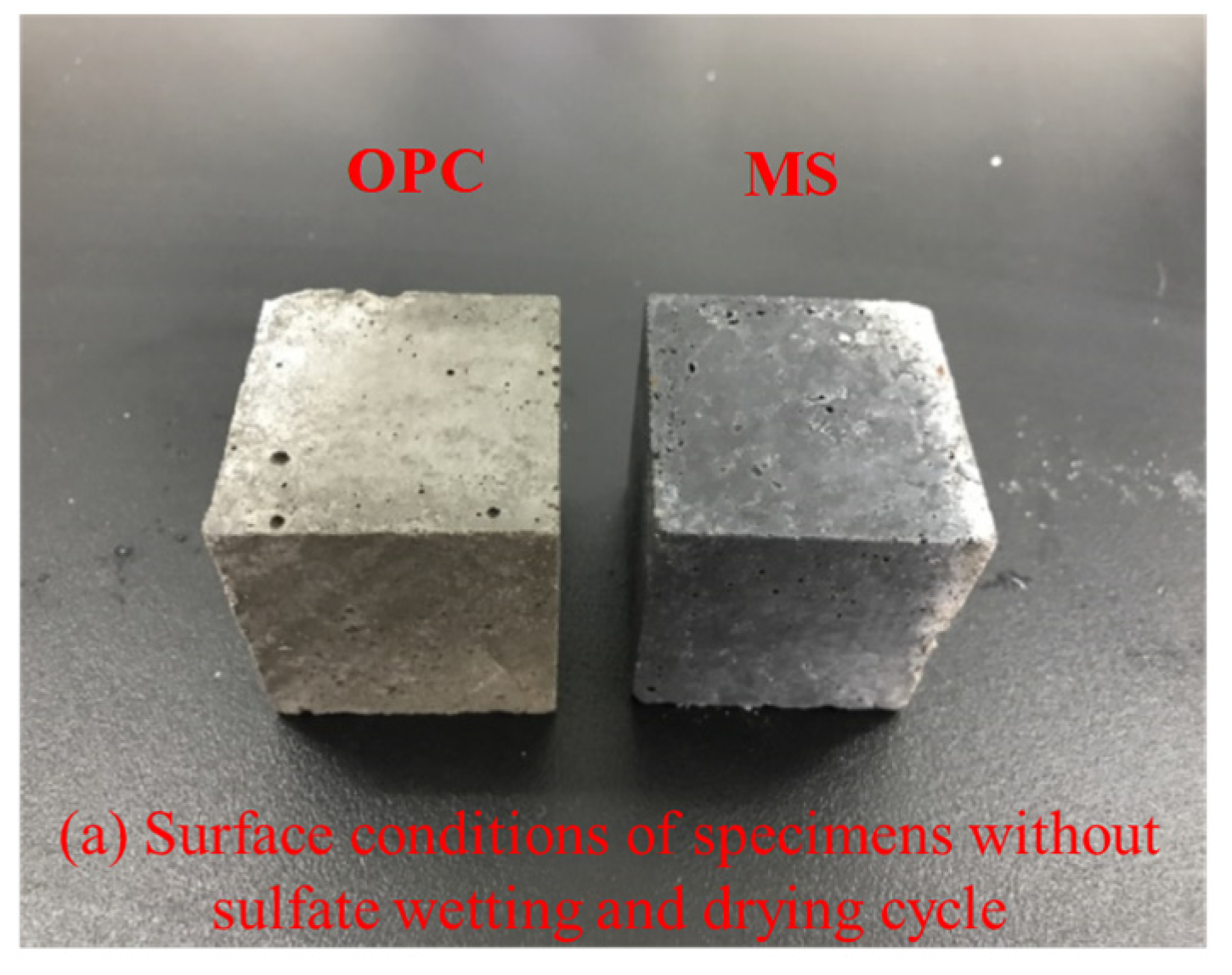
3.3.1. Surface Damage of the Specimen
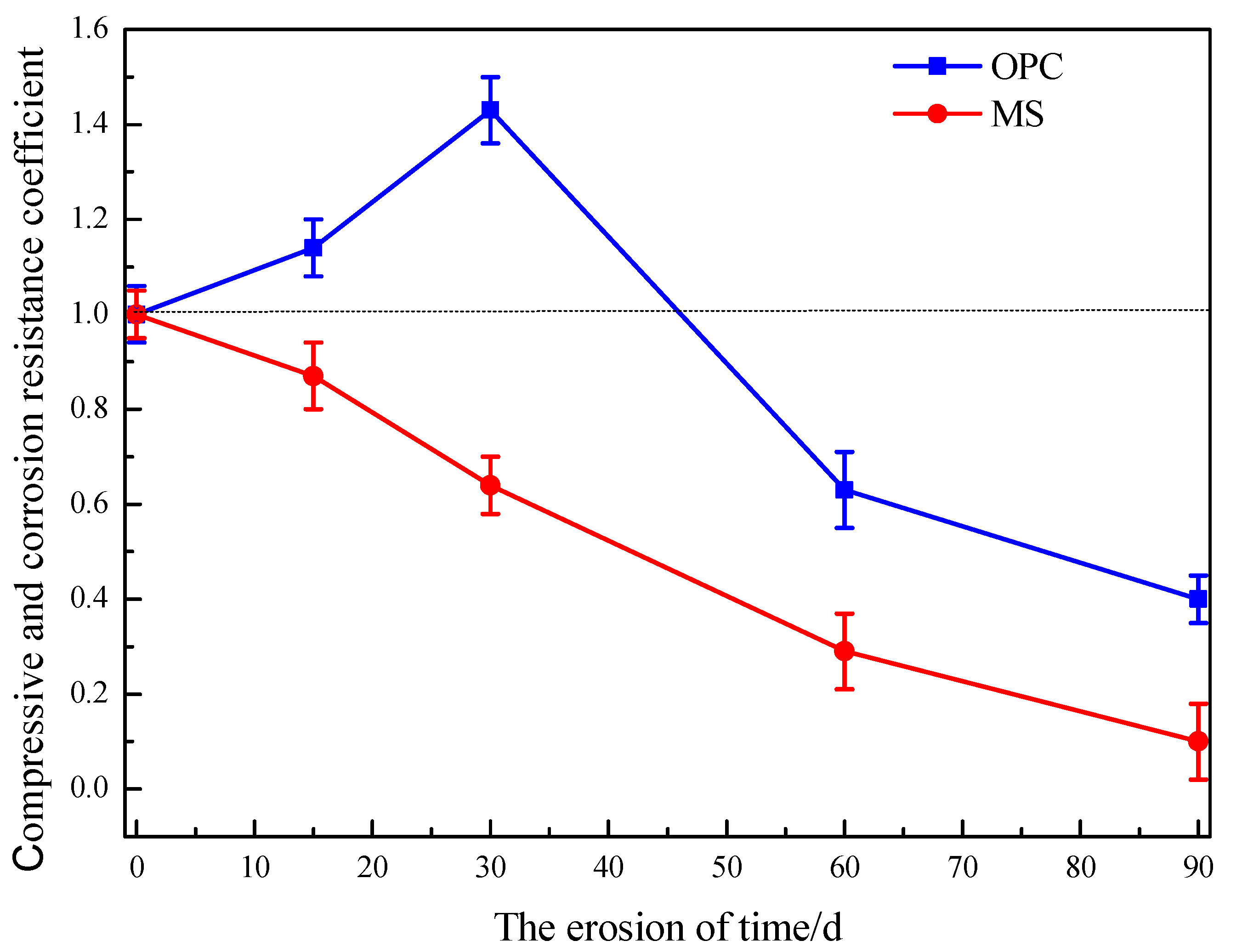
3.3.2. Mechanical Properties Analysis
4. Conclusions
- MS specimens cured for 56 d were more stable than uncured specimens soaked in a sulfate solution. The reaction process of magnesium oxide-based cementified materials could be delayed, to a certain extent, under the environment of sulfate erosion, but, after curing for 300 days, nevertheless, it completely reacted into magnesium silicate hydrate gel.
- SO42− did not react with hydration products of magnesium silicate hydrate gel, so MS specimens were more stable than OPC specimens under a sulfate erosion environment. However, its compressive strength was not as good as the OPC sample.
- Under the condition of dry and wet cycle sulfate erosion, the damage degree of magnesium silicate hydrate was obvious, and the strength loss was large, which might be due to the destruction of the structure of the magnesium oxide-based cementing system under the condition of dry water loss, which evaporates the internal bound water and causes the damage of the specimen.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, Y.; Zou, Y.; Zou, X.; Jiang, Y.; Li, F.; Ma, W.; Yan, H.; Hua, R. Effect of Aluminum Incorporation on the Reaction Process and Reaction Products of Hydrated Magnesium Silicate. Front. Mater. 2022, 8, 594. [Google Scholar] [CrossRef]
- Zhang, T.; Li, T.; Zhou, Z.; Li, M.; Jia, Y.; Cheeseman, C. A novel magnesium hydroxide sulfate hydrate whisker-reinforced magnesium silicate hydrate composites. Compos. B Eng. 2020, 198, 108203. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, Z.; Li, M.; He, Z.; Jia, Y.; Cheeseman, C.; Shi, C. Effect of hydrated magnesium carbonate grown in situ on the property of MgO-activated reactive SiO2 mortars. J. Sustain. Cem. Based Mater. 2022, 11, 286–296. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, B.; Wu, Z.; Zhang, T. Effect of CaO on the reaction process of MgO-SiO2-H2O cement pastes. Mater. Lett. 2017, 192, 48–51. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Y.; Hong, W. Facile synthesis of porous hydrated magnesium silicate adsorbent from ordinary silica gel. Mater. Lett. 2020, 272, 127886. [Google Scholar] [CrossRef]
- Du, J.; Tang, Z.; Li, G.; Yang, H.; Li, L. Key inhibitory mechanism of external chloride ions on concrete sulfate attack. Constr. Build. Mater. 2019, 225, 611–619. [Google Scholar] [CrossRef]
- Binici, H.; Aksoğan, O. Sulfate resistance of plain and blended cement. Cem. Concr. Compos. 2005, 28, 39–46. [Google Scholar] [CrossRef]
- Dehwah, H.A.; Maslehuddin, M.; Austin, S.A. Long-term effect of sulfate ions and associated cation type on chloride-induced reinforcement corrosion in Portland cement concretes. Cem. Concr. Compos. 2002, 24, 17–25. [Google Scholar] [CrossRef]
- Hu, S.; Yin, Y. Fracture properties of concrete under freeze–thaw cycles and sulfate attack. Constr. Build. Mater. 2022, 350, 128856. [Google Scholar] [CrossRef]
- Xu, C.; Gao, X.; Li, X.; Zhang, K. Estimation of the Occurrence Time of Thaumasite Sulfate Attack on Tunnel Lining Concrete. Adv. Civ. Eng. 2020, 2020, 6656304. [Google Scholar] [CrossRef]
- Scd, A.; Rak, B. Influence of graphene oxide on sulfate attack and carbonation of concrete containing recycled concrete aggregate. Constr. Build. Mater. 2020, 250, 118883. [Google Scholar]
- Liu, D.; Chen, H.; Tang, Y.; Gong, C.; Jian, Y.; Cao, K. Analysis and Prediction of Sulfate Erosion Damage of Concrete in Service Tunnel Based on ARIMA Model. Materials 2021, 14, 5904. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Yu, Z.; Chen, L.; Zheng, Y. Research on Sulfate Attack Mechanism of Cement Concrete Based on Chemical Thermodynamics. Adv. Mater. Sci. Eng. 2020, 2020, 6916039. [Google Scholar] [CrossRef]
- Sun, D.; Huang, C.; Cao, Z.; Wu, K.; Zhang, L. Reliability assessment of concrete under external sulfate attack. Case Stud. Constr. Mater. 2021, 15, e00690. [Google Scholar] [CrossRef]
- Ma, B.; Gao, X.; Byars, E.A.; Zhou, Z. Thaumasite formation in a tunnel of Bapanxia Dam in Western China. Cem. Concr. Res. 2006, 36, 716–722. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, T.; Zou, D.; Zhou, A. Compressive strength assessment of sulfate-attacked concrete by using sulfate ions distributions. Constr. Build. Mater. 2021, 293, 123550. [Google Scholar] [CrossRef]
- Tixier, R.; Mobasher, B. Modeling of Damage in Cement-Based Materials Subjected to External Sulfate Attack. I: Formulation. J. Mater. Civ. Eng. 2003, 15, 305–313. [Google Scholar] [CrossRef]
- Guo, J.; Wang, K.; Guo, T.; Yang, Z.; Zhang, P. Effect of Dry-Wet Ratio on Properties of Concrete Under Sulfate Attack. Materials 2019, 12, 2755. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; García-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
- Liao, K.; Zhang, Y.; Zhang, W.; Wang, Y.; Zhang, R. Modeling constitutive relationship of sulfate-attacked concrete. Constr. Build. Mater. 2020, 260, 119902. [Google Scholar] [CrossRef]
- Salamoni, N.; Rohden, A.B. Durability analysis of concrete foundations exposed to external sulfate attacks in the south of Santa Catarina, Brazil. J. Build. Pathol. Rehabil. 2022, 7, 66. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, W.; Hou, L.; Deng, D. Effect of carbonation on physical sulfate attack on concrete by Na2SO4. Constr. Build. Mater. 2018, 193, 211–220. [Google Scholar] [CrossRef]
- Yao, M.; Li, J. Effect of the degradation of concrete friction piles exposed to external sulfate attack on the pile bearing capacity. Ocean Eng. 2019, 173, 599–607. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, B.; Wu, Z.; Han, J.; Zhang, T.; Vandeperre, L.J. Role of sodium hexametaphosphate in MgO/SiO2 cement pastes. Cem. Concr. Res. 2016, 89, 63–71. [Google Scholar]
- Jiang, L.; Niu, D. Study of deterioration of concrete exposed to different types of sulfate solutions under drying-wetting cycles. Constr. Build. Mater. 2016, 117, 88–98. [Google Scholar]
- Liu, F.; Zhang, T.; Luo, T.; Zhou, M.; Ma, W. Study on the Deterioration of Concrete under Dry-Wet Cycle and Sulfate Attack. Materials 2020, 13, 4095. [Google Scholar] [CrossRef]
- Talero, R. Performance of metakaolin and Portland cements in ettringite formation as determined by ASTMC 452-68: Kinetic and morphological differences. Cem. Concr. Res. 2005, 35, 1269–1284. [Google Scholar] [CrossRef]
- Alyami, M.H.; Alrashidi, R.S.; Mosavi, H.; Almarshoud, M.A.; Riding, K.A. Potential accelerated test methods for physical sulfate attack on concrete. Constr. Build. Mater. 2019, 229, 116920. [Google Scholar] [CrossRef]
- He, R.; Zheng, S.; Gan, V.; Wang, Z.; Fang, J.; Shao, Y. Damage mechanism and interfacial transition zone characteristics of concrete under sulfate erosion and Dry-Wet cycles. Constr. Build. Mater. 2020, 255, 119340. [Google Scholar] [CrossRef]
- Zhang, T.; Zou, J.; Wang, B.; Wu, Z.; Jia, Y.; Cheeseman, C.R. Characterization of Magnesium Silicate Hydrate (MSH) Gel Formed by Reacting MgO and Silica Fume. Materials 2018, 11, 909. [Google Scholar] [CrossRef]
- Kuenzel, C.; Zhang, F.; Ferrándiz-Mas, V.; Cheeseman, C.R.; Gartner, E.M. The mechanism of hydration of MgO-hydromagnesite blends. Cem. Concr. Res. 2018, 3, 123–129. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Hu, J.; Yue, T.; Niu, Y.; Wei, J.; Yu, Q. Characterization of reaction products and reaction process of MgO-SiO2-H2O system at room temperature. Constr. Build. Mater. 2014, 61, 252–259. [Google Scholar] [CrossRef]
- Lin, C.; Bai, Z.; Zhang, Z. Minerals and Related Compound Thermodynamic Data Manual, 1st ed.; Science Press: Beijing, China, 1985. (In Chinese) [Google Scholar]
- Ye, Q.; Shen, C.; Sun, S.; Chen, R.; Song, H. The sulfate corrosion resistance behavior of slag cement mortar. Constr. Build. Mater. 2014, 71, 202–209. [Google Scholar] [CrossRef]
- Qudoos, A.; Kim, H.G.; Ryou, J.S. Influence of Titanium Dioxide Nanoparticles on the Sulfate Attack upon Ordinary Portland Cement and Slag-Blended Mortars. Materials 2018, 11, 356. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, J.; Jiang, X.; Mu, S.; Wu, M.; Sui, S.; Wang, L.; Wang, F. The influence of sodium sulfate and magnesium sulfate on the stability of bound chlorides in cement paste. Constr. Build. Mater. 2019, 228, 116775. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, P.; Yu, Z. Study on degradation of macro performances and micro structure of concrete attacked by sulfate under artificial simulated environment. Constr. Build. Mater. 2020, 260, 119951. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Yu, Z.; Lu, Z.; Shi, W. Evolution of the dynamic properties of concrete in a sulfate environment. Constr. Build. Mater. 2020, 245, 118468. [Google Scholar] [CrossRef]
- Wang, K.; Guo, J.; Wu, H.; Yang, L. Influence of dry-wet ratio on properties and microstructure of concrete under sulfate attack. Constr. Build. Mater. 2020, 263, 120635. [Google Scholar] [CrossRef]
- Nehdi, M.L.; Bassuoni, M.T. Durability of self-consolidating concrete to combined effects of sulphate attack and frost action. Mater. Struct. 2008, 41, 1657–1679. [Google Scholar] [CrossRef]
- Boudache, S.; Loukili, A.; Emmanuel, R.; Izoret, L. Influence of initial material properties on the degradation of mortars with low expansion cements subjected to external sulfate attack. Mater. Struct. 2021, 54, 104. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, T.; Liu, H. Performance evolution of the interfacial transition zone (ITZ) in recycled aggregate concrete under external sulfate attacks and dry-wet cycling. Constr. Build. Mater. 2019, 229, 116938. [Google Scholar] [CrossRef]
- Qi, B.; Gao, J.; Chen, F.; Shen, D. Evaluation of the damage process of recycled aggregate concrete under sulfate attack and wetting-drying cycles. Constr. Build. Mater. 2017, 138, 254–262. [Google Scholar] [CrossRef]
- Liu, F.; You, Z.; Diab, A.; Liu, Z.; Zhang, C.; Guo, S. External sulfate attack on concrete under combined effects of flexural fatigue loading and drying-wetting cycles. Constr. Build. Mater. 2020, 249, 118224. [Google Scholar] [CrossRef]
- Wojciech, P. Analysis of carbonate and sulphate attack on concrete structures. Eng. Fail. Anal. 2017, 79, 606–614. [Google Scholar]



| MgO | CaO | SiO2 | Fe2O3 | Al2O3 | Na2O | K2O | SO3 | Cl | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|
| MgO | 98.21 | 0.89 | 0.36 | 0.05 | 0.19 | - | - | 0.02 | 0.55 | 1.7 |
| SF | 1.12 | 0.59 | 95.9 | 0.40 | 0.24 | 0.38 | 1.64 | 0.27 | - | 2.0 |
| Cement Type | Water-Cement Ratio | Cement-Sand Ratio | Admixture |
|---|---|---|---|
| MS | 1:2 | 1:1 | Na-HMP |
| OPC | 1:2 | 1:1 | - |
| Soaking Duration (d) | 0–56 | 56–90 | 90–300 |
|---|---|---|---|
| NC-MS-1 | H2O | Na2SO4 | Na2SO4 |
| NC-MS-2 | Na2SO4 | Na2SO4 | Na2SO4 |
| Minerals or Species | (kJ/mol) | (kJ/mol) | (kJ/mol) |
|---|---|---|---|
| MgO(S) | 26.95 | −601.50 | −569.23 |
| Mg(OH)2(S) | 63.14 | −924.54 | −833.56 |
| M3S2H2(Chrysotile) | 221.30 | −4361.66 | −4034.24 |
| M3S4H(Talc) | 260.80 | −5915.90 | −5536.27 |
| Mg2+(aq) | −138.00 | −466.85 | −454.89 |
| OH−(aq) | −10.71 | −230.03 | −157.34 |
| H+(aq) | 0.00 | 0.00 | 0.00 |
| SiO2(glassy state) | 47.41 | −903.20 | −850.59 |
| H2O(l) | 69.95 | −285.83 | −237.19 |
| H3SiO4−(aq) | 112.55 | −1426.16 | −1253.98 |
| H2SiO42−(aq) | −12.97 | −1396.62 | −1187.02 |
| Na2SO4(aq) | 149.49 | −1387.11 | −1269.35 |
| SO42−(aq) | 20.00 | −909.27 | −744.48 |
| Na+(aq) | 58.41 | −240.30 | −261.88 |
| MgSO4·6H2O(crystalline state) | 348.11 | −3085.99 | −2631.24 |
| MgSO4·7H2O(crystalline state) | 372.00 | −3388.70 | −2871.58 |
| Na2SiO3(s) | 113.81 | −1557.62 | −1463.65 |
| ID | Chemical Reaction | (kJ/mol) | (kJ/mol) | (kJ/mol) |
|---|---|---|---|---|
| 1 | MgO + 2H+ → Mg2+ + H2O | −95.00 | −151.18 | −122.50 |
| 2 | SiO2 + 2OH− → H2SiO42− | −38.95 | −33.38 | −21.75 |
| 3 | SiO2 + OH− + H2O → H3SiO4− | 5.91 | −7.11 | −8.86 |
| 4 | Mg2+ + 2OH− → Mg(OH)2 | 222.56 | 2.35 | −63.99 |
| 5 | 3Mg(OH)2 + 2SiO2 → 3MgO·2SiO2·2H2O + H2O | 7.03 | −67.47 | −69.57 |
| 6 | 3Mg(OH)2 + 4SiO2 → 3MgO·4SiO2·H2O + 2H2O | 21.67 | −101.14 | −107.60 |
| 7 | 3MgO + 2H2O + 2SiO2 → 3MgO·2SiO2·2H2O | −94.26 | −179.10 | −151.00 |
| 8 | 3MgO + H2O + 4SiO2 → 3MgO·4SiO2·H2O | −79.62 | −212.77 | −189.03 |
| 9 | MgO + SiO2 + Na2SO4 + 6H2O → MgSO4·6H2O + Na2SiO3 | −181.63 | −36.82 | 17.42 |
| MgO + SiO2 + Na2SO4 + 7H2O → MgSO4·7H2O + Na2SiO3 | −227.69 | −53.7 | 14.27 |
| Time (d) | OPC Mortar Test Block (MPa) | MS Mortar Test Block (MPa) | ||||
|---|---|---|---|---|---|---|
| Maintenance of 56 d | 58.76 | 57.44 | ||||
| Process Started | Water Immersion | Na2SO4 Soak | Compression and Corrosion Resistance Coefficient (Kf) | Water Immersion | Na2SO4 Soak | Compression and Corrosion Resistance Coefficient (Kf) |
| 7 d | 60.32 | 60.86 | 1.01 (±0.08) | 61.82 | 60.32 | 0.97 (±0.08) |
| 14 d | 61.58 | 63.45 | 1.03 (±0.09) | 62.43 | 63.48 | 1.02 (±0.08) |
| 28 d | 64.28 | 69.76 | 1.09 (±0.06) | 62.63 | 63.54 | 1.01 (±0.07) |
| 180 d | 50.25 | 65.73 | 1.31 (±0.07) | 60.19 | 54.91 | 0.85 (±0.08) |
| 270 d | 68.62 | 58.37 | 0.85 (±0.07) | 61.86 | 60.13 | 0.97 (±0.06) |
| Time (d) | OPC Mortar Test Block (MPa) | MS Mortar Test Block (MPa) | ||||
|---|---|---|---|---|---|---|
| Maintenance of 56 d | 58.76 | 57.44 | ||||
| Process Started | Water Immersion | Na2SO4 Soak | Compression and Corrosion Resistance Coefficient (Kf) | Water Immersion | Na2SO4 Soak | Compression and Corrosion Resistance Coefficient (Kf) |
| 15 d | 34.10 | 38.73 | 1.14 (±0.06) | 46.25 | 40.10 | 0.87 (±0.07) |
| 30 d | 39.98 | 57.02 | 1.43 (±0.07) | 43.02 | 27.53 | 0.64 (±0.06) |
| 60 d | 50.80 | 32.24 | 0.63 (±0.08) | 44.48 | 12.89 | 0.29 (±0.08) |
| 90 d | 51.86 | 20.81 | 0.40 (±0.05) | 45.76 | 4.66 | 0.10 (±0.08) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Zou, X.; Jiang, Y.; Zou, Y.; Song, S.; Qin, J.; Wang, Y.; Zhu, L. Study on the Stability of Low-Carbon Magnesium Cementitious Materials in Sulfate Erosion Environments. Materials 2023, 16, 4042. https://doi.org/10.3390/ma16114042
Jia Y, Zou X, Jiang Y, Zou Y, Song S, Qin J, Wang Y, Zhu L. Study on the Stability of Low-Carbon Magnesium Cementitious Materials in Sulfate Erosion Environments. Materials. 2023; 16(11):4042. https://doi.org/10.3390/ma16114042
Chicago/Turabian StyleJia, Yuan, Xinmei Zou, Yaoting Jiang, Yuxin Zou, Shuanglin Song, Jianyun Qin, Yongjing Wang, and Lihua Zhu. 2023. "Study on the Stability of Low-Carbon Magnesium Cementitious Materials in Sulfate Erosion Environments" Materials 16, no. 11: 4042. https://doi.org/10.3390/ma16114042




